As microbial genomics makes increasingly important contributions to clinical and public health microbiology, the interpretation of whole-genome sequence data by nonspecialists becomes essential. In the absence of capsule-based vaccines, two protein-based vaccines have been used for the prevention of invasive serogroup B meningococcal disease (IMD) since their licensure in 2013 and 2014. These vaccines have different components and different levels of coverage of meningococcal variants. Hence, decisions regarding which vaccine to use in managing serogroup B IMD outbreaks require information about the index case isolate, including (i) the presence of particular vaccine antigen variants, (ii) the expression of vaccine antigens, and (iii) the likely susceptibility of its antigen variants to antibody-dependent bactericidal killing.
KEYWORDS: meningococcal disease, Neisseria meningitidis, vaccines, Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR), meningococcal antigen typing system (MATS), meningococcal antigen surface expression (MEASURE) assay, serum bactericidal activity assay, outbreaks, whole-genome sequencing, public health
ABSTRACT
As microbial genomics makes increasingly important contributions to clinical and public health microbiology, the interpretation of whole-genome sequence data by nonspecialists becomes essential. In the absence of capsule-based vaccines, two protein-based vaccines have been used for the prevention of invasive serogroup B meningococcal disease (IMD) since their licensure in 2013 and 2014. These vaccines have different components and different levels of coverage of meningococcal variants. Hence, decisions regarding which vaccine to use in managing serogroup B IMD outbreaks require information about the index case isolate, including (i) the presence of particular vaccine antigen variants, (ii) the expression of vaccine antigens, and (iii) the likely susceptibility of its antigen variants to antibody-dependent bactericidal killing. To obtain this information requires a multitude of laboratory assays, impractical in real-time clinical settings, where the information is most urgently needed. To facilitate assessment for public health and clinical purposes, we synthesized genomic and experimental data from published sources to develop and implement the Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index, which is publicly available on PubMLST (https://pubmlst.org). Using whole-genome sequences or individual gene sequences obtained from IMD isolates or clinical specimens, the MenDeVAR Index provides rapid evidence-based information on the presence and possible immunological cross-reactivity of different meningococcal vaccine antigen variants. The MenDeVAR Index enables practitioners who are not genomics specialists to assess the likely reactivity of vaccines for individual cases, outbreak management, or the assessment of public health vaccine programs. The MenDeVAR Index has been developed in consultation with, but independently of, both the 4CMenB (Bexsero; GSK) and rLP2086 (Trumenba; Pfizer, Inc.) vaccine manufacturers.
INTRODUCTION
Microbial whole-genome sequencing (WGS) has advanced our understanding of microbial evolution, diversity, and pathogenicity. Since the first bacterial genome was sequenced in 1995, the technology has developed from dideoxynucleotide terminator (Sanger) sequencing to multiplexed WGS platforms (1–3). Concomitantly, the cost of WGS has decreased substantially, increasing its availability and affordability worldwide; however, DNA sequencing itself is a first step, with multidisciplinary expertise required to exploit these complex large data sets to address particular questions and translate the results to public health action. Genomic technologies are increasingly incorporated into public health and clinical microbiology laboratories, where identifying and typing microorganisms are critical to informing infectious disease management in individuals and populations. Extracting information from genomic data is important, but it is equally important to communicate these data promptly and effectively to relevant practitioners (4). Here, we describe a generalizable framework for assimilating sequence data with phenotypic information, linking genotype to phenotype with the results presented in an easy-to-understand format for use by nonspecialists.
Invasive meningococcal disease (IMD), caused by Neisseria meningitidis, is a serious infection with significant mortality and morbidity (5, 6). Diagnosis of IMD is either through bacterial culture and capsular group serotyping or, in the absence of culture, by PCR testing, with additional discrimination provided by characterization of capsule-encoding and protein antigen-encoding genes (7). IMD generally occurs sporadically, but it can occur in clusters and outbreaks, due to the transmission of hyperinvasive meningococcal variants generally or among individuals living in closed or semiclosed communities, such as schools, universities, military barracks, and extended households. Increasingly, real-time WGS of meningococcal isolates can direct public health investigations and interventions.
Prevention of IMD is possible by immunization, delivered either by routine programs or in response to clusters or outbreaks. When they occur, such outbreaks are a public health priority, requiring the rapid identification of individuals at high risk from the meningococcal variant identified in the index case. Prophylactic antibiotics are provided to close contacts to prevent outbreak strain transmission, and vaccination is offered where appropriate (8). While highly immunogenic conjugate protein-polysaccharide vaccines are available against invasive meningococci expressing capsular serogroups A, C, W, and Y (9), there are none against serogroup B meningococci, which are a major cause of IMD outbreaks and clusters in many countries. In 2013 and 2014, two protein-based meningococcal vaccines were licensed to assist in the prevention of serogroup B IMD. The particular protein antigens contained in the two vaccines, 4CMenB (Bexsero; GSK) and rLP2086 (Trumenba; Pfizer, Inc.), were different and not specific to serogroup B meningococci. These antigens also displayed immunologically significant protein sequence diversity (10, 11). Therefore, the two vaccines exhibit different degrees of possible protection against heterologous vaccine antigens, and consequently, there could be a need for frontline clinical and public health specialists to assess each vaccine rapidly in the context of a particular scenario, to inform decisions about vaccine implementation.
Using WGS to provide clinically applicable information requires systematic and reproducible characterization of genetic variation. Multilocus sequence typing (MLST), based on housekeeping genes, is the most widely used approach to characterizing bacterial variants, facilitating communication among laboratories internationally and the identification of hyperinvasive meningococci (12). Typing of bacterial genetic diversity of medically important features, such as polysaccharide capsules (13, 14), antimicrobial resistance genes (15), and vaccine antigens (16), can be achieved through similar gene-by-gene approaches (17). For example, the Bexsero antigen sequence typing (BAST) scheme was established to characterize and describe vaccine antigen variants, using data derived through WGS or sequencing of individual genes (16).
Both vaccines contain factor H binding protein (fHbp): one recombinant peptide variant in Bexsero (peptide 1) and two native lipidated peptide variants in Trumenba (peptides 45 and 55) (11). Bexsero also contains the recombinant proteins neisserial heparin-binding antigen (NHBA [peptide 2]) and Neisseria adhesin A (NadA [peptide 8]), combined with the PorA-containing (variable region 2 [VR2]; peptide 4) outer membrane vesicle from the MeNZB vaccine (10). The BAST scheme catalogues peptide presence/absence and variation, using deduced peptide sequences, but cannot infer protein expression or cross-reactivity. The meningococcal antigen typing system (MATS) laboratory assay was devised to estimate the proportion of diverse serogroup B disease strains prevented by Bexsero, by assessing protein expression and cross-reactivity (18); however, MATS is not widely or immediately available in clinical settings and is time- and resource-intensive. Genetic MATS (gMATS) was developed to predict Bexsero strain coverage using sequence and phenotypic MATS data. At the time of writing, this algorithm was not available on an accessible, integrated platform for genome sequence data analysis, nor had it been updated to accommodate the description of additional variants (19).
