Cefiderocol (formerly S-649266) is a novel siderophore-conjugated cephalosporin with activity against a broad array of multidrug-resistant (MDR), aerobic Gram-negative bacilli. The siderophore component binds iron and uses active iron transport for drug entry into the bacterial periplasmic space. The cephalosporin moiety is the active antimicrobial component, structurally resembling a hybrid between ceftazidime and cefepime. Like other β-lactam agents, the principal bactericidal activity of cefiderocol occurs via inhibition of bacterial cell wall synthesis by binding of penicillin-binding proteins (PBPs) and inhibiting peptidoglycan synthesis, leading to cell death.
KEYWORDS: antimicrobial susceptibility testing, broth microdilution, cefiderocol, disk diffusion
ABSTRACT
Cefiderocol (formerly S-649266) is a novel siderophore-conjugated cephalosporin with activity against a broad array of multidrug-resistant (MDR), aerobic Gram-negative bacilli. The siderophore component binds iron and uses active iron transport for drug entry into the bacterial periplasmic space. The cephalosporin moiety is the active antimicrobial component, structurally resembling a hybrid between ceftazidime and cefepime. Like other β-lactam agents, the principal bactericidal activity of cefiderocol occurs via inhibition of bacterial cell wall synthesis by binding of penicillin-binding proteins (PBPs) and inhibiting peptidoglycan synthesis, leading to cell death. Iron concentrations need to be taken into consideration when in vitro antimicrobial susceptibility to cefiderocol is determined. Broth microdilution (BMD) and disk diffusion methods have been developed to determine in vitro activity of cefiderocol. For BMD, cation-adjusted Mueller-Hinton broth (CAMHB) requires iron depletion to provide MICs predictive of in vivo activity. A method to prepare iron-depleted CAMHB (ID-CAMHB) has been described by the Clinical and Laboratory Standards Institute (CLSI). For disk diffusion, standard Mueller-Hinton agar is recommended, presumably because iron is bound in the medium. Currently, clinical FDA and European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints and investigational (research-use-only) CLSI breakpoints exist for interpreting cefiderocol susceptibility results for certain Gram-negative bacilli. Cefiderocol does not have clinically relevant activity against Gram-positive or anaerobic organisms. FDA or EUCAST breakpoints should be applied to interpret results for Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii complex for patient care until the investigational status has been removed from CLSI breakpoints. Further clinical outcome data are required to assess the effectiveness of cefiderocol for treatment of other Acinetobacter species (non-baumannii complex) and Stenotrophomonas maltophilia at this time, and, as such, antimicrobial susceptibility testing of these organisms should be limited to research use in the scenario of limited treatment options.
INTRODUCTION
Internationally, over 700,000 people (including ∼162,000 Americans) die from antimicrobial-resistant infections annually (1). This number is projected to grow to 10 million deaths per year by 2050 (2). Arguably, the greatest antimicrobial resistance threat is that of multidrug-resistant (MDR) Gram-negative bacilli, including carbapenem-resistant Enterobacterales, alongside Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. These organisms have been identified as serious or urgent public health threats by the U.S. Centers for Disease Control and Prevention (CDC) (3).
Recently, several new antimicrobial agents have been approved for treatment of Gram-negative pathogens, including agents within already well-established classes of antimicrobials, such as new β-lactam–β-lactamase inhibitor (BL-BLI) combinations (e.g., ceftazidime-avibactam, ceftolozane-tazobactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam), a new aminoglycoside (plazomicin), and a new tetracycline (eravacycline) (4). These agents have been developed with unique features to overcome some resistance mechanisms in MDR organisms. However, there are gaps in their activity, such as the activity of BL-BLI combinations against some but not all carbapenemases (e.g., meropenem-vaborbactam and ceftazidime-avibactam have activity against class A carbapenemases, with ceftazidime-avibactam having additional activity against class D OXA-48-like carbapenemases, but neither has activity against metallo-β-lactamase [MBL] carbapenemases). Furthermore, the newer non-β-lactam agents have limited activity against non-glucose-fermenting Gram-negative bacilli.
Cefiderocol (formerly S-649266; Shionogi & Co., Ltd.), is a novel siderophore-conjugated cephalosporin with activity against a broad array of Gram-negative bacilli, including MDR strains. Thorough reviews of the chemistry, pharmacokinetics, pharmacodynamics, animal models, and clinical trials on cefiderocol (5, 6) were recently published. The focus of this minireview is on laboratory considerations for antimicrobial susceptibility testing of cefiderocol.
WHAT IS CEFIDEROCOL?
Cefiderocol (commercial names, Fetroja in the United States and Fetcroja in the European Union) is an injectable siderophore cephalosporin approved by the U.S. Food and Drug Administration (FDA) on 14 November 2019 for treatment of complicated urinary tract infections caused by selected Enterobacterales and P. aeruginosa. On 23 April 2020, the European Medicines Agency (EMA) Committee for Medical Products for Human Use (CMPH) authorized cefiderocol for medical use for treatment of infections caused by aerobic Gram-negative bacilli in adults with limited treatment options (7). Recently (28 September 2020), cefiderocol was approved by the FDA for the additional indication for treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia for the Enterobacterales, P. aeruginosa, and Acinetobacter baumannii complex.
