Summary
Obesity epidemic responsible for increase in diabetes, heart diseases, infections and cancer shows no signs of abating. Obesity in children is also on rise, indicating the urgent need of strategies for prevention and intervention that must begin in early life. While originally posited that obesity results from the simple concept of consuming more calories, or genetics, emerging research suggests that the bacteria living in our gut (gut microbiome) and its interactions with immune cells and metabolic organs including adipose tissues (microbiome-immune-metabolic axis) play significant role in obesity development in childhood. Specifically, abnormal changes (dysbiosis) in the gut microbiome, stimulation of inflammatory cytokines, and shifts in the metabolic functions of brown adipose tissue and the browning of white adipose tissue are associated with increased obesity. Many factors from as early as gestation appear to contribute in obesity, such as maternal health, diet, antibiotic use by mother and/or child, and birth and feeding methods. Herein, using evidence from animal and human studies, we discuss how these factors impact microbiome-immune-metabolic axis and cause obesity epidemic in children, and describe the gaps in knowledge that are warranted for future research.
Keywords: childhood obesity, immune, metabolism, microbiota
1 |. INTRODUCTION
Obesity is on rise in both adults and children, posing significant a health risk for humans. Presently, one-third of children in United States are either overweight or obese.1 Obesity is increasing at 2.3% each year among school-aged children (aged 6–11 years), which is unacceptably high and indicates worrisome prospects for next generation health. Therefore, developing a better understanding about factors involved in the progression of obesity will devise ways to design successful preventive and therapeutic strategies to check the rise of obesity in our children.
Obesity is simply defined by the extra-accumulation of fat in the adipose tissues due to either excess caloric intake, reduced energy expenditure, or both. In mammals, white adipose tissue (WAT) accumulates such extra calories in the form of triglycerides,2 while brown adipose tissue (BAT) burns the fat to produce heat in maintaining body temperature. WAT is the major culprit in the pathogenesis of obesity, as it accumulates fat/triglycerides and keeps expanding; while BAT consumes fat to produce heat, thus combating obesity.2 Individuals with lower BAT functionality have a higher risk of weight gain and obesity, while those carrying more active BAT remain relatively resistant to weight gain despite consuming excess calories. Although BAT remains relatively more active in children, it remains unknown whether decreased BAT activity contributes to childhood obesity.
In addition, the sedentary lifestyle due to digitalization and increased consumption of less nutritious and highly purified diets in the Western world plays an important role in the epidemic of obesity.3 A series of studies over the past decade has confirmed that the microbes living in our gut (the gut microbiome) not only associate with obesity but are one of the causative factors for the obesity epidemic. The changing of lifestyle and diets both impact the gut microbiome that contributes to the progression of obesity in both children and adults. The abnormal alterations (dysbiosis) in the gut microbiome result in abnormalities in nutrient absorption and metabolism, xenobiotic/drug metabolism, intestinal barrier functions controlling gut permeability to stop bacterial translocation, and activation of inflammatory immune functions.4 This dysbiosis in the gut microbiome and change in immune functions not only impacts children with obesity, but can also be passed from parental unhealthy gut microbiome and immune memories because of their unhealthy eating and lifestyle habits as well as overall health status.
Additionally, the population of healthy bacteria in the microbiome during infancy is one of the crucial determinants for the risk of obesity. Infants are born sterile (although recent studies showing the presence of bacteria in prenatal niches including placenta, amnion and meconium challenge this notion); however, their microbiomes start developing immediately after birth. The gut microbiome of infants born vaginally harbor the bacteria of maternal birth canal and vagina, which are typically beneficial bacteria. In contrast, the gut of infants born through Cesarean-section (C-section) is relatively populated by bacteria from maternal skin. Babies born by C-section have a higher risk of developing childhood obesity compared to babies who are vaginally delivered.5 In addition, infants who are formula-fed have higher incidences of childhood obesity and harbor different gut microbiomes compared to infants who are breastfed, suggesting that early-life feeding method impacts the risk of childhood obesity. Breastfed infants are expected to receive healthy nutrients like human milk oligosaccharides (HMOs) that promote healthy bacteria in the infant’s gut. The use of antibiotics during infancy and early childhood also significantly increases the risk of childhood obesity by inducing gut dysbiosis and affecting normal functioning of microbiome-associated metabolism regulation and immune system. These early life processes are determinants of the maturation of the gut microbiome, immune, and metabolic functions that regulate obesity incidences from childhood through adult life. In this review, we describe and discuss the interactions between gut microbiome, immune, and metabolic functions that can impact the WAT versus BAT functioning, thereby contributing to childhood obesity (Figure 1).
FIGURE 1.
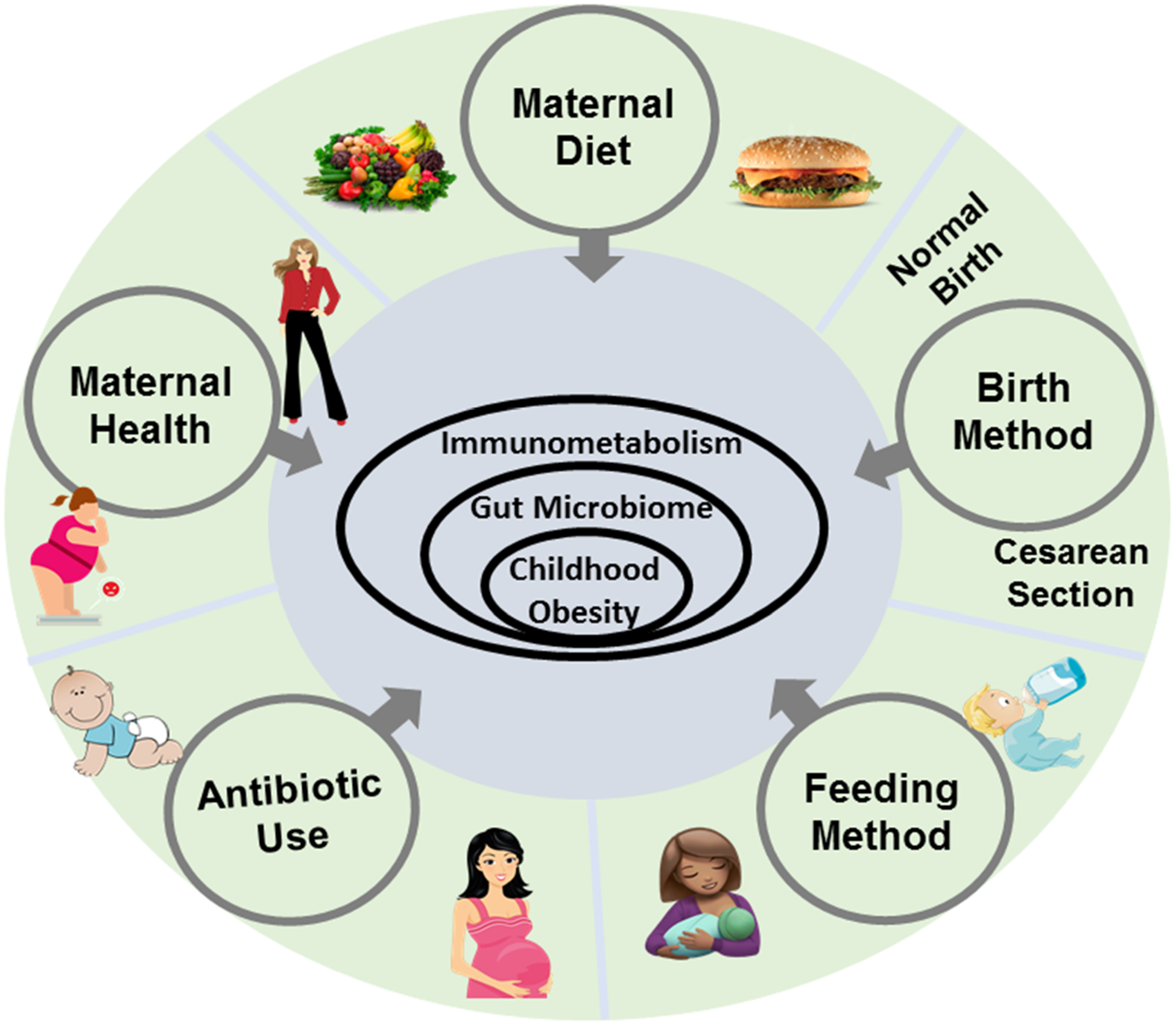
Factors contributing to the obesity epidemic
1.1 |. Microbiome-immune-metabolic (MIM) axis regulating WAT and BAT functioning
Several factors are involved in the induction of childhood obesity. Here, we specifically discuss the interactions among microbiome, immune, metabolic cells, and adipocytes, which are known to play a crucial role in the obesity epidemic. BAT is abundant during infancy. Brown adipocytes uniquely express a mitochondrial uncoupling protein 1 (UCP-1) protein,6 that uncouples the electron transport chain to produce heat instead of ATP formation.7 Thus, BAT helps in preventing obesity by consuming calories.8 Although the abundance of BAT in adulthood has been debated, recent studies demonstrate that there are physiologically relevant amounts of functional BAT present in adults.9 The BAT stimulation using cold, and/or any other means, enhances the burning of calories and weight reduction; thus, the stimulation of BAT is of significant importance to develop novel therapies against obesity. Conversely, WAT stores fat in the form of triglycerides10 and also plays an important role in regulating the whole body metabolic functions by releasing several adipokines such as leptin, adiponectin and resistin that regulate appetite and energy expenditure.10 However, extra accumulation of fat in WAT develops low-grade inflammation and insulin resistance that instigates type 2 diabetes. In leaner and metabolically efficient individuals, brown adipocyte-like cells (also called Beige cells) are dispersed into the WAT, but upon extra caloric intake and high fat accumulation, WAT begins to replace BAT/Beige cells. Increasing the abundance of beige cells in WAT by exercise, cold exposure, and other stimulations is linked to the reduction of obesity and type 2 diabetes. Therefore, the “conversion of WAT to BAT” remains an area of great interest to control obesity in adults11; however, its importance in children remains largely unknown. Also, the interactions of the gut microbiota and immune cell with WAT versus BAT cells are not well known.
