Abstract
Introduction:
Ampullary adenocarcinoma (AAC) is a rare neoplasm. We sought to determine the clinicopathologic factors contributing to the overall survival (OS) and recurrence-free (RFS) survival.
Methods:
Patients (pts) with resected AAC were identified from 1996 to 2015 and reviewed for clinicopathologic factors and correlated with outcome.
Results:
We identified and evaluated 106 pts diagnosed with AAC. The median age was 70.2 years (range 41–86) and 60 (56.6%) were male. Overall, 105 pts (99.1%) had a pancreaticoduodenectomy. An R0 resection was achieved in 101 (95%) pts. Median follow-up was 19 months with a median OS of 49.3 months. Lymph node metastasis and poorly differentiated tumors adversely affected OS on multivariate analysis (MVA). Twenty patients (18.9%) developed recurrence. The median RFS was 27 months. RFS was adversely affected by lymph node count and metastasis, tumor differentiation, and histological subtype on MVA. Survival was not affected by the addition of adjuvant therapy. Retrieval of ≤12 lymph nodes and lymph node ratio ≥0.10 resulted in worse OS on Kaplan–Meier analysis.
Conclusions:
Our data show retrieval of ≤12 nodes, involvement of nodes with AAC, moderately or poorly differentiated tumors, and pancreaticobiliary subtype adversely affected survival, while the use of adjuvant therapy demonstrated no significant benefit.
Keywords: ampullary adenocarcinoma, clinicopathologic factors, survival, lymph node, ratio
INTRODUCTION
Ampullary adenocarcinoma (AAC) is a rare neoplasm, accounting for approximately 0.2–0.5% of all gastrointestinal cancers [1,2]. Historically, AAC have been grouped with other periampullary tumors, which include distal cholangiocarcinoma, pancreatic, and duodenal adenocarcinoma. Even though they are rare tumors, there has been an increase in the incidence and diagnosis [1]. A National Cancer Institute Survival, Epidemiology, and End Results (SEER) study, which evaluated 5,625 patients with AAC spanning from 1973 to 2005, showed the incidence has been increasing by 0.9% per year. The 5-year survival in the study for localized and regional disease was 45% and 31%, respectively [3]. Other authors have reported 5-year survival rates as high as 65% following radical resection with a pancreaticoduodenectomy (PD) [4].
The current gold standard of treatment for AAC is surgical resection, with PD being the procedure of choice among surgeons. Brown et al. reported a potential 80% long-term survival rate in node-negative ampullary carcinoma patients undergoing PD and a potential 20% cure rate in node-positive patients. These findings led them to recommend PD for all adenocarcinoma patients [5]. Other studies have suggested that ampullectomy or even endoscopic resection is feasible for early-stage cancers. However, many studies recommend radical resection for all ampullary cancers, especially since a 30% lymph node metastasis rate has been demonstrated in T1 lesions [4,6,7].
Lymph node involvement has been shown to be an important prognostic indicator for patients who underwent PD for periampullary tumors [8–10]. Hurtuk et al. first reported on the prognostic impact of lymph node ratio (LNR) in periampullary cancers and showed an increased LNR was associated with decreased survival rates in pancreatic (P = 0.03) and ampullary cancers (P = 0.04), but not in duodenal adenocarcinoma or cholangiocarcinoma [11]. They also state the number of lymph nodes needed to accurately assess the LNR should be at least 10 or more, thereby avoiding the potential for stage migration effect and inaccurate survival assessments [11].
Other prognostic factors have been studied and include adjuvant therapy, postoperative complications, and tumor differentiation. Multiple studies evaluating adjuvant therapy in the literature report conflicting results, with some showing no benefit in overall survival (OS) or disease-free survival (DFS), while others state there may be a potential benefit in 3-year local control rate [12,13]. Wu et al. analyzed over 1,000 patients and demonstrated longer median OS in patients without postoperative complications after PD for periampullary tumors. Interestingly, all the patients in this cohort received adjuvant therapy of some type, although no association was made between the having complications and receiving adjuvant therapy [14]. Last, poor tumor differentiation has been shown to be significantly associated with worse survival in multiple studies [8–10,15]. This study aims to evaluate patient characteristics and clinicopathologic factors for resected AAC at our institution and correlate those with survival and outcome.
