Lyme disease is a tick-borne infection caused by the bacteria Borrelia burgdorferi. Current diagnosis of early Lyme disease relies heavily on clinical criteria, including the presence of an erythema migrans rash. The sensitivity of current gold-standard diagnostic tests relies upon antibody formation, which is typically delayed and thus of limited utility in early infection. We conducted a study of blood and skin biopsy specimens from 57 patients with a clinical diagnosis of erythema migrans.
KEYWORDS: Lyme disease, diagnosis, polymerase chain reaction, host response, immunology
ABSTRACT
Lyme disease is a tick-borne infection caused by the bacteria Borrelia burgdorferi. Current diagnosis of early Lyme disease relies heavily on clinical criteria, including the presence of an erythema migrans rash. The sensitivity of current gold-standard diagnostic tests relies upon antibody formation, which is typically delayed and thus of limited utility in early infection. We conducted a study of blood and skin biopsy specimens from 57 patients with a clinical diagnosis of erythema migrans. Samples collected at the time of diagnosis were analyzed using an ultrasensitive, PCR-based assay employing an isothermal amplification step and multiple primers. In 75.4% of patients, we directly detected one or more B. burgdorferi genotypes in the skin. Two-tier testing showed that 20 (46.5%) of those found to be PCR positive remained serologically negative at both acute and convalescent time points. Multiple genotypes were found in three (8%) of those where a specific genotype could be identified. The 13 participants who lacked PCR and serologic evidence for exposure to B. burgdorferi could be differentiated as a group from PCR-positive participants by their levels of several immune markers as well as by clinical descriptors such as the number of acute symptoms and the pattern of their erythema migrans rash. These results suggest that within a Mid-Atlantic cohort, patient subgroups can be identified using PCR-based direct detection approaches. This may be particularly useful in future research such as vaccine trials and public health surveillance of tick-borne disease patterns.
INTRODUCTION
Lyme disease is the most commonly reported tick-borne disease in the United States and is widespread across temperate regions of North America, Europe, and parts of Asia (1). In the United States, the number and geographic distribution of cases has increased steadily since the discovery of Lyme disease in the 1970s (2). Currently, the Centers for Disease Control and Prevention (CDC) estimate over 300,000 new cases of Lyme disease in the United States each year (3). At this time, the states with the highest incidence rates are regionally localized to the Northeast and the upper Midwest, with cases also present along areas of the West Coast (2, 4).
The diagnosis of early Lyme disease and its subsequent treatment are currently guided by clinical signs and symptoms. These criteria in acute disease include the hallmark erythema migrans (EM) skin lesion and/or symptoms such as fever, fatigue, myalgia, and arthralgia, as well as an increased likelihood of exposure to infected Ixodes ticks (5). Although the stereotypical “bullseye” appearance of the EM lesion is most easily recognizable, EM commonly has a wide variety of clinical presentations (6, 7). In addition, EM lesions are not specific to infection with Borrelia burgdorferi and can occur after the bite of Amblyomma americanum (the lone star tick), where they are associated with an idiopathic illness called southern tick-associated rash illness (STARI) (8).
Lyme disease in the United States is caused by Ixodes tick-mediated infection with the spirochete Borrelia burgdorferi sensu stricto (9). B. burgdorferi sensu stricto displays genetic heterogeneity, and many distinct genotypes and serotypes have been identified (10). The genotype/serotypes causing Lyme disease can vary based on geographic location, and associations between infecting genotype and disease virulence have been reported (11–13). Following transmission, B. burgdorferi sensu stricto can disseminate from the initial site of infection in the skin to other areas of the skin as well as to distal sites such as the central nervous system, heart, and joints (1). While dissemination of B. burgdorferi sensu stricto occurs hematogenously, detection of bacteria in blood is difficult because bacteremia is transient only during acute infection and typically occurs with only a small number of organisms present per microliter of blood (14, 15).
Antibody-based tests are frequently used in clinical practice to confirm a diagnosis of early Lyme disease despite significant sensitivity limitations (16, 17). Therefore, there is an increasing need for diagnostics that directly detect the presence of B. burgdorferi sensu stricto in the acute phase of Lyme disease. Direct detection technologies include culture of B. burgdorferi sensu stricto, antigen-based detection, or molecular diagnostics (i.e., DNA/RNA detection) (18). Molecular approaches offer the greatest potential for rapid, sensitive, and specific detection of B. burgdorferi sensu stricto to inform diagnosis and guide treatment. In the current study, we report our experience testing for the presence of B. burgdorferi sensu stricto using PCR and electrospray ionization mass spectrometry (PCR/ESI-MS) (19, 20) on skin and whole-blood specimens from patients diagnosed clinically with EM. In addition, we compare the clinical and immunologic characteristics of patients with and without detectable B. burgdorferi sensu stricto.
MATERIALS AND METHODS
Study participants.
The current study sample drew from a broader, longitudinal cohort study of patients with early Lyme disease. Consecutive, ambulatory study participants were recruited from general internal medicine, primary care, and urgent care settings during the spring, summer, and fall of 2012 to 2015. Patients were eligible to participate if they had a physician-diagnosed EM greater than 5 cm present at enrollment and if they were either antibiotic naive or had started doxycycline treatment less than 72 h prior to enrollment. Patients were excluded if they had a history of Lyme disease, exposure to the Lyme disease vaccine, a history of chronic fatigue syndrome, fibromyalgia, alcohol or drug abuse, major psychiatric illness, or a history of other significant immune-mediated processes such as cancer, HIV, or autoimmune diseases. The study was approved by the Johns Hopkins Medicine Institutional Review Board. Written informed consent was obtained from all participants prior to enrollment into the study.
Clinical characterization.
At the first study visit during acute infection (V1), a 2-mm skin biopsy sample was taken from the leading edge of the EM, and 20 ml of whole blood and serum were obtained. Standard, two-tier (enzyme-linked immunosorbent assay [ELISA] followed by an IgM/IgG Western blot) antibody testing was performed by a single commercial laboratory (Quest Diagnostics). Complete blood count (CBC) and complete metabolic panel (CMP) results were also obtained. Demographic and clinical information were recorded by a trained interviewer. All participants were treated with 3 weeks of doxycycline, considered standard of care for early Lyme disease (5). At the second study visit approximately 3 weeks later (V2) and at a final study visit 6 months later (V3), specimens were collected and repeat antibody testing was performed by the same laboratory. All antibody results were interpreted for overall positivity status using CDC criteria, which incorporates an estimate of disease duration at the time the sample was obtained (21). At V3, a second 2-mm skin biopsy specimen was obtained from approximately the same location as the original biopsy specimen.
