Abstract
Background:
Phosphorylated neurofilament heavy subunit (pNF-H) is a constituent protein of the nerve axon, which leaks into the peripheral blood in various central nervous disorders. This study examined the time course of pNF-H value up to 1 month after injury and investigated the correlation with clinical outcome.
Methods:
Serum pNF-H concentration was measured on admission, and at 24 h, 72 h, 1 week, 2 weeks, and 1 month after injury in 20 patients, 15 males and 5 females aged 35–68 years (mean 52 years), with traumatic brain injury (TBI) transported to our hospital between April 2016 and March 2017. The clinical outcome at discharge was evaluated by Glasgow Outcome Scale.
Results:
The pNF-H value showed no increase in patients without brain parenchymal injury, but pNF-H value increased depending on the severity of brain damage. pNF-H value peaked at 2 weeks after injury. Two patients with peak value exceeding 10,000 unit had very severe injury and died during hospitalization. Peak pNF-H value was 3210 ± 1073 unit in 12 patients with good outcome and 9884 ± 2353 unit in 8 patients with poor outcome (P = 0.0119).
Conclusion:
Serum pNF-H level tended to increase and peak at 2 weeks after injury, and the peak pNF-H value was correlated with clinical outcome after TBI. The temporal profile of blood pNF-H seems to be useful to predict the clinical outcome after TBI.
Keywords: Biomarker, Phosphorylated neurofilament, Traumatic brain injury

INTRODUCTION
Severity of cognitive and/or neuronal dysfunction can be determined by the extent of neuronal damage to the brain tissue from both primary and secondary insults following traumatic brain injury (TBI). Prediction of the clinical outcome after TBI may be possible based on various proteins as useful biomarkers.[6,11] Phosphorylated neurofilament heavy subunit (pNF-H) has been reported as a useful marker for functional evaluation in spinal cord injury and acutely worsening compression myelopathy.[3,4,10] In addition, serum pNF-H value in the acute phase of TBI has a significant correlation with clinical outcome.[1,2,9] However, serum pNF-H was only measured up to 72 h after injury and serial changes of pNF-H level for the longer period were not elucidated. The present study examined serial changes in the serum pNF-H level to evaluate pNF-H as a functional predictor of outcome after TBI.
MATERIALS AND METHODS
A total of 20 patients with moderate to severe head injury were transported to our hospital from April 2016 to March 2017. On admission, consciousness level, neurological findings, and systemic abnormal findings were immediately checked. Under systemic monitoring, tracheal intubation was performed if needed. Respiratory and circulatory dynamics were stabilized if possible and head computed tomography (CT) performed to evaluate the surgical indication. Intracranial pressure monitoring sensors were also placed in patients requiring surgery based on the standard treatment guidelines. After surgery, hypothermia treatment was also performed if brain pressure management was insufficient. Serial head CT was performed after surgery.
The content of the clinical trial was examined and approved by the Ethics Committee in our hospital. Written consent forms were obtained from a total of 20 patients, 15 males and 5 females aged 35–68 years (mean 52 years). Serum pNF-H value was measured on admission, and at 24 h, 72 h, 7 days, 14 days, and 1 month after TBI, and the change pattern was observed. Blood samples were examined as soon as possible after collection. Serum pNF-H level was measured by enzyme-linked immunosorbent assay utilizing antigen-antibody reaction using a commercially available kit (BioVendor, Modrice, Czech Republic). A standard concentration curve was prepared using a standard sample (human pNF-H protein) contained in the kit, and the concentration was determined. Two measurements were performed for each sample, and the average value was used as the blood concentration. Cognitive function, neurological findings, activities of daily living, and performance status such as modified Rankin scale were evaluated at 3 months and 6 months after injury. Clinical outcome at discharge was evaluated using the Glasgow Outcome Scale in five stages of good recovery (GR), moderate disability (MD), severe disability (SD), vegetative state (VS), and death (D).