PATIENTS AND METHODS
After obtaining Institutional Review Board approval, a retrospective series of consecutive patients with AAC were identified from a single institutional database from 1996 to 2015. Demographic and clinicopathologic characteristics (age at diagnosis, sex, presenting symptoms, stage, tumor, and histological differentiation, subtype, lymphovascular invasion [LVI], and perineural invasion [PNI]) and outcome data were abstracted from the database and chart review (Table I). Adjuvant treatment was also evaluated in the study, although these treatment modalities were not uniformly recorded for all patients. The primary outcome was survival, which was calculated from time of surgery to date of last follow-up or death. Recurrence was documented as local, regional, or distant. Local recurrence was defined as recurrence within the tumor bed or along the retroperitoneal margin. Regional recurrence was defined as recurrence in retroperitoneal, paracaval, and peripancreatic lymph nodes.
TABLE I.
Patient Demographics and Tumor Characteristics
| Characteristics | Entire cohort, N = 106 |
|---|---|
| Age at diagnosis (years) | |
| Median (range) | 70 (41–86) |
| Gender (%) | |
| Male | 60 (56.6) |
| Presenting symptom (%) | |
| Jaundice/increased LFT’s | 57 (53.7) |
| Abdominal pain | 32 (30.2) |
| Bleeding | 9 (8.5) |
| Pancreatitis | 2 (1.9) |
| Incidental finding | 6 (5.7) |
| Nodal stage (%) | |
| N0 | 56 (52.8) |
| N1 | 50 (47.2) |
| AJCC TNM stage (%) | |
| 0 | 1 (1.0) |
| IA | 9 (8.5) |
| IB | 24 (22.6) |
| IIA | 18 (17.0) |
| IIB | 41 (38.7) |
| III | 13 (12.2) |
| Tumor differentiation (%) | |
| Well | 16 (15.1) |
| Moderate | 70 (66) |
| Poor | 20 (18.9) |
| Histological differentiationa | |
| Intestinal | 50 (47.2) |
| Pancreaticobiliary | 30 (28.3) |
| LVI (%) | |
| Present | 54 (50.9) |
| Absent | 52 (49.1) |
| PNI (%)a | |
| Present | 31 (29.2) |
| Absent | 72 (67.9) |
| Adjuvant therapy | |
| Chemotherapy only | 10 (9.4) |
| Chemotherapy + RTb | 33 (31.2) |
| None | 63 (59.4) |
LFT’s, liver function tests; LVI, lymphovascular invasion; PNI, perineural invasion; RT, radiation therapy.
Data are unavailable or missing for histological differentiation in 26 cases and for PNI in 3 cases.
Only one patient received RT without chemotherapy.
Diagnostic workup for all patients included cross-sectional imaging, which consisted of a computed tomography (CT) scan of the chest, abdomen, and pelvis. Some patients had additional imaging which consisted of positron emission tomography (PET) scan. Patients were also evaluated with endoscopic ultrasound (EUS), although this was only routinely performed on patients diagnosed with AAC from 2014 and on. Those with documented clinical or radiographic evidence of distant metastatic disease were excluded from this study. Patients with an additional diagnosis of high grade dysplasia identified on final pathology were not excluded.
All pathology specimens were evaluated by a board-certified pathologist. All available original tissue biopsies obtained at an outside institution were reexamined prior to surgical intervention per standard protocol at our institution. LNR was calculated as the number of positive nodes divided by the total number of nodes retrieved and examined. The lymph node count (LNC) was dichotomized at 12, which allowed for the greatest significance on final survival analysis. Tumors were staged according to the 7th edition of the American Joint Committee on Cancer staging system. Postoperative complications that occurred within the first 30 days after the initial operation were recorded and defined using the Clavien–Dindo classification of surgical complications.