To perform genomic vaccine antigen analysis comprehensively requires an understanding of sequencing technology, genomic data quality control, and gene/peptide curation and analysis. As of mid-2020, these skills were developing among health care scientists/clinicians, but were far from universal (4). Given the need to assess breadth of vaccine reactivity and to ensure genomic data are harnessed to maximize clinical and public health benefit, we developed the Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index, publicly accessible on the PubMLST Neisseria website (20). By synthesizing published, peer-reviewed, experimental data with sequence data, the MenDeVAR Index provides a means for public health and clinical practitioners to extract easily understood, relevant information from genomic data in real time.
MATERIALS AND METHODS
Vaccine antigen typing.
Allele-based typing schemes for each of the antigens included in Bexsero and Trumenba have been published. The BAST scheme was developed as a multilocus, rapid, and scalable method to catalogue deduced peptide diversity of meningococcal vaccine antigens (16). The scheme includes five peptide components contained in the Bexsero vaccine: fHbp, NHBA, NadA, and PorA VR1 and VR2. Typing of Trumenba vaccine antigen fHbp was available with cross-referencing to the subfamily A and B nomenclature on the PubMLST Neisseria website (21, 22). Novel peptide variants are curated in real time after submission to PubMLST; these curated databases form the basis of the MenDeVAR Index.
Literature search.
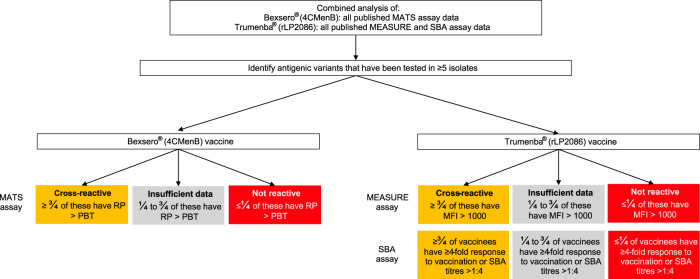
Determining the extent to which either protein-based vaccine is protective against a given meningococcus requires an assessment for each vaccine component of the protein sequence variant present, its surface expression, its likely recognition by vaccine-induced antibodies, and finally the likelihood of bactericidal killing of the meningococcus in the presence of vaccinee serum. These factors were assessed using published experimental studies for each vaccine. For Bexsero, the MATS assay was used, which was established to assess the breadth of vaccine coverage to diverse meningococcal strains (18, 23). MATS determines the antigenic variants of fHbp, NHBA, and NadA through sandwich enzyme-linked immunosorbent assay (ELISA) and their reactivity to pooled toddler serum (postvaccination with three doses and booster), based on a collection of reference strains tested in serum bactericidal activity (SBA) assays. For Trumenba, the meningococcal antigen surface expression (MEASURE) assay (24), a flow cytometric measurement of fHbp surface expression, was used. Additionally, SBA assays using serum from individuals immunized with Trumenba (2 or 3 doses on various dosing schedules) were included, as there was only one vaccine antigen. Only antigens tested in these assays were analyzed as contributing to a cross-protective vaccine effect for the MenDeVAR Index (Fig. 1).
FIG 1.
The Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index algorithm used to identify which antigens are included as cross-reactive in the combined analysis of published experimental data from the meningococcal antigen typing system (MATS) (18), the meningococcal antigen surface expression (MEASURE) assay (24), and the serum bactericidal activity (SBA) assay (27). RP, relative potency; PBT, positive bactericidal threshold; MFI, mean fluorescence intensity.
For Bexsero, a literature search using the terms “meningococcal antigen typing system,” “Neisseria meningitidis,” and “vaccine” on 14 May 2020 yielded 44 studies published in English. There were 13 studies eligible for assessment (see Table S1 in the supplemental material), pertaining to capsular group B IMD isolates (MATS is only validated for serogroup B), with data of sufficient detail to assess individual antigens and their predicted vaccine coverage. For Trumenba, a literature search using the terms “meningococcal antigen surface expression (MEASURE) assay,” “Neisseria meningitidis,” and “vaccine” on 14 May 2020 yielded 12 studies published in English. One study contained MEASURE assay data for individual antigenic variants (see Table S2 in the supplemental material). Additionally, a literature search using the terms “serum bactericidal activity assay,” “Neisseria meningitidis,” “vaccine,” and “bivalent” on 14 May 2020 yielded 28 studies published in English. Fifteen studies contained data to assess individual antigenic variants and their likelihood of providing protection using SBA assays (Table S2).
Criteria for defining cross-reactive antigens in the MenDeVAR Index.
To index the experimental data, thresholds were determined to define antigenic variants as either likely cross-reactive or not cross-reactive, and the proportion of isolates with a given antigenic variant considered covered/protected through experimental assays was calculated. For each assay (MATS, MEASURE, and SBA), thresholds previously defined by the developers or the research community were employed. For the MATS assay, an antigenic variant was considered “covered” (i.e., would be susceptible to a vaccine-induced immune response) where the relative potency (RP) was greater than the positive bactericidal threshold (PBT) (18). For the MEASURE assay, an antigenic variant was considered “covered” if the mean fluorescent intensity (MFI) was >1,000 (24). For the SBA assay, antigenic variants were assessed through host immunogenicity, resulting in likely protection from infection. The accepted serological measure indicating likely protection by immunization with meningococcal vaccines is either a ≥4-fold rise in antibody titers between pre- and postvaccination sera or a titer of >1:4 (25, 26). From the combined analysis of the experimental studies, if an antigenic variant had been tested in ≥5 isolates and ≥3/4 of them were covered/protected, then the variant was considered cross-reactive (amber). If an antigenic variant had been tested in ≥5 isolates, and ≥3/4 of them were not covered/protected, then the variant was considered not cross-reactive (red) (Fig. 1).
Development of data visualization for the MenDeVAR Index.
For ease of data presentation, a red, amber, green “traffic light” data interpretation was employed: “green” was assigned to meningococcal variants with ≥1 antigenic vaccine variant, based on exact peptide sequence match, “amber” was assigned to isolates with ≥1 antigenic variant demonstrated as cross-reactive in experimental studies, and “red” was assigned to isolates where all antigenic variants were not exact matches and had been shown to not elicit cross-reactivity to vaccine variants. The designation “gray” was assigned to variants possessing antigenic variants untested in experimental assays at the time of writing or where such tests did not meet the threshold chosen to indicate cross-reactivity. The MenDeVAR Index status of the variants, especially those designated “gray” will be updated in light of the above criteria as further published information becomes available.