Photographs of the EM rash were also taken at V1. These photographs were later reviewed independently by both a nurse practitioner and a research coordinator and evaluated for shape (round, oval, or irregular), color (red or blue/red), pattern (ring within a ring, central darkness, central lightness, or uniform), the presence of vesicles, and the presence of a visible tick bite puncture. Any interrater discordant assessments were resolved by one of the authors (J. N. Aucott).
PCR/ESI-MS.
Nucleic acids from EDTA whole-blood (from V1) specimens were extracted from four 5-ml aliquots as previously described (19). An extraction control was added to each sample as utilized previously (22). DNA extraction from skin biopsy specimens was carried using a modified Qiagen column-based protocol with a bead beating step as previously described (23) with the substitution of the Qiagen DNeasy columns (Qiagen, Valencia, CA) for the Qiagen Virus MinElute columns (Qiagen, Valencia, CA).
The entire volume of extracted nucleic acids was employed in the isothermal amplification step prior to PCR. Both the PCR and isothermal amplification steps target seven B. burgdorferi sensu stricto genome targets (rpoC, rplB, leuS, flaB, ospC, hbb, and gyrB), and the primer sequences and conditions for isothermal amplification were previously described (20). Following isothermal amplification, an aliquot of the DNA was then dispensed into eight broad-range PCRs. PCR cycling conditions and reaction setup, including the addition of a low-copy-number synthetic positive control, were as previously described (10). Following PCR, the amplicons were characterized by electrospray ionization mass spectrometry (PCR/ESI-MS) to detect B. burgdorferi sensu stricto DNA, identify the multilocus genotype, and generate an inferred OspC type as previously described (10).
Based on the PCR/ESI-MS skin biopsy specimen and blood sample results, three groups were generated for the clinical and immunologic analyses. Group 1 was PCR/ESI-MS negative on both skin and blood samples (PCRneg/neg), group 2 had evidence of B. burgdorferi sensu stricto in the skin but it was not found to have disseminated to the blood at a high enough level to be detected (PCRpos/neg), and group 3 was found to be PCR/ESI-MS positive on both skin and blood samples (PCRpos/pos).
Cytokine/chemokine assays.
Multiplex analysis of the following 38 cytokines, chemokines, and acute-phase markers was performed: eotaxin, fibroblast growth factor (FGF) basic, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), human thymus and activation-regulated chemokine (HTARC), gamma interferon (IFN-γ), interleukin 1β (IL-1β), IL-1 receptor α (IL-1rα), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, IL-31, IL-33, interferon-inducible protein of 10 kDa (IP-10), monocyte chemotactic peptide 1 (MCP-1) (monocyte chemotactic and activating factor [MCAF]), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-3β, platelet-derived growth factor ββ (PDGF-ββ), RANTES (regulated on activation, normal T cell expressed and secreted), soluble CD40 L (sCD40L), tumor necrosis factor alpha (TNF-α), and vascular endothelial growth factor (VEGF). Cytokines were measured using Bio-Plex cytokine arrays and the Bio-Plex 200 System (Bio-Rad Laboratories). All tests at the participant level for each specific immune mediator were run by the same system in a single batch. All tests were run as recommended by the manufacturer using previously described optimized assay protocols (24). Data processing was performed using Bio-Plex manager software version 4.4.1, and serum cytokine concentrations were interpolated from standard curves for each respective cytokine.
These data were transformed using the log of the ‘‘ratio to average’’ for ease of interpretation. This was calculated by setting all values less than 1 pg/ml to 1 pg/ml and then calculating the log base 2 of the (value)/(average value in the cohort). As a result, 0 represents an average value, 1 represents a value which is 2 times the average, and 2 represents a value which is 4 times the average.
Statistical analyses.
All analyses were performed using SAS Software (version 9.4; SAS Institute Inc., Cary, NC, USA) or R (version 3.6.1), and all figures were produced using Microsoft Excel (version 2016), Microsoft Power Point (version 2016), or GraphPad Prism (version 8.1.0; GraphPad Software, San Diego, CA, USA). Group comparisons were performed using chi-square or Fisher’s exact tests for categorical variables. For normally distributed continuous variables, group comparisons were performed using t test or one-way analysis of variance tests, and means and standard deviations are presented. For nonnormally distributed continuous variables, group comparisons were performed using Wilcoxon rank sum or Kruskal-Wallis tests, and median (interquartile range [IQR]) [range] is presented. A P value of <0.05 was considered statistically significant.
Data availability.
Any data, metadata, or methods used to replicate findings in this article will be made available upon request in a timely fashion. Please address requests to Alison Rebman (arebman1@jhmi.edu).
RESULTS
Participant cohort.
A total of 57 participants with complete skin and blood PCR/ESI-MS and two-tier serology data were enrolled during the acute infection and diagnosis time frame (V1). Of these 57 participants, 57 (100%) returned at V2, and 47 (82.5%) returned at V3. Of the 47 participants with a V3 visit, 39 (83.0%) provided a second skin biopsy sample. The overall demographics and clinical characteristics of our study sample are shown in Table 1. The mean age was approximately 49 years, and 39.3% were female. The proportion of patients with evidence of dermatologic dissemination (i.e., multiple EM present) and abnormal lymphocyte and liver function tests is similar to those of other previously reported cohorts (25–27). Although participants were allowed to enter the study having initiated doxycycline treatment for early Lyme disease, the majority (41/57 [71.9%]) were antibiotic naive at V1, and none had taken doxycycline for more than 48 h at V1.
TABLE 1.