Cefiderocol is a siderophore conjugate molecule, a single molecular structure with two distinct roles; a siderophore and a cephalosporin.
The siderophore moiety is the “undercover cloak” that allows the antimicrobial access into the periplasmic space to reach its target, penicillin-binding proteins (PBPs) (8). It exploits a Trojan horse mechanism to enter the periplasmic space using active iron transport by binding iron. Iron is an essential nutrient for bacterial growth, replication, and metabolism, and therefore, bacteria secrete siderophores naturally to scavenge iron from the human host. Iron homeostasis is important for health in humans, with both iron deficiency and overload being associated with morbidity and in some cases increasing susceptibility to infection (9). Iron bound to the siderophore component of cefiderocol utilizes iron transport systems in Gram-negative bacilli to gain entry into the periplasmic space (10), where the iron atom disassociates from the siderophore and crosses the cytoplasmic membrane into the cytoplasm, leaving cefiderocol in the periplasmic space, where the cephalosporin can apply its antimicrobial effects (6).
The cephalosporin component of cefiderocol is the active antimicrobial component and structurally resembles a hybrid between ceftazidime and cefepime, with cefiderocol differing by the presence of a catechol group on the side chain at position 3, responsible for binding iron (6). Like other β-lactam agents, the principal bactericidal activity of cefiderocol occurs by inhibiting bacterial cell wall synthesis by binding PBPs, primarily PBP3 (similar to other oxyimino-cephalosporins), and inhibiting peptidoglycan synthesis, causing cell death (6).
WHAT MAKES CEFIDEROCOL DIFFERENT FROM OTHER β-LACTAM AGENTS?
The spectrum of activity of cefiderocol differs from that of most β-lactam agents. Cefiderocol has activity against aerobic Gram-negative bacilli but does not demonstrate clinically relevant activity against Gram-positive or anaerobic organisms. The lack of activity against anaerobes may be partially explained by a lower reliance on siderophore-iron transporter systems for growth under anaerobic conditions (11). Thus, it is thought that cefiderocol does not enter such cells as efficiently. The mechanism behind the lack of activity against Gram-positive organisms is currently unknown.
That said, cefiderocol has the ability to overcome most common mechanisms of β-lactam resistance among Gram-negative bacilli, including porin deficiency, upregulation of efflux pump expression, and production of β-lactamases, including carbapenemases. As mentioned above, cefiderocol gains access into the cell primarily through active iron transport, whereas other available β-lactams rely solely on passive diffusion through porin-mediated channels (12). Exploiting iron transport allows high concentrations of the drug to be delivered to the site of cephalosporin action and allows it to overcome porin-mediated resistance mechanisms that other β-lactam agents may be susceptible to (e.g., carbapenem resistance in P. aeruginosa due to OprD porin deficiency). Furthermore, cefiderocol is relatively stable to nearly all β-lactamase enzymes, including both serine- and metallo-β-lactamases, providing a broad spectrum of activity. The bulkier chlorocatechol side chain at position C-3 of cefiderocol is thought to provide steric hindrance to β-lactamase binding and reduce its capacity to enter efflux pumps (11, 13). This observation is based on circumstantial evidence in relation to differences in activity (i.e., MICs) between cefiderocol, structural analogues, and similar agents (e.g., ceftazidime and cefepime) when assessed against isolates with upregulated efflux, reduced porins, or the presence of various β-lactamases. As the agent is a poor substrate for efflux pumps, it can overcome efflux-mediated resistance.
IN VITRO ACTIVITY OF CEFIDEROCOL
Cefiderocol has in vitro activity against carbapenem-resistant Gram-negative bacilli, including serine- and metallo-β-lactamase carbapenemase-producing organisms and MDR non-glucose-fermenting Gram-negative bacilli, such as P. aeruginosa, A. baumannii, and S. maltophilia (14–17).
In testing of all Enterobacterales, P. aeruginosa, A. baumannii, and S. maltophilia, the MIC required to inhibit growth of 90% of organisms (MIC90) was found to be ≤2 μg/ml based on surveillance studies from 2014 to 2016 (Table 1). However, high MICs (128 to >256 μg/ml) were observed for some isolates of A. baumannii, Enterobacter cloacae, Proteus mirabilis, Providencia rettgeri, and Morganella morganii (14, 18, 19). When testing was limited to meropenem-resistant organisms, a ≥4-fold increase was observed for the Enterobacterales. For MDR (i.e., resistant to meropenem, amikacin, and ciprofloxacin) P. aeruginosa and A. baumannii, MIC90s were 4- to 8-fold higher when samples were enriched for resistant versus susceptible isolates. However, in systematic surveillance studies where the subset of non-meropenem-susceptible isolates was evaluated, a ≤2-fold difference in MIC90 was observed for P. aeruginosa and A. baumannii (17, 18). Further, the MIC90s for extended-spectrum-β-lactamase (ESBL)-producing Gram-negative bacilli were 2- to 4-fold higher than for non-ESBL-producing Gram-negative bacilli, and New Delhi metallo-β-lactamase (NDM)-producing Enterobacterales had a MIC90 of 8 μg/ml; although the MIC90 was elevated among MDR Gram-negative bacilli, cefiderocol was more active than ceftolozane-tazobactam, ceftazidime-avibactam, and colistin (18, 20, 21). Several recent studies reaffirmed the potent activity of cefiderocol against MDR Gram-negative organisms with various mechanisms of resistance or among high-risk clones (20–23).