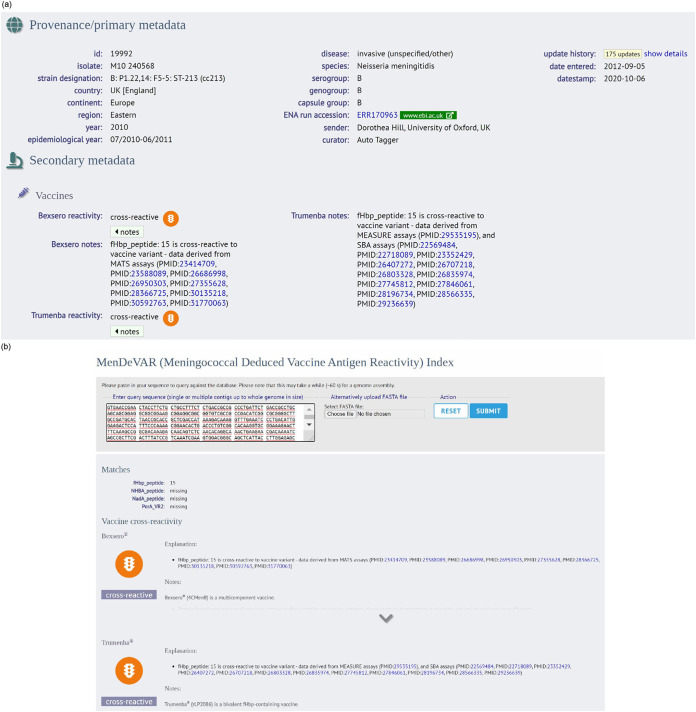
The MenDeVAR Index was implemented on the PubMLST Neisseria website on the isolate record (Fig. 2a). In addition, WGS data or individual gene sequences can be used to make a direct query on https://pubmlst.org/bigsdb?db=pubmlst_neisseria_mendevar, which outputs the MenDeVAR Index result, without the need to create isolate records or upload WGS data to the database (Fig. 2b). A written description is provided to aid those with color vision deficits, where “green” means “exact,” “amber” means “cross-reactive,” “red” means “none,” and gray means “insufficient data.” Additional supporting information is provided: (i) the antigenic determinant of the reactivity index, (ii) the assay used to determine cross-reactivity, (iii) specific references to studies including those antigens, and (iv) caveats to interpretation (Table 1). The MenDeVAR Index was also integrated with the BAST scheme (16), so the index appears as part of the BAST profile on the isolate record. Users can also perform a batch profile query selecting the BAST scheme that outputs the MenDeVAR Index (https://pubmlst.org/bigsdb?db=pubmlst_neisseria_seqdef&l=1&page=batchProfiles).
FIG 2.
(a) The Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index as it appears on the isolate record page of the https://pubmlst.org/neisseria/ website. The provenance data show the PubMLST ID no. is 19992 and state that this is a serogroup B meningococcal isolate from the eastern region of the United Kingdom, collected from invasive disease in 2010. The MenDeVAR Index is shown under the secondary metadata heading and shows this isolate contains cross-reactive antigens for both vaccines, with fHbp peptide 15 the antigen used to determine this through the MATS assay for Bexsero and the MEASURE and SBA assays for Trumenba; the reference is shown with the PubMed ID no. (PMID). (b) The Web interface to search using the genome sequence, individual genes, or whole-genome data to output the MenDeVAR Index.
TABLE 1.
The caveats that are listed on the PubMLST Neisseria website when interpreting the MenDeVAR Indexa
| Parameter | Caveat(s) for both vaccines (unless otherwise stated) |
|---|---|
| Source of data | These data combine multiple sources of information, including peptide sequence identity through whole-genome sequencing, experimental assays developed as indirect measures of the breadth of vaccine protection against diverse meningococci, and assays developed to assess immunogenicity. |
| Protein expression | We have not inferred protein expression from genomic data; therefore, there may be isolates that possess genes but do not express the protein in vivo. |
| Cross-reactivity definition | An antigenic variant was considered cross-reactive if it had been tested in ≥5 isolates/subjects and was above the accepted threshold in ≥3/4 of those isolates. This was established through combined analysis of published experimental studies (PubMLST ID no. [PMID] provided for each variant), not from genomic data. |
| Meningococcal isolate source | These assays were based on serogroup B disease isolates for both vaccines. |
| Experimental assays | |
| Bexsero | MATS assay |
| Trumenba | MEASURE assay, SBA assay |
| Age of vaccinees | |
| Bexsero vaccine | For MATS assay development, Bexsero vaccine recipients were infants who had received 3 doses of vaccine and then a booster at 12 mo. |
| The pooled sera used for the MATS assay were taken from the toddlers at 13 mo of age. | |
| Trumenba vaccine | The age of vaccine recipients in the experimental studies varies widely, ranging from toddlers to adults, and needs to be taken into consideration when interpreting results. |
| Vaccine studies used different schedules and doses of vaccines. |
MenDeVAR, Meningococcal Deduced Vaccine Antigen Reactivity; MATS, meningococcal antigen typing system; MEASURE, meningococcal antigen surface expression; SBA, serum bactericidal activity.
Case studies.
To exemplify the application of the MenDeVAR Index, two published IMD outbreaks/clusters were analyzed: an IMD outbreak among a semiclosed, Irish Traveller community from 2010 to 2013 (27) and a university IMD cluster in the United States in 2016 (28). Both WGS data available through PubMLST and published antigenic variants determined through WGS were examined.
RESULTS
Cross-reactive vaccine antigens.
For Bexsero vaccine, MATS studies (29–41) were identified through literature searches. With the exception of two studies (34, 40) that used a PBT for fHbp of 0.012, all other antigen RPs were assessed against PBTs of 0.021 for fHbp, 0.294 for NHBA, and 0.009 for NadA (18). For each antigenic variant of fHbp, NHBA, and NadA, the proportion of isolates with RP > PBT was calculated. For fHbp, there were 139 peptides examined by MATS assay, 28 (20.1%) tested in ≥5 isolates. For NHBA, there were 110 peptides, 30 (27.3%) tested in ≥5 isolates. For NadA, there were 22 peptides, 5 (22.7%) tested in ≥5 isolates; peptide 8 is an exact sequence match but has been experimentally tested in only 3 isolates to date. For Trumenba vaccine, each antigen tested by the MEASURE assay in one study (24) was evaluated. For fHbp, there were 9 peptides examined by MEASURE assay, 6 of which were tested in ≥5 isolates (Table 2). From SBA studies (42–56), there were 23 fHbp peptides examined by SBA assay, 23 (100.0%) tested in ≥5 isolates; peptide 55 is an exact sequence match but has not been experimentally tested to date.
TABLE 2.
Vaccine antigen variants for the protein-based meningococcal vaccines Bexsero (4CMenB) and Trumenba (rLP2086) and their designation by the MenDeVAR Indexa
Vaccine antigen variants for the protein-based meningococcal vaccines Bexsero (4CMenB) and Trumenba (rLP2086) are designated by color by the Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index as follows: green, exact matches to the sequence variants; amber, cross-reactive in experimental studies; red, not cross-reactive in experimental studies; gray, insufficient data. fHbp, factor H binding protein; NHBA, neisserial heparin-binding antigen; NadA, Neisseria adhesin A; PorA VR2, porin A variable region.