Statistical analysis
Values are expressed as mean ± standard deviation. Serial changes in the pNF-H level were examined using two-factor analysis of variance. Clinical outcome at each follow-up period was compared with the preoperative scores by the Student’s t-test. Results with P < 0.05 were considered statistically significant. SPSS statistical software (IBM Corp., Armonk, New York) was used for statistical analyses.
RESULTS
Solitary brain injury was found in 16 patients and complicated injuries in 4 patients (pneumothorax 1, rib fracture 1, femoral fracture 1, and pulmonary contusion 1). Head CT findings showed acute subdural hematoma (ASDH) in 7, acute epidural hematoma (AEDH) in 5, cerebral contusion in 2, ASDH with brain contusion in 3, traumatic subarachnoid hemorrhage in 2, and diffuse axonal injury in one case. The brain pressure sensor was removed 1 week after surgery. Three patients required hypothermia treatment. The clinical outcome at discharge was GR in 4, MD in 8, SD in 4, VS in 2, and D in 2 cases. Two patients died in this series.
The pNF-H value showed no increase in patients without brain parenchymal injury, however, pNF-H value increased depending on the severity of brain damage. The pNF-H value increased during the 72 h after injury but normalized 1 week after the injury in patients with traumatic subarachnoid hemorrhage. In patients with AEDH, slight increase in pNF-H value was seen immediately after injury and was maintained for about 2 weeks. Increase in pNF-H value was observed immediately within 24 h after the injury, peaked at 2 weeks after the injury, and the increased level was maintained until 1 month after the injury in 12 patients with brain parenchymal injury (ASDH in 7 and brain contusion injury in 5). Two patients with peak value exceeding 10,000 unit had very severe injury and died during hospitalization. In general, serial changes in the pNF-H value showed significant increase and peaked at 2 weeks after injury. The peak pNF-H level remains elevated up to 1 month after injury [Figure 1].The increase in pNF-H value showed a significant difference between good outcome (GR and MD) groups and the poor outcome (SD, VS, and death). Peak pNF-H value was 3210 ± 1073 unit in 12 patients with good outcome and 9884 ± 2353 unit in 8 patients with poor outcome (P = 0.0119) [Figure 2].
Figure 1:
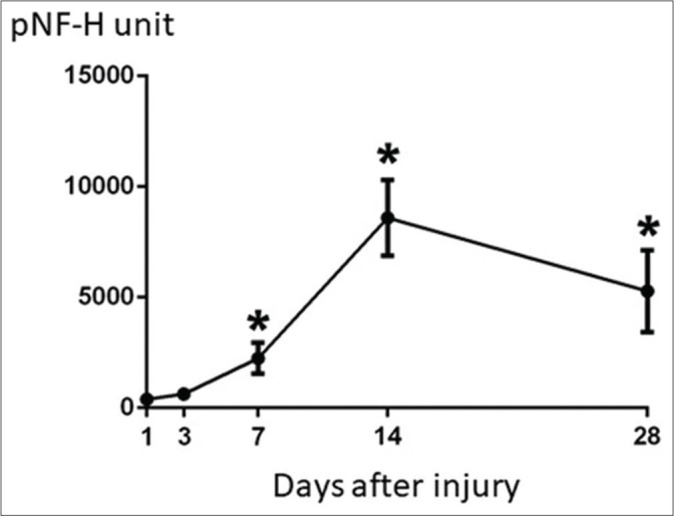
The peak phosphorylated neurofilament heavy subunit level remained elevated up to 1 month after injury.
