Abstract
Nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the rate-limiting step in NAD synthesis and is up-regulated in several human malignancies, including breast, colon, prostate, thyroid, gastric, and several hematopoietic malignancies. In some malignancies, such as gastric, thyroid, and prostate carcinomas, higher NAMPT expression correlates with deeper tumor invasion, increased metastatic potential and chemotherapy resistance. We employed tissue microarray immunohistochemistry to examine NAMPT expression in benign skeletal and smooth muscle, leiomyomas, leiomyosarcomas (graded low-, intermediate-, and high-grade), and spindle, embryonal, pleomorphic, and alveolar rhabdomyosarcomas. We found low to intermediate NAMPT expression in benign tissue, leiomyomas, leiomyosarcomas (low- and intermediate-grades), and spindle cell rhabdomyosarcomas. In contrast, high-grade leiomyosarcomas and embryonal, alveolar, and pleomorphic rhabdomyosarcomas showed high NAMPT expression. Herein we show for the first time that NAMPT is overexpressed in certain sarcoma types and the level of NAMPT expression correlates with tumor behavior.
Keywords: NAMPT, smooth muscle, skeletal muscle, leiomyoma, leiomyosarcoma, rhabdomyosarcoma
Rhabdomyosarcomas (RMS) are rare mesenchymal tumors that are derived from myogenic precursor cells that have histological features of skeletal muscle in different embryological stages of development (1, 2). About 250 RMS occur in the US each year, and these are most often in the pediatric population. Genitourinary system tumors are common in children under 5 years of age, head and neck tumors between the ages of 5-9 years, and RMS of the extremities and trunk are seen in children over 10 years old (1–3). Currently the World health Organization (WHO) employs molecular and histological criteria to classify RMS into three groups or types: embryonal (ERMS, with botryoid and spindle cell variants), pleomorphic (PRMS), and alveolar [ARMS (2)]. Histologically, ERMS are comprised of small, round cells and hyperchromatic nuclei with intermixed polygonal cells with eosinophilic cytoplasm which often contain cross striations, i.e. ‘strap cells’. ARMS have uniform cells arranged in variably sized nests separated by fibrous tissue septa, which can resemble a pulmonary alveolar pattern (1–3). PRMS are the least common subtype and typically consist of large anaplastic cells with lobate hyperchromatic nuclei and frequent multipolar mitotic figures (1–3). A fourth subtype, sclerosing RMS, occurs in adults and is characterized by hyaline sclerosis and a pseudovascular growth pattern; this entity is not included in the WHO system (4). By immunohistochemistry, RMS are positive for desmin, muscle-specific actin, myogenin, and myogenic differentiation 1 (MyoD1) (1–4).
Leiomyomas (LM) are benign smooth muscle neoplasms commonly occurring in the myometrium and gastrointestinal tract, although they can occur in almost any location (5). Leiomyosarcomas (LMS) are malignant neoplasms arising from smooth muscle cells, or committed precursor mesenchymal stem cells, that can arise anywhere in the body (6). Both neoplasms are spindle cell proliferations forming smooth muscle bundles and fascicles with characteristic elongated and blunt-ended nuclei (5, 6). LMS range from low to intermediate to high-grade based on their histological criteria with varying degrees of nuclear hyperchromasia and pleomorphism, mitotic activity, tumor necrosis, metastatic potential, and clinical aggressiveness (5, 6). Immunohistochemically, 70% of LM and LMS are positive for smooth muscle actin, desmin, and h-caldesmon, although none of these immunostains are specific for smooth muscle differentiation (7).