Statistical Analysis
Demographic data and clinical variables were collected and analyzed. Five-year OS, recurrence-free survival (RFS), as well as median survival times were calculated using the Kaplan–Meier method of estimation. Kaplan–Meier plots were generated and survival curves were compared with the log-rank test. Chi-squared test was used to compare categorical variables and LN groups; Wilcoxon rank sum test was used for continuous variables, both with the exact method using Monte Carlo estimation. Univariate analysis (UVA) and multivariate analysis (MVA) were performed with Cox proportional hazard models. Statistical significance was determined by a P-value of ≤0.05. All variables with a P-value of ≤0.10 in the UVA were included in the MVA. All analysis was done in R version 3.1.0 (a statistical computing environment).
RESULTS
Patients and Tumor Characteristics
A total of 106 patients met study criteria and were included for review and analysis. Patient demographics and tumor characteristics are shown in Table I. The median age for the cohort was 70.2 years (range 41–86), and the majority of the patients were male (56.6%). The majority of patients presented with jaundice and increased liver function enzymes (LFT’s) (53.7%), while the remaining patients presented with abdominal pain (30.2%), bleeding (8.5%), and pancreatitis (1.9%). The other 5.7% were diagnosed as an incidental finding (Table I). All but one patient underwent a PD, and of those, 101 (95%) had an R0 resection. Overall, 59 patients (55.7%) had stage II disease, while the remaining 33 (31.1%) and 13 (12.2%) had stages I and III disease, respectively. LVI and PNI were also present in 51% and 29.2% of patients, respectively. Forty-three patients (40.6%) received some form of adjuvant therapy as depicted in Table I. Of the 43, 10 (23%) received chemotherapy-only consisting of 5-fluorouracil, capecitabine, gemcitabine, or docetaxel. Thirty-two (74%) received chemoradiation and one (2%) received radiation-alone. Histological differentiation was reported in 80 patients. Of those, 30 patients (28.3%) had pancreaticobiliary differentiation, while 50 (47.2%) had intestinal differentiation.
Survival Analysis
The median follow-up for the entire cohort was 19 months, with a range of 0.6–133 months. At the time of last follow-up, 56 patients (52.8%) were alive (49 without evidence of disease, 7 with recurrent disease). Fifty patients (47.2%) had died, and of those, 11 died of disease while the remaining 39 died of other or unknown causes. The median OS for the entire cohort was 49.3 months, while the median RFS was 27 months.
Overall Survival
The 5-year OS for the entire cohort was 43% as demonstrated by Kaplan–Meier survival analysis. Improved OS was seen with well differentiated tumors compared to moderate and poorly differentiated, P = 0.006 (Fig. 1). Kaplan–Meier OS was also stratified by LNC and LNR. The median number of LNC at the completion of resection was 14 with a range of 0–44. Analysis of LNC was dichotomized at 12. Forty-nine patients (43%) had ≤12 lymph nodes and 57 (57%) had >12 lymph nodes. A LNC of ≤12 adversely affected OS, while the median OS was not reached for a LNC of >12 as depicted in Figure 2 (P = 0.01). LNR was dichotomized at 0.10; 28 (26.7%) had a LNR of ≥0.1 (range 0.10–1.00), while 78 (73.3%) had a LNR of <0.1. A LNR of <0.1 showed significant improvement in OS compared to ≥0.1, P < 0.001 (Fig. 3). The median OS for a LNR <0.1 was not reached. Age, LNC, lymph node metastasis, tumor differentiation, LVI, and PNI were variables found to be associated with OS on UVA (Table II). LNC (HR = 0.20, CI = 0.08–0.48, P < 0.001), moderately and poorly differentiated tumors (HR = 28.77, CI = 2.50–331.08, P = 0.007), and pancreaticobiliary subtype (HR = 2.80, CI = 1.27–6.18, P = 0.01) were significant predictors and adversely affected OS on MVA (Table III). Lymph node metastasis was not significant on MVA (HR = 1.27, CI = 0.98–1.64, P = 0.07).
Fig. 1.