TABLE 1.
Cefiderocol MICs required to inhibit the growth of 90% of Gram-negative bacilli (MIC90)a
| Organism(s)b | No. of isolates tested | MIC (μg/ml) |
|
|---|---|---|---|
| Range | MIC90 | ||
| Enterobacterales | |||
| Escherichia coli | 5,139 | ≤0.002–8 | 0.5 |
| Meropenem not-susceptible E. coli | 72 | 0.015–4 | 2 |
| Klebsiella pneumoniae | 4,627 | ≤0.002–8 | 1 |
| Meropenem not-susceptible K. pneumoniae | 689 | 0.004–32 | 4 |
| Klebsiella oxytoca | 1,434 | ≤0.002–4 | 0.25 |
| Meropenem not-susceptible K. oxytoca | 31 | 0.03–4 | 1 |
| Enterobacter cloacae | 1,800 | ≤0.002–128 | 1 |
| Klebsiella (formerly Enterobacter) aerogenes | 1,017 | ≤0.002–8 | 0.5 |
| Meropenem not-susceptible Enterobacter speciesc | 159 | 0.06–32 | 8 |
| Citrobacter freundii complex | 931 | ≤0.002–8 | 0.5 |
| Citrobacter koseri | 517 | 0.008–8 | 0.5 |
| Meropenem not-susceptible Citrobacter species | 32 | 0.015–8 | 2 |
| Serratia marcescens | 2,382 | ≤0.002–32 | 0.5 |
| Meropenem not-susceptible S. marcescens | 38 | 0.015–4 | 2 |
| Proteus mirabilis | 819 | ≤0.002–>256 | 0.12 |
| Proteus vulgaris | 537 | ≤0.002–0.5 | 0.12 |
| Providencia rettgeri | 341 | ≤0.002–>256 | 0.12 |
| Morganella morganii | 697 | ≤0.002–8 | 0.25 |
| Non-glucose-fermenting Gram-negative bacilli | |||
| Pseudomonas aeruginosa | 4,942 | ≤0.002–8 | 0.5 |
| MDR P. aeruginosa | 262 | 0.002–32 | 2 |
| Acinetobacter baumannii | 2,896 | ≤0.002–>256 | 2 |
| MDR A. baumannii | 368 | 0.015–>256 | 8 |
| Stenotrophomonas maltophilia | 1,173 | ≤0.002–64 | 0.25 |
| MDR S. maltophilia | 218 | 0.015–>256 | 0.25 |
Adapted from reference 19.
The not-susceptible category includes isolates that tested intermediate and resistant. MDR, multidrug resistant, i.e., resistant to meropenem, amikacin, and ciprofloxacin.
Includes Klebsiella aerogenes.
MECHANISMS OF RESISTANCE TO CEFIDEROCOL
Although resistance to cefiderocol among Gram-negative bacilli remains low (<10%, depending on the organism, breakpoints applied, and study), a few large surveillance programs (SIDERO-WT and SIDERO-CR) have found that A. baumannii and Enterobacterales are the most common among a small proportion of total organisms to demonstrate elevated cefiderocol MICs (>4 μg/ml) (14, 15, 18, 24). Molecular characterization of these isolates has shown resistance in A. baumannii to be associated with the ESBLs PER (Pseudomonas-extended resistant) and VEB (Vietnam extended‐spectrum β‐lactamase) and resistance among E. cloacae and K. pneumoniae to be associated with the metallo-β-lactamase NDM (17). When these isolates were tested in combination with a BLI (avibactam for serine β-lactamases and dipicolinic acid for MBL), the cefiderocol MICs decreased, supporting a role for β-lactamase production in elevated cefiderocol MICs (17). Importantly, although these β-lactamases likely contribute to cefiderocol resistance, the presence of these enzymes on their own might be insufficient to cause resistance, as some isolates harboring these mechanisms are susceptible to cefiderocol.
Elevated MICs of cefiderocol have been reported for S. maltophilia and P. aeruginosa (Table 1). In an experimental frequency-of-resistance study, development of resistance in P. aeruginosa was found to be lower for cefiderocol than ceftazidime; cefiderocol resistance was reported to occur due to mutations in iron transport-related genes (pvdS and fecI), including the regulation of pyoverdine (25). To date, the mechanism(s) of resistance to cefiderocol among S. maltophilia isolates has not been defined.
