Abstract
There is strong, but not complete, concordance between immunohistochemical stains for p16ink4a and human papilloma virus in situ hybridization. Warty, basaloid, or mixed warty basaloid tumor subtypes are significantly more common in p16ink4a-positive patients. In p16ink4a-negative patients, positive p53 status is associated with nodal metastasis (pN+). In pN+ patients, cancer-specific survival (CSS) was significantly worse in patients with negative p16ink4a and p53 expression. p16ink4a status is a significant predictor for improved CSS.
Background:
Because of the low incidence of penile carcinoma (PC), the value of p16ink4a, p53, and human papilloma virus (HPV) infection status in clinical practice remains unclear. Herein, we report our experience with potential clinical utility of these markers in men with PC treated at our institution.
Patients and Methods:
Tissue microarrays of 57 cases of invasive penile squamous cell carcinomas were immunohistochemically stained for p16 and p53. HPV in situ hybridization (ISH) for high-risk subtypes was also performed. Association between marker status, nodal disease, overall (OS) and cancer-specific survival (CSS) were assessed.
Results:
p16 and HPV ISH were positive in 23 (40%) and 24 (42%) of the cohort, respectively. The proportion of warty, basaloid, or mixed warty basaloid tumor subtypes were significantly greater in the p16-positive patients (48% vs. 3%; P < .01). p53 expression was negative in 31 (54%) cases. Only in p16-negative patients, positive p53 status was associated with pN+ disease (odds ratio, 4.4 [95% confidence interval (CI), 1.04-18.6]). In Kaplan–Meier analysis, the unadjusted estimated OS was insignificantly longer in p16-positive patients (median OS, 75 vs. 27 months; P = .27) and median CSS was not reached (P = .16). In a multivariable Cox proportional hazard model, when controlling for pathological nodal status and adjuvant chemotherapy, p16 status was a significant predictor for improved CSS (hazard ratio, 0.36 [95% CI, 0.13-0.99]). The worst CSS was seen in pN+ patients with double negative p16 and p53 expression (8 vs. 34 months; P = .01).
Conclusion:
In this current cohort, p53 and p16 status showed clinical utility in predicting nodal disease as well as survival.
Keywords: Papillomavirus infections, Penile neoplasms, Prognosis, Survival, Tumor suppressor protein p53
Introduction
Penile carcinoma (PC) is a relatively uncommon malignancy in the developed countries; however, advanced disease is associated with poor outcomes. Two major etiologies for development of PC have been proposed. In human papilloma virus (HPV)-induced disease, viral E7 and E6 oncoproteins disrupt cell cycle checkpoints and apoptosis, by binding to host retinoblastoma and p53 proteins. The subsequent inactivation of these regulatory pathways leads to autonomous cell proliferation, without Gap 1 delay. Ultimately, this process leads to accumulation of p16ink4a in HPV-infected cells. Immunohistochemical staining can detect the overexpression of p16ink4a and this can serve as a marker for transcriptionally active HPV infection. p16ink4a overexpression is only seen in high-risk HPV genotypes.1,2 Therefore, it has been suggested that the detection of HPV DNA alone does not prove that a certain cancer is HPV-induced and p16 expression, exhibiting continuous strong nuclear and cytoplasmic staining of the entire epithelium is important to prove evidence of transcriptionally active infection.1
Penile carcinoma in the absence of HPV infection is linked to p53 mutations. The proposed precursor lesion in this setting is differentiated penile intraepithelial neoplasia, which can be concurrently seen in the presence of invasive disease. This latter lesion often shows p53 overexpression in the absence of p16 expression.1 On the basis of these described pathways, it has been suggested that all p53-positive tumors should stain negative for p16 and conversely, HPV-induced lesions should not express p53.1 However, these findings have not been replicated by others,3 and additionally, other alternative molecular and genetic pathways for development of PC have also been proposed.4
Clinically, p16 expression has been shown to be associated with improved cancer-specific survival (CSS)5 and additionally, p16ink4a/high-risk human papillomavirus status has been shown to be associated with reduced recurrence rates, particularly in patients with pathological involvement of nodes.6
The objective of this current study was to assess clinical utility of p16ink4a and p53 staining in a contemporary cohort of patients treated for PC, and evaluate the reproducibility of results from previous publications in routine clinical practice.
Patients and Methods
Patient Population
This study was approved by our institutional review board. Men, who received treatment for invasive PC from 1999 to 2013 at our institution and had had tissue available for analysis, were included in this study. Clinical data was collected from our electronic medical records and tumor registry. This included age, race, clinical T and N stage, tumor histology, Grade, presence of lymphovascular invasion, pathological T and N stage, use of adjuvant therapies, and survival data. Survival was calculated form the date of surgery to the date of death or last follow-up. Overall and CSS were assessed. Patients who were treated for PC and were clinically node-negative and did not undergo inguinal lymphadenectomy, were considered to be pathologically node-negative if they remained relapse-free during the course of their follow-up. Histopathology results are reported according to the 2009 (seventh edition) tumor, node, metastases classification for penile cancer.