Demographic and clinical characteristics of 56 participantsa with clinically diagnosed early Lyme disease by PCR/ESI-MS-defined subgroup
| Characteristic | No. (%) of participants with characteristic or value for characteristicb
|
P valuec
|
||||||
|---|---|---|---|---|---|---|---|---|
| All groups (n = 56) | Group 1 PCRneg/neg) (n = 13) | Group 2 (PCRpos/neg) (n = 25) | Group 3 (PCRpos/pos) (n = 18) | Overall | Group 1 vs group 2 | Group 2 vs group 3 | Group 1 vs group 3 | |
| Seasond | ||||||||
| 2012 | 1 (1.8) | 1 (7.7) | 0 (0.0) | 0 (0.0) | ||||
| 2013 | 26 (46.4) | 7 (53.8) | 10 (40.0) | 9 (50.0) | 0.15 | 0.12 | 0.08 | 1.00 |
| 2014 | 22 (39.3) | 3 (23.1) | 14 (56.0) | 5 (27.8) | ||||
| 2015 | 7 (12.5) | 2 (15.4) | 1 (4.0) | 4 (22.2) | ||||
| Age (yr) | 49.1 ± 15.1 | 45.6 ± 18.7 | 48.8 ± 12.5 | 51.9 ± 16.0 | 0.53 | 0.53 | 0.49 | 0.32 |
| Female gender | 22 (39.3) | 6 (46.2) | 8 (32.0) | 8 (44.4) | 0.60 | 0.49 | 0.40 | 0.92 |
| Duration of illness (days) | 5.0 (4.0–10.0) [1.0–60.0] | 5.0 (4.0–6.0) [2.0–18.0] | 6.0 (4.0–10.0) [3.0–60.0] | 6.0 (4.0–13.0) [1.0–21.0] | 0.23 | 0.13 | 0.92 | 0.16 |
| Antibiotic treatment (h) | 0.0 (0.0–2.0) [0.0–48.0] | 0.0 (0.0–0.0)[0.0–36.0] | 0.0 (0.0–14.0)[0.0–36.0] | 0.0 (0.0–0.0)[0.0–48.0] | 0.30 | 0.40 | 0.15 | 0.67 |
| No. of symptoms | 5.0 (3.0–10.0) [0.0–26.0] | 2.0 (0.0–3.0) [0.0–6.0] | 5.0 (3.0–10.0) [0.0–26.0] | 9.5 (4.0–13.0) [0.0–20.0] | 0.0002 | 0.002 | 0.19 | 0.0007 |
| Seropositive | ||||||||
| Acute, V1 | 11 (19.6) | 0 (0.0) | 5 (20.0) | 6 (33.3) | 0.06 | 0.14 | 0.48 | 0.03 |
| Acute or convalescent, V1/V2e | 23 (41.1) | 0 (0.0) | 13 (52.0) | 10 (55.6) | 0.007 | 0.006 | 0.57 | 0.002 |
| Lymphocyte count < 1.1 × 103/μlf | 12/55 (21.8) | 1 (7.7) | 6 (24.0) | 5 (27.8) | 0.37 | 0.39 | 0.73 | 0.20 |
| Elevated liver function testg | 16 (28.6) | 1 (7.7) | 8 (32.0) | 7 (38.9) | 0.15 | 0.13 | 0.64 | 0.10 |
| Saw and/or removed the tick | 4 (7.1) | 2 (15.4) | 1 (4.0) | 1 (5.6) | 0.44 | 0.27 | 1.00 | 0.56 |
One participant in group 1 who was misclassified by the PCR/ESI-MS test was removed from this analysis.
Mean ± standard deviation for normally distributed variables, median (IQR) [range] for nonnormally distributed continuous variables, and n (%) for categorical variables are presented. Groups are distinguished by and labeled with their PCRskin/blood result status.
All P values are two sided.
The one participant enrolled in 2012 was not included in analyses of season by group.
None of the participants who were seronegative at V2 were positive at the V3 visit; therefore, final serology status could be determined using the V1 and V2 time points only.
One participant in group 3 was missing a baseline lymphocyte count.
Elevated liver function test was defined as any one of the following: aspartate aminotransferase above 35 U/liter, alanine transaminase above 40 U/liter, or alkaline phosphatase above 130 U/liter for males and above 115 U/liter for females.
Direct detection of B. burgdorferi sensu stricto by PCR/ESI-MS.
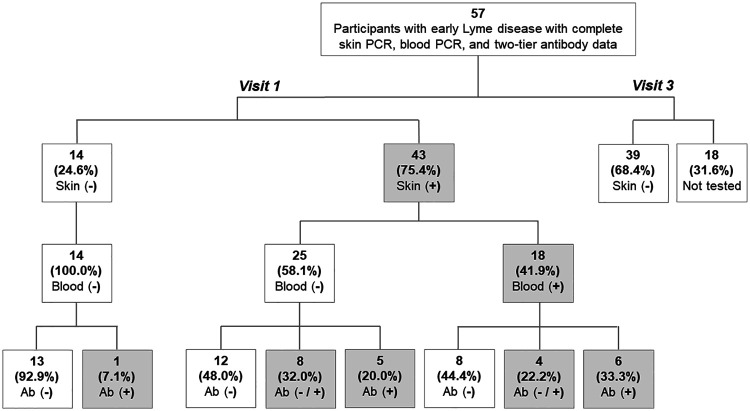
Of the 57 participants seen at V1, 43 (75.4%) had a positive skin biopsy sample for B. burgdorferi sensu stricto, 18 (31.6%) had a positive blood sample for B. burgdorferi sensu stricto, and 12 (21.1%) were two-tier antibody positive (Fig. 1). Thirteen participants (22.8%) were found to be negative on all three measures. An additional 12 (21.1%) of participants were found to be two-tier antibody positive at V2, and no additional participants were two-tier antibody positive at V3. All of the participants with a positive blood sample also had a positive skin biopsy sample; however, the reverse was not true, as an additional 25 participants were skin biopsy sample positive and blood sample negative. Among the 43 skin and/or blood PCR-positive participants, 20 (46.5%) remained antibody negative at both V1 and V2. Of the 39 participants who provided a second skin biopsy sample at V3, we did not find evidence of B. burgdorferi sensu stricto in any of the samples.
FIG 1.