Multiple types of studies have demonstrated a high barrier to the development of resistance to cefiderocol (19). That being said, multiple mechanisms of mutation-derived resistance to cefiderocol have been reported and include mutations in genes associated with (i) siderophore regulation and synthesis, (ii) iron uptake, (iii) two-component regulatory systems, and (iv) penicillin-binding proteins (6, 19). Furthermore, a recent study demonstrated structural changes in the AmpC β-lactamase (AmpC R2 loop deletion) of E. cloacae, resulting in reduced susceptibility to both cefiderocol and ceftazidime-avibactam (26).
WHAT ANTIMICROBIAL SUSCEPTIBILITY TESTING METHODS ARE AVAILABLE TO CLINICAL LABORATORIES?
Cefiderocol relies on active iron transport for entry into the periplasm to access PBPs. Iron transporters are upregulated under iron-depleted conditions as occurs in vivo, and this is likely advantageous for the agent’s in vivo activity (17). As such, iron concentrations in media also need to be taken into consideration when the in vitro susceptibility of cefiderocol is being determined, in order to mimic the in vivo state to accurately predict efficacy.
Broth microdilution (BMD) and disk diffusion methods have been developed to determine in vitro activity for cefiderocol. Development of gradient diffusion strips for cefiderocol and the addition of the agent to automated antimicrobial susceptibility testing (AST) panels have proven more difficult and have not yet been achieved (as of October 2020). However, MIC test strips (Liofilchem, Abruzzi, Italy), Etests (bioMérieux, Marcy l'Etoile, France), and automated AST panels with cefiderocol added (e.g., MicroScan, Vitek, and Phoenix) are under development. Agar dilution susceptibility testing results do not match those of broth microdilution, and therefore, agar dilution is not recommended (27).
Standard cation-adjusted Mueller-Hinton medium is not controlled for iron concentration, and iron concentrations may vary by manufacturer. For BMD, cation-adjusted Mueller-Hinton broth (CAMHB) requires iron depletion to provide reproducible MICs to predict in vivo activity (17). Initial studies presented to the Clinical and Laboratory Standards Institute (CLSI) demonstrated higher MICs when cefiderocol was tested with standard CAMHB (>0.03 μg/ml iron) than with iron-depleted media (≤0.03 μg/ml iron); the reason for this is thought to be that iron transport and accordingly uptake of cefiderocol are increased in iron-depleted states (28, 29). This finding is recapitulated in the study by Albano et al. for Enterobacterales, Burkholderia cepacia complex, A. baumannii, and P. aeruginosa, but interesting not clearly for S. maltophilia (27).
The CLSI has approved iron-depleted CAMHB (ID-CAMHB) and provides instructions as to how to prepare it for determining cefiderocol MICs (see appendix I in reference 30). The CLSI-endorsed ID-CAMHB method supplants all previous medium preparation methods, including the use of Chelex-treated Iso-Sensitest broth, because of the limited number of manufacturers of such media and those using 20 μM human apotransferrin, which resulted in MICs that did not correlate with in vivo efficacy (12, 16, 31). The CLSI method requires first preparing standard CAMHB, then chelating cations with a resin, filtering out the resin, checking the pH, adjusting the pH (if required), and finally reconstituting the noniron cations (i.e., calcium, magnesium, and zinc cations). Iron is not reintroduced, so that the iron concentration is at or below 0.03 μg/ml, creating an iron-depleted medium. This is similar to the concentration of non-transferrin-bound iron in human serum (0.01 μg/ml; the mean level in healthy volunteers [19, 32]). Preparing ID-CAMHB is cumbersome and introduces an additional barrier to commercial manufacturers for inclusion on automated antimicrobial susceptibility testing system panels, which have yet to be developed.
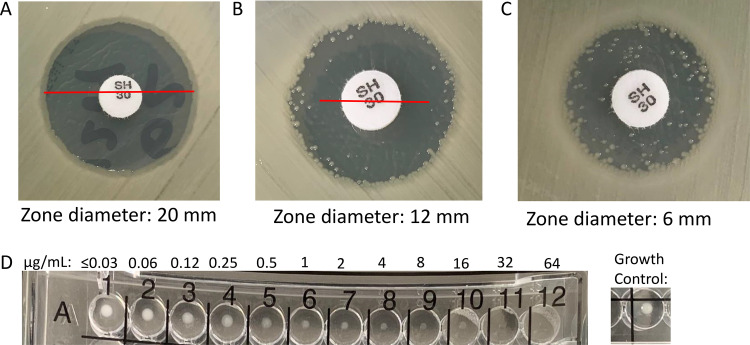
Reading BMD cefiderocol results can also be tricky for certain organisms where trailing may occur. This is most evident with Acinetobacter species, where up to 30% of isolates will demonstrate trailing. Trailing endpoints or unusual growth patterns are not atypical for Acinetobacter spp. and have been previously reported for 33% (64/195) of isolates when beta-lactam AST was carried out by BMD (33). When trailing is observed, it should be ignored. Trailing is characterized as multiple wells of tiny or faint growth relative to the growth control. The MIC should be read as the first well where growth is significantly reduced compared to the positive growth control (Fig. 1). A recent study (31) demonstrated reproducibility of the ID-CAMHB method by using CAMHB from 3 manufacturers to test 19 clinical isolates of Gram-negative bacilli (including 9 A. baumannii isolates) over 10 replicates. When analyzed by individual medium lot, more than 95% of MIC results were within one doubling dilution. When all medium lots were combined, 92.2% of MIC results were within one doubling dilution and 99.8% were within two doubling dilutions (31).