RMS, LM, and LMS carry several different molecular alterations (2). Amongst RMS, the ARMS are often more aggressive. Eighty percent carry t(2;13) or t(1;13) translocations, resulting in novel malignancy-promoting paired box gene 3-forkhead transcription factor (PAX3-FKHR) and paired box gene 7-forkhead transcription factor (PAX7-FKHR) hybrid transcription factors (2). PRMS typically occur in adults, carry a poor prognosis, and demonstrate complex cytogenetic aberrations (2). In contrast, spindle cell RMS are associated with favorable clinical outcomes and genetically heterogenous features, some involving nuclear receptor coactivator 2 (NCOA2) gene rearrangements and MYOD1 mutations (8–10). Amongst smooth muscle neoplasms, LM growth appears to depend on estrogen and progesterone-initiated signal transduction pathways leading to cell proliferation via tyrosine kinase activating growth factors (11). LMS have complex karyotypes with few consistent changes, although the phosphatase and tensin homolog (PTEN) and retinoblastoma protein (pRb) loci are often lost at 10q and 13q, respectively (12).
Nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the rate-limiting step in the nicotinamide adenine dinucleotide (NAD) salvage pathway and is up-regulated in many different human malignancies, where it promotes cell growth and survival, DNA synthesis, mitochondrial biogenesis, and angiogenesis (13). In several malignancies, higher NAMPT expression correlates with a more aggressive clinical course (14–23). Based on this, we hypothesized that NAMPT expression would be increased in LM, LMS, and RMS compared to benign muscle tissue, and NAMPT protein expression would correlate with increased tumor clinical aggressiveness and histological grade. Herein we investigated NAMPT expression in benign muscle and LM, LMS of different grades, and RMS.
Materials and Methods
Tissue microarrays (TMAs), catalog numbers SO2082a and SO281, were purchased from US Biomax, Inc. (Rockville, MD, USA). Together the TMAs contained 23 spindle RMS, 33 embryonal RMS, 36 PRMS, and 27 ARMS, 18 skeletal and 10 smooth muscle samples, 30 LM, 26 LMS (low-, intermediate-, and high-grade), one example of proliferative myositis, and three epithelioid LMS. Each case in the TMAs came in duplicate cores with a 1.0 mm diameter.
NAMPT immunohistochemistry (IHC).
The concentration of primary NAMPT antibody was optimized to normal kidney as control tissue. The staining of the TMA was performed in the Tissue Core Histology Lab Facility at the Moffitt Cancer Center. The microarray slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson, AZ, USA) as per the manufacturer’s protocol with proprietary reagents. Briefly, slides were deparaffinized on the automated system with EZ Prep solution (#950-100; Ventana Medical Systems). The heat-induced antigen retrieval method was used in Cell Conditioning 1 (#950-124; Ventana Medical Systems). Mouse monoclonal antibody to human NAMPT (#ALX-804-717; Enzo life Sciences, Plymouth Meeting, PA, USA) was used at a 1:1000 concentration in Dako antibody diluent (#S0809; Dako, Carpenteria, CA, USA) and incubated for 60 min. The Ventana anti-mouse or rabbit secondary antibodies were used for 16 min. The detection system used was the Ventana OmniMap kit. Slides were then dehydrated and coverslipped as per standard laboratory protocol.
Evaluation of NAMPT staining.
Relative NAMPT protein expression was determined as immunostain intensity scored on a 0-3 scale as follows: no staining as 0, light staining as 1, moderate staining as 2, and heavy staining as 3. The percentage of cells stained was measured, with no detectable staining as 0, 1-33% as 1, 34-66% as 2, and 67-100% as 3. The final IHC score was the product of the percentage of cells stained multiplied by the intensity score, allowing for a maximal score of 9 and a minimal score of 0. Nuclear and cytoplasmic NAMPT staining was seen in all tissue samples examined, although at low levels in benign skeletal and smooth muscle. We therefore measured and quantified NAMPT staining in the nuclear and cytoplasmic compartments. In all cases, the levels of staining were the same for the duplicate tissue cores.
Statistical analysis.
The standard error of the mean (SEM) IHC score was calculated by using the standard deviation for the staining scores of each tumor type and dividing this number by the square root of the sample size.
