Abstract
Background:
Adjuvant chemotherapy is the standard of care for resected pancreatic ductal adenocarcinoma (PDAC). It is estimated that only 40–80% eligible patients initiate intended adjuvant chemotherapy. Completion rates are largely unknown.
Methods:
A retrospective analysis of outcomes of patients with resected PDAC over an 8-year period at H. Lee Moffitt Cancer Center (MCC) was performed.
Results:
From a total of 309 patients, 299 were included for further analysis. 242 (81%) initiated adjuvant therapy (AT) and 195 (65%) completed the intended course. The median time-to-initiation of AT was 53 days (7.6 weeks). The most common reasons for early discontinuation of AT (n = 47) were toxicity (n = 29), disease recurrence (n = 9), patient decision (n = 4), unrelated comorbidities (n = 3), and death (n = 1). Completion of AT was an independent predictor of overall survival (OS) and recurrence-free survival (RFS) on multivariable analysis (OS: HR 0.41, CI 0.27–0.61, p < 0.001; RFS: HR 0.52, CI 0.36–0.76, p < 0.001). Factors associated with early termination of AT were vascular resection (OR 0.29, CI 0.13–0.67, p = 0.004) and administration of AT with local oncologist as opposed to MCC (OR 0.41, CI 0.21–0.82, p = 0.010).
Conclusion:
Completion of AT is associated with improved survival in patients with resected PDAC. Factors associated with an inability to complete AT include vascular resection and administration of AT with local care team in the patient’s community.
Introduction
Surgical resection combined with adjuvant chemotherapy is recommended as standard of care for localized pancreatic adenocarcinoma4 based on large randomized trials demonstrating improved disease-free and overall survival.5-9 Despite this, recurrence rates of 50–90% have been reported.2,3
In recent years, there has been increased emphasis on value-based medicine and high-quality cancer care.10 Bilimoria and colleagues created a list of 43 high-validity pancreatic cancer-specific quality indicators and encouraged hospitals to use these to monitor and improve care. Listed among these metrics were administration of adjuvant chemotherapy (with or without radiation) and time to initiation of AT relative to surgical resection.11 In 2007, an analysis using NCDB data reported that only 40% of patients received any adjuvant chemotherapy.12 Based on linked data from NSQIP and the NCDB, 62% of patients initiated AT following an uncomplicated post-operative course, but this rate decreased to 44% in the setting of a serious complication.13 Many experts in the field now recommend that receipt of AT be monitored as a quality metric.14
Additional efforts have focused on the significance of timing of AT. Retrospective analysis of the ESPAC-3 trial and data from the Central Pancreas Consortium (CPC) both report that delay in initiation of AT was not associated with worse survival.15,16 It is hypothesized that the more sensitive quality metric is successful completion of intended AT. It is easy to track whether and when adjuvant chemotherapy is initiated, but information on completion rates, treatment interruptions, and dose reductions are not available in typical administrative databases. The only reliable insights we have into completion rates for AT are from clinical trials, which are unlikely to reflect cancer care in the “real world.” We hypothesize that successful completion of intended AT is associated with both the quality of care delivery and tumor biology, making it a useful metric of patient outcome.
The aim of this study was to capitalize on the granularity of a single institution experience to assess outcomes associated with initiation and completion of intended oncologic therapy. Additionally the aim was to identify barriers to successful initiation and completion of intended therapy.
Methods
Study population
An IRB-approved, retrospective cohort study of 309 patients who underwent surgical resection for pancreatic adenocarcinoma over an 8-year period from January 2008–December 2015 at MCC was performed (Fig. 1). All patients who were treated with curative intent and underwent pancreatoduodenectomy, distal pancreatectomy, or total/completion pancreatectomy were included. Minimally invasive and open approaches were included, as were patients treated with neoadjuvant therapy (NAT). Patients found to have stage IV disease on final pathology of frozen section specimens were excluded. Patients were also excluded from analysis if there was insufficient AT data.
Figure 1.