FIG 1.
Nuances of disk diffusion (30 μg) and broth microdilution (performed using iron-depleted cation-adjusted Mueller-Hinton broth) interpretations of cefiderocol susceptibility testing. (A) Clear zone of inhibition (20 mm); (B) zone of inhibition with colonies near the outer zone of inhibition (colony-free zone diameter, 12 mm); (C) zone of inhibition with colonies growing throughout the zone of inhibition (zone diameter, 6 mm); (D) Acinetobacter baumannii demonstrating the trailing effect (MIC, 2 μg/ml). The MIC should be read as the first well where growth is significantly reduced. The growth levels in wells A7 to A10 demonstrate trailing and are considered significantly reduced compared to the growth control well.
Thermo Fisher Scientific has developed a lyophilized Sensititre BMD Gram-negative panel to include cefiderocol, which received FDA clearance on 9 March 2020. Plates are reconstituted with standard CAMHB with a proprietary chelator added to the cefiderocol-containing wells to chelate the iron in the medium, obviating the need to prepare and use ID-CAMHB for reconstitution of the cefiderocol wells.
In contrast to BMD, disk diffusion methods for cefiderocol (30-μg disk content) have been developed to be performed on standard Mueller-Hinton agar (MHA). MHA with various concentrations of iron (0.03 to 10 μg/ml) was assayed to determine cefiderocol zone diameters, with only small variations in results (29). It is thought that iron is sufficiently bound in the agar, mimicking an iron-depleted state, and does not interfere with determining reproducible zone diameter results (34). As such, iron depletion of MHA is not required; the medium usually contains around 0.5 μg/ml of iron. It has been noted that colonies within the zone of inhibition may occur and that zone diameters should be determined using the colony-free inner zone (Fig. 1) (35). To date (October 2020), there is only one brand of cefiderocol (30 μg) disks (Hardy Diagnostics) that has received U.S. FDA clearance. Liofilchem and MAST (Liverpool, United Kingdom) also have CE-marked disks (30 μg) available for use in the European Union. A recent study demonstrated comparable activity between research-use-only (RUO) MASTDISCS and FDA-cleared HardyDisks, with ≥75% categorical agreement compared to lyophilized BMD panels when a variety of MDR Gram-negative bacilli were tested (23). The categorical agreement ranged from 75% to 90%, with 8 to 25% minor errors, 0 to 19% major errors, and 0 to 20% very major errors and varied based on disk, organisms, and breakpoints evaluated. Most major errors and very major errors occurred when the EUCAST breakpoints were applied, due to the lack of an intermediate category, whereas the lowest categorical agreement was obtained with the now obsolete FDA breakpoints, driven by a high number of minor errors. Categorical agreement was highest for carbapenem-resistant Enterobacterales (84 to 91%) compared to MDR non-glucose fermenters (36 to 90%), across FDA, EUCAST, and CLSI interpretative criterion recommendations, with reduced agreement largely attributable to A. baumannii complex isolates (23). Disk diffusion is likely to be the most practical method applied by clinical laboratories until cefiderocol is included on commercially available automated AST system panels that are currently still under development.
Once a method is chosen for AST, verification must be completed prior to implementing the AST device for clinical care. Furthermore, if an FDA-cleared device is applied outside the uses defined in the package insert or reviewed by the FDA 510K submission (i.e., for organisms that do not have FDA breakpoints or were not evaluated in the diagnostic clinical trial), further validation is required to establish the performance characteristics. The CDC & FDA AR Isolate Bank is currently working on adding the cefiderocol results to already established organism panels so that laboratories that acquired a relevant panel previously may not need to request a new panel for verification of cefiderocol AST devices (D. M. Sievert, personal communication) (36).
HOW SHOULD THE ANTIMICROBIAL SUSCEPTIBILITY TESTING RESULTS FOR CEFIDEROCOL BE INTERPRETED?
Currently, clinical FDA and European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints and investigational CLSI breakpoints exist for interpreting cefiderocol susceptibility results for certain Gram-negative bacilli (Table 2) (30, 37, 38). CLSI published investigational MIC and associated disk zone diameter breakpoints prior to FDA approval of the agent for the Enterobacterales, P. aeruginosa, Acinetobacter species, and S. maltophilia. Investigational breakpoints are breakpoints used for research use only when there are no other therapies available for a severely ill patient. Investigational breakpoints should not be used for interpretation of AST results for routine clinical care purposes.