Emerging evidence indicates that immune cells and adipocytes interact to regulate metabolic functions that impact obesity. In general, to fight with infections or other threats, immune cells disperse energy to release cytokines and cell proliferation by consuming glucose and other sources of energy from the body.12 However, upon surplus energy situations, adipocytes also start releasing inflammatory cytokines that bi-directionally influence the functions of adipose tissue resident immune cells such as macrophages,13 and this condition is called low-grade inflammation. In these conditions, adipocytes do not function properly and cause different forms of cellular stress which activate the inflammatory signaling pathways, which in turn cause insulin resistance in adipocytes. Thus, this alteration of adipocyte functioning and metabolism can lead to weight gain, even once the overnutrition has ceased.14 In contrast, the role of macrophages is pivotal in the process of non-shivering thermogenesis, stimulating brown adipocytes to increase UCP-1 expression.15 Specifically, the browning of adipose tissue is caused by increased type 1 (M1) and 2 (M2) cytokines. Type 1 cytokines activate classical (M1) macrophages, and type 2 cytokines polarize macrophages to the alternative M2 state.16 There are also specific links between adipose tissues and immunometabolic pathways. For instance, mice deficient in Smad3, a protein involved in transforming growth factor-beta (TGF-β) signaling, are more resistant to weight gain when exposed to high-fat diet (HFD). Smad3 deficiency in mice enhances conversion of white to brown adipocytes in WAT milieu.17 This demonstrates the interactions of immune cells such as macrophages and adipocytes that can play a dual role in both gaining and losing weight. The detailed immune cell and adipocyte interactions have been reviewed elsewhere.
The gut microbiome can play a key role in the immune-adipocyte interactions regulating obesity. Studies over the past decade have confirmed that the gut microbiome is not only associated with obesity but is also a causative factor, having the ability to increase its risk. The gut microbiome and its metabolites impact WAT versus beige functioning and abundance. For example, beige adipose tissue growth and insulin sensitivity are higher in gut microbiome-depleted leptin-deficient (Lepob/ob) and diet-induced obese (DIO) mouse models using antibiotics, or in germ-free (GF) mice.18 These mice exhibit higher numbers of multi-lobular, small lipid-droplet containing UCP-1 expressing cells compared to control mice.18 Host-microbiome derived metabolites such as bile acids (BA) contribute to the browning of adipose tissue in mouse models by modulating beneficial effects on gut hormones and modulating healthier microbiomes characterized with decreased Firmicutes and increased Bacteroidetes, which in turn are associated with increased thermogenesis and better regulation of glucose and lipid metabolism.19 Such effects have also been demonstrated in humans orally receiving chenodeoxycholic acid (CDCA; a bile acid) that significantly increased BAT activity and UCP-1 expression compared to the placebo group.20 Short-chain fatty acids (SCFAs) such as acetate, propionate and butyrate are the other major gut microbiome-derived metabolites that control host metabolism. Butyrate is also a browning agent that increases BAT functioning and stimulates peroxisome proliferator-activated receptor gamma (PPAR-γ) coactivator (PGC)-1α which enhances fatty acid metabolism and stimulates adaptive thermogenesis by the upregulation of UCP-1.21,22 Butyrate simulates the metabolite-sensing G-protein coupled receptors (GCPRs) known as free fatty acid receptors 2 and 3 (FFAR2/3). FFAR2/3 signaling stimulated by butyrate increases the production of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) which increase insulin secretion and affect energy intake/expenditure.23,24 In addition, SCFA-stimulated FFAR2/3 signaling is also involved in the regulation of immune cell functions such as anti-inflammatory properties by suppressing interleukin-8 (IL-8) which is known for inducing diabetes and causing inflammation when present in elevated quantities25,26 and increasing Treg cells.27 Altogether, these examples support our notion that gut microbiome, immune, and metabolic (MIM) cells, including adipocytes, closely interact and regulate functions of each other, which can impact obesity pathology. While the role of the MIM axis in obesity is emerging considerably in context to adulthood obesity, its role in childhood obesity is not well developed. Figure 2 depicts the interactions between the gut microbiome, immune-metabolism, and adipose tissue modulating obesity versus lean phenotypes, and further sections will describe the role of this MIM axis in childhood obesity.
FIGURE 2.
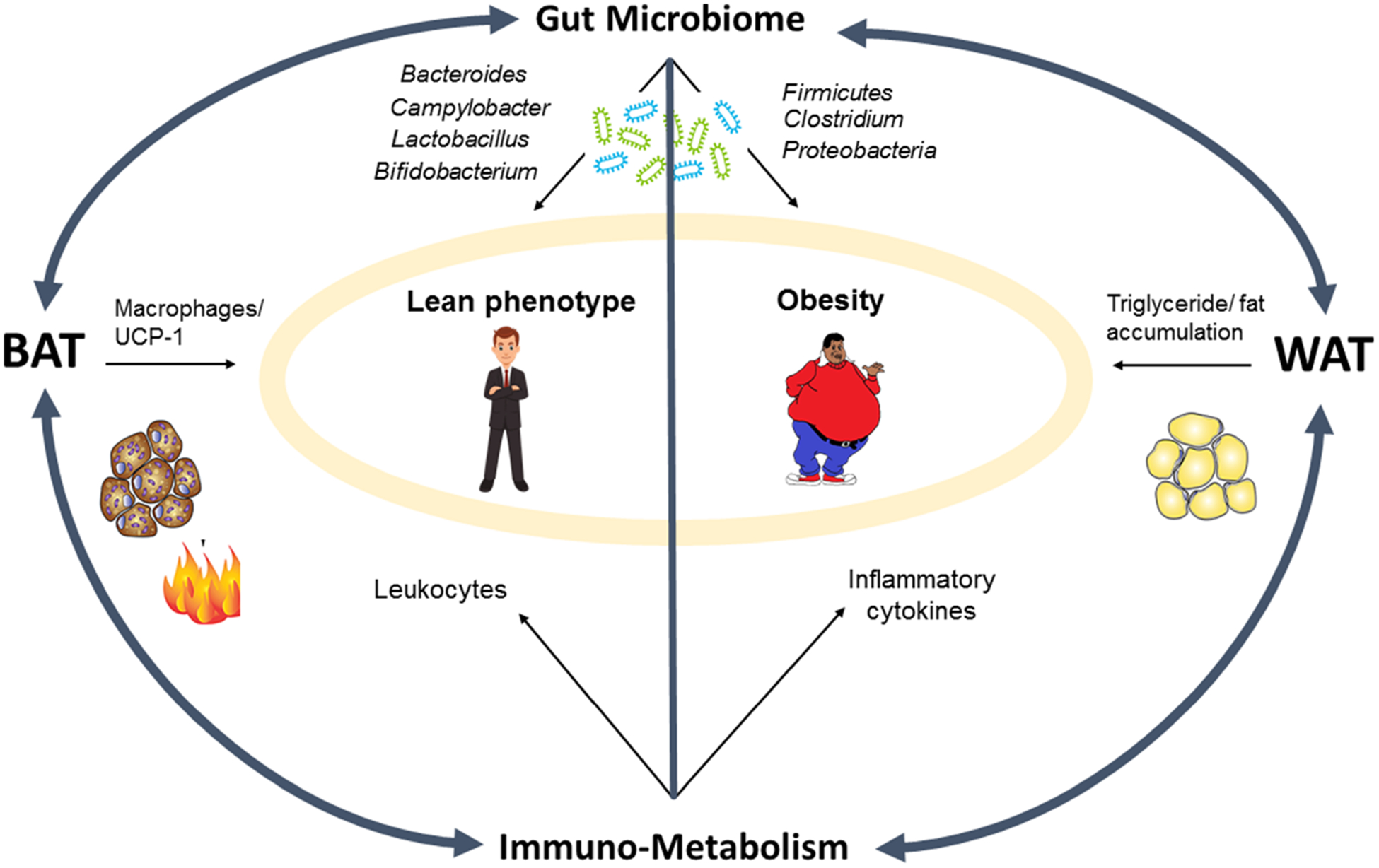
Microbiome-immune-metabolic axis involvement in obesity pathology
1.2 |. Maternal health contributing to childhood obesity and influencing MIM axis
Overall maternal health is crucial for the risk of obesity in offspring later in life. Factors including maternal obesity, diabetes, malnutrition, medications, and lifestyle (during, before, and after pregnancy) can impact the offspring’s MIM axis.28 Almost two-thirds of American child-bearing aged women have obesity.29 Maternal obesity and diabetes are known to pose high risks of developing these ailments in their children30,31 (Figure 3). This is supported by the facts that: (i) mothers with obesity/diabetes even before pregnancy (prepartum) have increased expression of harmful genes in reproductive cells including ovum, that are imprinted and passed to the offspring; (ii) mothers who become obese or diabetic during pregnancy (peripartum) can induce gene imprinting in the developing embryonic cells; (iii) mothers with obesity/diabetes can pass harmful metabolites through placental barriers that can influence the risk of obesity and diabetes in offspring; (iv) mothers that develop obesity soon after delivery (postpartum) can also pass harmful metabolites, fat, proteins and bacteria through breastmilk to the offspring, impacting the MIM axis.32 Thus, prepartum, peripartum, intrapartum and postpartum health of the mother can impact the risk of obesity in offspring. However, more research is warranted to investigate factors that can influence maternal and offspring relationship in the risk of obesity. Some studies are described below and summarized in Table 1.