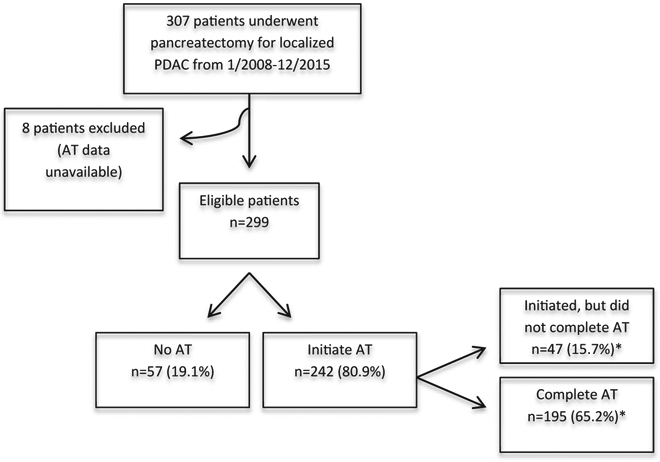
Study design. Abbreviations PDAC, pancreatic ductal adenocarcinoma; AT, adjuvant therapy. *Percentage is of eligible patients.
Description of postoperative outcomes and covariates
Primary outcomes were completion of AT, RFS and OS. Preoperative variables included age, sex, BMI, Charlson Comorbidity Index CCI,17 diabetes, pre-operative lab values, presence of a biliary stent, and neoadjuvant treatment approach. Operative data assessed included vascular resection, minimally invasive versus open approach, estimated blood loss, and operative time. Pathologic variables assessed were tumor size, margin status, lymph node yield and involvement, presence of lymphovascular and perineural invasion, and final AJCC TNM pathologic stage.
Postoperative complications were recorded based on Clavien-Dindo classification18 and pancreatic-specific complications included fistula, hemorrhage, delayed gastric emptying, biliary, and chyle leak. Outcome variables included length of stay, 90-day readmission, and survival data were recorded. OS was defined as the time from first treatment to death, and RFS as the time from resection to recurrence or last contact or death. Data was recorded on whether patients initiated AT, and if so, whether it was completed. Initiation and completion data was collected for both chemotherapy and radiation. In addition, the presence of dose reduction and treatment interruptions was also recorded for both chemotherapy and radiation. Completion of AT was specifically defined as completion of all treatment as planned by treating oncologist. Clinic notes were reviewed to confirm the treatment plan prior to initiation of therapy. Most commonly, the plan for AT consisted of 6 months of gemcitabine-based chemotherapy, but there was heterogeneity among the treatment regimens over the duration of the study. Early recurrence defined as within 4 months of resection occurred in 11 patients (4%). Approximately half of these patients continued with palliative intent chemotherapy and the remaining patients received no further treatment. Patients that developed an early recurrence were classified as not completing AT. The most common treatment plan for patients treated in the NAT setting was two months of upfront therapy followed by four months of AT. Total neoadjuvant therapy was not offered during the study period. Location of AT (MCC versus outside facility) was also documented. Date of last follow up was identified in EMR based on last clinic visit or date of death.
Statistical analysis
Data analyses were performed using SAS version 9.4. Continuous variables were expressed as median and categorical variables were expressed as percentages. Continuous variables were compared with the Mann–Whitney U test and categorical variables were compared using the chi-square or Fisher exact test as appropriate. Multivariable models were designed to include factors the investigators considered likely to be associated with the primary outcomes. Univariable and multivariable Cox proportional models were constructed to analyze association of predictive variables with OS and RFS. To avoid overfitting the data, we analyzed 12 variables, noting that there were 192 events for RFS and 170 events for OS. Analysis of factors associated with failure to complete AT was conducted using univariable and multivariable logistic regression model. Backward elimination of nonsignificant factors (p < 0.25) was utilized in both the Cox and logistic regression models. Relative risks were reported as odds ratio (OR) or hazard ration (HR) with a 95% confidence interval (CI). An association was considered statistically significant if p < 0.05.