Skin PCR/ESI-MS, blood PCR/ESI-MS, and two-tier antibody test results among 57 participants with early Lyme disease. The denominator used to generate the percentages shown in each box is drawn from the box on the figure directly above it rather than the overall total. Shaded boxes indicate positive results. Visit 1 is at baseline enrollment during acute infection, and visit 3 is 6 months following the end of standard of care treatment for early Lyme disease. Two-tier antibody (Ab) status is characterized as follows: Ab (−), two-tier negative on both acute and convalescent testing; Ab (−/+), two-tier negative on acute testing and positive on convalescent testing; Ab (+), two-tier positive on acute testing.
Notably, one participant who was negative on both skin and blood PCR/ESI-MS testing was found to be strongly two-tier antibody positive at V1, consistent with recent infection (ELISA, >5.00; IgM, 3/3 reactive bands; IgG, 4/10 reactive bands). This participant also had strong clinical evidence for infection, with disseminated lesions and a new onset seventh nerve palsy. Therefore, we considered this participant misclassified and removed this participant from subsequent clinical and immunologic group comparisons. However, sensitivity analyses with this participant were performed and are reported below.
Genotype testing.
We determined B. burgdorferi sensu stricto genotypes for 37/43 (86.0%) of the participants with a positive skin sample (Fig. 2A). Of these participants, 3 (8.1%) were found to have multiple genotypes present in the skin. Genotype detection was lower (10/18 [55.6%]) in the blood samples than in the skin biopsy samples (Fig. 2B). However, when a genotype was identified in a blood sample, it was always concordant with the one found in the skin sample from the same participant. None of the 10 participants for which we could detect a genotype in the blood sample were found to have multiple genotypes present.
FIG 2.
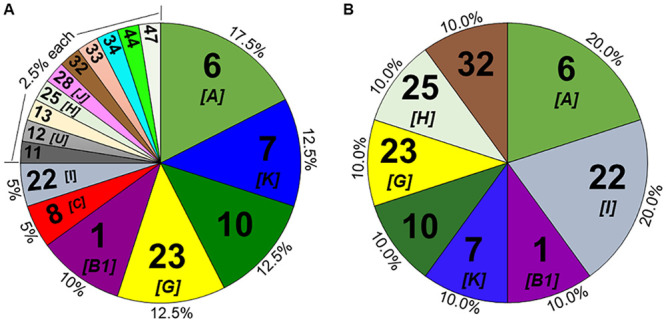
B. burgdorferi genotype prevalence in skin (A) and blood (B) samples from participants with early Lyme disease. Genotype percentages are shown on the chart’s outer edge with our genotype number shown inside. Where determined, the corresponding inferred OspC type is also included in brackets. The denominator used to generate the percentages is the total number of genotypes determined (n = 40 for skin biopsy specimen and n = 10 for blood samples), which accounts for the multiple genotypes found in the skin of three participants. Any genotypes found in blood samples were concordant with the ones found in the skin sample from the same participant.
Clinical characteristics.
Participants were characterized by PCR/ESI-MS status into three groups, and their demographic and clinical characteristics were compared across groups (Table 1). We found that the number of presenting symptoms and final two-tier antibody status were the only variables that were statistically significantly different by group. Participants were also compared by group on the characteristics of their EM rash (Table 2), and we found that the primary pattern of the EM, as well as whether or not a tick bite puncture was present were statistically significantly different by group. While they did not reach statistical significance, trends were observed for EM size as well as presence of vesicles in the EM. When pairwise comparisons were performed, it was predominantly the PCRneg/neg group that accounted for the group differences, while the PCRpos/neg and PCRpos/pos groups were more similar. In sensitivity analyses, we included the one misclassified PCRneg/neg participant who was initially removed and found similar results with less significant P values, given that this participant presented clinically more similar to the PCRpos/pos group.
TABLE 2.
Erythema migrans (EM) characteristics of 56 participantsa with clinically diagnosed early Lyme disease, by PCR/ESI-MS-defined subgroup
| EM characteristic | No. (%) of participants with the characteristic or value for characteristicb
|
P valuec
|
||||||
|---|---|---|---|---|---|---|---|---|
| All groups (n = 56) | Group 1 (PCRneg/neg) (n = 13) | Group 2 (PCRpos/neg) (n = 25) | Group 3 (PCRpos/pos) (n = 18) | Overall | Group 1 vs group 2 | Group 2 vs group 3 | Group 1 vs group 3 | |
| Disseminated EM | 15 (26.8) | 3 (23.1) | 5 (20.0) | 7 (38.9) | 0.37 | 1.00 | 0.17 | 0.45 |
| EM size (cm2) | 94.5 (49.0–165.5) [16.0–900.0] | 60.0 (36.0–100.0) [24.0–256.0] | 108.0 (60.0–225.0) [16.0–900.0] | 98.5 (44.0–162.0) [25.0–240.0] | 0.08 | 0.04 | 0.28 | 0.25 |
| EM shape | ||||||||
| Round | 31 (55.4) | 10 (76.9) | 13 (52.0) | 8 (44.4) | 0.18 | 0.14 | 0.62 | 0.07 |
| Oval/irregular | 25 (44.6) | 3 (23.1) | 12 (48.0) | 10 (55.6) | ||||
| EM color | ||||||||
| Red | 43 (76.8) | 12 (92.3) | 19 (76.0) | 12 (66.7) | 0.24 | 0.39 | 0.50 | 0.19 |
| Blue/red | 13 (23.2) | 1 (7.7) | 6 (24.0) | 6 (33.3) | ||||
| EM pattern | 0.01 | 0.009 | 0.58 | 0.005 | ||||
| Ring within a ring | 14 (25.0) | 3 (23.1) | 5 (20.0) | 6 (33.3) | ||||
| Central darkness | 20 (35.7) | 4 (30.8) | 9 (36.0) | 7 (38.9) | ||||
| Central lightness | 8 (14.3) | 6 (46.2) | 2 (8.0) | 0 (0.0) | ||||
| Uniform | 14 (25.0) | 0 (0.0) | 9 (36.0) | 5 (27.8) | ||||
| EM vesicles present | 7 (12.5) | 0 (0.0) | 6 (24.0) | 1 (5.6) | 0.08 | 0.08 | 0.21 | 1.00 |
| EM tick bite puncture present | 26 (46.4) | 11 (84.6) | 8 (32.0) | 7 (38.9) | 0.007 | 0.005 | 0.64 | 0.01 |
One participant in group 1 who was misclassified by the PCR/ESI-MS test was removed from this analysis. Groups are distinguished by and labeled with their PCRskin/blood result status.