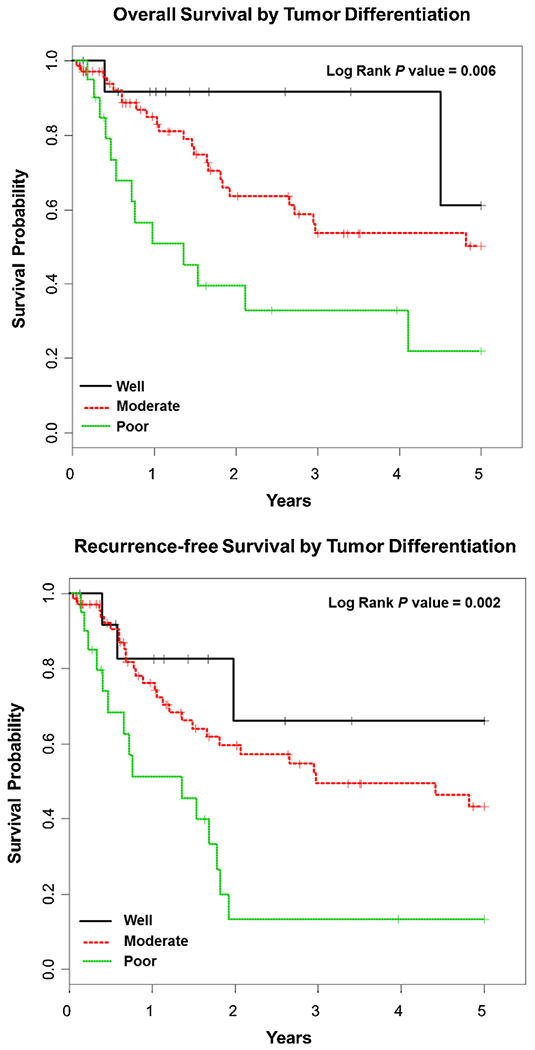
Kaplan–Meier overall survival and recurrence-free survival curves based on tumor differentiation: well (black), moderate (red), and poor (green).
Fig. 2.
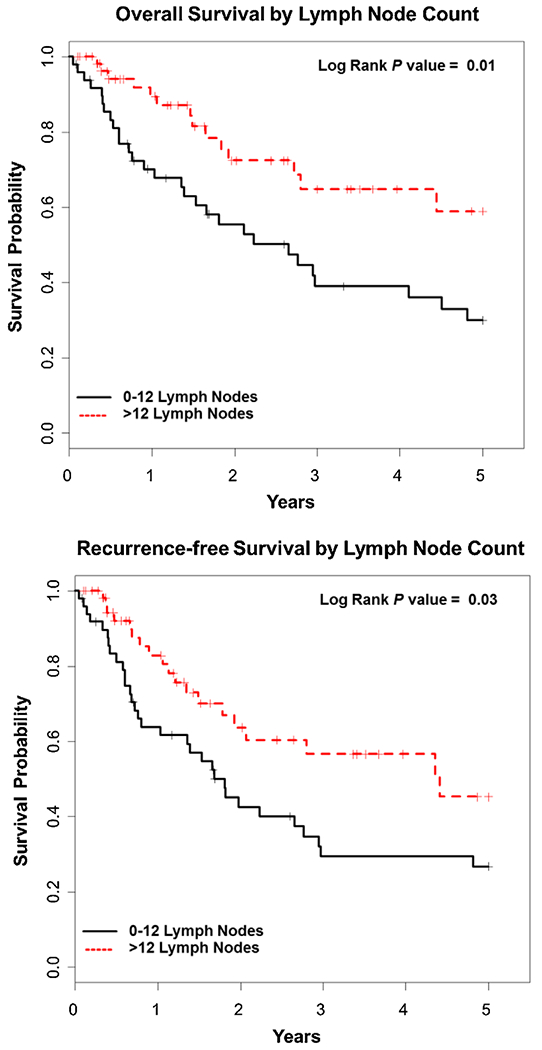
Kaplan–Meier overall survival and recurrence-free survival curves based on lymph node count: 0–12 (black) and >12 (red).