Results
Patient characteristics
In total 309 patients were identified. Two were excluded due to stage 4 disease and eight due to insufficient data. Of the 299 patients with PDAC that underwent pancreatectomy with curative intent, 242 (81%) initiated AT and 57 (19%) received no AT. Among the 242 patients that initiated AT, 47 (16%) were not able to complete the intended course. This yielded an overall completion rate of 65% (n = 195). A total of 196 (66%) patients underwent upfront resection and the remaining 103 (34%) were treated with NAT.
Median time-to-initiation of chemotherapy was 7.6 weeks (53 days). 45% (n = 133) of patients were treated with adjuvant chemotherapy and 37% (n = 109) underwent adjuvant chemotherapy and radiation. The remaining patients received no AT. The most commonly initiated adjuvant regimens were gemcitabine monotherapy (n = 108) and gemcitabine with 5-FU based chemoradiation (n = 88). 80% of patients treated with adjuvant chemotherapy alone received gemcitabine monotherapy and 81% of patients with adjuvant chemotherapy and radiation received gemcitabine with 5-FU based chemoradiation. The most common neoadjuvant regimen was gemcitabine, docetaxel, and capecitabine with stereotactic beam radiation (GTX + SBRT). The median duration of adjuvant chemotherapy (AC) was 18.7 weeks (131 days) and adjuvant chemoradiation (ACR) was 21 weeks (147 days).
Baseline patient characteristics are reported in Table 1 with data included for the overall cohort, patients who did not receive AT, patients who needed to terminate AT prematurely, and patients who completed all intended AT. Several factors were associated with omission of AT, including age (p = 0.001), Charlson comorbidity index (p = 0.002), grade 3–4 surgical complications (p = 0.001), and 90-day readmission (p = 0.001).
Table 1.
Perioperative characteristics divided according to receipt of adjuvant therapy
| Patient characteristics (% or median) | Overall Cohort |
No AT |
Initiated AT |
Initiated, but did not complete AT |
Completed AT |
|---|---|---|---|---|---|
| N = 299 | N = 57 | N = 242 | N = 47 | N = 195 | |
| Age, years | 68 | 74 | 67 | 71 | 67 |
| Male (%) | 57.3 | 65.5 | 54.3 | 53.2 | 54.9 |
| Race (%) | |||||
| White | 90.6 | 96.5 | 90.1 | 93.6 | 89.2 |
| Black | 4.2 | 1.7 | 4.9 | 4.3 | 5.1 |
| Other | 5.2 | 1.7 | 5.0 | 2.1 | 5.6 |
| Body mass index, Kg/m2 | 26.1 | 26.5 | 26.1 | 25.8 | 26.3 |
| Albumin, g/dL | 4.1 | 4 | 4.1 | 4.1 | 4.1 |
| Comorbidity index | 4 | 5 | 4 | 5 | 4 |
| Diabetes (%) | 27.7 | 37.9 | 25.5 | 25.5 | 25.6 |
| Resection (%) | |||||
| Whipple | 72.9 | 74.1 | 72.8 | 61.7 | 75.4 |
| Distal | 21.5 | 19.0 | 21.4 | 27.7 | 20.0 |
| Total/Completion | 5.6 | 6.9 | 5.8 | 10.6 | 5.1 |
| Neoadjuvant (%) | 34.4 | 31.0 | 35.0 | 38.3 | 34.9 |
| Vascular resection (%) | 12.2 | 12.4 | 12.8 | 25.5 | 9.7 |
| Estimated blood loss (cc) | 300 | 350 | 300 | 300 | 275 |
| Operative time (min) | 404 | 457.5 | 398 | 380 | 404 |
| Number of LN resected | 18.5 | 15 | 19 | 21 | 19 |
| Positive 1 Margin (%) | 10 | 8.6 | 9.9 | 12.8 | 9.2 |
| Pathologic Stage (%) | |||||
| 0 (ypT0N0) | 1.0 | 1.7 | 0.82 | 0 | 1.0 |
| 1A | 6.2 | 8.6 | 6.3 | 6.4 | 6.2 |
| 1B | 5.5 | 8.6 | 4.9 | 8.5 | 4.6 |
| 2A | 29.3 | 37.9 | 28.4 | 31.9 | 27.7 |
| 2B | 56.8 | 41.4 | 59.7 | 59.6 | 59.5 |
| 3 | 1.0 | 1.7 | 0.82 | 0 | 1.0 |
| Grade 3–4 complication (%) | 13.6 | 29.3 | 10.7 | 10.6 | 10.8 |
| Length of stay, days | 10 | 12.5 | 10 | 10 | 10 |
| 90-day readmission (%) | 17.7 | 32.8 | 14.0 | 10.6 | 14.9 |
| 90-day mortality (%) | 2.9 | 13.8 | 0.4 | 2.1 | 0.0 |
| AT therapy at MCC (%) | 55.3 | – | 56.0 | 36.2 | 61 |
AT, adjuvant therapy. LN, lymph nodes. Stage 0 patients had complete pathologic response. MCC, Adjuvant therapy performed at Moffitt Cancer Center as opposed to with local care team.