Mean ± standard deviation for normally distributed variables, median (IQR) [range] for nonnormally distributed continuous variables, and n (%) for categorical variables are presented.
All P values are two sided.
Serum cytokine profiling.
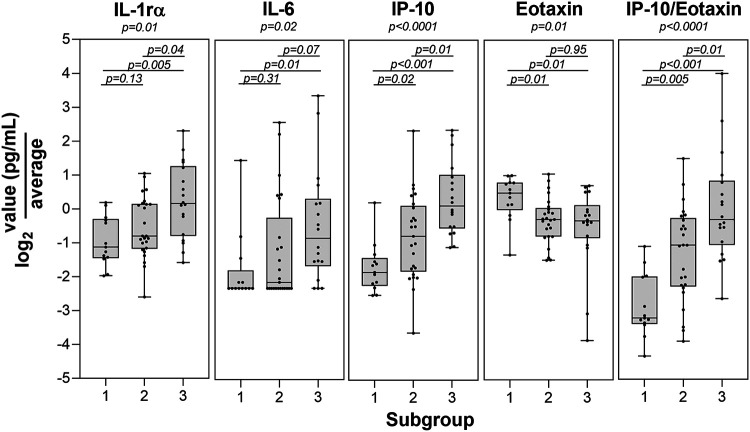
We examined the levels of 38 immune mediators in serum across the three PCR/ESI-MS skin and blood result status groups to look for group differences. This analysis found that IL-1rα, IL-6, IP-10 (CXC chemokine ligand 10 [CXCL10]), and eotaxin were statistically significantly different across the three groups at V1 (Fig. 3). Since IP-10 is thought to be driven by IFN-γ and reflects a predominantly Th1 response and eotaxin predominantly reflects a Th2 response, we also calculated a ratio of IP-10/eotaxin, which was also statistically significantly different by group. These results hold at a false discovery rate of 0.2. Results from pairwise comparisons showed that PCR/ESI-MS group status differentiated all three groups on IP-10 levels, uniquely identified the PCRneg/neg group on eotaxin levels, and uniquely identified the PCRpos/pos group on IL-1rα levels. These differences found at V1 did not persist at the later V2 or V3 time points (P > 0.23 for all overall group comparisons at both visits; P > 0.09 for all pairwise comparisons at both visits). In a sensitivity analysis where the one initially removed PCRneg/neg misclassified participant was added back, these results remained consistent.
FIG 3.
Statistically significant immune mediator differences among PCR/ESI-MS-defined patient subgroups. Levels of the four immune mediators found to be statistically significant by subgroup are shown (group 1, PCR skin and blood negative [PCRneg/neg]; group 2, PCR skin positive/blood negative [PCRpos/neg]; group 3, PCR skin and blood positive [PCRpos/pos]). Also shown is the calculated ratio of IP-10/eotaxin.
DISCUSSION
In this study, we utilized a PCR-based methodology (PCR/ESI-MS) to directly detect B. burgdorferi sensu stricto from patients with a clinically diagnosed EM of early Lyme disease and characterized these patients clinically and immunologically based on their skin and blood sample results. This approach identified several patient subgroups of interest within our cohort, most notably (i) those with B. burgdorferi sensu stricto detected who failed to seroconvert on the current two-tier serologic assay and (ii) those for whom no microbiologic or serologic evidence for infection could be found.
Similar to findings from previous studies (28), PCR/ESI-MS of EM skin specimens was found to be more sensitive for identifying B. burgdorferi sensu stricto infection than testing on whole blood or through commercially available two-tier serology. In our study, 21% were two-tier seropositive at V1, an additional 21% seroconverted between V1 and V2, and no additional participants seroconverted between V2 and V3, for a 42% overall seropositive rate in the sample. This rate was somewhat higher (53%) among those with microbiologically confirmed infection with B. burgdorferi sensu stricto, and higher still among those with disseminated EM at V1 (63%). The sensitivity limitations of the serologic test are often appreciated in the academic literature (17); however, this is not always the case in clinical practice. Our findings illustrate again that in early disease, physicians should avoid relying on two-tier test results in order to limit misdiagnoses, treatment delays, and the complication of documentation for potential later persistent symptoms posttreatment (29). Furthermore, the low rate of seroconversion in our sample also lead to the identification of a subgroup of patients who were PCR/ESI-MS positive but who remained seronegative on antibody tests at both V1 and V2 time points. This phenomenon has been described previously, often in the context of patients with EM treated promptly with antibiotics (17). We did find that the PCRneg/neg group presented with shorter duration of illness at V1, although it was not statistically significant.
Future research is needed to understand the subset of participants with clinically diagnosed EM who were found to be negative on PCR/ESI-MS and serology measures. The rate of this subgroup (23%) is similar to other, larger studies which have tested PCR positivity in clinically diagnosed EM samples in the United States and in Europe (30–32). Sampling error could have been present if the biopsy specimen was taken too far from the leading edge or if B. burgdorferi sensu stricto was present below the level of detection for this assay. We concluded that one participant in the PCRneg/neg group was likely misclassified given the strong clinical and serologic evidence for infection for this participant. It is unclear how this misclassification occurred, and similar factors could have contributed to misclassification of additional members of this subgroup.
It is also possible that diagnostic error of large tick bite reactions or sequelae of other infecting pathogens could have been present. Specifically, our Mid-Atlantic location introduces the possibility of Amblyomma americanum-associated infections, such as STARI (8). While we did not intend to assess the frequency of STARI in this cohort and do not have proof that any of our patients had A. americanum tick bites preceding their rash, several of the significant clinical differences we observed in the PCRneg/neg group, such as the predominance of EM central clearing and the overall lower severity of illness, are similar to those detailed by Wormser et al. in their comparison of Lyme disease and STARI (33). Furthermore, it is important to note that the range of A. americanum was thought to be confined to the southeastern United States, but it has recently been found to have significantly expanded into several northern states and is predicted to expand into southern Canada (34). At this time, an infectious driver of STARI has not been identified. In our study, additional tests were run on PCRneg/neg specimens, and no known pathogens were identified. These tests included a broad-range bacterial assay previously characterized on blood samples from septic patients (35) and a broad-range vector-borne assay previously used for detection of tick-borne viruses, bacteria, and protozoans (36, 37).