Fig. 3.
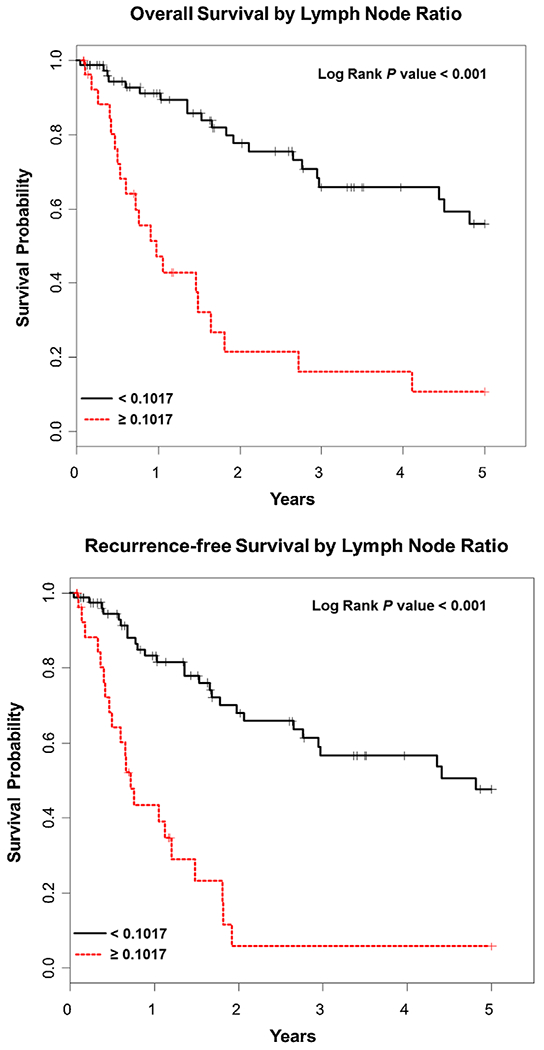
Kaplan–Meier overall survival and recurrence-free survival curves based on lymph node ratio: <0.10 (black) and ≥0.10 (red).
TABLE II.
Univariate Analysis of Factors Associated With Overall and Recurrence-Free Survival
| Overall survival |
Recurrence-free survival |
|||
|---|---|---|---|---|
| Variable | Hazard ratio 95%CI | P-value | Hazard ratio 95%CI | P-value |
| Age | 1.06 (1.03–1.10) | <0.001 | 1.05 (1.02–1.08) | 0.002 |
| LN counta | 0.43 (0.23–0.82) | 0.01 | 0.53 (0.30–0.94) | 0.03 |
| LN metastasis | 1.17 (1.05–1.31) | 0.007 | 1.29 (1.14–1.47) | <0.001 |
| Tumor differentiationb | 2.7 (1.38–5.27) | 0.004 | 2.8 (1.49–5.23) | 0.001 |
| LVI | 1.90 (1.02–3.52) | 0.04 | 2.24 (1.25–4.02) | 0.07 |
| PNI | 1.93 (1.02–3.67) | 0.04 | 1.75 (0.97–3.21) | 0.007 |
| Post-operative complications | 1.31 (0.70–2.43) | 0.40 | 1.65 (0.93–2.90) | 0.11 |
| Histological subtypec | 1.89 (0.93–3.84) | 0.08 | 1.45 (0.76–2.78) | 0.26 |
LN, lymph node; LVI, lymphovascular invasion; PNI, perineural invasion.
Dichotomized at 12.
Compares well differentiated tumors to moderate and poorly differentiated tumors.
Compares pancreaticobiliary and intestinal subtypes.
TABLE III.