Overall and recurrence-free survival
Median OS for the entire cohort was 24 months. 30-day mortality was 1.9% and 90-day mortality was 2.9%. Patients who received no AT had the worst median survival (14 months). Patients who initiated AT had an OS of 26 months. Patients who initiated, but did not complete AT had an OS of 15 months while those who completed AT had median OS of 28 months.
During the follow up period, 69% of patients presented with recurrent disease. Sites of first metastatic recurrence included liver (n = 65), lung (n = 38), peritoneum/omentum (n = 21), multifocal (n = 13), and other (n = 18). Among patients with disease recurrence, 16% (n = 29) presented with isolated local recurrence. Analysis of factors associated with survival are shown in Table 2.
Table 2.
Analysis of factors associated with recurrence-free and overall survival
| Patient Characteristics | Univariable Analysisa |
Multivariable Analysisb |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Recurrence-Free Survival | ||||
| Charlson Comorbidity Index | 1.09 (0.99–1.20) | 0.075 | ||
| Age (years) | 1.01 (1.00–1.03) | 0.159 | ||
| Tumor Size (mm) | 1.25 (1.13–1.37) | <0.001 | 1.11 (1.002–1.230) | 0.046 |
| Pre-Op CA 19-9c | 1.60 (1.35–1.90) | <0.001 | 1.30 (1.072–1.577) | 0.008 |
| Neoadjuvant Therapy | 0.49 (0.36–0.67) | <0.001 | 0.647 (0.432–0.969) | 0.035 |
| Number of Positive LNsd | 1.70 (1.48–1.95) | <0.001 | 1.447 (1.231–1.701) | <0.001 |
| Vascular Resection | 1.07 (0.69–1.67) | 0.761 | 1.541 (0.934–2.542) | 0.091 |
| Positive Margin | 1.26 (0.77–2.05) | 0.355 | ||
| Grade 3–4 Complication | 1.02 (0.63–1.66) | 0.941 | ||
| Time to Initiation of AT >9 wks | 1.09 (0.79–1.5) | 0.613 | ||
| Completion of AT | 0.47 (0.33–0.66) | <0.001 | 0.52 (0.359–0.755) | <0.001 |
| AT Performed Locally | 1.24 (0.92–1.66) | 0.153 | ||
| Overall Survival | ||||
| Charlson Comorbidity Index | 1.16 (1.05–1.29) | 0.003 | 1.133 (1.017–1.263) | 0.024 |
| Age (years) | 1.01 (1.00–1.03) | 0.154 | ||
| Tumor Size (mm) | 1.21 (1.10–1.34) | <0.001 | 1.12 (1.006–1.246) | 0.038 |
| Pre-Op CA 19-9c | 1.4 (1.19–1.66) | <0.001 | 1.204 (0.993–1.459) | 0.059 |
| Neoadjuvant Therapy | 0.55 (0.40–0.77) | <0.001 | 0.761 (0.511–1.134) | 0.180 |
| Number of Positive LNsd | 1.54 (1.34–1.79) | <0.001 | 1.341 (1.131–1.590) | <0.001 |
| Vascular Resection | 1.00 (0.63–1.58) | 0.987 | ||
| Positive Margin | 1.52 (0.92–2.51) | 0.106 | 1.547 (0.910–2.628) | 0.107 |
| Grade 3–4 Complication | 1.30 (0.81–2.10) | 0.283 | ||
| Time to Initiation of AT > 9 wks | 1.09 (0.78–1.53) | 0.601 | ||
| Completion of AT | 0.40 (0.28–0.58) | <0.001 | 0.410 (0.274–0.614) | <0.001 |
| AT Performed Locally | 1.32 (0.97–1.79) | 0.076 | 1.291 (0.915–1.820) | 0.146 |
Abbreviations: HR, hazard ratio; OR, odds ratio; CI, confidence interval; AT, adjuvant therapy.