FIGURE 3.
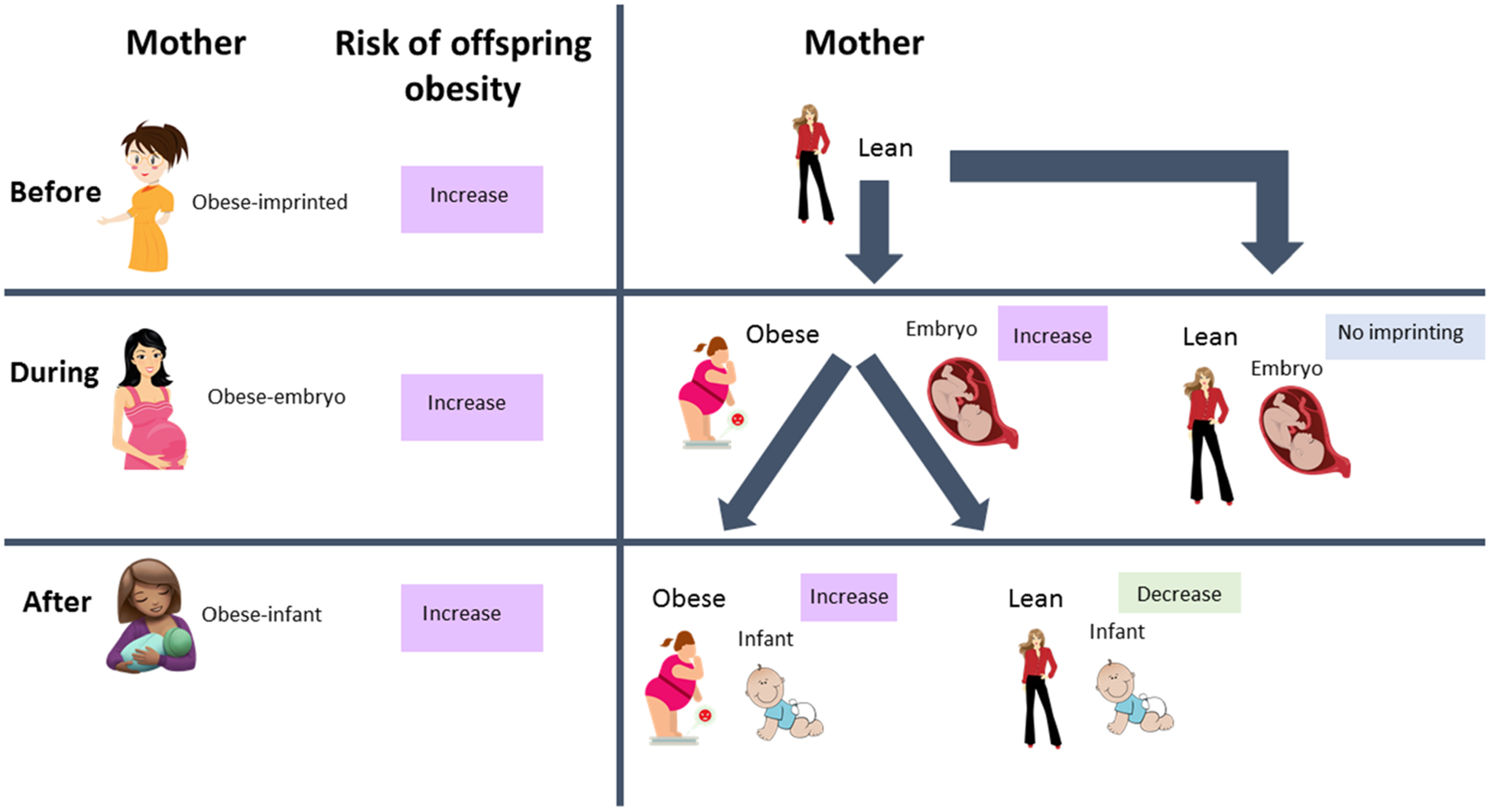
Interactions of the microbiome-immune-metabolic axis with maternal and early-life health in the risk of obesity
TABLE 1.
Summary of major studies and their findings of factors involved in childhood obesity impacting MIM axis
| Model type | Major Findings | Refs. |
|---|---|---|
| Maternal Health Contributing to Childhood Obesity and the MIM Axis | ||
| Mouse | -mice colonized with stool microbes from 2-week old infants born to mothers with obesity had impaired macrophage function, excess weight gain, and accelerated NAFLD compared to mice with microbes of infants for normal weight mothers | 35 |
| Rat | -Offspring of dams subjected to exercise improved insulin/glucose metabolism compared to sedentary dams | 45 |
| Mouse | -dams with obesity- either sedentary or wheel running lifestyle both had offspring with increased WAT and body weight | 38 |
| -Offspring of sedentary dams increased IL-6 in WAT and hypothalamus | ||
| Rat | -offspring of HFD-fed dams increased lipogenesis/fatty acid synthesis genes in WAT compared to lean dams | 33 |
| -adult offspring of HFD-fed dams increased leptin mRNA causing higher leptin resistance, higher hypertrophy in adipose tissue, more adipogenesis and adiposity, compared to offspring of control dams | 34 | |
| -Maternal dam exercise protected against offspring leptin increase and helped to protect against triglyceride increase compared to dams with sedentary lifestyle | 36 | |
| Human | -Infants from mothers with insufficient GWG had lower fat mass and percent body fat | 39 |
| -Infants from mothers with excessive GWG had higher fat mass and fat-free mass with no change in percent body fat | ||
| -infants of mothers with obesity had less microbiome diversity, more likely to be with obesity | 40 | |
| -offspring of mothers who smoked or consumed alcohol increased risk of late onset overweight | 41 | |
| -Mothers who exercise 150 min per week, no smoking, moderate alcohol consumption, maintained healthy BMI, and ate a high quality diet significantly reduced offspring’s risk of developing obesity | 42 | |
| Contribution of Maternal Diet to the Risk of Childhood Obesity via the MIM axis | ||
| -offspring of HFD fed mothers transitioning to normal fat diet one or five weeks before pregnancy had adipocyte hypertrophy, increased macrophage infiltration, and increased inflammatory cytokine expression compared to normal fat diet fed mothers | 47 | |
| Sheep | -offspring of overfed ewes had increased UCP-1 levels and higher thermogenic activity compared to normal diet offspring | 46 |
| Mouse | -decreased thermogenesis in BAT and WAT in offspring of HFD compared to control | 48 |
| - HFD offspring recovered thermogenic activity if mother supplemented with dietary resveratrol | ||
| -decreased body weight in offspring of low protein fed mothers and increased BAT thermogenesis by 2–6 times compared to control diet | 49 | |
| Human | -infants born to mothers with HFD had significantly less Bacteroides compared to infants of mothers with normal diet | 50 |
| -each kg GWG increased childhood BMI by 0.0220 and the odds ratio of developing obesity or overweight by 1.007 | 51 | |
| -offspring of mothers with GWG and leptin levels had increased birth weight and lower leptin levels | 52 | |
| Contribution of Antibiotics to the Risk of Childhood Obesity via the MIM axis | ||
| Rat | -pups born to mothers fed antibiotics gained less weight and had increased expression of PYY hormone compared to control pups | 55 |
| Human | -infants born to mothers who took intrapartum antibiotics had significantly less lactobacillus transferred from the mother compared to non-antibiotic mothers | 57 |
| -infants born from C section had less bifidobacterial and Bacteroides and more C difficile compared to infants who are vaginally born | 78 | |
| - antibiotic use during pregnancy did not affect infant microbiome composition | ||
| -infants only formula fed increased E coli, C difficile, Bacteroides, and Lactobacilli compared to breastfed infants | ||
| -infants exposed to prenatal antibiotic use significantly high BMI at age 2 compared to control | 56 | |
| -even stronger associations with high BMI at age 2 if prenatal antibiotics taken during first or second trimester of pregnancy | ||
| -children exposed to antibiotics during second or third trimester had 84% higher risk of obesity and increased BMI and % body fat | 58 | |
| -increased maternal usage of antibiotics led to increased risk of childhood obesity | 59 | |
| -second trimester maternal usage of antibiotics had the strongest increase in childhood obesity rates | ||
| Mouse | -mice fed antibiotics increased adiposity and body weight, increased ratio of Firmicutes to Bacteroides compared to control | 61 |
| - Mice fed antibiotics at 13 weeks old (STAT) had significantly increased body weight gain, fat mass, severe insulin resistance, and increase risk of non-alcoholic fatty liver disease compared to control | 62 | |
| -STAT mice had reduced microbiome diversity and increased inflammatory cytokines TNFa α and IL-6 compared to control | ||
| -ratio of leptin to ghrelin significantly increased in STAT mice compared to control | ||
| Pig | -both antimicrobial mixtures ZAC and ZCT increased body weight gain and decreased bile acid ratios associated with decreased bile salt hydrolase | 63 |
| -ZAC increased Bacteroidetes and decreased Firmicutes, Lactobacillus, and Clostridium and ZCT did not affect microbiome diversity | ||
| Human | -infants fed antibiotics, H2RA, or PPI had higher risk of obesity regardless of strength | 64 |
| - additional 30 days of antibiotics, H2RA, or PPI strengthened association of infant with obesity | ||
| -any usage of antibiotics in ages 2–3 years did not increase risk of overweight or obesity at age 5 | 65 | |
| -at least 4 courses of antibiotics in ages 2–3 years significantly increased risk of obesity at age 5 | ||
| -antibiotics up to 6 months of age increased risk of obesity in children of normal weight mothers and decreased risk of obesity in children of mothers with obesity/overweight | 66 | |
| Birth Method’s Impact on the MIM axis | ||
| Lamb | -lambs born through C section had lower metabolic rate compared to lambs from vaginal birth | 67 |
| -lambs born through C section relied more on shivering thermogenesis when in cool environment and had lower UCP-1 levels compared to lambs born through vaginal birth | ||
| Mouse | -mice born through C section were 33% heavier than vaginally born mice at age 15 weeks | 68 |
| -female mice demonstrated a stronger association between birth method and weight gain | ||
| -C-section offspring significantly lower gut microbiome diversity | ||
| Human | - infants born by C section have significantly lower gut microbiome diversity compared to infants who are vaginally born | 69 |
| -both geographic regions’ infants born by C section significantly greater weight gain compared to vaginally born | 70 | |
| - girls and boys of Yucatec Maya descent and only Toba/Qom boys significantly higher weight compared to children who are vaginally born at 4 yr | ||
| -infants born through C section significantly associated with overweight/obesity at age 17 | 71 | |