TABLE 2.
U.S. FDA, EUCAST, and investigational CLSI cefiderocol breakpointsa
| Organism | MIC breakpoint (μg/ml) |
Disk zone diameter breakpoint (mm) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Investigational CLSI MIC (21) |
Obsolete FDA clinical (38)b
|
Current FDA clinical (38)e
|
EUCAST |
Investigational CLSI (21) |
Obsolete FDA (38)b
|
Current FDA (38)e
|
EUCAST |
|||||||||||||||
| S | I | R | S | I | R | S | I | R | S | R | S | I | R | S | I | R | S | I | R | S | R | |
| Enterobacteralesc | ≤4 | 8 | ≥16 | ≤2 | 4 | ≥8 | ≤4 | 8 | ≥16 | ≤2 | >2 | ≥16 | 12–15 | ≤11 | ≥18 | 14–17 | ≤13 | ≥16 | 9–15 | ≤8 | ≥22 | <22 |
| P. aeruginosa | ≤4 | 8 | ≥16 | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 | ≤2 | >2 | ≥18 | 13–17 | ≤12 | ≥25 | 19–24 | ≤18 | ≥22 | 13–21 | ≤12 | ≥22 | <22 |
| Acinetobacter sp. | ≤4 | 8 | ≥16 | ≤1 | 2 | ≥4 | ≤2c | >2c | ≥15 | 11–14 | ≤10 | ≥19 | 12–18 | ≤11 | ≥17d | —d | ||||||
| S. maltophilia | ≤4 | 8 | ≥16 | ≤2c | >2c | ≥17 | 13–16 | ≤12 | ≥20d | —d | ||||||||||||
S, susceptible; I, intermediate; R, resistant.
Accessed 13 February 2020. These breakpoints are now considered obsolete as revised breakpoints were published on 28 September 2020. The FDA breakpoints for the Enterobacterales (listed as Enterobacteriaceae on the FDA website) are specific for Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae complex in patients with complicated urinary tract infections (cUTI).
EUCAST set non-species-specific PK/PD breakpoints of ≤2 μg/ml for S and >2 μg/ml for R that can be applied to Acinetobacter baumannii and S. maltophilia.
EUCAST provided disk correlates associated with the susceptible PK/PD breakpoint for A. baumannii and S. maltophilia. Although the PK/PD breakpoint for S is set at ≤2 μg/ml, there were no S. maltophilia isolates tested by EUCAST with MICs of >0.5 μg/ml. Thus, the disk correlate for S. maltophilia is for isolates with MICs of ≤0.5 μg/ml.
Accessed 25 October 2020. The FDA breakpoints were revised on 28 September 2020 and included breakpoints for Acinetobacter baumannii complex. The Enterobacterales (listed as Enterobacteriaceae on the FDA website) are further specified for Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae complex, and Serratia marcescens in patients with hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia.
In November 2019, the FDA approved the agent for treatment of complicated urinary tract infections (19). Additional clinical trials have been completed for the treatment of carbapenem-resistant Gram-negative bacillus infections (CREDIBLE-CR trial) and nosocomial pneumonia caused by Gram-negative bacilli (APEKS-NP trial) (6). Initially, Shionogi proposed the same breakpoints as the CLSI investigational breakpoints to the FDA, but the FDA advisory review panel had concerns over mortality data at lower MICs for particular organisms (i.e., A. baumannii, S. maltophilia, and P. aeruginosa). The FDA panel was unsure if the mortality results represented a true safety signal or if they represented noise due to the small sample size and the severity of infections of patients enrolled in the study (39). As such, the breakpoints set by the FDA were more conservative than the investigational CLSI breakpoints and were initially limited to the Enterobacterales and P. aeruginosa (19). Not surprisingly, a few studies have noted higher rates of cefiderocol not-susceptible results applying FDA over CLSI breakpoints (applying the now obsolete breakpoints) (21, 23). Based on additional clinical trial data generated by the CREDIBLE-CR and APEKS-NP trials, the FDA breakpoints were revised (28 September 2020) when the agent was approved for the additional indications for treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. This resulted in changes to the breakpoints for the Enterobacterales and P. aeruginosa and the addition of breakpoints for Acinetobacter baumannii complex. Of note, the FDA breakpoints for the Enterobacterales (listed as Enterobacteriaceae on the FDA website) are specific for E. coli, K. pneumoniae, P. mirabilis, Serratia marcescens, and E. cloacae complex. If laboratories apply the FDA Enterobacterales breakpoints outside the listed organisms (e.g., Klebsiella oxytoca and Citrobacter species), consideration should be given to adding a comment advising the use of off-label breakpoints for that organism.