In the current study, individual pairwise comparisons indicated that the PCRneg/neg group appeared to contribute the most to many of the overall clinical and immunologic group differences we observed. Interestingly, elevated levels of eotaxin at V1, a mediator typically associated with Th2 and/or eosinophilic type of immune processes and low levels of IP-10, a chemokine associated with Th1 responses and known to be elevated in acute Lyme disease, are observed in this group (38–40). Eotaxin has been strongly associated with allergic and asthmatic processes of the respiratory and gastrointestinal tract as well as allergic skin disease (41). This suggests that this patient subgroup, while having many features that clinically overlap with early Lyme disease, may represent a unique clinical or microbiologic subgroup phenotype driven by distinct immune-mediated processes.
We observed the trend that PCR/ESI-MS-positive participants were more likely to have a higher disease burden at V1, with a greater number of symptoms, higher antibody titers to B. burgdorferi sensu stricto, and higher levels of several inflammatory mediators, including IP-10 (CXCL10), which has been found to be a signature of the acute phase of infection (24, 42–44). These findings are all consistent with possible increased representation of B. burgdorferi strains such as RST1 in these participants, which has been associated with greater inflammation and more virulent infection (42). IP-10 and IL-1rα levels could also distinguish the PCRpos/pos group not only from the PCRneg/neg group but also from the PCRpos/neg group as well. This suggests a possible immunologic distinction between those with skin-restricted or otherwise not yet disseminated strains. Finally, we also observed that several EM-related factors differed by group, in particular the pattern, presence of an observable tick bite puncture, and presence of vesicles. Notably, these differences were not explained by various degrees of antibiotic exposure at the V1 visit, and we were able to identify B. burgdorferi sensu stricto in skin samples taken up to 48 h from initiation of therapy.
A high degree of B. burgdorferi sensu stricto genotype diversity was found in PCR/ESI-MS-positive skin samples, and we hypothesize that this heterogeneity may account for the presence of PCRpos/pos and PCRpos/neg subgroups because some genotypes may be able to disseminate more readily from the initial site of the infection to other areas of the body, including peripheral blood and areas that may be difficult to treat, such as joints. Moreover, genotype information can serve as a control across blood/skin and against contamination, as all genotypes detected in the blood of a subject were also detected in the skin biopsy specimen (Fig. 2). In this study, the B. burgdorferi sensu stricto genotyping was utilized as a research tool, and the method employed is capable of detecting high degrees of genotypic diversity without affecting PCR results (10). Unfortunately, given the number of genotypes found and the small number of participants represented within each genotype, we were unable to test for clinical or immunologic differences by specific genotypes. In addition, we detected multiple genotypes in three (8%) of those participants with a classified genotype: two in the PCRpos/neg group and one in the PCRpos/pos group. The presence of multiple coinfecting genotypes of B. burgdorferi sensu stricto has been reported before in both humans and ticks (19, 45); however, the clinical implications of this are unknown. In post hoc analyses comparing those with a single genotype detected to those with multiple genotypes detected, we did not find a clear pattern of more severe disease as reflected by differences in lymphocyte count, liver function abnormalities, or dissemination of EM. We did find a trend toward a higher number of reported symptoms among those with multiple genotypes, although this was not statistically significant (the first value is the median value, IQR shown in parentheses, and range shown in brackets; 12.0 (5.0 to 18.0) [5.0 to 18.0] versus 6.0 (4.0 to 12.0) [0.0 to 20.0], P = 0.32) and significantly smaller EM rash sizes among those with multiple genotypes (32.0 (16.0 to 54.0) [16.0 to 54.0] versus 143.0 (60.0 to 225.0) [25.0 to 900.0], P = 0.02). However, the sample size among those with multiple genotypes is very small, and therefore, additional research is warranted.
Finally, we did not find evidence of B. burgdorferi sensu stricto in skin biopsy specimens repeated 6 months after completion of antibiotic therapy. This finding is similar to other, earlier studies in which B. burgdorferi sensu stricto could not be cultured following antibiotic treatment (46, 47). We therefore conclude that B. burgdorferi sensu stricto nucleic acid does not remain in host skin for prolonged periods of time after antibiotic therapy. However, this is expected, as B. burgdorferi sensu stricto is known to rapidly disseminate from the skin to other organs, and we were unable to assess these distant sites following early dissemination for the persistence of nucleic acid. This finding does not rule out the presence of B. burgdorferi sensu stricto or bacterial fragments in other tissue sites following antibiotic treatment, as has been suggested by others (48).
This study has several limitations, most notably the number of participants in our sample. In addition, while our rigorous participant inclusion and exclusion criteria created a more homogenous sample, it may have also limited our understanding of participants whose immune response and clearance of the bacteria may be different (i.e., those with prior exposure to Lyme disease, those with immunosuppressive conditions or medications, etc.). Relatedly, a subset of patients with Lyme disease will not have an observable EM at the time of early infection (49), which may reflect additional factors such as host skin color, location of the EM, or host immunologic or bacterial genotype diversity. Any extrapolation of our findings to clinical management of patients should take into account these considerations and this level of patient variability.
PCR-based methodologies on EM skin biopsy samples can identify the greatest number of patients and can separate those with microbiologically confirmed infection from those without microbiologically confirmed infection. Therefore, they may have a key role in future studies where differentiating EM from the rash of STARI is important, such as vaccine trials or public health surveillance initiatives. In addition, biopsy of the EM may potentially improve early diagnostics for Lyme disease. While not an attractive sample type for routine diagnostics because of the need for an invasive biopsy, molecular testing of EM skin biopsy specimens could be used during the development of a routine test that utilizes a less invasive sample type, such as whole blood, by aiding in determining true-positive results and true-negative results. Direct detection of nucleic acid in the skin may be especially useful for aiding in diagnoses of small lesions that may not reach the specific case definition of 5 cm and may therefore be missed (50) or of atypical lesions that may be confused with other diagnoses in consultative dermatology or infectious diseases practices (51). PCR/ESI-MS and other direct detection approaches can help identify a larger group of patients to decrease misdiagnosis and delayed treatment.