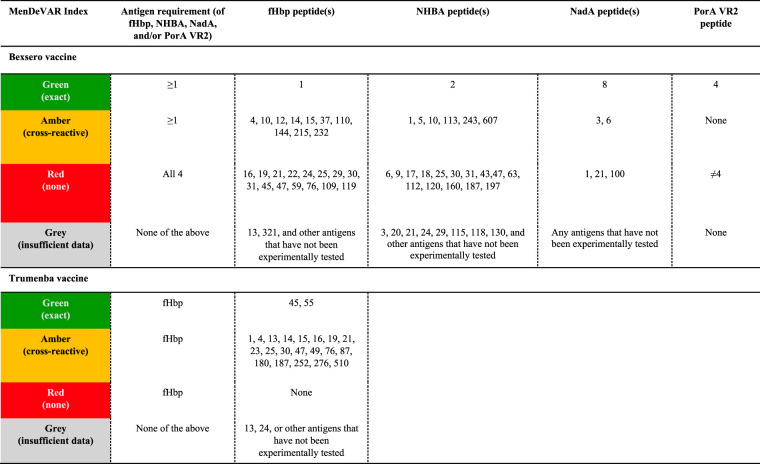
Antigenic variants that did not meet either the cross-reactive or not cross-reactive threshold were designated “gray,” indicating that insufficient data were available to make an assessment for this variant. This included (i) variants tested in ≥5 isolates, with between 1/4 and 3/4 covered/protected (Table 2), (ii) variants tested in <5 isolates (for Bexsero vaccine, 111 fHbp peptides, 80 NHBA peptides, and 17 NadA peptides, and for Trumenba vaccine, 3 fHbp peptides tested by MEASURE assay), or (iii) variants not tested in experimental assays.
Designation of isolates with the MenDeVAR Index.
A meningococcal variant was designated “green” if it contained a ≥1 exact sequence match to the vaccine antigenic variants. For Bexsero, this corresponded to fHbp peptide 1, NHBA peptide 2, NadA peptide 8, and PorA VR2 4 (16, 57). Similarly, for Trumenba, this corresponded to fHbp peptide 45 or 55 (11) (Table 2). The “amber” designation was used if a meningococcus contained ≥1 antigenic variant deemed cross-reactive from experimental studies from any of the fHbp, NHBA, or NadA peptides (Table 2). PorA peptides are not considered cross-reactive (58). Finally, the “red” designation was used for meningococci where none of the antigens present were exact matches with the vaccine antigens and the antigen variants had been shown experimentally not to cross-react with antibodies elicited by the vaccine (Table 2).
MenDeVAR Index: exemplar case studies. (i) Irish Traveller community outbreak.
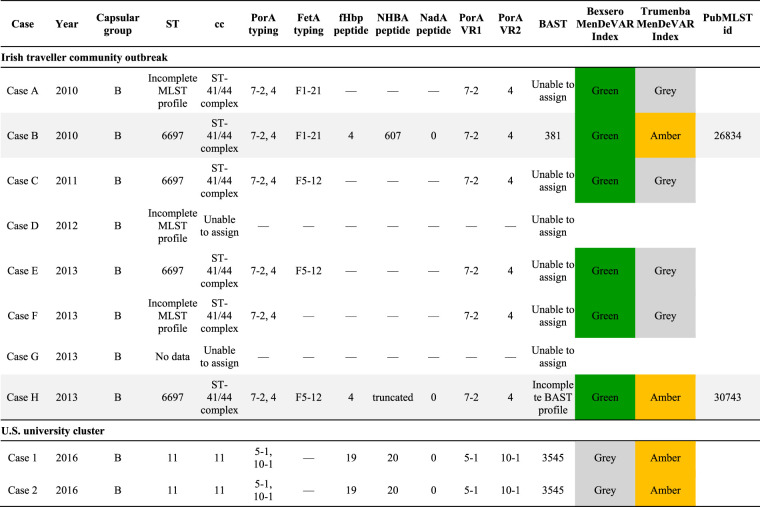
Retrospective analysis of a published IMD outbreak in the Republic of Ireland from 2010 to 2013 (27) exemplified the potential use of the MenDeVAR Index in the context of a community outbreak, where a variety of clinical specimens were available. A total of eight cases were identified over 42 months (Table 3). The initial meningococcus, from case A, was not cultured, but identification and typing data were acquired by PCR amplification and sequencing of MLST loci and fine-typing of antigen-encoding genes porA and fetA. PorA VR2 antigen 4 was present, an exact peptide sequence match to Bexsero. There were insufficient data to inform the use of Trumenba, which contains only fHbp proteins. At the time of identification, case A was considered to be a sporadic case and the appropriate public health action was antibiotic prophylaxis for close contacts. Using the MenDeVAR Index, the disease-associated meningococcus would have been designated “green” for Bexsero and “gray” for Trumenba. Of the seven cases subsequently linked to this case, only two were successfully cultured and underwent WGS (cases B and H), but five could have a MenDeVAR Index inferred from fine-typing of antigen PorA, with respect to Bexsero (Table 3). Additional molecular fHbp typing of isolates would inform the use of Trumenba, in a setting where the PorA is not variant 4. These data identified 75% (6/8) of isolates, two with WGS, with sufficient information to designate as MenDeVAR Index “green” for Bexsero and two WGS isolates with “amber” for Trumenba (Table 3).
TABLE 3.
Two examples of outbreak/clusters from published literature, showing the molecular typing data used to determine the MenDeVAR Indexa
MenDeVAR, Meningococcal Deduced Vaccine Antigen Reactivity; ST, sequence type; cc, clonal complex; PorA VR, porin A variable region; FetA, enterobactin receptor FetA; fHbp, factor H binding protein; NHBA, neisserial heparin-binding antigen; NadA, Neisseria adhesin A; BAST, Bexsero antigen sequence type; —, no data.
(ii) U.S. university cluster.
A cluster of IMD occurring in the United States in 2016 (28) was examined to demonstrate the use of the MenDeVAR Index in an institutional outbreak. In this cluster, two undergraduate students at a New Jersey university were diagnosed with serogroup B IMD, with meningococci isolated from the cerebrospinal fluid of both (28). These isolates were examined in real time by WGS through the local public health department, and both were sequence type 11 (clonal complex 11) and indistinguishable (Table 3). Antigenic variant data provided in the publication were assessed, which provided data equivalent to those obtained by determining the antigenic variants through PCR and sequencing, if WGS had not been available. The meningococci causing the cluster harbored fHbp variant 2 peptide 19, an antigen that is cross-reactive with Trumenba (amber) but not cross-reactive with Bexsero (red). The meningococcal cluster strains also had (i) no nadA gene present (“red”), (ii) PorA 10-1 (“red”), and (iii) NHBA peptide 20, for which there is insufficient data to determine cross-reactivity with confidence (“gray”). The MenDeVAR Index therefore designated these isolates “amber” for Trumenba and “gray” for Bexsero, the latter based solely on the NHBA variant present, with the remaining antigens “red.” This information could have directed public health specialists to using Trumenba early after IMD cluster definition was met, preventing delays in health protection interventions, including mass vaccination campaigns, frequently required in university settings.