Multivariate Analysis of Factors Associated With Overall and Recurrence-Free Survival
| Overall survival |
Recurrence-free survival |
|||
|---|---|---|---|---|
| Variable | Hazard ratio 95%CI | P-value | Hazard ratio 95%CI | P-value |
| Age | 1.04 (0.97–1.12) | 0.20 | 1.02 (0.97–1.08) | 0.40 |
| LN counta | 0.20 (0.08–0.48) | <0.001 | 0.22 (0.10–0.49) | <0.001 |
| LN metastasis | 1.27 (0.98–1.64) | 0.07 | 1.71 (1.28–2.27) | <0.001 |
| Tumor differentiationb | 28.77 (2.50–331.08) | 0.007 | 22.93 (3.06–171.72) | 0.002 |
| LVI | 2.16 (0.58–8.02) | 0.25 | 2.39 (0.70–8.11) | 0.16 |
| PNI | 1.94 (0.70–5.36) | 0.20 | 1.63 (0.61–4.36) | 0.33 |
| Histological subtypec | 2.80 (1.27–6.18) | 0.01 | 2.11 (1.04–4.27) | 0.04 |
LN, lymph nodes; LVI, lymphovascular invasion; PNI, perineural invasion.
Dichotomized at 12.
Compares well differentiated tumors to moderate and poorly differentiated tumors.
Compares pancreaticobiliary and intestinal subtypes.
Recurrence-Free Survival
Twenty patients (19%) developed recurrence. Of those, 13 (65%) developed distant recurrence, while the other 7 (35%) developed local and regional recurrence (four local, four regional). The 5-year RFS for the entire cohort was 36% as demonstrated by Kaplan–Meier survival analysis. The median RFS of moderately and poorly differentiated tumors were significantly worse compared to well differentiated tumors, P = 0.002 (Fig. 1). A significantly worse RFS was seen with a LNC <12 (P = 0.03) and a LNR of ≥0.10 (P < 0.001), respectively (Fig. 3). Age, LNC, metastasis, tumor differentiation, and PNI were variables found to be associated with RFS on UVA. LNC (HR = 0.22, CI = 0.10–0.49, P < 0.001), lymph node metastasis (HR = 1.71, CI = 1.28–2.27, P < 0.001), moderately and poorly differentiated tumors (HR = 22.93, CI = 3.06–171.72, P = 0.002), and pancreaticobiliary subtype (HR = 2.11, CI = 1.04–4.27, P = 0.04) were significant predictors and of RFS on MVA (Table III).
Forty-five patients (42%) experienced postoperative complications, which were defined as 6-grade I, 13-grade II, and 26-grade IIIa according to the Clavien–Dindo classification. No life threatening complications (grade IV) were recorded. Of those 45, 19 (42.2%) were reported to have either a pancreatic or bile leak. No postoperative mortalities were documented. Postoperative complications were not significant for OS or RFS (Table II). The use of adjuvant therapy did not impact OS or RFS (data not shown).
DISCUSSION
The goal of this study was to determine significant prognostic factors that have the greatest effect on survival and outcome in patients with resected AAC. AAC is a rare tumor of the periampullary region and accounts for about 6% of all periampullary malignancies [16]. In previous studies, AAC has been analyzed in combination with pancreatic adenocarcinoma, bile duct, or duodenal cancers [1]. More recently, some studies have selected out only patients diagnosed with AAC that have undergone surgical resection. These studies have proposed multiple prognostic factors along with variable outcomes. Most of these studies also reported varied regimens of adjuvant therapy, but rarely comment on the specifics of the regimen or the significance on survival or outcome [15]. In our series, we review prognostic factors and their relation to survival in a population of exclusively resected AAC.
The current American Joint Committee on Cancer Staging recommends retrieval of at least 12 LNs for adequate surgical staging [17]. This number has been validated in studies evaluating resected AAC, which show improved survival with greater than 12 lymph nodes [18]. Other studies report patients being understaged if less than 10 nodes are retrieved. We dichotomized the number of lymph nodes retrieved and examined at 10. Based on our analysis, we demonstrated that the extraction of less than 12 nodes lead to decreased OS and RFS. Lymph node metastasis was not significant for OS on MVA; however, it was statistically significant for RFS in our study.