Figure 2:
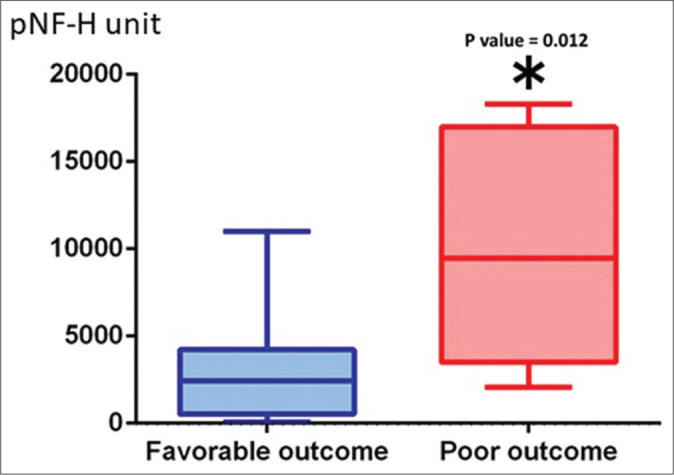
The increase in phosphorylated neurofilament heavy subunit value showed a significant difference between the poor outcome (severe disability, vegetative state, and death) and good outcome (good recovery and moderate disability) groups.
DISCUSSION
The neurofilaments (NFs), the intermediate filaments of the neuronal cytoskeleton, provide mechanical stability to the cell. NFs are relatively scarce in the neuronal cell body and dendrites and are mainly distributed in the nerve axon. NFs have three types of subunits, NF-L (low), NF-M (medium), and NF-H (heavy). pNF-H contains a heavily phosphorylated carboxyl terminal “sidearm” domain which helps determine the interfilament spacing distances. pNF-H has high resistance to protease, so pNF-H released from damaged axons does not decompose but remains unchanged in the body.[7] pNF-H is specifically expressed in the neuronal body. Therefore, detection of pNF-H in the blood or cerebrospinal fluid could be a useful biomarker to evaluate neuronal damage.[5] The serum pNF-H level increases after injury and remains high for several days after injury in the spinal cord injury model.[8] In addition, pNF-H has been reported as a useful predictor after spinal cord injury.[3,4,10]
However, the value of pNF-H as a blood biomarker in brain injury was established. pNF-H level rises in the blood with time from the injury, so should have a longer detection period and higher sensitivity than other markers such as S100B. Serum pNF-H may be useful as a predictive marker for the outcome of patients after TBI.[1,2,9] pNF-H may be released from damaged neurons after TBI in large enough amounts to allow detection in the blood and these levels may reflect the degree of injury severity.[1] An enzyme-linked immunosorbent assay capable of detecting nanogram amounts of pNF-H was used to test the blood of rats subjected to experimental TBI using a controlled cortical impact model. The measurement of blood levels of pNF-H is a convenient method for assessing neuropathology following TBI in the acute stage. The serum profile of pNF-H was measured in patients suffering with moderate TBI with and without CT findings on days 1 and 3 after injury and suggested that detection of pNF-H may be useful in determining which individuals require CT imaging to assess the severity of their injury.[2] The serum pNF-H value at 24 h after injury was a good predictive marker of death at 6 months (P < 0.001) after injury with a cutoff value of 240 pg/ml.[9] The serum pNF-H value at 72 h after injury was correlated with an unfavorable outcome (VS or death) at 6 months (P < 0.01) with a cutoff value of 80 pg/mL. Collectively, these results indicate that serum pNF-H value is a useful predictive marker for patient outcome after TBI. These studies investigated the correlation between clinical outcome and expression of pNF-H in the acute phase up to 72 h after injury. Therefore, serial changes of pNF-H have not yet been elucidated after TBI.
The present study clarified that the increase in pNF-H level after TBI peaked at about 2 weeks–1 month after injury, and the upward trend was maintained for 1 month after injury. The peak of the pNF-H level in the subacute phase, even immediately after injury depending on the severity of the TBI, is very indicative. These serial changes suggest that pNF-H may be involved in repair of the damaged neuronal tissue. Further investigation will be needed to clarify its involvement in damaged brain tissue after TBI.
CONCLUSION
Serum pNF-H increased in patients with the brain tissue damage after TBI and peaked at about 2 weeks to 1 month after injury, which significantly correlated with the clinical outcome after TBI. In addition, the pNF-H upward trend was maintained for 1 month after injury. Therefore, measurement of the peak pNF level may be a useful blood biomarker for functional prognostic prediction after moderate-tosevere TBI.