N = 215–228, RFS; N222-235 OS.
N = 199, RFS; N = 205 OS.
log10 transformation.
Square root transformation.
Predictors of inability to complete AT
Reasons sited for failure to initiate chemotherapy or failure to complete it once initiated are detailed in Table 3A. Interruptions in therapy and dose reductions are detailed in Table 3B. Analysis of predictors of failure to complete AT is summarized in Table 4.
Table 3.
(A) Reason for failure to initiate AT or early discontinuation of AT. (B) Dose reductions in patients that completed AT
| (A) Reason for failure to initiate AT or early discontinuation of AT | |||||
|---|---|---|---|---|---|
| Reason for failure to initiate AT (n = 57) | n | % | Reason for early discontinuation of AT (n = 47) | n | % |
| Surgical complication | 25 | 44.8 | Toxicity | 29 | 61.7 |
| Early Recurrence | 4 | 6.9 | Recurrence | 7 | 14.8 |
| Patient Decision | 11 | 19.0 | Patient Decision | 4 | 8.5 |
| Poor functional status (age > 70) | 15 | 25.9 | Poor performance status/unrelated comorbidities | 3 | 6.4 |
| Poor functional status (age<70) | 2 | 3.4 | Death | 1 | 2.1 |
| Reason Unclear | 3 | 6.4 | |||
| (B) Dose reductions and interruptions in patients that completed adjuvant therapy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chemotherapy (n = 195) |
Radiation (n = 92) |
|||||||
| Interruption |
Dose Reduction |
Interruption |
Dose Reduction |
|||||
| n | % | n | % | n | % | n | % | |
| Overall Cohort | 50 | 25.6 | 39 | 20 | 11 | 12.0 | 8 | 8.7 |
AT, Adjuvant therapy.
Note: These numbers were extracted from medical record and likely underestimate the true incidence of dose reductions and interruptions.
Table 4.
Analysis of Factors Associated with Failure to Complete Adjuvant Therapy Once it has been Initiated
| Patient Characteristics | Completion of AT |
Univariable Analysis |
Multivariable Analysis |
|||
|---|---|---|---|---|---|---|
| n | % | OR (95% CI) | P | OR (95% CI) | P | |
| Charlson Comorbidity Index | 242 | – | 0.84 (0.68–1.04) | 0.108 | ||
| Neoadjuvant Therapy | ||||||
| Yes | 85 | 35.1% | 0.85 (0.44–1.64) | 0.628 | ||
| No | 157 | 64.9% | ||||
| Number of LNs Examined | 242 | – | 0.92 (0.69–1.21) | 0.535 | ||
| Number of Positive LNsa | 242 | – | 0.88 (0.66–1.18) | 0.393 | ||
| Vascular Resection | ||||||
| Yes | 31 | 12.8% | 0.32 (0.14–0.71) | 0.005 | 0.29 (0.13–0.67) | 0.004 |
| No | 211 | 87.2% | ||||
| Grade 3–4 Complication | ||||||
| Yes | 26 | 10.7% | 1.01 (0.36–2.85) | 0.979 | ||
| No | 216 | 89.3% | ||||
| Time to Initiation of AT | ||||||
| >9 weeks | 66 | 27.4% | 0.73 (0.37–1.47) | 0.378 | ||
| ≤9 weeks | 175 | 72.6% | ||||
| Location of AT | ||||||
| Local | 104 | 43.3% | 0.39 (0.20–0.76) | 0.005 | 0.41 (0.21–0.82) | 0.010 |
| Moffitt Cancer Center | 136 | 56.7% | ||||
Abbreviations: HR, hazard ratio; OR, odds ratio; CI, confidence interval; AT, adjuvant therapy.