| - infants born by C section lower birth weight and increased adiposity at age 6 weeks through 15 years | 72 | |
| -at age 11, offspring born by C section 1.83 times more risk of developing overweight/obesity compared to offspring born by normal birth | ||
| -stronger correlation between birth method and BMI only for mothers with high BMI (weaker correlation for normal weight mothers) | ||
| Feeding Method’s Impact on the MIM axis | ||
| Mouse | -mice who gained weight with HFD had reduced weight gain and adiposity when diet supplemented with bovine-milk oligosaccharides and increased gut microbiome diversity | 74 |
| -mice fed caprine milk oligosaccharides (CMO) had increased weight gain and body fat compared to control 30 days post weaning | 75 | |
| -CMO mice had increased gut microbiome diversity (increased Bifidobacterium) | ||
| Human | -breastfeeding at 1 month decreased risk of childhood obesity by 53% | 76 |
| - infants with high birth weight had significantly decreased risk of obesity if breastfed | 77 | |
| -gut microbiome significantly associated with birth method and feeding method | 79 | |
| -a few days of formula feeding among mostly breastfeeding still results in a gut microbiome like that of an exclusively formula fed infant | ||
| -infant serum influenced by fatty acid composition of breast milk | 82 | |
| -higher milk protein content increases risk of obesity | ||
| -high protein content in breastmilk or infant formula increases risk of childhood obesity | ||
1.2.1 |. Animal Studies
Animal studies also indicate that mothers with obesity status can raise offspring’s risk for obesity.33 Offspring from dams with obesity demonstrate increased hypertrophy in adipose tissues and leptin resistance with enhanced adipogenesis.34 Interestingly, even the mice colonized with fecal microbiota of infants from mothers with obesity had increased weight gain and non-alcoholic fatty liver disease (NAFLD) rate as well as impaired macrophage function in the liver and increased Firmicutes to Bacteroidetes ratio,35 indicating that the gut microbiome that was passed from mothers with obesity to infants passed along a strong risk of obesity for infants. Additionally, a rat study demonstrated significantly increased expression of lipogenesis/fatty acid synthesis genes in the WAT of adult offspring born from mothers with obesity compared to mothers that were lean.33Maternal lifestyle and daily level of activity can also impact the infant’s obesity risk. Vega et al36 demonstrated that maternal obesity increases fat mass, triglycerides, and leptin levels in male offspring while exercise protects against the increase of these obesity measures in offspring. When pregnant rats with both obese and lean phenotypes are subjected to volunteer exercise, their offspring maintain better insulin sensitivity and glucose homeostasis compared to offspring from mothers that are not subjected to any exercise.37 Another study reported that the offspring of sedentary mothers with obesity had increased expression of inflammatory interleukin-6 (IL-6) in WAT and hypothalamus while the offspring of mothers with obesity who exercised were protected against this elevated IL-6 expression.38 Overall, these studies suggest that maternal obesity has a negative impact on childhood obesity. Unfortunately, the precise factors and mechanisms remain largely unknown; however, the MIM axis factors can be important in childhood obesity and further comprehensive research is warranted in this area.
1.2.2 |. Human Studies
Human studies also demonstrate that the offspring from mothers with peripartum or intrapartum obesity/overweight are more likely to have obesity/overweight.39 Infants of mothers with less gestational weight gain (GWG) have lower fat mass, while offspring with excessive GWG have greater fat mass.39 The microbiome is significantly less diverse in children from mothers with obesity, demonstrating that environmental factors like microbiome may contribute to human childhood obesity.40 Maternal smoking and alcohol consumption is also associated with increased risk of late onset overweight in offspring.41 Emerging studies have determined that maternal exercise significantly reduces offspring’s risk of developing obesity.42 However, well controlled and large-scale studies are missing to indicate which factors of mothers with obesity are significantly contributing to childhood obesity that can be targeted to curve the childhood obesity epidemic.
1.3 |. Maternal diet contributing to the risk of childhood obesity via MIM axis
Maternal diet during pregnancy and breastfeeding is critical as mother’s nutrition is directly transferred to the infant/child through placental circulation and breast milk, respectively. Maternal diet during pregnancy can impact the risk of obesity in progeny in several ways during embryo development. Unhealthy diet can modulate the maternal gut microbiome and produce detrimental metabolites exposing embryos by crossing placental barriers43 and can impact the epigenetic signatures affecting the molecular and cell mechanisms of highly proliferative and developmentally active embryos to imprint postnatal susceptibility to obesity. Therefore, alterations to the embryonic environment in nutrient availability, gut microbiome metabolite exposure, and/or immunological cytokines can imprint risk of obesity in children. Figure 4 illustrates the interactions between maternal diet and offspring MIM axis.
FIGURE 4.
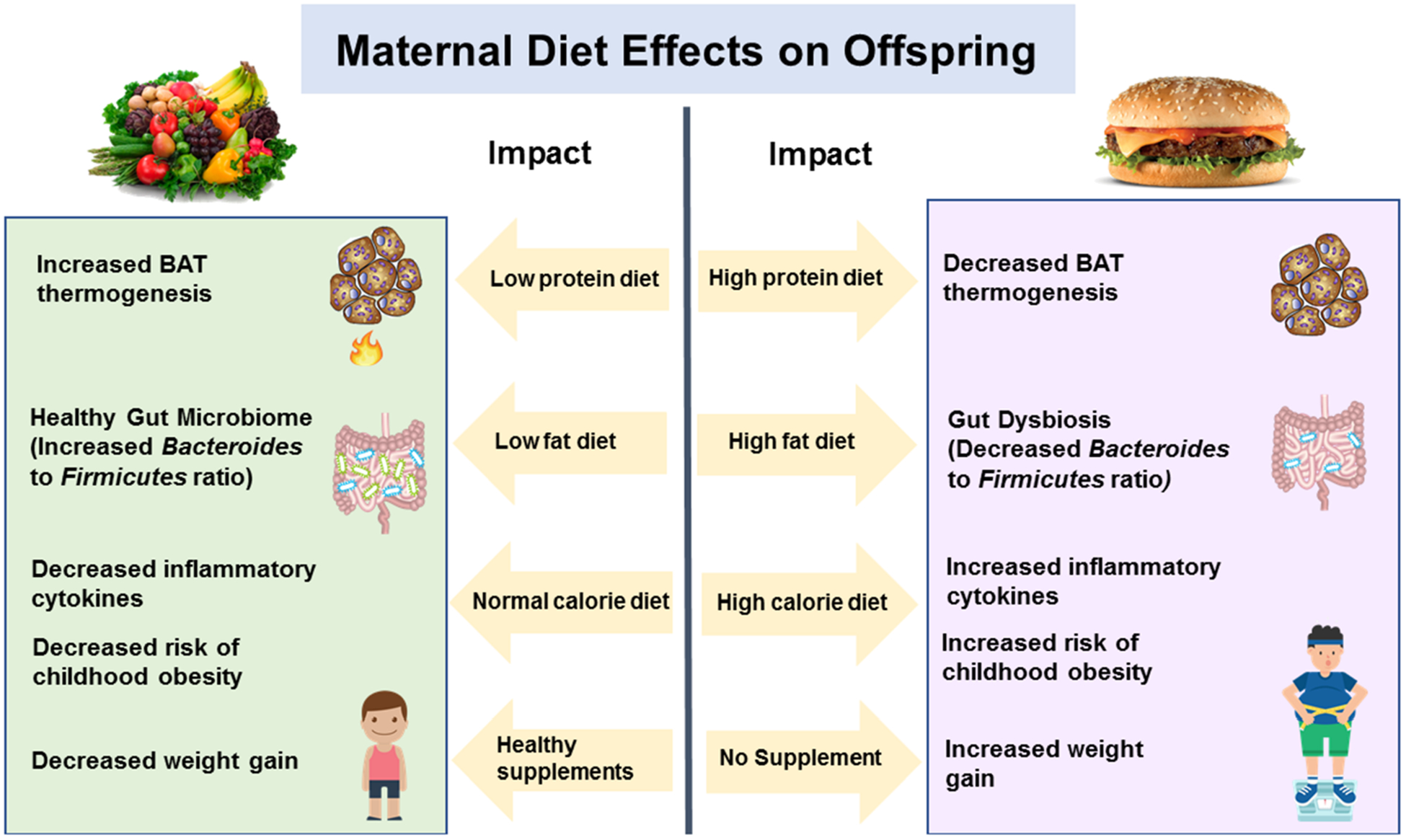
Effects of materinal diet on microbiome-immune-metabolic axis and obesity risk
1.3.1 |. Animal Studies
The impact of maternal diet on the risk of childhood obesity in offspring are reported in several animal models, and a few of them are reviewed here. Ma et al44 reported that although the host diet is a major modulator of the gut microbiome, mothers fed with HFD during pregnancy and lactation had progeny with a microbiome signature similar to animals with obesity, suggesting that the mother’s diet passes effects to offspring microbiome and obesity phenotype. Another study demonstrated that the offspring from HFD-fed rats have significantly (2-times) higher WAT and total adiposity, compared to control counterparts,37 again suggesting that maternal diet is highly important for progeny metabolic health.45 In contrast, when ewes mothers were overfed during pregnancy, the offspring exhibited increased UCP-1 levels and higher thermogenic activity,46 indicating that maternal overnutrition could lead to stimulated BAT in offspring. Summerfield et al47 found that the closer the mothers ate HFD to pregnancy, the higher the adipocyte hypertrophy, macrophage infiltration with increased inflammation, and over-activation of JNK signaling in WAT of progeny. During breastfeeding, the negative effects of maternal HFD on offspring can be recovered if maternal diets are supplemented with dietary resveratrol which promotes beige adipogenesis.48 Maternal low-protein diets decrease offspring birth weight by increasing the BAT thermogenesis by 2–6 times compared to the level exhibited in control rat offspring.49 Overall, these studies indicate that the maternal diet (in terms of not only the fat contents but also the protein content) also influences the risk of obesity in offspring linked to MIM axis modulation.