LNR has been recently investigated as a potential standardize prognostic factor for resected AAC, although this has been difficult to accomplish as many studies use different cut-off values [11,19–22]. This inconsistency has led to multiple values being used, but the general trend in the literature shows that a higher LNR predicts a worse survival. One of the earlier studies evaluating LNR by Hurtuk et al. categorized the patient’s LNR into four groups: LNR = 0, ≤0.2, ≤0.4, and >0.4. The authors demonstrated a worse prognosis as the LNR increased [11]. A more recent study from Taiwan used a cut-off value of 0.056. They showed a LNR > 0.056 was significantly associated with worse OS and disease-free survival [20]. In our series of patients, we were able to demonstrate an LNR of >0.10 to be a significant predictor of OS and RFS.
Some authors have also evaluated histologic differentiation and node positivity as predictors of tumor aggression and survival. Kim et al. reviewed 104 patients who underwent curative resection for AAC over a 14-year period. They demonstrated that presence of node metastases (P = 0.003) and poor differentiation (P = 0.039) correlated with worse survival [23]. An early study from Memorial Sloan Kettering Cancer Center reviewed 101 patients with resected AAC. The authors reported positive nodes and poor tumor differentiation to be factors predictive for worse survival [16]. Similar to these studies, we have also shown moderately and poorly differentiated and node positive tumors to negatively impact outcome by resulting in worse OS and RFS.
The role of adjuvant therapy in AAC has yet to be determined. Several studies have shown a potential benefit in resected AAC with lymph node involvement, although the data have been limited by retrospective design and small sample size [24–26]. Westgaard et al. contributes small sample sizes to selection bias, which are caused by physicians selecting only patients with a poor prognosis to receive adjuvant therapy [2]. Some studies group AAC with other periampullary cancers, making it difficult to determine a true benefit in a homogenous population of only AAC [24]. In this study, there was no association with OS or RFS in patients who received adjuvant therapy. However, many patients did not receive adjuvant therapy and of those that did, multiple different regimens were used based on the physician’s discretion.
The authors recognize that this single institutional study has a number of limitations. Some of the limitations in our study are derived from the inherent flaws of its retrospective design and missing data points for some patients. One missing variable for many patients included the specific histologic subtype, either intestinal or pancreaticobiliary within ampullary adenocarcinoma. Other missing data points include the administration of adjuvant therapy or the specific type, particularly in the earlier cases in our series. The last limitation is the small number of patients analyzed and the relatively short follow-up of our patient population.
CONCLUSIONS
Although AAC is rare, pathologic features such as moderate and poor tumor differentiation and more advanced stage (i.e., lymph node involvement) predict worse survival. Meticulous surgical technique is imperative to improved outcome, not only for complete resection and avoidance of postoperative complications, but for oncologic benefit in obtaining a greater number of lymph nodes. Larger studies are needed to determine whether adjuvant therapy or novel targeted therapies can be of benefit in this patient population.
REFERENCES
- 1.Carter JT, Grenert JP, Rubenstein L, et al. : Tumors of the ampulla of vater: Histopathologic classification and predictors of survival. J Am Coll Surg 2008;207:210–218. [DOI] [PubMed] [Google Scholar]
- 2.Westgaard A, Pomianowska E, Clausen OPF, et al. : Intestinal-type and pancreaticobiliary-type adenocarcinoma: How does ampullary carcinoma differ from other periampullary malignancies? Ann Surg Oncol 2013;20:430–439. [DOI] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J, Schwartz AM, Batich K, et al. : Cancers of the ampulla of vater: Demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol 2009; 100:598–605. [DOI] [PubMed] [Google Scholar]