EUCAST recently published an addendum to their breakpoint tables (version 10.0) with MIC and disk breakpoints for cefiderocol, which are yet again different from CLSI and FDA breakpoints (Table 2). Similar to the initial FDA breakpoints, EUCAST set clinical breakpoints for the Enterobacterales and P. aeruginosa only. The EUCAST committee felt there was insufficient evidence to support a clinical breakpoint for A. baumannii and S. maltophilia. However, EUCAST set non-species-specific pharmacokinetic-pharmacodynamic (PK-PD) MIC breakpoints that can be applied to interpret results for A. baumannii and S. maltophilia, among other difficult-to-treat aerobic, Gram-negative bacilli. Additionally, EUCAST provided disk correlates associated with the susceptible non-species-specific PK-PD breakpoint for A. baumannii and S. maltophilia (37).
As CLSI set the provisional breakpoints with limited clinical outcome data, the FDA or EUCAST breakpoints should be applied by laboratories until CLSI re-evaluates the data with the expanded clinical outcomes. The breakpoints will be revisited by CLSI and at a minimum will have the investigational status removed; a revision is expected in 2021 (J. Lewis, personal communication).
WHICH ORGANISMS SHOULD BE CONSIDERED FOR CEFIDEROCOL ANTIMICROBIAL SUSCEPTIBILITY TESTING?
Because cefiderocol is a new agent, and because of the increased emphasis on antimicrobial stewardship to limit the development of resistance, this agent should be reserved for treatment of MDR Gram-negative bacilli, including Enterobacterales and non-glucose fermenters. This is in line with the FDA and EMA approval of the agent for treatment caused by cefiderocol-susceptible Gram-negative organisms, in patients who have limited or no alternative treatment options. Ultimately (as with all other antimicrobials), the initiative to test and report cefiderocol is a decision best made by each laboratory in consultation with the clinical practice (e.g., infectious diseases leadership, pharmacy, and antimicrobial stewardship team).
SUMMARY
In summary, cefiderocol is a new catechol-substituted siderophore cephalosporin with activity against a broad range of MDR Gram-negative bacilli, including serine and MBL carbapenemase producers and non-glucose fermenters. To date, an iron-depleted CAMHB BMD, a lyophilized BMD panel, and disk diffusion methods are available for antimicrobial susceptibility testing of cefiderocol. FDA or EUCAST breakpoints should be applied to interpret results for the Enterobacterales, P. aeruginosa, and A. baumannii complex for patient care until the investigational status has been removed from CLSI breakpoints. Further clinical outcome data are required to assess the effectiveness of cefiderocol for treatment of Acinetobacter species (non-baumannii complex) and S. maltophilia at this time, and as such, AST of these organisms should be limited to research use only in the scenario of limited treatment options.
ACKNOWLEDGMENTS
Patricia J. Simner served as an advisory board member as part of a microbiology panel for Shionogi Inc. Patricia J. Simner reports grants and personal fees from Accelerate Diagnostics, OpGen Inc. and BD Diagnostics, grants from bioMérieux, Inc., Affinity Biosensors, and Hardy Diagnostics, and personal fees from Roche Diagnostics, outside the submitted work. Patricia J. Simner receives travel reimbursement from ASM, CAP, and CLSI. Robin Patel reports grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics, Ltd., and Shionogi. Robin Patel reports grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, ContraFect, TenNor Therapeutics, Ltd., and Shionogi. Robin Patel is a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST and Qvella; monies are paid to Mayo Clinic. In addition, Robin Patel has a patent on Bordetella pertussis/Bordetella parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Robin Patel receives travel reimbursement from ASM and IDSA, an editor’s stipend from IDSA, and honoraria from the NBME, Up-to-Date and the Infectious Diseases Board Review Course.
Footnotes
For companion articles on this topic, see https://doi.org/10.1128/JCM.01649-20 and https://doi.org/10.1128/JCM.00966-20.
REFERENCES
- 1.Burnham JP, Olsen MA, Kollef MH. 2019. Re-estimating annual deaths due to multidrug-resistant organism infections. Infect Control Hosp Epidemiol 40:112–113. doi: 10.1017/ice.2018.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Neil J. 2016. Tackling drug-resistant infections globally: final report and recommendations. The Review on Antimicrobial Resistance.
- 3.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
- 4.Doi Y. 2019. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis 69:S565–S575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JJ, McCarthy MW. 2018. Cefiderocol: a novel siderophore cephalosporin. Expert Opin Invest Drugs 27:193–197. doi: 10.1080/13543784.2018.1426745. [DOI] [PubMed] [Google Scholar]
- 6.Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel MA, Lagace-Wiens PRS, Walkty AJ, Noreddin A, Lynch Iii JP, Karlowsky JA. 2019. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs 79:271–289. doi: 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 7.European Medicine Company. 2020. Fetcroja. https://www.ema.europa.eu/en/medicines/human/EPAR/fetcroja#overview-section. Accessed July 2020.