1.3.2 |. Human Studies
Several human studies also show that maternal diet can increase obesity in children by impacting the MIM axis. Chu et al50 reported that infants of mothers who ate a high calorie diet had significantly lower levels of Bacteroides, indicating a dysbiotic microbiome, compared to normal control. A study matching GWG with offspring BMI determined that childhood BMI increases for each kilogram of GWG from maternal overnutrition,51 indicating that regardless of diet type, any form of overnutrition by mothers can increase obesity risk in the offspring. Another study determined that higher GWG and maternal leptin levels are associated with higher birth weight and lower leptin levels in offspring, the two factors known to increase the child’s risk of developing obesity later in life.52 Although these studies indicate that maternal diet increases the risk of obesity in children, more comprehensive and planned clinical studies are needed to conclude these observations and find definitive factors that can be implemented in clinical practice to check the growing rate of childhood obesity.
1.4 |. Impact of antibiotics use on MIM axis and childhood obesity risk
Most clinically available antibiotics are broad-spectrum and can dramatically modulate the gut microbiome composition and functions.53 After antibiotic treatment, pathogenic bacteria can repopulate faster than beneficial bacteria; therefore, a single antibiotic use can leave a long-term impact on the gut microbiome. Figure 5 depicts the effects of antibiotic use on childhood obesity.
FIGURE 5.
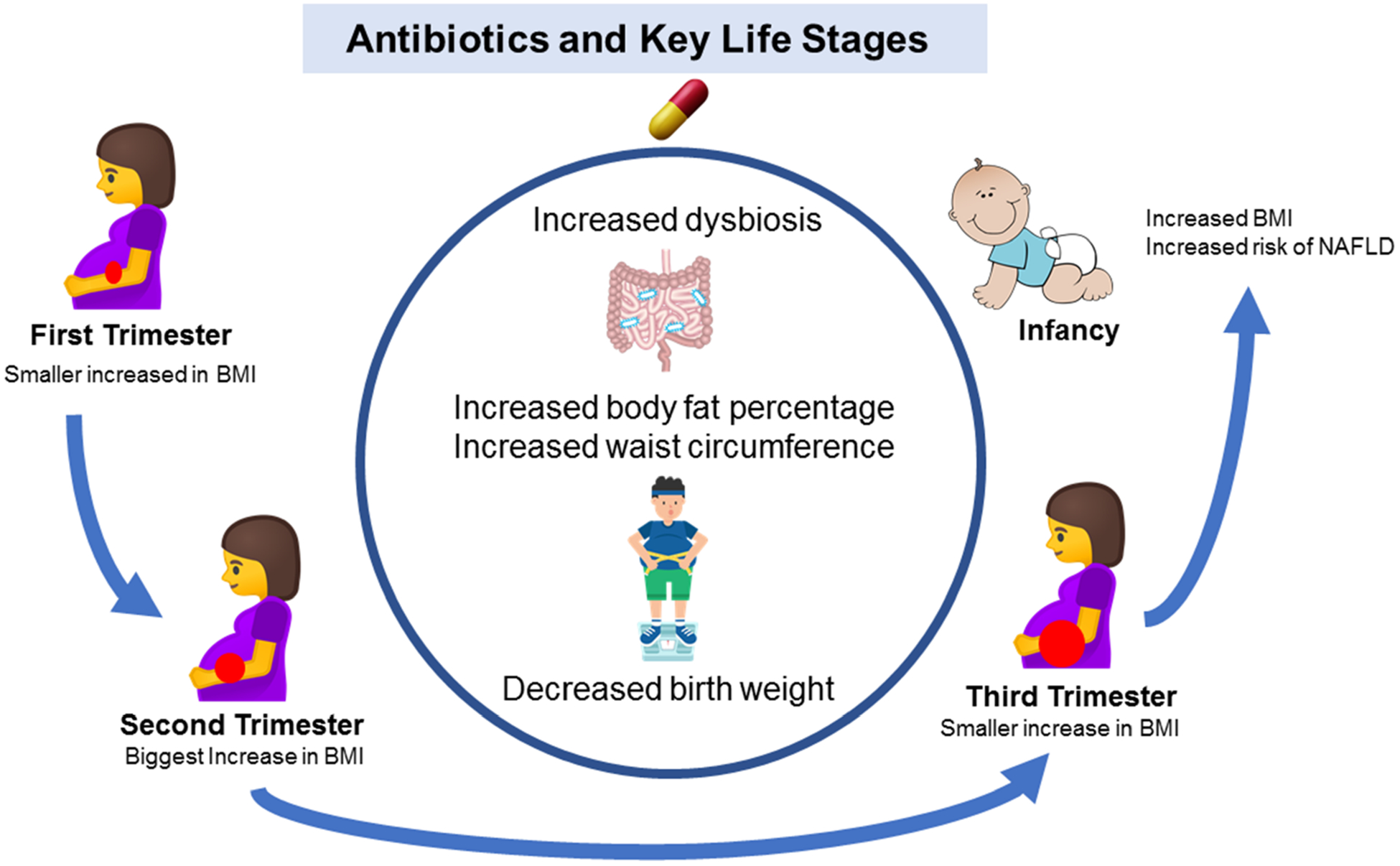
Antibiotic use by mother and early-life impact on microbiome-immune-metabolic axis and the risk of obesity
A). Use of antibiotics during pregnancy:
Antibiotics significantly decrease the diversity of the gut microbiome. When mothers take antibiotics before and/or during pregnancy, infants only receive a partial set of bacteria from the mother instead of a fully diverse healthy microbiome. This can lead to future overweight/obesity. Studies demonstrating the impact of maternal antibiotic use on obesity in offspring are discussed below:
Animal Studies: To the best of our search, we found only minimal animal studies demonstrating the impact of antibiotics during pregnancy on the risk of childhood obesity. However, a study by Li et al54 found that the expression of BAT UCP-1 in mice fed antibiotic cocktails was significantly reduced. Additionally, these mice had a significantly decreased amount of M2 (beneficial) macrophages in the BAT. This decreased amount suggests that the depletion of the gut microbiome by antibiotics in adult mothers can reduce BAT and pose a higher risk for obesity that can be passed to the next generation. Contradictory evidence from a rat study demonstrated that pups born from mothers fed antibiotics from pregnancy through weaning gain less weight throughout adulthood,55 suggesting that the use of antibiotics during pregnancy can be beneficial. However, such studies will need further comprehensive investigation to establish these facts.
Human Studies: A study found that the offspring of women who used antibiotics during pregnancy have significantly higher BMI/obesity.56 Keski-Nisula et al. found that the use of antibiotics during pregnancy causes a significantly reduced transfer of beneficial bacteria (such as Lactobacillus) to infants.57 Mueller et al. found that the offspring of mothers taking antibiotics during second or third trimester pregnancy had an 84% higher risk of offspring obesity, higher BMI, fat mass and waist circumference.58 Another study determined that increased frequency of antibiotic exposure increases the risk for developing childhood obesity, especially during second trimester, which causes the strongest increase in childhood obesity rates.59 Maternal antibiotic use reduces infant microbial diversity, and timing of antibiotics plays a fundamental role. Administration of antibiotics during pregnancy negatively affects the infant microbiome while antibiotic use before pregnancy appears to have minimal to no effect. However, more research is requisite to validate these impacts and correlations.