DISCUSSION
As bacterial genome sequencing has become increasingly accessible, the prospect of using genomic data for the benefit of public and individual health has become a reality. This opportunity is, however, fraught with challenges, including (i) the large and complex genomic data sets involved, (ii) the expertise required to understand the uses and limitations of WGS technologies, (iii) the increasing number and complexity of analysis tools, (iv) the requirement for skills with command line interfaces, (v) insufficient bioinformatics or genomic epidemiology training among health care practitioners and scientists, and (vi) the diversity of the information sources that need to be integrated.
Genome sequence data provide information on the presence or absence of genes associated with clinically relevant phenotypes: e.g., antibiotic susceptibility, pathogenicity, or vaccine antigens. The first step in exploiting this information is to extract relevant data for the identification of the genes and the protein variants they encode (typing). The second step is to index these types to the relevant phenotypic data. The third step is to present the result in an accessible format for non-genomics specialists to inform clinical decision-making. Here, we demonstrated the MenDeVAR Index, which combines these steps into a system for rapid, real-time assessment of protein-based meningococcal vaccine antigens for public health and clinical microbiology application.
The epidemiology of IMD varies geographically. Sporadic cases occur in countries where IMD is endemic, with clusters and outbreaks associated with high-density living conditions, such as universities, military, or travelling communities (59). Endemic and hyperendemic serogroup B IMD is problematic in many industrialized regions (60), and in the absence of group B polysaccharide vaccines, protein-based vaccines (10, 11) have been developed. When IMD clusters or outbreaks emerge, it is essential to identify contacts and implement public health interventions rapidly. These include antibiotics and vaccinations, the latter, especially, requiring timely serogroup determination of the outbreak strain to ensure deployment of the appropriate vaccine (8). For serogroup B outbreaks, characterization of peptide antigens is required to assess whether vaccination with Bexsero and/or Trumenba is likely to prevent disease (8). At the time of writing, this assessment was only possible using the laboratory assays established during the clinical development of these vaccines to assess their breadth of antigenic coverage, namely, the MATS, MEASURE, and SBA assays (18, 24, 26). These assays, however, required growth of the causative isolate, were confined to reference laboratories in a limited number of countries, and were time-consuming and expensive to perform (28, 61). Consequently, they could not be relied upon to inform timely public health interventions. At the same time, WGS has become increasingly accessible to microbiology laboratories, often in real time or near real time. Furthermore, where meningococcal cultures were not available, PCR of fine-typing and fHbp antigens provided information that complements the phenotypic data compiled within the MenDeVAR Index. Interpreted by local microbiologists and epidemiologists in the context of other pertinent information, the MenDeVAR Index offers a pragmatic assessment of likely susceptibility of outbreak strains to vaccine-induced immunity, based on published data.
For the development of the MenDeVAR Index, robust, pragmatic criteria were used to assess the weight of evidence of potential antigenic cross-reactivity from four different sources. The SBA titer remained the accepted immune correlate of protection for assessing meningococcal vaccine efficacy; however, the SBA assay cannot be performed for routine IMD case isolates investigated as part of a public health response for many reasons, including the availability of expertise, resources, time, human complement, and infant sera. The use of MATS and MEASURE assays, as means of assessing the breadth of antigenic coverage, generated the best data available. Data from MEASURE assays, however, were limited at the time of assessment, and the MATS assay was suggested to provide a conservative estimate compared to the SBA titer (36, 38, 41). SBA data were not included for Bexsero, which as a multicomponent vaccine could induce multiple antibody responses. Although the gMATS assay also used genotypic predictors of MATS phenotype and predicted cross-reactivity in agreement with the MenDeVAR Index using similar criteria (fHbp peptides 1, 4, 10, 12, 14, 15, 37, 110, 144, 215, 224, and 232; NHBA peptides 1, 5, 10, 113, 243, 607) (19), the gMATS assay was only applicable to one of the two available protein-based vaccines. Moreover, it excluded NadA antigens as predictors, included some unpublished data, and had not been updated. The MenDeVAR Index can assist public health and microbiology specialists by compiling and indexing the complex data available in the published evaluation of hundreds of meningococcal antigenic variants—a total of 29 studies at the time of writing. The MenDeVAR Index is accessible through a user-friendly webpage (https://pubmlst.org/bigsdb?db=pubmlst_neisseria_mendevar) that facilitates the submission of WGS data as single or multiple contigs or as part of an isolate record on the PubMLST Neisseria website.
The case studies explored here demonstrated how the MenDeVAR Index can be used as outbreaks developed, with the Irish outbreak showing how multiple types of information can be used effectively. Had the MenDeVAR Index been available at the time, it would have supported the use of the Bexsero vaccine in this outbreak setting. The U.S. university cluster demonstrated the difficulties faced by public health specialists in combining complex data sets from multiple sources in real time to inform intervention strategies. This cluster was investigated by U.S. Centers for Disease Control and Prevention, and the isolates were sent for laboratory testing at U.S. universities, which is not a routine procedure. These analyses identified relatively low fHbp protein expression and low binding of NHBA peptide 2 antisera to the outbreak strain, suggesting reduced likelihood of bactericidal killing (28). Based on these data, along with additional information about persistence of antibody responses postvaccination, immunization of ∼35,000 university students with Trumenba was recommended. The public health team acknowledged that WGS data indicated the presence or absence of particular antigenic variants, which could be compared to the respective vaccine antigens. When variants were not exact sequence matches, however, there was no additional information available to indicate potential cross-protection offered by the vaccine. In the case of this cluster, the MenDeVAR Index would have supported the use of Trumenba solely on the basis of WGS data.
There are limitations to using the MenDeVAR Index, as it is based on WGS data linked to information from published in vitro MATS, MEASURE, and SBA serological studies (Table 1). These assays are not perfect surrogates of protection for a variety of reasons, including the age groups used to establish the assays and the provenance of the isolates used in their development. Furthermore, at the time of writing, the expression of the antigens could not be reliably inferred or predicted from WGS data, although some fHbp promoter and intergenic regions had been correlated with protein expression (62, 63). Finally, the MenDeVAR Index applies only to possible direct protection against IMD, with no information available about possible herd immunity due to the lack of evidence to suggest either vaccine impacted oropharyngeal carriage of serogroup B meningococci (64–66).
In conclusion, we present a generalizable multilocus gene-by-gene framework for interpreting complex genomic data sets that can be used by practitioners to address clinical questions in a timely manner. Specifically, the MenDeVAR Index combines genomic and experimental data to provide a rational, evidence-based estimate of the likelihood that either of the meningococcal protein-based vaccines offers protection against a given meningococcus. To ensure broad accessibility, the MenDeVAR Index is implemented with a “red,” “amber,” and “green” interpretive interface that is easy to use and informative for practitioners without expertise in genomic analysis. In the light of new published evidence, the MenDeVAR Index can be regularly reevaluated using the criteria described here, adjusting antigenic variant designations accordingly, to ensure that public health and clinical microbiologists globally benefit from the latest research findings.