Results
Following IHC processing, we were left with eight spindle RMS, 30 embryonal RMS, 26 PRMS and 27 ARMS, 18 skeletal and 10 smooth muscle samples, 30 LM, 26 LMS (five low-, 14 intermediate-, and seven high-grade), and three epithelioid LMS. Some cases were lost in IHC processing; hence we analyzed a fewer number of cases than were on the TMAs. The cases of proliferative myositis and epithelioid LMS were not analyzed due to the low number of samples provided. Examples of NAMPT IHC of sample tissues are shown in Figure 1. The number of cases examined and the quantified IHC results are given in Table I.
Figure 1.
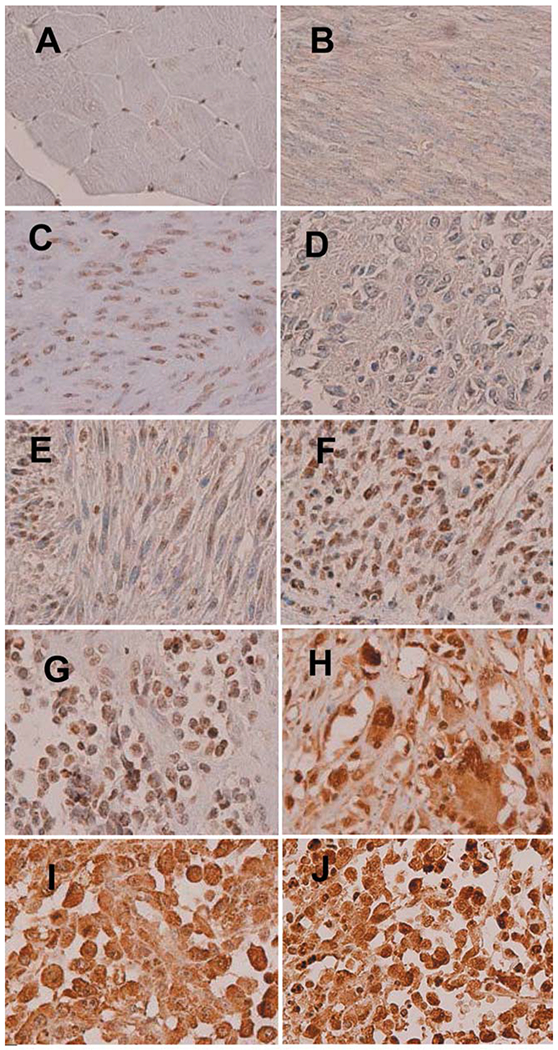
Representative nicotinamide phosphoribosyltransferase (NAMPT) immunostaining of skeletal muscle (A); smooth muscle (B); leiomyoma (C); low-grade leiomyosarcoma (D); intermediate-grade leiomyosarcoma (E); high-grade leiomyosarcoma (F); epithelioid leiomyosarcoma (G); and pleomorphic (H), alveolar (I) and embryonal rhabdomyosarcomas (J). All photos are high-power views (×400).
Table I.
Relative nicotinamide phosphoribosyltransferase (NAMPT) staining in the two tissue microarrays comparing benign skeletal and smooth muscle tissue to leiomyomas, different leiomyosarcoma grades, and different rhabdomyosarcoma subtypes.
| Tissue type | Sample number | Average NAMPT IHC score | SEM |
|---|---|---|---|
| Skeletal muscle | 18 | 0.22 | 0.10 |
| Smooth muscle | 10 | 0.99 | 0.31 |
| Leiomyoma | 30 | 1.74 | 0.19 |
| Leiomyosarcoma (total) | 26 | 3.42 | 0.42 |
| Low-grade | 5 | 2.00 | 0.63 |
| Intermediate-grade | 14 | 2.50 | 0.72 |
| High-grade | 7 | 6.29 | 0.49 |
| Spindle rhabdomyosarcoma | 8 | 2.38 | 0.75 |
| Alveolar rhabdomyosarcoma | 28 | 8.00 | 0.69 |
| Pleomorphic rhabdomyosarcoma | 26 | 7.12 | 0.49 |
| Embryonal rhabdomyosarcoma | 30 | 7.10 | 0.47 |
IHC: Immunohistochemistry; SEM: standard error of the mean.