Square root transformation.
Discussion
This study suggests that completion, and not just initiation, of intended AT is associated with a significant survival advantage among patients with PDAC. A retrospective analysis of the ESPAC-3 trial reported that completion of all six intended cycles of AT was an independent predictor of survival after pancreatectomy.15 This study is the first to explore this finding outside of a clinical trial in patients treated at a high volume cancer center in the United States. As completion data is not typically recorded in large databases, “real world” completion rates of AT for pancreatic cancer are largely unknown. Overall a completion rate of 65% was achieved, which is similar to the ESPAC-3 trial (68% completion rate.) 19% of patients in the current study received no AT and 16% did not complete the intended course. A single institution analysis of 113 patients that underwent resection with curative intent for PDAC at Seoul National University Bundang Hospital also reported a similar completion rate of 63%.19 To the authors’ knowledge, these are the only reports in the literature that include data on completion of AT for pancreatic cancer.
The underutilization of both surgery and AT for stage I/II PDAC is well documented.11,12,20 An AT initiation rate of 40% is frequently cited.12 Under-utilization is often amplified among elderly, poor, and minority patients.21-23 However, recent reports are somewhat more encouraging with linked data from the NCDB and NSQIP reporting AT initiation rate of 58%13 and analysis from the CPC reporting AT initiation rate of 80%.16 The MCC rate of 81% is in line with the CPC data.
This study is the first to independently evaluate clinical predictors of early termination of AT. Vascular resection/reconstruction and pursuit of AT with a local care team in the patient’s community are independent predictors of early termination of AT on multivariable analysis. Interestingly, vascular resection was not associated with an inability to initiate AT in this series, but was highly associated with an inability to complete therapy once it was initiated. These findings may be driven by the aggressive tumor biology and disease burden in patients who require vascular resection. As performance of a vascular resection did not limit patients’ ability to initiate chemotherapy, it is possible the association with completion is not related to the increased surgical complexity with resultant late-term complications. Notably, reasons for early termination of therapy were reviewed in these patients and were similar in nature and frequency to those in the overall cohort. Further analysis is needed to better explain these findings.
Pursuit of AT therapy with a local oncologist in the patient’s community was also significantly associated with inability to complete the full course of AT. This study represents the first report of such an association. A precise explanation for this finding is difficult to pinpoint. A recent NCDB analysis of 32,521 patients with resected PDAC reported that patients from remote areas are less likely to initiate AT and had worse survival.24 One explanation is that there is significant selection bias among patients treated a tertiary care centers. Specifically, patients with greater social and financial resources, and better baseline health may seek AT at NCI-designated cancer centers. Completion of AT is physically, psychosocially, and financially challenging for patients and the option of completing therapy closer to home relieves some of this burden and should be encouraged when appropriate. Additional resources and support may be needed to allow more patients to complete the intended course of therapy.
Treatment at MCC may favor patients who live in the Tampa metropolitan area as opposed to more rural regions. Nationally, patients treated at NCI-designated cancer centers tend to be younger, white, and have fewer comorbidities compared with patients treated at other centers.25,26 While many factors, including physician bias and financial incentives, can drive decision-making in cancer care and could influence these findings, investigation of these was beyond the scope of this work. Several factors were not significantly associated with decreased completion rates. In agreement with other reports,13,27,28 grade 3–4 post-operative complications were associated with omission of all AT. However, surgical complications were not associated with completion of AT once it was begun.