Supplementary Material
ACKNOWLEDGMENTS
This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/) sited at the University of Oxford (20). The development of this site has been funded by the Wellcome Trust (grant no. 218205/Z/19/Z) and the European Union. Charlene Rodrigues was supported by a grant from the Thrasher Research Fund.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae RD. Science 269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 3.Forde BM, O'Toole PW. 2013. Next-generation sequencing technologies and their impact on microbial genomics. Brief Funct Genomics 12:440–453. doi: 10.1093/bfgp/els062. [DOI] [PubMed] [Google Scholar]
- 4.Black A, Maccannell DR, Sibley TR, Bedford T. 2020. Ten recommendations for supporting open pathogen genomic analysis in public health. Nat Med 26:832–841. doi: 10.1038/s41591-020-0935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladhani SN, Flood JS, Ramsay ME, Campbell H, Gray SJ, Kaczmarski EB, Mallard RH, Guiver M, Newbold LS, Borrow R. 2012. Invasive meningococcal disease in England and Wales: implications for the introduction of new vaccines. Vaccine 30:3710–3716. doi: 10.1016/j.vaccine.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Viner RM, Booy R, Johnson H, Edmunds WJ, Hudson L, Bedford H, Kaczmarski E, Rajput K, Ramsay M, Christie D. 2012. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol 11:774–783. doi: 10.1016/S1474-4422(12)70180-1. [DOI] [PubMed] [Google Scholar]
- 7.Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol 55:887–896. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 8.Public Health England. 2013. Chapter 22. Meningococcal, p 24 In Ramsay M. (ed), Immunisation against infectious disease. Public Health England, London, United Kingdom. [Google Scholar]
- 9.Finne J, Leinonen M, Makela PH. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 10.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. 2012. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30:B87–B97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H-Q, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, Scott AA, Alexander K, Mason K, Miller L, DaSilva I, Mack M, Zhao X-J, Pride MW, Andrew L, Murphy E, Hagen M, French R, Arora A, Jones TR, Jansen KU, Zlotnick GW, Anderson AS. 2010. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28:6086–6093. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 12.Maiden MCJ, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marjuki H, Topaz N, Rodriguez-Rivera LD, Ramos E, Potts CC, Chen A, Retchless AC, Doho GH, Wang X. 2018. Whole-genome sequencing for characterization of capsule locus and prediction of serogroup of invasive meningococcal isolates. J Clin Microbiol 57:e01609-18. doi: 10.1128/JCM.01609-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croucher NJ, Finkelstein JA, Pelton SI, Mitchell PK, Lee GM, Parkhill J, Bentley SD, Hanage WP, Lipsitch M. 2013. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet 45:656–663. doi: 10.1038/ng.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison OB, Clemence M, Dillard JP, Tang CM, Trees D, Grad YH, Maiden MC. 2016. Genomic analyses of Neisseria gonorrhoeae reveal an association of the gonococcal genetic island with antimicrobial resistance. J Infect 73:578–587. doi: 10.1016/j.jinf.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brehony C, Rodrigues CM, Borrow R, Smith A, Cunney R, Moxon ER, Maiden MC. 2016. Distribution of Bexsero antigen sequence types (BASTs) in invasive meningococcal disease isolates: implications for immunisation. Vaccine 34:4690–4697. doi: 10.1016/j.vaccine.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiden MC, Jansen van Rensburg MJ, Bray JE, Earle SG, Ford SA, Jolley KA, McCarthy ND. 2013. MLST revisited: the gene-by-gene approach to bacterial genomics. Nat Rev Microbiol 11:728–736. doi: 10.1038/nrmicro3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, Muzzi A, Andrews W, Chen J, Santos G, Santini L, Boucher P, Serruto D, Pizza M, Rappuoli R, Giuliani MM. 2010. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A 107:19490–19495. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzzi A, Brozzi A, Serino L, Bodini M, Abad R, Caugant D, Comanducci M, Lemos AP, Gorla MC, Křížová P, Mikula C, Mulhall R, Nissen M, Nohynek H, Simões MJ, Skoczyńska A, Stefanelli P, Taha M-K, Toropainen M, Tzanakaki G, Vadivelu-Pechai K, Watson P, Vazquez JA, Rajam G, Rappuoli R, Borrow R, Medini D. 2019. Genetic meningococcal antigen typing system (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine 37:991–1000. doi: 10.1016/j.vaccine.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 20.Jolley KA, Bray JE, Maiden MCJ. 2018. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res 3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brehony C, Wilson DJ, Maiden MC. 2009. Variation of the factor H-binding protein of Neisseria meningitidis. Microbiology 155:4155–4169. doi: 10.1099/mic.0.027995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. 2004. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RM, Donnelly CA, Gupta S. 1997. Vaccine design, evaluation, and community based use for antigenically variable infectious agents. Lancet 350:1466–1470. doi: 10.1016/S0140-6736(97)03255-8. [DOI] [PubMed] [Google Scholar]
- 24.McNeil LK, Donald RGK, Gribenko A, French R, Lambert N, Harris SL, Jones TR, Li S, Zlotnick G, Vogel U, Claus H, Abad R, Vazquez JA, Borrow R, Findlow J, Taha MK, Deghmane AE, Caugant DA, Kriz P, Musilek M, Wang X, Vuong J, Mayer LW, Pride MW, Jansen KU, Anderson AS. 2018. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rLP2086, a novel prophylactic vaccine. mBio 9:e00036-18. doi: 10.1128/mBio.00036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borrow R, Balmer P, Miller E. 2005. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 23:2222–2227. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 26.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, Miller E, Plikaytis B, van Alphen L, Poolman J, Rappuoli R, Danzig L, Hackell J, Danve B, Caulfield M, Lambert S, Stephens D. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine 24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 27.Mulhall RM, Brehony C, O'Connor L, Meyler K, Jolley KA, Bray J, Bennett D, Maiden MC, Cunney R. 2016. Resolution of a protracted serogroup B meningococcal outbreak with whole genome sequencing shows interspecies genetic transfer. J Clin Microbiol 54:2891–2899. doi: 10.1128/JCM.00881-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeters HM, Dinitz-Sklar J, Kulkarni PA, MacNeil JR, McNamara LA, Zaremski E, Chang HY, Lujan E, Granoff D, Lasky M, Montana B. 2017. Serogroup B meningococcal disease vaccine recommendations at a university, New Jersey, USA, 2016. Emerg Infect Dis 23:867–869. doi: 10.3201/eid2305.161870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abad R, Medina V, Stella M, Boccadifuoco G, Comanducci M, Bambini S, Muzzi A, Vazquez JA. 2016. Predicted strain coverage of a new meningococcal multicomponent vaccine (4CMenB) in Spain: analysis of the differences with other European countries. PLoS One 11:e0150721. doi: 10.1371/journal.pone.0150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R. 2013. The disease burden of invasive meningococcal serogroup B disease in Canada. Pediatr Infect Dis J 32:e20–e25. doi: 10.1097/INF.0b013e3182706b89. [DOI] [PubMed] [Google Scholar]