Statistics
All data are expressed in median (interquartile range [IQR]). Categorical variables were compared using the χ2 test and continuous variables with the Mann–Whitney U test. Survival analysis was performed using the Kaplan–Meier method and group comparisons were carried out using the log rank test. The Cox regression proportional hazard model was used for multivariate analysis, predicting overall survival (OS) and CSS. Logistic regression was used to asses the association for binary outcomes.
Tissue Analysis
A tissue microarray (TMA) for 57 cases of invasive penile squamous cell carcinoma was constructed and the unstained slides from the TMA were stained with immunohistochemical stains for p16ink4a (Ventana; clone E6H4 mouse, prediluted) and p53 (Ventana clone BP53-11 mouse, prediluted). Presence of HPV was detected with in situ hybridization (ISH; Family 16 probe, prediluted) which was used to assess for high-risk HPV subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. The TMA slides were scored semiquantitatively by a specialized genitourinary pathologist. For p53 assessment, staining intensity (weak, moderate, and strong) as well as percentage of cells staining positive was reported. The H score was calculated using a combination of staining intensity and extent of staining according to the following formula: H score = 1 × percentage of tumor cells with weak staining + 2 × percentage of tumor cells with moderate staining + 3 × percentage of tumor cells with strong staining, resulting in a total score of 0 to 300. p53 expression was considered to be negative, when no staining or weak staining of ≤ 5% (ie, H score ≤ 5) was observed. Significant p53 expression was defined as expression of moderate intensity at 50% or greater or any strong p53 expression (H score of ≥ 150). For p16ink4a and HPV ISH, the results were recorded as negative or positive. Overexpression of p16ink4a was defined as continuous strong nuclear and cytoplasmic staining of the entire carcinoma.
Results
From 1999 to 2013, 57 patients with accessible tissue for analysis were identified. Median age for this cohort was 60 years (IQR, 53-73 years). Thirty-nine patients (68%) were treated with partial penectomy, 12 (23%) with total penectomy, and the remainder with less invasive local excision. Most patients (63%) had clinically N0 disease at the time of diagnosis. Pathologically, 24 patients (42%) had pT3 to T4 disease and 54% were pN+.
p16ink4a and HPV Findings
p16ink4a and HPV ISH were positive in 23 (40%) and 24 (42%) of the cohort, respectively. There was discordance between p16ink4a and HPV ISH results for 3 patients. Two HPV ISH-positive patients were p16ink4a-negative and 1 p16ink4a-positive patient exhibited negative HPV ISH expression.
p16ink4a-positive patients were insignificantly older (median, 60 vs. 65 years; P = .11), and there were no other significant differences between recorded clinical characteristics of the p16-positive and -negative cohorts (Table 1).
Table 1.
Patient Characteristics According to p16 Status
| Characteristic | p16-Negative | p16-Positive | P |
|---|---|---|---|
| Age at Time of Surgery | |||
| Median (IQR) | 58 (51-69) | 66 (57-75) | .11 |
| Race | |||
| White | 26 (76) | 18 (78) | 1 |
| Non-white | 8 (24) | 5 (22) | |
| Primary Surgical Procedure | |||
| Wide local excision | 4 (12) | 1 (4) | .20 |
| Partial penectomy | 20 (59) | 19 (83) | |
| Total penectomy | 10 (29) | 3 (13) | |
| Tumor Grade | |||
| Low | 22 (65) | 13 (57) | .37 |
| High | 8 (23) | 9 (39) | |
| Unknown | 4 (12) | 1 (4) | |
| Clinical Stage | |||
| cN0 | 22 (76) | 14 (70) | .75 |
| cN+ | 7 (24) | 6 (30) | |
| Pathology | |||
| T stage | |||
| 1 | 10 (30) | 7 (30) | .79 |
| 2 | 9 (26) | 7 (30) | |
| 3 | 13 (38) | 9 (39) | |
| 4 | 2 (6) | 0 (0) | |
| Histological subtype | |||
| Warty and/or basaloid | 1 (3) | 11 (48) | <.001 |
| Other | 33 (97) | 12 (52) | |
| LVI | |||
| Negative | 16 (47) | 9 (39) | .60 |
| Positive | 18 (53) | 14 (61) | |
| Nodal Stage | |||
| 0 | 16 (47) | 10 (44) | 1.00 |
| 1 | 4 (12) | 2 (9) | |
| 2 | 13 (38) | 7 (30) | |
| 3 | 1 (3) | 4 (17) | |
| Adjuvant Therapy | |||
| No | 23 (68) | 15 (65) | 1.00 |
| Yes | 11 (32) | 8 (35) | |
| HPV ISH | |||
| Negative | 32 (94) | 1 (4) | <.001 |
| Positive | 2 (6) | 22 (96) | |
| p53 Score | |||
| Negative (H < 5) | 17 (50) | 14 (61) | .59 |
| Positive (H > 5) | 17 (50) | 9 (39) |
Data are presented as n (%) except where otherwise noted.