ACKNOWLEDGMENTS
The work was supported by the Bay Area Lyme Foundation, the Global Lyme Alliance, the Steven and Alexandra Cohen Foundation, and NIH P30-AR070254.
M.R.M., H.E.C., T.M., R.L., D.J.E., and M.W.E were all employees of Ibis Biosciences, an Abbott Company, which developed the PCR/ESI-MS assays and instrumentation used in these studies; assays described are for research use only. M.J.S. is a member of the Scientific Advisory Board of the Global Lyme Alliance.
REFERENCES
- 1.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019. Lyme disease data and surveillance. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/lyme/datasurveillance/index.html. [Google Scholar]
- 3.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascoe EL, Stephenson N, Abigana A, Clifford D, Gabriel M, Wengert G, Brown R, Higley M, Bloch EM, Foley JE. 2019. Human seroprevalence of tick-borne Anaplasma phagocytophilum, Borrelia burgdorferi, and Rickettsia species in northern California. Vector Borne Zoonotic Dis 19:871–878. doi: 10.1089/vbz.2019.2489. [DOI] [PubMed] [Google Scholar]
- 5.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 6.Tibbles CD, Edlow JA. 2007. Does this patient have erythema migrans? JAMA 297:2617–2627. doi: 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 7.Nadelman RB. 2015. Erythema migrans. Infect Dis Clin North Am 29:211–239. doi: 10.1016/j.idc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Masters EJ, Grigery CN, Masters RW. 2008. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect Dis Clin North Am 22:361–376, viii. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 10.Crowder CD, Matthews HE, Schutzer S, Rounds MA, Luft BJ, Nolte O, Campbell SR, Phillipson CA, Li F, Sampath R, Ecker DJ, Eshoo MW. 2010. Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS One 5:e10650. doi: 10.1371/journal.pone.0010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, Nadelman RB, Ludin S, Schwartz I. 2008. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis 198:1358–1364. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Ojaimi C, Wu H, Saksenberg V, Iyer R, Liveris D, McClain SA, Wormser GP, Schwartz I. 2002. Disease severity in a murine model of lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis 186:782–791. doi: 10.1086/343043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun 67:3518–3524. doi: 10.1128/IAI.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman JL, Bradley JF, Ross AE, Goellner P, Lagus A, Vitale B, Berger BW, Luger S, Johnson RC. 1995. Bloodstream invasion in early Lyme disease: results from a prospective, controlled, blinded study using the polymerase chain reaction. Am J Med 99:6–12. doi: 10.1016/S0002-9343(99)80097-7. [DOI] [PubMed] [Google Scholar]
- 15.Wormser GP. 2006. Hematogenous dissemination in early Lyme disease. Wien Klin Wochenschr 118:634–637. doi: 10.1007/s00508-006-0688-9. [DOI] [PubMed] [Google Scholar]
- 16.Moore A, Nelson C, Molins C, Mead P, Schriefer M. 2016. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis 22:1169–1177. doi: 10.3201/eid2207.151694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. 2005. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schutzer SE, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, Gerald NJ, Gomes-Solecki M, Kintrup M, Ledizet M, Levin AE, Lewinski M, Liotta LA, Marques A, Mead PS, Mongodin EF, Pillai S, Rao P, Robinson WH, Roth KM, Schriefer ME, Slezak T, Snyder JL, Steere AC, Witkowski J, Wong SJ, Branda JA. 2019. Direct diagnostic tests for Lyme disease. Clin Infect Dis 68:1052−1057. doi: 10.1093/cid/ciy614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosel MR, Carolan HE, Rebman AW, Castro S, Massire C, Ecker DJ, Soloski MJ, Aucott JN, Eshoo MW. 2019. Molecular testing of serial blood specimens from patients with early Lyme disease during treatment reveals changing coinfection with mixtures of Borrelia burgdorferi genotypes. Antimicrob Agents Chemother 63:e00237-19. doi: 10.1128/AAC.00237-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. 2012. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. 2011. Two-tiered testing decision tree. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/lyme/healthcare/clinician_twotier.html. [Google Scholar]
- 22.Metzgar D, Frinder M, Lovari R, Toleno D, Massire C, Blyn LB, Ranken R, Carolan HE, Hall TA, Moore D, Hansen CJ, Sampath R, Ecker DJ. 2013. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol 51:2670–2678. doi: 10.1128/JCM.00966-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowder CD, Rounds MA, Phillipson CA, Picuri JM, Matthews HE, Halverson J, Schutzer SE, Ecker DJ, Eshoo MW. 2010. Extraction of total nucleic acids from ticks for the detection of bacterial and viral pathogens. J Med Entomol 47:89–94. doi: 10.1603/033.047.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, Aucott JN. 2014. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS One 9:e93243. doi: 10.1371/journal.pone.0093243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horowitz HW, Dworkin B, Forseter G, Nadelman RB, Connolly C, Luciano BB, Nowakowski J, O’Brien TA, Calmann M, Wormser GP. 1996. Liver function in early Lyme disease. Hepatology 23:1412–1417. doi: 10.1002/hep.510230617. [DOI] [PubMed] [Google Scholar]
- 26.Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Molla I, Dornbush R, Visintainer P, Nowakowski J. 2015. Long-term assessment of health-related quality of life in patients with culture-confirmed early Lyme disease. Clin Infect Dis 61:244–247. doi: 10.1093/cid/civ277. [DOI] [PubMed] [Google Scholar]
- 27.Wormser GP, Aguero-Rosenfeld ME, Cox ME, Nowakowski J, Nadelman RB, Holmgren D, McKenna D, Bittker S, Zentmaier L, Cooper D, Liveris D, Schwartz I, Horowitz HW. 2013. Differences and similarities between culture-confirmed human granulocytic anaplasmosis and early Lyme disease. J Clin Microbiol 51:954–958. doi: 10.1128/JCM.02929-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wormser GP, Nowakowski J, Nadelman RB, Visintainer P, Levin A, Aguero-Rosenfeld ME. 2008. Impact of clinical variables on Borrelia burgdorferi-specific antibody seropositivity in acute-phase sera from patients in North America with culture-confirmed early Lyme disease. Clin Vaccine Immunol 15:1519–1522. doi: 10.1128/CVI.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebman AW, Crowder LA, Kirkpatrick A, Aucott JN. 2015. Characteristics of seroconversion and implications for diagnosis of post-treatment Lyme disease syndrome: acute and convalescent serology among a prospective cohort of early Lyme disease patients. Clin Rheumatol 34:585–589. doi: 10.1007/s10067-014-2706-z. [DOI] [PubMed] [Google Scholar]