The median time-to-initiation of AT was 7.6 weeks in this series, which is similar to what was reported by the CPC.16 Patients were divided into two groups according to whether they initiated AT before or after 9 weeks. There was no significant difference in completion rates or OS between patients that initiated AT before or after 9 weeks. The optimal timing of AT has remained an area of uncertainty, with some reports demonstrating no significant difference in OS with later initiation of AT,15,16,29 while others have reported better survival outcomes with earlier initiation.19,30 Earlier initiation of chemotherapy may eradicate residual tumor cells and combat the high rates of distant failure among patients with PDAC. However, a less aggressive approach allows patients to fully recover in hopes of better tolerating a full course of AT with minimal interruptions in therapy or dose reductions. Although time to initiation of AT has been identified as a useful quality metric in other malignancies, such as breast and colon cancer,31-34 there is insufficient data to support its use as a quality metric for PDAC.
Receipt of NAT was not associated with completion of AT, but was associated with improved OS and RFS. It is our institutional practice to recommend AT to all patients regardless of whether they were treated in a neoadjuvant setting. No patients in this study were treated with a total neoadjuvant approach. Given the national trend toward increased utilization of NAT, we felt our analysis would be more relevant to current clinical practice if patients who were treated in the neoadjuvant setting were included. Despite a paucity of randomized trials to support a neoadjuvant approach, several groups have advocated for increased utilization of NAT regardless of primary tumor extent.35-37 This remains an area of significant debate.37,38
In summary, this study has identified completion and not just initiation of intended AT as a useful quality metric associated with improved OS for patients with resected PDAC. Both initiation and completion of AT should be carefully monitored. Controversy remains over the reliability of time-to-initiation of AT as a quality metric and the available data do not support its use. Two predictors of early termination of AT were identified, including performance of vascular resection/reconstruction and receipt of ATwith local care team. These findings should be taken into consideration when counseling patients about AT. Furthermore, clinicians must ensure that patients social, financial, and medical resources are optimized in attempt to maximize the number of patients that are able to complete the recommended AT.
This study is exposed to the usual limitations of a retrospective single-institution analysis. There is the potential for significant unmeasured bias and confounding. Data from a single center are unlikely to accurately reflect the entire spectrum of surgical management of PDAC at a wide range of centers. In addition, significant advances have been made in recent years with respect to chemotherapy. Thus, both the NAT and AT regimens administered to patients in this study vary considerably from what is utilized today. However, as more effective systemic options are developed, it is expected that the findings supporting the importance of completing AT will only become more relevant.
Footnotes
Conflicts of interest
None to declare
This study was presented at the AHPBA 2018 annual meeting, March 2018, Miami, Florida, USA
References
- 1.Siegel RL, Miller KD, Jemal A. (2018) Cancer statistics, 2018. CA A Cancer J Clin 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH et al. (2007) Impact of resectio status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 246:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh AA, Maiga A, Bentrem D, Squires MH, Kooby DA, Maithel SK et al. (2016) Adjuvant therapy in pancreas cancer: does it influence patterns of recurrence? J Am Coll Surg 222:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW et al. (2017) Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15:1028–1061. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K et al. (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H et al. (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K et al. (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D et al. (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304:1073–1081. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM et al. (2017) Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 10.Abbott DE, Martin G, Kooby DA, Merchant NB, Squires MH, Maithel SK et al. (2016) Perception Is Reality: quality metrics in pancreas surgery - a Central Pancreas Consortium (CPC) analysis of 1399 patients. HPB 18:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Bentrem DJ, Lillemoe KD, Talamonti MS, Ko CY. (2009) Pancreatic Cancer Quality Indicator Development Expert Panel AeCoS. Assessment of pancreatic cancer care in the United States based on formally developed quality indicators. J Natl Cancer Inst 101:848–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP et al. (2007) Multimodality therapy for pancreatic cancer in the U.S. : utilization, outcomes, and the effect of hospital volume. Cancer 110:1227–1234. [DOI] [PubMed] [Google Scholar]