B). Use of antibiotics during infancy:
Antibiotic use during infancy increases the risk of obesity by inducing microbiome dysbiosis.60 Compared to older children and adults, the microbiome of the infant is much more sensitive to antibiotic-induced alterations. Specifically, infants struggle to replenish bacteria that have been killed by antibiotics. These observations are supported by the following animal and human studies:
Animal studies: Cho et al. demonstrated that infant mice that were subjected to an antibiotic treatment had increased adiposity with an increased detrimental ratio of Firmicutes to Bacteroides.61 It has also been shown that life-long sub-therapeutic antibiotic treatment (STAT) in mice significantly increases fat mass, insulin resistance, NAFLD, gut microbiome dysbiosis and expression of proinflammatory cytokines later in life.62 A pig study demonstrated that feeding two different combinations of antibiotics increase body-weight gain and adiposity; however, one combination of antibiotics caused dysbiosis while the other did not.63 Thus, early life antibiotic exposure can enhance the risk of obesity later in life. However, more comprehensive studies are needed to address the impact of types and course of antibiotics during early life on the risk of obesity.
Human Studies: Stark et al. found that antibiotic exposure in early life significantly increases the risk of early childhood obesity regardless of the strength or type of antibiotic, and longer exposure increases this risk.64 Another study demonstrated that specifically the number of antibiotic courses – rather than mere exposure – during childhood determines the risk of childhood obesity, where children having at least four course of antibiotics had significant increases in the risk of childhood obesity.65 Interestingly, administration of antibiotics up to six months in infants of normal weight mothers significantly increases the risk of developing obesity, while antibiotic administration decreases the risk of obesity in infants born to mothers with obesity/overweight.66 This difference might account for the fact that antibiotics might have disrupted the normal microbiome in infants from mothers with normal weight, which might have induced obesity; on other hand, such antibiotics may have killed bad bacteria in infants from mothers with obesity/overweight and protected them from obesity.
1.5 |. Impact of birth method on childhood obesity and MIM axis
The risk of childhood obesity is strongly linked with birth methods that impact the MIM axis. For example, the gut of infants born through C-section is colonized by microbes primarily from the mother’s skin and the hospital environment whereas the gut of infants born vaginally harbors mother’s vaginal and perianal microbiome (Figure 6). The microbial community’s origin depending on birth method largely impacts the risk of childhood obesity, as described below:
Animal Studies: A study showed that the lambs born through C-section exhibit lower metabolic rate and UCP-1 expression in BAT compared to vaginally born counterparts and rely more on shivering thermogenesis.67 A mouse study also demonstrated that mice born through C-section were 33% heavier and had less diverse gut microbiome than normal birth mice.68 However, due to limited need for C-sections in animal models, there are limited studies discussing its impact on offspring obesity; therefore, more research is needed in this area to find the definite factors that can impact childhood obesity risk.
Human Studies: Infants born through C-section demonstrate significantly lower gut microbiome diversity and richness, which may also be predictive of obesity.69 Veile et al70 compared populations in two geographic regions and determined that C-section significantly increased weight gain for both boys and girls in one region, while in the other region, just boys born through C-section exhibited significant weight gain. Thus, while C-section causes weight gain, factors including geographical region as well as the gender can affect the impact of C-section on offspring weight gain. Bar-Meir et al71 found significant association of C-section with overweight/obesity at 17 years. Additionally, Blustein et al72 found that by 11 years of age, infants born via C-section had 1.83 times higher risk of developing obesity compared to offspring born vaginally. The risk of childhood obesity is compounded with C-section in mothers with obesity. However, these data are scattered and hence further, better-plannedmultifactorialstudiesareneededtoestablishthesefacts.
FIGURE 6.
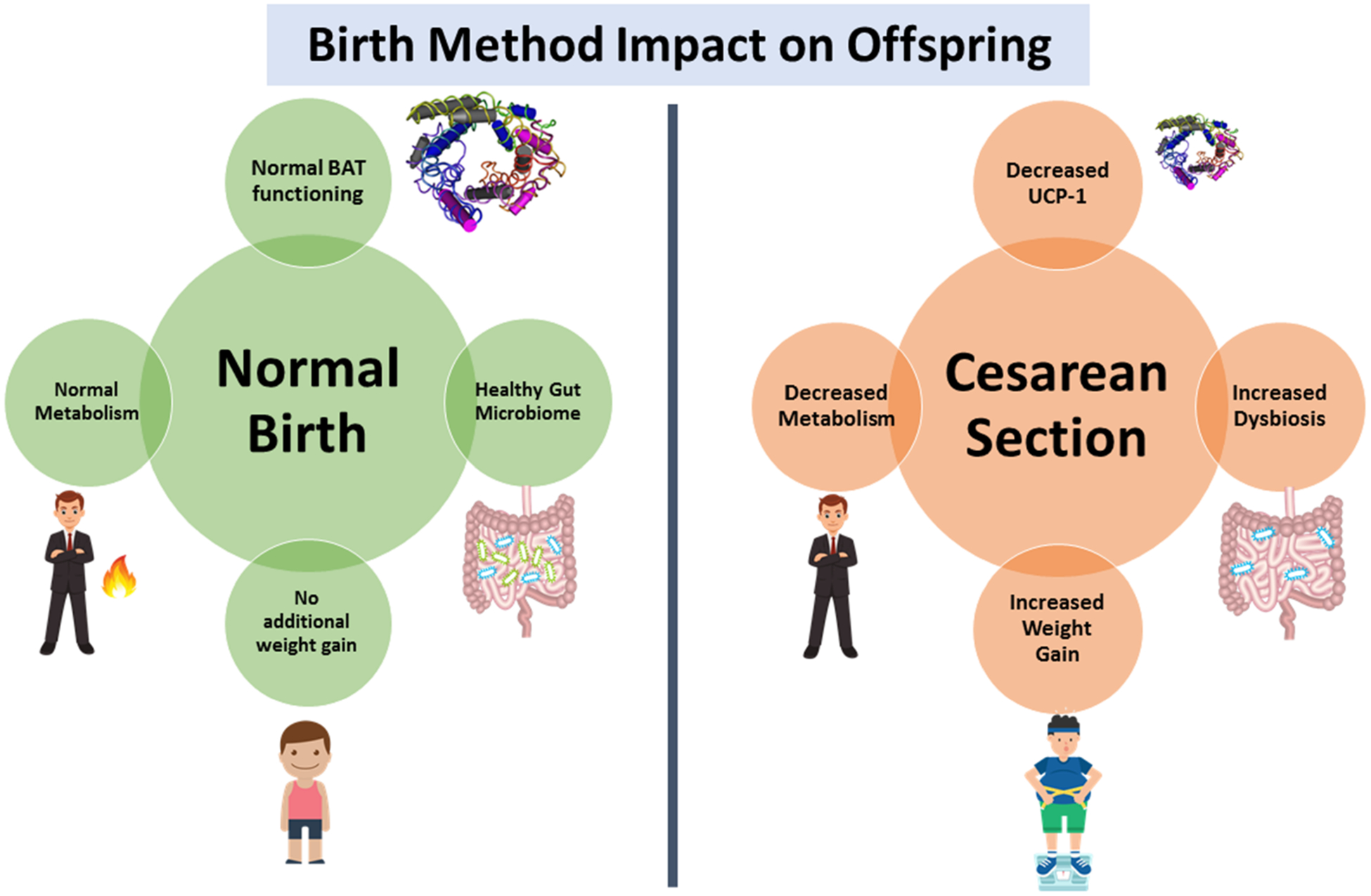
Impact of birth method on microbiome-immune-metabolic axis and risk of obesity
1.6 |. Impact of Feeding Method on Childhood Obesity and MIM axis
As mothers have become increasingly busy with new responsibilities at the workplace, increasing rates of formula feeding have become more common. The mother’s milk harbors a microbiome of breast and skin; unfortunately, formula lacks this microbiome, thereby resulting in an imbalanced microbiome in the infant’s gut. One of the most common components of the mother’s milk are human milk oligosaccharides (HMOs), which are a nutritional source of healthy bacteria. For example, Bifidobacteria are commonly enhanced in breast-fed infants and promote a healthy gut microbiome and are associated with reduced obesity.73 As research has identified the beneficial effects of these ingredients, a new generation of infant formulas have begun to include HMOs and Bifidobacteria. Effects of breastfeeding versus formula-feeding options on offspring health are depicted in Figure 7 and Table 1.
FIGURE 7.