- 8.Simner PJ, McAdam AJ. 2020. What's special about cefiderocol? A micro-comic strip. J Clin Microbiol 58:e00256-20. doi: 10.1128/JCM.00256-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MGP. 2019. The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin Infect Dis 69:S529–S537. doi: 10.1093/cid/ciz825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. 2017. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller-Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 88:198–200. doi: 10.1016/j.diagmicrobio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. 2017. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 62:e01454-17. doi: 10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, Rhomberg PR, Jones RN, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother 71:670–677. doi: 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Yamawaki K. 2019. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 69:S538–S543. doi: 10.1093/cid/ciz826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. 2019. In vitro activity of defiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int J Antimicrob Agents 53:456–466. doi: 10.1016/j.ijantimicag.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Kazmierczak KM, Tsuji M, Wise MG, Hackel M, Yamano Y, Echols R, Sahm DF. 2019. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-beta-lactamase-producing isolates (SIDERO-WT-2014 study). Int J Antimicrob Agents 53:177–184. doi: 10.1016/j.ijantimicag.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of a siderophore cephalosporin, s-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamano Y. 2019. In vitro activity of cefiderocol against a broad range of clinically important Gram-negative bacteria. Clin Infect Dis 69:S544–S551. doi: 10.1093/cid/ciz827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 62:e01968-17. doi: 10.1128/AAC.01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shionogi, Inc. 2019. Cefiderocol FDA briefing document. https://www.fda.gov/media/131703/download.
- 20.Delgado-Valverde M, Conejo MDC, Serrano L, Fernandez-Cuenca F, Pascual A. 2020. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother 75:1840–1849. doi: 10.1093/jac/dkaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden AR, Adam HJ, Baxter M, Walkty A, Lagace-Wiens P, Karlowsky JA, Zhanel GG. 2020. In vitro activity of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacilli isolated from patients in Canadian intensive care units. Diagn Microbiol Infect Dis 97:115012. doi: 10.1016/j.diagmicrobio.2020.115012. [DOI] [PubMed] [Google Scholar]
- 22.Iregui A, Khan Z, Landman D, Quale J. 2020. Activity of cefiderocol against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii endemic to medical centers in New York City. Microb Drug Resist 97:722–726. doi: 10.1089/mdr.2019.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris CP, Bergman Y, Tekle T, Fissel J, Tamma PD, Simner PJ. 2021. Cefiderocol antimicrobial susceptibility testing against multidrug-resistant Gram-negative bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol 59:e01649-20. doi: 10.1128/JCM.01649-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother 61:e00093-17. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito A, Nishikawa Ishii R, Kuroiwa M, Kurihara N, Sakikawa I, Ota T, Rokushima M, Tsuiji M, Sato T, Yamano Y. 2018. Mechanisms of cefiderocol high MIC mutants obtained in non-clinical studies, abstr 696. IDWeek 2018, San Francisco, CA.
- 26.Kawai A, McElheny CL, Iovleva A, Kline EG, Sluis-Cremer N, Shields RK, Doi Y. 2020. Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother 64:e00198-20. doi: 10.1128/AAC.00198-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albano M, Karau MJ, Schuetz AN, Patel R. 2020. Comparison of agar dilution to broth microdilution for testing in vitro activity of cefiderocol against Gram-negative bacilli. J Clin Microbiol 59:e00966-20. doi: 10.1128/JCM.00966-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2017. June, 2017 AST agenda summary minutes. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing; thirtieth informational supplement. M100-S30 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 31.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2019. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn Microbiol Infect Dis 94:321–325. doi: 10.1016/j.diagmicrobio.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki K, Ikuta K, Tanaka H, Ohtake T, Torimoto Y, Fujiya M, Kohgo Y. 2011. Improved quantification for non-transferrin-bound iron measurement using high-performance liquid chromatography by reducing iron contamination. Mol Med Rep 4:913–918. doi: 10.3892/mmr.2011.518. [DOI] [PubMed] [Google Scholar]
- 33.Swenson JM, Killgore GE, Tenover FC. 2004. Antimicrobial susceptibility testing of Acinetobacter spp. by NCCLS broth microdilution and disk diffusion methods. J Clin Microbiol 42:5102–5108. doi: 10.1128/JCM.42.11.5102-5108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley IA, Basker MJ. 1988. Conventional laboratory agar media provide an iron‐limited environment for bacterial growth. FEMS Microbiology Lett 50:35–39. doi: 10.1111/j.1574-6968.1988.tb02907.x. [DOI] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2019. June, 2019 AST agenda summary minutes. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 36.Centers for Disease Control and Prevention. 2020. CDC & FDA Antibiotic Resistance (AR) Isolate Bank. CDC, Atlanta, GA Accessed 30 April 2020.
- 37.EUCAST. 2020. Breakpoints for cefiderocol from EUCAST. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf.
- 38.U.S. Food and Drug Administration. 2019. Cefiderocol injection. https://www.fda.gov/drugs/development-resources/cefiderocol-injection. Accessed 13 February 2020.
- 39.Food and Drug Administration Center for Drug Center for For Drug Evaluation and Research. 2019. Final summary minutes of the Antimicrobial Drugs Advisory Committee Meeting. https://www.fda.gov/media/133853/download. Accessed July 2020.