Footnotes
How to cite this article: Otani N, Morimoto Y, Kinoshita M, Ogata T, Mori K, Kobayashi M, et al. Serial changes in serum phosphorylated neurofilament and value for prediction of clinical outcome after traumatic brain injury. Surg Neurol Int 2020;11:387.
Contributor Information
Naoki Otani, Email: otani.naoki@nihon-u.ac.jp.
Yuji Morimoto, Email: moyan@ndmc.ac.jp.
Manabu Kinoshita, Email: manabu@ndmc.ac.jp.
Toru Ogata, Email: togata16@gmail.com.
Kentaro Mori, Email: kentaro.mori@mt.strins.or.jp.
Masato Kobayashi, Email: kobayashi.masato@nihon-u.ac.jp.
Takeshi Maeda, Email: maeda.takeshi@nihon-u.ac.jp.
Atsuo Yoshino, Email: yoshino.atsuo@nihon-u.ac.jp.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Anderson KJ, Scheff SW, Miller KM, Roberts KN, Gilmer LK, Yang C, et al. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J Neurotrauma. 2008;25:1079–85. doi: 10.1089/neu.2007.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatson JW, Barillas J, Hynan LS, Diaz-Arrastia R, Wolf SE, Minei JP. Detection of neurofilament-H in serum as a diagnostic tool to predict injury severity in patients who have suffered mild traumatic brain injury. J Neurosurg. 2014;121:1232–8. doi: 10.3171/2014.7.JNS132474. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa K, Okazaki R, Ishii K, Ueno T, Izawa N, Tanaka Y, et al. Phosphorylated neurofilament subunit NF-H as a biomarker for evaluating the severity of spinal cord injury patients, a pilot study. Spinal Cord. 2012;50:493–6. doi: 10.1038/sc.2011.184. [DOI] [PubMed] [Google Scholar]
- 4.Kato S, Chikuda H, Ohya J, Hayakawa K, Takeshita K, Tanaka S, et al. Phosphorylated neurofilament subunit levels in the serum of cervical compressive myelopathy patients. J Clin Neurosci. 2015;22:1638–42. doi: 10.1016/j.jocn.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Lewis SB, Wolper RA, Miralia L, Yang C, Shaw G. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2008;28:1261–71. doi: 10.1038/jcbfm.2008.12. [DOI] [PubMed] [Google Scholar]
- 6.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, Van Der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78:1428–33. doi: 10.1212/WNL.0b013e318253d5c7. [DOI] [PubMed] [Google Scholar]
- 7.Petzold A, Shaw G. Comparison of two ELISA methods for measuring levels of the phosphorylated neurofilament heavy chain. J Immunol Methods. 2007;30:34–40. doi: 10.1016/j.jim.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Shaw G, Yang C, Ellis R, Anderson K, Parker Mickle J, Scheff S, et al. Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun. 2005;336:1268–77. doi: 10.1016/j.bbrc.2005.08.252. [DOI] [PubMed] [Google Scholar]
- 9.Shibahashi K, Doi T, Tanaka S, Hoda H, Chikuda H, Sawada Y, et al. The serum phosphorylated neurofilament heavy subunit as a predictive marker for outcome in adult patients after traumatic brain injury. J Neurotrauma. 2016;33:1826–33. doi: 10.1089/neu.2015.4237. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T, Ohori Y, Ito J, Hoshikawa S, Yamamoto S, Nakamura K, et al. Hyperphosphorylated neurofilament NF-H as a biomarker of the efficacy of minocycline therapy for spinal cord injury. Spinal Cord. 2011;49:333–6. doi: 10.1038/sc.2010.116. [DOI] [PubMed] [Google Scholar]
- 11.Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, et al. GFAP and S100B are biomarkers of traumatic brain injury: An observational cohort study. Neurology. 2010;75:1786–93. doi: 10.1212/WNL.0b013e3181fd62d2. [DOI] [PubMed] [Google Scholar]