- 4.Song J, Liu H, Yang C, et al. : Long-term prognosis of surgical treatment for early ampullary cancers and implications for local ampullectomy. BMC Surg 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KM, Tompkins AJ, Yong S, et al. : Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg 2005;140:529–532. [DOI] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Olino K, et al. : Clinicopathologic analysis of ampullary neoplasms in 450 patients: Implications for surgical strategy and long-term prognosis. J Gastrointest Surg 2010;14:379–387. [DOI] [PubMed] [Google Scholar]
- 7.Kawabata Y, Ishikawa N, Moriyama I, et al. : What is an adequate surgical management for pTis and pT1 early ampullary carcinoma? Hepatogastroenterology 2014;61:12–17. [PubMed] [Google Scholar]
- 8.Kim RD, Kundhal PS, McGilvray ID, et al. : Predictors of failure after pancreaticoduodenectomy for ampullary carcinoma. J Am Coll Surg 2006;202:112–119. [DOI] [PubMed] [Google Scholar]
- 9.Hsu HP, Yang TM, Hsieh YH, et al. : Predictors for patterns of failure after pancreaticoduodenectomy in ampullary cancer. Ann Surg Oncol 2007;14:50–60. [DOI] [PubMed] [Google Scholar]
- 10.Jarufe NP, Coldham C, Mayer AD, et al. : Favourable prognostic factors in a large UK experience of adenocarcinoma of the head of the pancreas and periampullary region. Dig Surg 2004;21:202–209. [DOI] [PubMed] [Google Scholar]
- 11.Hurtuk MG, Hughes C, Shoup M, et al. : Does lymph node ratio impact survival in resected periampullary malignancies? Am J Surg 2009;197:348–352. [DOI] [PubMed] [Google Scholar]
- 12.Showalter TN, Zhan T, Anne PR, et al. : The influence of prognostic factors and adjuvant chemoradiation on survival after pancreaticoduodenectomy for ampullary carcinoma. J Gastrointest Surg 2011;15:1411–1416. [DOI] [PubMed] [Google Scholar]
- 13.Palta M, Patel P, Broadwater G, et al. : Carcinoma of the ampulla of vater: Patterns of failure following resection and benefit of chemoradiotherapy. Ann Surg Oncol 2012;19:1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu W, He J, Cameron JL, et al. : The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21:2873–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazaryan A, Kalmadi S, Almhanna K, et al. : Predictors of clinical outcomes of resected ampullary adenocarcinoma: A single-institution experience. Eur J Surg Oncol 2011;37:791–797. [DOI] [PubMed] [Google Scholar]
- 16.Howe JR, Klimstra DS, Moccia RD, et al. : Factors predictive of survival in ampullary carcinoma. Ann Surg 1998;228:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, et al. : The AJCC cancer staging manual, 7th edition New York (NY): Springer; 2010. [Google Scholar]
- 18.Partelli S, Crippa S, Capelli P, et al. : Adequacy of lymph node retrieval for ampullary cancer and its association with improved staging and survival. World J Surg 2013;37:1397–1404. [DOI] [PubMed] [Google Scholar]
- 19.Falconi M, Crippa S, Dominguez I, et al. : Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of vater carcinoma. Ann Surg Oncol 2008;15:3178–3186. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CH, Chen TD, Tsai CY, et al. : Prognostic value of the metastatic lymph node ratio in patients with resectable carcinoma of the ampulla of vater. Medicine 2015;94:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhan H, Xu J, Wang L, et al. : Lymph node ratio is an independent prognostic factor for patients after resection of pancreatic cancer. World J Surg Oncol 2015;13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Lee KG, Ha TK, et al. : Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg 2011;77:322–3299. [PubMed] [Google Scholar]
- 23.Kim WS, Choi DW, Choi SH, et al. : Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol 2012;105:266–272. [DOI] [PubMed] [Google Scholar]
- 24.Jabbour SK, Mulvihill D: Defining the role of adjuvant therapy: Ampullary and duodenal adenocarcinoma. Semin Radiat Oncol 2014;24:85–93. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia S, Miller RC, Haddock MG, et al. : Adjuvant therapy for ampullary carcinomas: The Mayo Clinic experience. Int J Radiat Oncol Biol Phys 2006;66:514–519. [DOI] [PubMed] [Google Scholar]
- 26.Narang AK, Miller RC, Hsu CC, et al. : Evaluation of adjuvant chemoradiation for ampullary adenocarcinoma: The John’s Hopkins Hospital-Mayo Clinic collaborative study. Radiat Oncol 2011;6:126. [DOI] [PMC free article] [PubMed] [Google Scholar]