- 31.Budroni S, Kleinschmidt A, Boucher P, Medini D. 2016. Pooled-sera hSBA titres predict individual seroprotection in infants and toddlers vaccinated with 4CMenB. Vaccine 34:2579–2584. doi: 10.1016/j.vaccine.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Mowlaboccus S, Perkins TT, Smith H, Sloots T, Tozer S, Prempeh LJ, Tay CY, Peters F, Speers D, Keil AD, Kahler CM. 2016. Temporal changes in BEXSERO antigen sequence type associated with genetic lineages of Neisseria meningitidis over a 15-year period in Western Australia. PLoS One 11:e0158315. doi: 10.1371/journal.pone.0158315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulhall RM, Bennett D, Cunney R, Borrow R, Lucidarme J, Findlow J, Jolley KA, Bray J, Maiden MCJ, Moschioni M, Serino L, Stella M, Medini D. 2018. Potential coverage of the 4CMenB vaccine against invasive serogroup B Neisseria meningitidis isolated from 2009 to 2013 in the Republic of Ireland. mSphere 3:e00196-18. doi: 10.1128/mSphere.00196-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh SR, Newbold L, Slater S, Stella M, Moschioni M, Lucidarme J, De Paola R, Giuliani M, Serino L, Gray SJ, Clark SA, Findlow J, Pizza M, Ramsay ME, Ladhani SN, Borrow R. 2017. Meningococcal serogroup B strain coverage of the multicomponent 4CMenB vaccine with corresponding regional distribution and clinical characteristics in England, Wales, and Northern Ireland, 2007–08 and 2014–15: a qualitative and quantitative assessment. Lancet Infect Dis 17:754–762. doi: 10.1016/S1473-3099(17)30170-6. [DOI] [PubMed] [Google Scholar]
- 35.Rajam G, Stella M, Kim E, Paulos S, Boccadifuoco G, Serino L, Carlone G, Medini D. 2017. Meningococcal antigen typing system (MATS)-based Neisseria meningitidis serogroup B coverage prediction for the MenB-4C vaccine in the United States. mSphere 2:e00261-17. doi: 10.1128/mSphere.00261-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, Carannante A, Deghmane AE, Fazio C, Frosch M, Frosi G, Gilchrist S, Giuliani MM, Hong E, Ledroit M, Lovaglio PG, Lucidarme J, Musilek M, Muzzi A, Oksnes J, Rigat F, Orlandi L, Stella M, Thompson D, Pizza M, Rappuoli R, Serruto D, Comanducci M, Boccadifuoco G, Donnelly JJ, Medini D, Borrow R. 2013. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 13:416–425. doi: 10.1016/S1473-3099(13)70006-9. [DOI] [PubMed] [Google Scholar]
- 37.Waśko I, Hong E, De Paola R, Stella M, Moschioni M, Taha M-K, Skoczyńska A. 2016. High predicted strain coverage by the multicomponent meningococcal serogroup B vaccine (4CMenB) in Poland. Vaccine 34:510–515. doi: 10.1016/j.vaccine.2015.11.070. [DOI] [PubMed] [Google Scholar]
- 38.Abad R, Biolchi A, Moschioni M, Giuliani MM, Pizza M, Vazquez JA. 2015. A large portion of meningococcal antigen typing system-negative meningococcal strains from Spain is killed by sera from adolescents and infants immunized with 4CMenB. Clin Vaccine Immunol 22:357–360. doi: 10.1128/CVI.00669-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green LR, Lucidarme J, Dave N, Chan H, Clark S, Borrow R, Bayliss CD. 2018. Phase variation of NadA in invasive Neisseria meningitidis isolates impacts on coverage estimates for 4C-MenB, a MenB vaccine. J Clin Microbiol 56:e00204-18. doi: 10.1128/JCM.00204-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kesanopoulos K, Bratcher HB, Hong E, Xirogianni A, Papandreou A, Taha MK, Maiden MCJ, Tzanakaki G. 2018. Characterization of meningococcal carriage isolates from Greece by whole genome sequencing: implications for 4CMenB vaccine implementation. PLoS One 13:e0209919. doi: 10.1371/journal.pone.0209919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stella M, Giuliani M, Biolchi A, Tomei S, De Paola R, Bai X, Borrow R, Lucidarme J, La Gaetana R, Toneatto D, Pizza M, Serino L, Mori E, Giuliani MM. 2020. Does vaccination with 4CMenB convey protection against meningococcal serogroup B strains not predicted to be covered by MATS? A study of the UK clonal complex cc269. Hum Vaccin Immunother 16:945–948. doi: 10.1080/21645515.2019.1688039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris SL, Donald RG, Hawkins JC, Tan C, O'Neill R, McNeil LK, Perez JL, Anderson AS, Jansen KU, Jones TR. 2017. Neisseria meningitidis serogroup B vaccine, bivalent rLP2086, induces broad serum bactericidal activity against diverse invasive disease strains including outbreak strains. Pediatr Infect Dis J 36:216–223. doi: 10.1097/INF.0000000000001399. [DOI] [PubMed] [Google Scholar]
- 43.Lujan E, Partridge E, Giuntini S, Ram S, Granoff DM. 2017. Breadth and duration of meningococcal serum bactericidal activity in health care workers and microbiologists immunized with the MenB-FHbp vaccine. Clin Vaccine Immunol 24:e00121-17. doi: 10.1128/CVI.00121-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taha M-K, Hawkins JC, Liberator P, Deghmane A-E, Andrew L, Hao L, Jones TR, McNeil LK, O’Neill RE, Perez JL, Jansen KU, Anderson AS. 2017. Bactericidal activity of sera from adolescents vaccinated with bivalent rLP2086 against meningococcal serogroup B outbreak strains from France. Vaccine 35:1530–1537. doi: 10.1016/j.vaccine.2017.01.066. [DOI] [PubMed] [Google Scholar]
- 45.Ostergaard L, Vesikari T, Absalon J, Beeslaar J, Ward BJ, Senders S, Eiden JJ, Jansen KU, Anderson AS, York LJ, Jones TR, Harris SL, O'Neill R, Radley D, Maansson R, Pregaldien JL, Ginis J, Staerke NB, Perez JL, B1971009 and B1971016 Trial Investigators. 2017. A bivalent meningococcal B vaccine in adolescents and young adults. N Engl J Med 377:2349–2362. doi: 10.1056/NEJMoa1614474. [DOI] [PubMed] [Google Scholar]
- 46.Marshall HS, Richmond PC, Beeslaar J, Jiang Q, Jansen KU, Garces-Sanchez M, Martinon-Torres F, Szenborn L, Wysocki J, Eiden J, Harris SL, Jones TR, Lee SS, Perez JL, B1971009 and B1971016 Trial Investigators. 2017. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 17:58–67. doi: 10.1016/S1473-3099(16)30314-0. [DOI] [PubMed] [Google Scholar]