- 13.Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS et al. (2014) Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 260:372–377. [DOI] [PubMed] [Google Scholar]
- 14.Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN. (2014) Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol 110:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P et al. (2014) Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 32:504–512. [DOI] [PubMed] [Google Scholar]
- 16.Xia BT, Ahmad SA, Al Humaidi AH, Hanseman DJ, Ethun CG, Maithel SK et al. (2017) Time to initiation of adjuvant chemotherapy in pancreas cancer: a multi-institutional experience. Ann Surg Oncol 24:2770–2776. [DOI] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196. [DOI] [PubMed] [Google Scholar]
- 19.Kim HW, Lee JC, Lee J, Kim JW, Kim J, Hwang JH. (2017) Early versus delayed initiation of adjuvant treatment for pancreatic cancer. PLoS One 12e0173960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. (2007) National failure to operate on early stage pancreatic cancer. Ann Surg 246:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eubanks A, Pepe J, Veldhuis P, de la Fuente SG. (2018) Age as a prognostic indicator for adjuvant therapy in patients who underwent pancreatic resections for cancer. J Geriatr Oncol 9:362–366. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MM, Simons JP, Ng SC, McDade TP, Smith JK, Shah SA et al. (2009) Racial differences in cancer specialist consultation, treatment, and outcomes for locoregional pancreatic adenocarcinoma. Ann Surg Oncol 16:2968–2977. [DOI] [PubMed] [Google Scholar]
- 23.Cheung MC, Yang R, Byrne MM, Solorzano CC, Nakeeb A, Koniaris LG. (2010) Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer 116:723–733. [DOI] [PubMed] [Google Scholar]
- 24.Bertens KA, Massman JD, Helton S, Garbus S, Mandelson MM, Lin B et al. (2018) Initiation of adjuvant therapy following surgical resection of pancreatic ductal adenocarcinoma (PDAC): are patients from rural, remote areas disadvantaged? J Surg Oncol 117:1655–1663. [DOI] [PubMed] [Google Scholar]
- 25.Merkow RP, Bentrem DJ, Chung JW, Paruch JL, Ko CY, Bilimoria KY. (2013) Differences in patients, surgical complexity, and outcomes after cancer surgery at National Cancer Institute-designated cancer centers compared to other hospitals. Med Care 51:606–613. [DOI] [PubMed] [Google Scholar]
- 26.Friese CR, Earle CC, Silber JH, Aiken LH. (2010) Hospital characteristics, clinical severity, and outcomes for surgical oncology patients. Surgery 147:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aloia TA, Aloia TE, Lee JE, Vauthey JN, Abdalla EK, Wolff RA et al. (2007) Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 204:347–355. [DOI] [PubMed] [Google Scholar]
- 28.Kim BJ, Caudle AS, Gottumukkala V, Aloia TA. (2016) The impact of postoperative complications on a timely return to intended oncologic therapy (RIOT): the role of enhanced recovery in the cancer journey. Int Anesthesiol Clin 54:e33–e46. [DOI] [PubMed] [Google Scholar]
- 29.Mirkin KA, Greenleaf EK, Hollenbeak CS, Wong J. (2016) Time to the initiation of adjuvant chemotherapy does not impact survival in patients with resected pancreatic cancer. Cancer 122:2979–2987. [DOI] [PubMed] [Google Scholar]
- 30.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N et al. (2013) Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol 71:419–429. [DOI] [PubMed] [Google Scholar]
- 31.Gagliato DeM, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V et al. (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farolfi A, Scarpi E, Rocca A, Mangia A, Biglia N, Gianni L et al. (2015) Time to initiation of adjuvant chemotherapy in patients with rapidly proliferating early breast cancer. Eur J Cancer 51:1874–1881. [DOI] [PubMed] [Google Scholar]
- 33.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. (2011) Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 305:2335–2342. [DOI] [PubMed] [Google Scholar]
- 34.Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR et al. (2006) Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer 107:2581–2588. [DOI] [PubMed] [Google Scholar]
- 35.Abbott DE, Tzeng CW, Merkow RP, Cantor SB, Chang GJ, Katz MH et al. (2013) The cost- effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol 20(Suppl 3):S500–S508. [DOI] [PubMed] [Google Scholar]
- 36.Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC et al. (2017) Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol 35:515–522. [DOI] [PubMed] [Google Scholar]
- 37.Russo S, Saif MW. (2016) Neoadjuvant therapy for pancreatic cancer: an ongoing debate. Therap Adv Gastroenterol 9:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinrich S, Lang H. (2017) Neoadjuvant therapy of pancreatic cancer: definitions and benefits. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]