Discussion
LM, LMS, and RMS exhibit increased hypoxia-inducible factor-1-alpha and -2-alpha expression and 27% of RMS have increased signal transducer and activator of transcription 3 (STAT3) activity – signal transduction cascades known to increase NAMPT expression (13, 24–30). In gastric, lung, endometrial and breast cancer, astrocytomas, and melanoma, increased NAMPT expression correlates with cellular dedifferentiation, greater depth of invasion, increased tumor growth, lymph node metastases, and a higher clinical TMN stage (13). Additionally, NAMPT expression has been correlated with increased resistance to chemotherapeutic agents (fluorouracil, doxorubicin, paclitaxel, etoposide, and phenylethyl isothiocyanate), reduced patient survival, and a worse prognosis (14–23).
Herein we showed that compared to benign smooth and skeletal muscle, there are progressively higher levels of NAMPT expression in LM, low- and intermediate-grade LMS, and high-grade LMS (Table I). LMS are graded based on tumor cellularity, degree of differentiation, presence of tissue necrosis, degree of nuclear atypia and the number of mitotic figures per 10 high-powered fields, with high-grade LMS having a worse prognosis than low- and intermediate-grade LMS (31, 32). LMS have been classified by two, three, and four-tier systems from well-differentiated to highly anaplastic, with each system having advantages and disadvantages with regard to predictive ability and reproducibility among pathologists (5, 6, 31–37). We found only a small difference in NAMPT expression between low- and intermediate-grade LMS, but noted a markedly higher expression in high-grade LMS. Although the clinical significance of this finding will require further study, the correlation of increased tumor dedifferentiation and histological grade with higher protein expression suggests that up-regulation of NAMPT might have prognostic significance. Generally, LMS, ERMS, PRMS and ARMS are clinically aggressive tumors, while the spindle cell variant of ERMS is associated with a better clinical outcome (8–10). In the present study, we showed that the spindle cell variant of ERMS exhibits a lower level of NAMPT expression, which correlates with less aggressive clinical behavior.
We showed for the first time that NAMPT is elevated in two sarcoma types, demonstrating that increased oncogenic NAMPT is not unique to carcinomas. Additionally, in LMS and RMS, the levels of NAMPT expression showed some correlation with known clinical outcomes for these sarcomas. Our data further suggest the NAMPT inhibitor KF866, which has had some success in treating human cancer, might have value in treating LMS and RMS, particularly those with poorer prognoses and higher NAMPT expression levels (38, 39).
Acknowledgements
The Authors thank the Histology Section of the Tissue Core at the Moffitt Cancer Center and Research Institute for the support in performing the IHC stains.
Footnotes
Conflicts of Interest
The Authors report no conflicts of interest.