- 47.Muse D, Christensen S, Bhuyan P, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O'Neill RE, Harris SL, Perez JL. 2016. A phase 2, randomized, active-controlled, observer-blinded study to assess the immunogenicity, tolerability and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with tetanus, diphtheria and acellular pertussis vaccine and serogroup A, C, Y and W-135 meningococcal conjugate vaccine in healthy US adolescents. Pediatr Infect Dis J 35:673–682. doi: 10.1097/INF.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 48.Senders S, Bhuyan P, Jiang Q, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O'Neill RE, Harris SL, Ginis J, Perez JL. 2016. Immunogenicity, tolerability and safety in adolescents of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with quadrivalent human papilloma virus vaccine. Pediatr Infect Dis J 35:548–554. doi: 10.1097/INF.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 49.Vesikari T, Wysocki J, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, Harris SL, O'Neill RE, York LJ, Perez JL. 2016. Immunogenicity, safety, and tolerability of bivalent rLP2086 meningococcal group B vaccine administered concomitantly with diphtheria, tetanus, and acellular pertussis and inactivated poliomyelitis vaccines to healthy adolescents. J Pediatr Infect Dis 5:180–187. doi: 10.1093/jpids/piv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiner DM, Bhuyan P, Eiden JJ, Ginis J, Harris S, Jansen KU, Jiang Q, Jones TR, O’Neill RE, York LJ, Perez JL. 2016. Immunogenicity, safety, and tolerability of the meningococcal serogroup B bivalent rLP2086 vaccine in adult laboratory workers. Vaccine 34:809–813. doi: 10.1016/j.vaccine.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Marshall HS, Richmond PC, Nissen MD, Wouters A, Baber J, Jiang Q, Anderson AS, Jones TR, Harris SL, Jansen KU, Perez JL. 2013. A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine 31:1569–1575. doi: 10.1016/j.vaccine.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 52.Vesikari T, Ostergaard L, Diez-Domingo J, Wysocki J, Flodmark CE, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, Harris SL, O'Neill RE, York LJ, Crowther G, Perez JL. 2016. Meningococcal serogroup B bivalent rLP2086 vaccine elicits broad and robust serum bactericidal responses in healthy adolescents. J Ped Infect Dis 5:152–160. doi: 10.1093/jpids/piv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nissen MD, Marshall HS, Richmond PC, Jiang Q, Harris SL, Jones TR, Jansen KU, Perez JL. 2013. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr Infect Dis J 32:364–371. doi: 10.1097/INF.0b013e31827b0d24. [DOI] [PubMed] [Google Scholar]
- 54.Richmond PC, Nissen MD, Marshall HS, Lambert SB, Roberton D, Gruber WC, Jones TR, Arora A. 2012. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 30:6163–6174. doi: 10.1016/j.vaccine.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 55.Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garces-Sanchez M, Martinon-Torres F, Beeslaar J, Szenborn L, Wysocki J, Eiden J, Harris SL, Jones TR, Perez JL. 2012. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 12:597–607. doi: 10.1016/S1473-3099(12)70087-7. [DOI] [PubMed] [Google Scholar]
- 56.Marshall HS, Richmond PC, Nissen MD, Jiang Q, Anderson AS, Jansen KU, Reynolds G, Ziegler JB, Harris SL, Jones TR, Perez JL. 2012. Safety and immunogenicity of a meningococcal B bivalent rLP2086 vaccine in healthy toddlers aged 18–36 months: a phase 1 randomized-controlled clinical trial. Pediatr Infect Dis J 31:1061–1068. doi: 10.1097/INF.0b013e31826327e4. [DOI] [PubMed] [Google Scholar]
- 57.Su EL, Snape MD. 2011. A combination recombinant protein and outer membrane vesicle vaccine against serogroup B meningococcal disease. Expert Rev Vaccines 10:575–588. doi: 10.1586/erv.11.32. [DOI] [PubMed] [Google Scholar]
- 58.Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Hoiby EA, Holst J, Nokleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman JT, Perkins BA. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527. doi: 10.1001/jama.281.16.1520. [DOI] [PubMed] [Google Scholar]
- 59.Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, Echaniz-Aviles G, Hakawi A, Kamiya H, Karachaliou A, Lucidarme J, Meiring S, Mironov K, Safadi MAP, Shao Z, Smith V, Steffen R, Stenmark B, Taha MK, Trotter C, Vazquez JA, Zhu B. 2019. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines 18:15–30. doi: 10.1080/14760584.2019.1557520. [DOI] [PubMed] [Google Scholar]
- 60.Sridhar S, Greenwood B, Head C, Plotkin SA, Safadi MA, Saha S, Taha MK, Tomori O, Gessner BD. 2015. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect Dis 15:1334–1346. doi: 10.1016/S1473-3099(15)00217-0. [DOI] [PubMed] [Google Scholar]
- 61.Clark SA, Lucidarme J, Angel G, Lekshmi A, Morales-Aza B, Willerton L, Campbell H, Gray SJ, Ladhani SN, Wade M, Ramsay M, Yates J, Finn A, Borrow R. 2019. Outbreak strain characterisation and pharyngeal carriage detection following a protracted group B meningococcal outbreak in adolescents in South-West England. Sci Rep 9:9990. doi: 10.1038/s41598-019-46483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biagini M, Spinsanti M, De Angelis G, Tomei S, Ferlenghi I, Scarselli M, Rigat F, Messuti N, Biolchi A, Muzzi A, Anderloni G, Brunelli B, Cartocci E, Buricchi F, Tani C, Stella M, Moschioni M, Del Tordello E, Colaprico A, Savino S, Giuliani MM, Delany I, Pizza M, Costantino P, Norais N, Rappuoli R, Masignani V. 2016. Expression of factor H binding protein in meningococcal strains can vary at least 15-fold and is genetically determined. Proc Natl Acad Sci U S A 113:2714–2719. doi: 10.1073/pnas.1521142113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cayrou C, Akinduko AA, Mirkes EM, Lucidarme J, Clark SA, Green LR, Cooper HJ, Morrissey J, Borrow R, Bayliss CD. 2018. Clustered intergenic region sequences as predictors of factor H binding protein expression patterns and for assessing Neisseria meningitidis strain coverage by meningococcal vaccines. PLoS One 13:e0197186. doi: 10.1371/journal.pone.0197186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marshall HS, McMillan M, Koehler AP, Lawrence A, Sullivan TR, MacLennan JM, Maiden MCJ, Ladhani SN, Ramsay ME, Trotter C, Borrow R, Finn A, Kahler CM, Whelan J, Vadivelu K, Richmond P. 2020. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med 382:318–327. doi: 10.1056/NEJMoa1900236. [DOI] [PubMed] [Google Scholar]
- 65.Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJ, Pollard AJ, Turner DP, Bazaz R, Ganguli A, Havelock T, Neal KR, Okike IO, Morales-Aza B, Patel K, Snape MD, Williams J, Gilchrist S, Gray SJ, Maiden MC, Toneatto D, Wang H, McCarthy M, Dull PM, Borrow R. 2014. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet 384:2123–2131. doi: 10.1016/S0140-6736(14)60842-4. [DOI] [PubMed] [Google Scholar]
- 66.Soeters HM, McNamara LA, Whaley M, Wang X, Alexander-Scott N, Kanadanian KV, Kelleher CM, MacNeil J, Martin SW, Raines N, Sears S, Vanner C, Vuong J, Bandy U, Sicard K, Patel M, Centers for Disease Control and Prevention. 2015. Serogroup B meningococcal disease outbreak and carriage evaluation at a college—Rhode Island, 2015. MMWR Morb Mortal Wkly Rep 64:606–607. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.