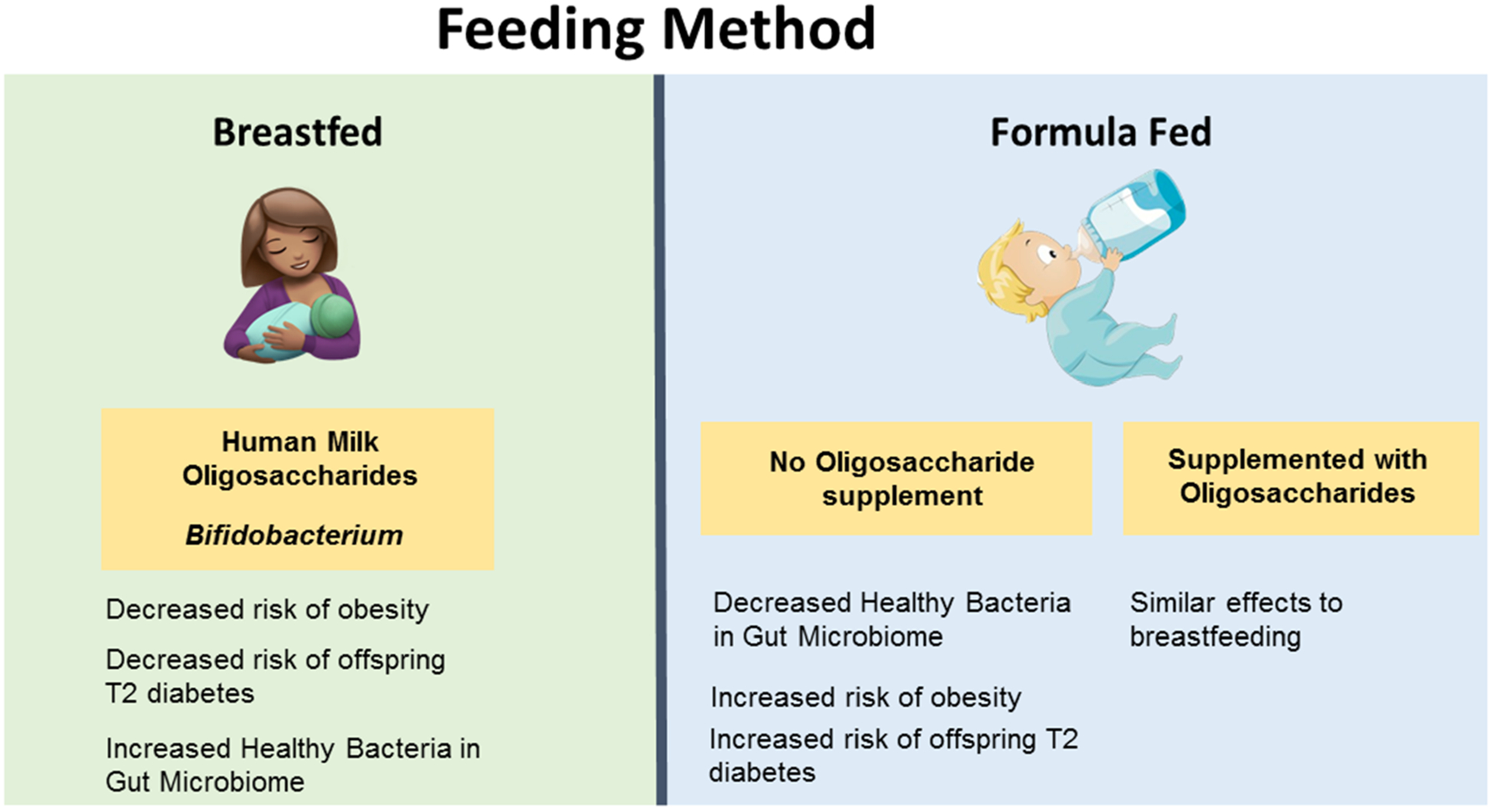
Interactions of microbiome-immune-metabolic axis with feeding method and risk of obesity
1.6.1 |. Animal Studies
Hamilton et al74 found that bovine-milk derived oligosaccharide feeding reduced weight gain and gut microbiome dysbiosis in HFD-fed mice. Another study showed that the offspring from dams fed with prebiotic caprine milk oligosaccharides (CMO) had increased gut microbiome diversity and Bifidobacterium abundance and decreased weight gain and body fat compared to control offspring 30 days after weaning.75 However, very limited studies have compared breast-fed versus formula-fed in animals; whereas, human studies are abundant, as discussed in the following section.
1.6.2 |. Human Studies
Wang et al76 found that infants who are breastfed are less likely to develop obesity later in life. Another study demonstrated that breastfeeding has protective effects in high birth weight (HBW) offspring, who have a higher risk for childhood obesity.77 Penders et al78 demonstrated that the infants exclusively fed with formula had significantly higher levels of C. difficile, E. coli and B. fragilis, bacteria that are commonly abundant in the gut of people with obesity, thus posing a risk of obesity. Another study demonstrated that infants fed a combination of breastmilk and formula develop a gut microbiome more similar to infants that were exclusively formula-fed, indicating that periodic formula feeding can strongly impact the infant’s gut microbiome.79 However, if a mother had to choose a type of formula, formulas containing oligosaccharides develop healthier offspring microbiomes consisting of more Lactobacillus and Bifidobacterium.80 An analysis of pooled studies demonstrated that infants that are breastfed are less likely to develop type 2 diabetes later in life compared to infants that are formula-fed.81 Another problem with infant formulas is that they typically contain higher proteins which promote lipogenesis and development of fat cells in the infant, leading to childhood obesity.82 Further studies demonstrated that the infants fed a lower protein formula weighed less compared to higher protein formula fed infants and have similar weight compared to breastfed infants.83 In addition, Liber and Szajewska73 found that a combination of three oligosaccharides proved to increase bifidobacteria in the infant gut compared to individual prebiotics. Not only does breastmilk contain healthy oligosaccharides and bacteria, but a recent study has determined that breastmilk also contains a lipid called alkylglycerol-type ether lipids (AKG-type) which maintains beige adipose tissue when adipose tissue macrophages metabolize the lipids and activate IL-6/STAT3 signaling in adipocytes.84Therefore, a combination of oligosaccharides, AKG-type, and bacteria in infant formulas could be an alternative to breast milk.
2 |. CONCLUSION AND FUTURE PERSPECTIVES
As obesity has become a worldwide problem, researchers have naturally searched to discover causative factors that could be modulated to improve this issue. Early life factors seem to play a large role in imprinting risk factors for future overweight and obesity. Thus, it has become crucial to analyze maternal, prenatal, infant, and childhood decisions made that could impact the child’s health, as early life overweight and obesity lead to significantly increased risk of persistent obesity in adulthood, contributing to the current obesity epidemic. Some of the important factors that could significantly alter the risk of childhood obesity include maternal health, use of antibiotics in both mother and children, and birth and feeding methods. Because these factors alter numerous biological processes, this article narrowed its focus to the impact of these risk factors on the microbiome-immune-metabolic (MIM) axis regulating white adipose tissue and brown adipose tissue functioning. The goal of analyzing the MIM axis, specifically, is to determine whether certain early life decisions mentioned above impact the child’s health and encourage future research into these areas and hopefully prevent future decisions of parents, physicians, and health care agencies that could cause increased risk for obesity. Specifically, this review identified potential risk factors for childhood obesity based on the evidence of gut microbiome dysbiosis, elevated inflammatory cytokine levels, decreased BAT functioning/increased WAT, elevated BMI, or increased risk for type 2 diabetes.
While research about childhood obesity has increased due to the obesity epidemic, specific studies analyzing the gut microbiome, immunometabolism, and BAT functioning are still lacking. For instance, there is good data discussing the effects of certain risk factors on a child’s weight gain and risk for obesity; however, there is less data discussing what specific problems are caused by the risk factor, such as microbiome dysbiosis, and immune and metabolic dysregulations. More research is needed to determine the specific mechanisms associated with risk factors in order to discover solutions to these problems. Additionally, animal studies providing mechanistic evidence of childhood obesity risk factors are lacking. More research should be done to investigate the impact on the risk of childhood obesity of both maternal and offspring use of antibiotics, birth method, and feeding method in animal models, delineating the factors that can be targeted to curve the childhood obesity epidemic. Studies analyzing BAT and cytokine functioning in particular are extremely limited, especially when analyzing the risk factors of birth methods and feeding method. Indeed, with these gaps in research filled, there will be a better understanding of specific mechanisms and factors causing childhood obesity, and the better understanding will aid in developing new preventative and interventional strategies to prevent/treat childhood obesity.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant R01 AG018915 and the Pepper Older Americans for Independence Center (P30AG21332) (HY), and the Department of Defense funding W81XWH-18-1-0118, W81XWH-18-PRARP-NIRA, R01AG018915 (RN, HY), as well funds and services provided from the Center for Diabetes, Obesity and Metabolism, Wake Forest Baptist Medical Center, and the National Center for Advancing Translational Sciences (NCATS), the National Institutes of Health-funded Wake Forest Clinical and Translational Science Institute (WF CTSI) through Grant Award Number UL1TR001420.
Footnotes
CONFLICTS OF INTEREST
No conflict of interest was declared.