References
- 1.Weiss SW and Goldblum JR: Rhabdomyosarcoma In: Enzinger and Weiss’s Soft Tissue Tumors. Weiss SW and Goldblum JR (eds.). St Louis, CV Mosby Co; Fourth Edition, pp. 785–835, 2001. [Google Scholar]
- 2.Parham DM, Barr FG and Montgomery E: Skeletal muscle tumors In: Fletcher CDM, Unni KK, Mertens F (eds). WHO Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Third Edition Lyon: IARC Press, pp. 141–154, 2006. [Google Scholar]
- 3.Ruymann FB and Grovas AC: Progress in the diagnosis and treatment of rhabdomyosarcoma and related soft tissue sarcomas. Cancer Invest 18: 223–241, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Mentzel T and Katenkamp D: Sclerosing, pseudovascular rhabdomyosarcoma in adults: clinicopathological and immunohistochemical analysis of three cases. Virchows Arch 436: 305–311, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, Doherty GP, Walsh C, McInnes M and Atri M: Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics 28: 1931–1948, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Edris B, Fletcher JA, West RB, van de Rijn Matt and Beck AH: Comparative gene expression profiling of benign and malignant lesions reveals candidate therapeutic compounds for leiomyosarcoma. Sarcoma 2012: 805614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher CDM, Bridge JA, Hogendoorn P and Merterns F: WHO Classification of Tumours of Soft Tissue and Bone. Fourth Edition Lyon: IARC publisher; 2013. [Google Scholar]
- 8.Carroll SJ and Nodit L: Spindle cell rhabdomyosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 137: 1155–1158, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Mosquera JM, Sboner A, Zhang L, Kitabayashi N, Chen CL, Sung YS, Wexler LH, LaQuaglia MP, Edelman M, Sreekantaiah C, Rubin MA and Antonescu CR: Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosomes Cancer 52: 10.1002/gcc.22050, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agaram NP, Chen CL, Zhang L, LaQuaglia MP, Wexler L and Antonescu CR: Recurrent MYOD1 mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas – evidence for a common pathogenesis. Genes Chromosomes Cancer 53: 779–787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Moore AB and Dixon D: Receptor tyrosine kinases and their hormonal regulation in uterine leiomyoma. Semin Reprod Med 28: 250–259, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Du X, Chen K, Ylipaa A, Lazar AJF, Trent J, Lev D, Pollock R, Hao X, Hunt K and Zhang W: Genetic aberrations in soft tissue leiomyosarcoma. Cancer Lett 275: 1–8, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shackelford RE, Mayhall K, Maxwell NM, Kandil E and Coppola D: Nicotinamide phosphoribosyltransferase in malignancy: a review. Genes Cancer 4: 447–456, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou T, Wang T and Garcia JGN: Expression of nicotinamide phosphoribosyltransferase-influenced genes predicts recurrence-free survival in lung and breast cancers. Sci Rep 4: 6107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folgueira MA, Carraro DM, Brentani H, Patrão DF, Barbosa EM, Netto MM, Caldeira JR, Katayama ML, Soares FA, Oliveira CT, Reis LF, Kaiano JH, Camargo LP, Vêncio RZ, Snitcovsky IM, Makdissi FB, Silva PJ, Góes JC and Brentani MM: Gene expression profile associated with response to doxorubicin-based therapy in breast cancer. Clin Cancer Res 11: 7434–7443, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Bae SK, Kim SR, Kim JG, Kim JY, Koo TH, Jang HO, Yun I, Yoo MA and Bae MK: Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1. FEBS Lett 580: 4105–4113, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Bi TQ, Che XM, Liao XH, Zhang DJ, Long L, Li HJ and Zhao W: Overexpression of NAMPT in gastric cancer and chemopotentiating effects of the NAMPT inhibitor FK866 in combination with fluorouracil. Oncol Rep 26: 1251–1257, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Long HL, Che XM, Bi TQ, Li HJ, Liu JS and Li DW: The expression of nicotinamide phosphoribosyl transferase and vascular endothelial growth factor-A in gastric carcinoma and their clinical significance. Zhonghua Wai Ke Za Zhi 50: 839–842, 2012. [PubMed] [Google Scholar]