- 30.Stupica D, Lusa L, Maraspin V, Bogovič P, Vidmar D, O’Rourke M, Traweger A, Livey I, Strle F. 2015. Correlation of culture positivity, PCR positivity, and burden of Borrelia burgdorferi sensu lato in skin samples of erythema migrans patients with clinical findings. PLoS One 10:e0136600. doi: 10.1371/journal.pone.0136600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liveris D, Wang G, Girao G, Byrne DW, Nowakowski J, McKenna D, Nadelman R, Wormser GP, Schwartz I. 2002. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J Clin Microbiol 40:1249–1253. doi: 10.1128/jcm.40.4.1249-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. 2011. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or lyme arthritis. Arthritis Rheum 63:2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wormser GP, Masters E, Nowakowski J, McKenna D, Holmgren D, Ma K, Ihde L, Cavaliere LF, Nadelman RB. 2005. Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions. Clin Infect Dis 41:958–965. doi: 10.1086/432935. [DOI] [PubMed] [Google Scholar]
- 34.Sagurova I, Ludwig A, Ogden NH, Pelcat Y, Dueymes G, Gachon P. 2019. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ Health Perspect 127:107014. doi: 10.1289/EHP5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzgar D, Frinder MW, Rothman RE, Peterson S, Carroll KC, Zhang SX, Avornu GD, Rounds MA, Carolan HE, Toleno DM, Moore D, Hall TA, Massire C, Richmond GS, Gutierrez JR, Sampath R, Ecker DJ, Blyn LB. 2016. The IRIDICA BAC BSI Assay: rapid, sensitive and culture-independent identification of bacteria and Candida in blood. PLoS One 11:e0158186. doi: 10.1371/journal.pone.0158186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honig V, Carolan HE, Vavruskova Z, Massire C, Mosel MR, Crowder CD, Rounds MA, Ecker DJ, Ruzek D, Grubhoffer L, Luft BJ, Eshoo MW. 2017. Broad-range survey of vector-borne pathogens and tick host identification of Ixodes ricinus from Southern Czech Republic. FEMS Microbiol Ecol 93:fix129. doi: 10.1093/femsec/fix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshoo MW, Crowder CD, Carolan HE, Rounds MA, Ecker DJ, Haag H, Mothes B, Nolte O. 2014. Broad-range survey of tick-borne pathogens in Southern Germany reveals a high prevalence of Babesia microti and a diversity of other tick-borne pathogens. Vector Borne Zoonotic Dis 14:584–591. doi: 10.1089/vbz.2013.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zlotnik A, Yoshie O. 2012. The chemokine superfamily revisited. Immunity 36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira MM, Wells TNC, Lukacs NW, Proudfoot AEI, Kunkel SL, Williams TJ, Hellewell PG. 1997. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J Clin Invest 100:1657–1666. doi: 10.1172/JCI119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. 1996. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest 97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amerio P, Frezzolini A, Feliciani C, Verdolini R, Teofoli P, Pità O, Puddu P. 2003. Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: therapeutical implications. Curr Drug Targets Inflamm Allergy 2:81–94. doi: 10.2174/1568010033344480. [DOI] [PubMed] [Google Scholar]
- 42.Strle K, Jones KL, Drouin EE, Li X, Steere AC. 2011. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol 178:2726–2739. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin JJ, Glickstein LJ, Steere AC. 2007. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum 56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 44.Strle K, Shin JJ, Glickstein LJ, Steere AC. 2012. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seinost G, Golde WT, Berger BW, Dunn JJ, Qiu D, Dunkin DS, Dykhuizen DE, Luft BJ, Dattwyler RJ. 1999. Infection with multiple strains of Borrelia burgdorferi sensu stricto in patients with Lyme disease. Arch Dermatol 135:1329–1333. doi: 10.1001/archderm.135.11.1329. [DOI] [PubMed] [Google Scholar]
- 46.Berger BW, Johnson RC, Kodner C, Coleman L. 1992. Failure of Borrelia burgdorferi to survive in the skin of patients with antibiotic-treated Lyme disease. J Am Acad Dermatol 27:34–37. doi: 10.1016/0190-9622(92)70152-6. [DOI] [PubMed] [Google Scholar]
- 47.Nadelman RB, Nowakowski J, Forseter G, Bittker S, Cooper D, Goldberg N, McKenna D, Wormser GP. 1993. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: report of a prospective study. Am J Med 94:583–588. doi: 10.1016/0002-9343(93)90208-7. [DOI] [PubMed] [Google Scholar]
- 48.Wormser GP, Nadelman RB, Schwartz I. 2012. The amber theory of Lyme arthritis: initial description and clinical implications. Clin Rheumatol 31:989–994. doi: 10.1007/s10067-012-1964-x. [DOI] [PubMed] [Google Scholar]
- 49.Steere AC, Dhar A, Hernandez J, Fischer PA, Sikand VK, Schoen RT, Nowakowski J, McHugh G, Persing DH. 2003. Systemic symptoms without erythema migrans as the presenting picture of early Lyme disease. Am J Med 114:58–62. doi: 10.1016/s0002-9343(02)01440-7. [DOI] [PubMed] [Google Scholar]
- 50.Aucott JN, Seifter A, Rebman AW. 2012. Probable late Lyme disease: a variant manifestation of untreated Borrelia burgdorferi infection. BMC Infect Dis 12:173. doi: 10.1186/1471-2334-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schutzer SE, Berger BW, Krueger JG, Eshoo MW, Ecker DJ, Aucott JN. 2013. Atypical erythema migrans in patients with PCR-positive Lyme disease. Emerg Infect Dis 19:815–817. doi: 10.3201/eid1905.120796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data, metadata, or methods used to replicate findings in this article will be made available upon request in a timely fashion. Please address requests to Alison Rebman (arebman1@jhmi.edu).