REFERENCES
- 1.Kumar S, Kelly AS. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin Proc. 2017;92(2):251–265. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacy-lglycerol metabolism in adipose tissue. Future Lipidol. 2007;2(2):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70(1):97–103. [DOI] [PubMed] [Google Scholar]
- 4.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller NT, Mao G, Bennet WL, et al. Does vaginal delivery mitigate or strengthen the intergenerational association of overweight and obesity? Findings from the Boston Birth Cohort. Int J Obes (Lond). 2017;41(4):497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Origins Trayhurn P. and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2017;134:62–70. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Bi S. Hypothalamic Regulation of Brown Adipose Tissue Thermogenesis and Energy Homeostasis. Front Endocrinol (Lausanne). 2015;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–E452. [DOI] [PubMed] [Google Scholar]
- 10.Keuper M On the role of macrophages in the control of adipocyte energy metabolism. Endocr Connect. 2019;8(6):R105–R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53(4):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity. 2017;47(3):406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol (Lausanne). 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38(4):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papackova Z, Palenickova E, Dankova H, et al. Kupffer cells ameliorate hepatic insulin resistance induced by high-fat diet rich in monounsatu-rated fatty acids: the evidence for the involvement of alternatively activated macrophages. Nutr Metab (Lond). 2012;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32(1):609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav H, Quijano C, Kamaraju AK, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14(1):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suarez-Zamorano N, Fabbiano S, Chevalier C, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21(12):1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierre JF, Martinez KB, Ye H, et al. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G286–G304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broeders EP, Nascimento EB, Havekes B, et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015;22(3):418–426. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. [DOI] [PubMed] [Google Scholar]
- 23.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology. 2005;146(2):879–888. [DOI] [PubMed] [Google Scholar]
- 25.Gibson P, Rosella O. Interleukin 8 secretion by colonic crypt cells in vitro: response to injury suppressed by butyrate and enhanced in inflammatory bowel disease. Gut. 1995;37(4):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cimini FA, Barchetta I, Porzia A, et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol. 2017;54(10):961–967. [DOI] [PubMed] [Google Scholar]
- 27.Bhaskaran N, Quigley C, Paw C, Butala S, Schneider E, Pandiyan P. Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection. Front Microbiol. 2018;9:1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segovia SA, Vickers MH, Reynolds CM. The impact of maternal obesity on inflammatory processes and consequences for later offspring health outcomes. J Dev Orig Health Dis. 2017;8(5):529–540. [DOI] [PubMed] [Google Scholar]
- 29.Mulligan CM, Friedman JE. Maternal modifiers of the infant gut microbiota: metabolic consequences. J Endocrinol. 2017;235(1):R1–R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 31.Maric-Bilkan C, Symonds M, Ozanne S, Alexander BT. Impact of maternal obesity and diabetes on long-term health of the offspring. Exp Diabetes Res. 2011;2011:163438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care. 2011;41(6):158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borengasser SJ, Zhong Y, Kang P, et al. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology. 2013;154(11):4113–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lecoutre S, Oger F, Pourpe C, et al. Maternal obesity programs increased leptin gene expression in rat male offspring via epigenetic modifications in a depot-specific manner. Mol Metab. 2017;6(8):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soderborg TK, Clark SE, Mulligan CE, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9(1):4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond). 2015;39(4):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raipuria M, Bahari H, Morris MJ. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS ONE. 2015;10(4):e0120980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae-Gartz I, Janoschek R, Kloppe CS, et al. Running Exercise in Obese Pregnancies Prevents IL-6 Trans-signaling in Male Offspring. Med Sci Sports Exerc. 2016;48(5):829–838. [DOI] [PubMed] [Google Scholar]
- 39.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS ONE. 2014;9(11):e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring). 2007;15(3):760–771. [DOI] [PubMed] [Google Scholar]
- 42.Dhana K, Haines J, Liu G, et al. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: results from two prospective cohort studies of mother-child pairs in the United States. BMJ. 2018;362:k2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabo AJ. Transferred maternal fatty acids stimulate fetal adipogenesis and lead to neonatal and adult obesity. Med Hypotheses. 2019;122:82–88. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5(1):3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Symonds ME, Bloor I, Ojha S, Budge H. The Placenta, Maternal Diet and Adipose Tissue Development in the Newborn. Ann Nutr Metab. 2017;70(3):232–235. [DOI] [PubMed] [Google Scholar]
- 46.Budge H, Bispham J, Dandrea J, et al. Effect of maternal nutrition on brown adipose tissue and its prolactin receptor status in the fetal lamb. Pediatr Res. 2000;47(6):781–786. [DOI] [PubMed] [Google Scholar]
- 47.Summerfield M, Zhou Y, Zhou T, et al. A long-term maternal diet transition from high-fat diet to normal fat diet during pre-pregnancy avoids adipose tissue inflammation in next generation. PLoS ONE. 2018;13(12):e0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou T, Chen D, Yang Q, et al. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol. 2017;595(5):1547–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claycombe KJ, Vomhof-DeKrey EE, Roemmich JN, Rhen T, Ghribi O. Maternal low-protein diet causes body weight loss in male, neonate Sprague-Dawley rats involving UCP-1-mediated thermogenesis. J Nutr Biochem. 2015;26(7):729–735. [DOI] [PubMed] [Google Scholar]
- 50.Chu DM, Antony KM, Ma J, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig DS, Rouse HL, Currie J. Pregnancy weight gain and childhood body weight: a within-family comparison. PLoS Med. 2013;10(10): e1001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Retnakaran R, Ye C, Hanley AJ, et al. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. CMAJ. 2012;184(12):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turta O, Rautava S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC Med. 2016;14(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li B, Li L, Li M, et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep. 2019;26(10):2720–37 e5. [DOI] [PubMed] [Google Scholar]
- 55.Tulstrup MV, Roager HM, Thaarup IC, et al. Antibiotic treatment of rat dams affects bacterial colonization and causes decreased weight gain in pups. Commun Biol. 2018;1(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassidy-Bushrow AE, Burmeister C, Havstad S, et al. Prenatal antimicrobial use and early-childhood body mass index. Int J Obes (Lond). 2018;42(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keski-Nisula L, Kyynarainen HR, Karkkainen U, Karhukorpi J, Heinonen S, Pekkanen J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013;102(5):480–485. [DOI] [PubMed] [Google Scholar]
- 58.Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond). 2015;39(4):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang B, Liu J, Zhang Y, et al. Prenatal Exposure to Antibiotics and Risk of Childhood Obesity in a Multicenter Cohort Study. Am J Epidemiol. 2018;187(10):2159–2167. [DOI] [PubMed] [Google Scholar]
- 60.Korpela K, Zijlmans MA, Kuitunen M, et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahana D, Trent CM, Kurtz ZD, et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 2016;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ipharraguerre IR, Pastor JJ, Gavalda-Navarro A, Villarroya F, Mereu A. Antimicrobial promotion of pig growth is associated with tissue-specific remodeling of bile acid signature and signaling. Sci Rep. 2018;8(1):13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stark CM, Susi A, Emerick J, Nylund CM. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68(1):62–69. [DOI] [PubMed] [Google Scholar]
- 65.Kelly D, Kelly A, O’Dowd T, Hayes CB. Antibiotic use in early childhood and risk of obesity: longitudinal analysis of a national cohort. World J Pediatr. 2019;15(4):390–397. [DOI] [PubMed] [Google Scholar]
- 66.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond). 2011;35(4):522–529. [DOI] [PubMed] [Google Scholar]
- 67.Clarke L, Heasman L, Firth K, Symonds ME. Influence of route of delivery and ambient temperature on thermoregulation in newborn lambs. Am J Physiol. 1997;272(6 Pt 2):R1931–R1939. [DOI] [PubMed] [Google Scholar]
- 68.Martinez KA 2nd, Devlin JC, Lacher CR, et al. Increased weight gain by C-section: Functional significance of the primordial microbiome. Sci Adv. 2017;3(10):eaao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. [DOI] [PubMed] [Google Scholar]
- 70.Veile A, Valeggia C, Kramer KL. Cesarean birth and the growth of Yucatec Maya and Toba/Qom children. Am J Hum Biol. 2019;31(2):e23228. [DOI] [PubMed] [Google Scholar]
- 71.Bar-Meir M, Friedlander Y, Calderon-Margalit R, Hochner H. Mode of delivery and offspring adiposity in late adolescence: The modifying role of maternal pre-pregnancy body size. PLoS ONE. 2019;14(1):e0209581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blustein J, Attina T, Liu M, et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond) 2013;37(7):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liber A, Szajewska H. Effect of oligofructose supplementation on body weight in overweight and obese children: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2014;112(12):2068–2074. [DOI] [PubMed] [Google Scholar]
- 74.Hamilton MK, Ronveaux CC, Rust BM, et al. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G474–G487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thum C, McNabb WC, Young W, Cookson AL, Roy NC. Prenatal caprine milk oligosaccharide consumption affects the development of mice offspring. Mol Nutr Food Res. 2016;60(9):2076–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, Collins C, Ratliff M, Xie B, Wang Y. Breastfeeding Reduces Childhood Obesity Risks. Child Obes. 2017;13(3):197–204. [DOI] [PubMed] [Google Scholar]
- 77.Lee JW, Lee M, Lee J, Kim YJ, Ha E, Kim HS. The Protective Effect of Exclusive Breastfeeding on Overweight/Obesity in Children with High Birth Weight. J Korean Med Sci. 2019;34(10):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. [DOI] [PubMed] [Google Scholar]
- 79.Madan JC, Hoen AG, Lundgren SN, et al. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016;170(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rastall RA. Bacteria in the gut: friends and foes and how to alter the balance. J Nutr. 2004;134(8):2022S–2026S. [DOI] [PubMed] [Google Scholar]
- 81.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84(5):1043–1054. [DOI] [PubMed] [Google Scholar]
- 82.Hellmuth C, Uhl O, Demmelmair H, et al. The impact of human breast milk components on the infant metabolism. PLoS ONE. 2018;13(6): e0197713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koletzko B, Broekaert I, Demmelmair H, et al. Protein intake in the first year of life: a risk factor for later obesity? The EU childhood obesity project. Adv Exp Med Biol. 2005;569:69–79. [DOI] [PubMed] [Google Scholar]
- 84.Yu H, Dilbaz S, Cossmann J, et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest. 2019;130:2485–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]