- 19.Wang B, Hasan MK, Alvarado E, Yuan H, Wu H and Chen WY: NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene 30: 907–921, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Tian W, Zhu Y, Wang Y, Teng F, Zhang H, Liu G, Ma X, Sun D, Rohan T and Xue F: Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol 129: 505–512, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Maldi E, Travelli C, Caldarelli A, Agazzone N, Cintura S, Galli U, Scatolini M, Ostano P, Miglino B, Chiorino G, Boldorini R and Genazzani AA: Nicotinamide phosphoribosyltransferase (NAMPT) is overexpressed in melanoma lesions. Pigment Cell Melanoma Res 26: 144–146, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Bułdak RJ, Bułdak Ł, Polaniak R, Kukla M, Birkner E, Kubina R, Kabała-Dzik A, Duława-Bułdak A and Żwirska-Korczala K: Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: an in vitro study. Oncol Rep 29: 771–778, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Reddy PS, Umesh S, Thota B, Tandon A, Pandey P, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V, Rao MR, Kondaiah P and Somasundaram K: PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol Ther 7: 663–668, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, Brunkan CS, Wolberger C, Imai S and Tabas I: Extracellular NAMPT promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem 283: 34833–34843, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae SK, Kim SR, Kim JG, Kim JY, Koo TH, Jang HO, Yun I, Yoo MA and Bae MK: Hypoxic induction of human visfatin gene is directly mediated by hypoxia-inducible factor-1. FEBS Lett 580: 4105–4113, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Won Y, Shin Y, Kim JH and Chun JS: Reciprocal activation of hypoxia-inducible factor (HIF)-2α and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthritis Cartilage 24: 134–145, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Shintani K, Matsumine A, Kusuzaki K, Matsubara T, Satonaka H, Wakabayashi T, Hoki Y and Uchida A: Expression of hypoxia-inducible factor (HIF)-1α as a biomarker of outcome in soft-tissue sarcomas. Virchows Archiv 449: 673–681, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kilic-Eren M, Boylu T and Tabor V: Targeting PI3K/Akt represses Hypoxia inducible factor-1α activation and sensitizes Rhabdomyosarcoma and Ewing’s sarcoma cells for apoptosis. Cancer Cell Int 3: 36, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis BJ, Risinger JI, G VR, Chandramouli VR, Bushe PR, Baird BD and Peddada SD: Gene expression in uterine leiomyoma from tumors likely to be growing (from Black women over 35) and tumors likely to be non-growing (from White women over 35). PLoS One 8: e63909, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CL, Loy A, Cen L, Chan C, Hsieh FC, Cheng G, Wu B, Qualman SJ, Kunisada K, Yamauchi-Takihara K and Lin J: Signal transducer and activator of transcription 3 is involved in cell growth and survival of human rhabdomyosarcoma and osteosarcoma cells. BMC Cancer 7: 111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajdu SI, Shiu MH and Brennan MF: The role of the pathologist in the management of soft tissue sarcomas. World J Surg 12: 326–331, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Gladdy RA, Qin LX, Moraco N, Agaram NP, Brennan MF and Samuel Singer S: Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol 20: 1851–1857, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lack EE, Steinberg SM, White DE, Kinsella T, Glatstein E, Chang AE and Rosenberg SA: Extremity soft tissue sarcomas: analysis of prognostic variables in 300 cases and evaluation of tumor necrosis as a factor in stratifying higher-grade sarcomas. J Surg Oncol 41: 263–273, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A, Jacquemier J, Duplay H, Sastre-Garau X and Costa J: Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 15: 350–362, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Brown FM and Fletcher CD: Problems in grading soft tissue sarcomas: Am J Clin Pathol 114(Suppl): S82–S89, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Cindre JM: Grading of soft tissue sarcomas: review and update. Arch Pathol Lab Med 130: 1448–1453, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Deyrup AT and Weiss SW: Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology 48: 42–50, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Hasmann M and Schemainda I: FK866, a highly specific non-competitive inhibitor of nicotinamide phosphoribo-syltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res 63: 7436–7442, 2003. [PubMed] [Google Scholar]
- 39.Pogrebniak A, Schemainda I, Azzam K, Pelka-Fleischer R, Nussler V and Hasmann M: Chemopotentiating effects of a novel NAD biosynthesis inhibitor, FK866, in combination with antineoplastic agents. Eur J Med Res 11: 313–321, 2006. [PubMed] [Google Scholar]