Abbreviations: HPV = human papilloma virus; IQR = interquartile range; ISH = in situ hybridization; LVI = lymphovascular invasion.
Pathologically, the 2 groups were also similar with respect to T stage, tumor grade, lymphovascular status or nodal stage. The proportion of warty, basaloid, or mixed warty basaloid tumor subtypes were significantly greater in the p16-positive patients (48% vs. 3%; P < .01). In this cohort, only 1 warty carcinoma was p16-negative, however, this patient stained positive in HPV ISH.
p53 Status
p53 score ranged from 0 to 300 and p53 status was negative (H ≤ 5) in 31 cases (54%). In the entire cohort, 17 patients (30%) were p53- and p16-negative. Significant p53 expression (H score, > 150) was only seen in 1 p16-positive patient (4%). Seventeen patients (65%) with positive p53 staining were also pathologically node-positive (pN+; Table 2). In p16-negative patients, positive p53 status (H > 5) was associated with pN+ disease (odds ratio [OR], 4.4; 95% confidence interval [CI], 1.04-18.6), however, this association was not seen in p16-positive patients (OR, 0.94; 95% CI, 0.17-5.07).
Table 2.
Patient Characteristics According to Pathological Nodal Status
| Characteristic | pN0 | pN+ |
|---|---|---|
| Age at Time of Surgery | ||
| Median (IQR) | 66 (53-77) | 59 (50-68) |
| Race | ||
| White | 19 (0.731) | 25 (81) |
| Non-white | 7 (27) | 6 (19) |
| Primary Surgical Procedure | ||
| Wide local excision | 3 (11) | 2 (6) |
| Partial penectomy | 22 (85) | 17 (55) |
| Total penectomy | 1 (4) | 12 (39) |
| Tumor Grade | ||
| Low | 16 (62) | 19 (61) |
| High | 5 (19) | 12 (39) |
| Unknown | 5 (19) | 0 (0) |
| Clinical Nodal Stage | ||
| cN0 | 17 (85) | 19 (65) |
| cN+ | 3 (15) | 10 (35) |
| Pathology | ||
| T-Stage | ||
| 1 | 10 (39) | 7 (23) |
| 2 | 5 (19) | 11 (35) |
| 3 | 10 (38) | 12 (39) |
| 4 | 1 (4) | 1 (3) |
| Histological subtype | ||
| Warty and/or basaloid | 5 (19) | 7 (23) |
| Other | 21 (81) | 24 (77) |
| LVI | ||
| Negative | 18 (69) | 7 (23) |
| Positive | 8 (31) | 24 (77) |
| Adjuvant Therapy | ||
| No | 21 (81) | 17 (55) |
| Yes | 5 (19) | 14 (45) |
| HPV ISH | ||
| Negative | 17 (65) | 16 (52) |
| Positive | 9 (35) | 15 (48) |
| p16 | ||
| Negative | 16 (62) | 18 (58) |
| Positive | 10 (38) | 13 (42) |
| p53 Score | ||
| Negative (H ≤ 5) | 17 (65) | 14 (45) |
| Positive (H > 5) | 9 (35) | 17 (55) |
Data are presented as n (%) except where otherwise noted.
Abbreviations: HPV = human papilloma virus; IQR = interquartile range; ISH = in situ hybridization; LVI = lymphovascular invasion.
Follow-Up and Survival
Sixteen patients (28%) received adjuvant therapies. This included 7 patients treated with systemic chemotherapy alone, 3 with radiation therapy alone, and 6 with a combination of chemotherapy and radiation therapy.
Median follow-up for the entire cohort was 22 months. Thirty patients (52%) died during the follow-up period, with 9 (16%) who died from noncancer-related causes. The median OS for the cohort was 55 (IQR, 14-94) months with the median CSS not reached (mean, 78 months).
In Kaplan-Meier analysis, the unadjusted estimated OS was insignificantly longer in p16-positive patients (median OS, 75 vs. 27 months; P = .27) and median CSS was not reached (NR; P = .16; Figure 1). p16 status had a more pronounced protective influence on CSS in p53-negative (H < 5) patients (p16 positive vs. p16 negative; median, NR; P = .07), than in p53-positive patients (median, NR; P = .89).
Figure 1. Kaplan–Meier Curve for Cancer-Specific Survival According to p16 Status.
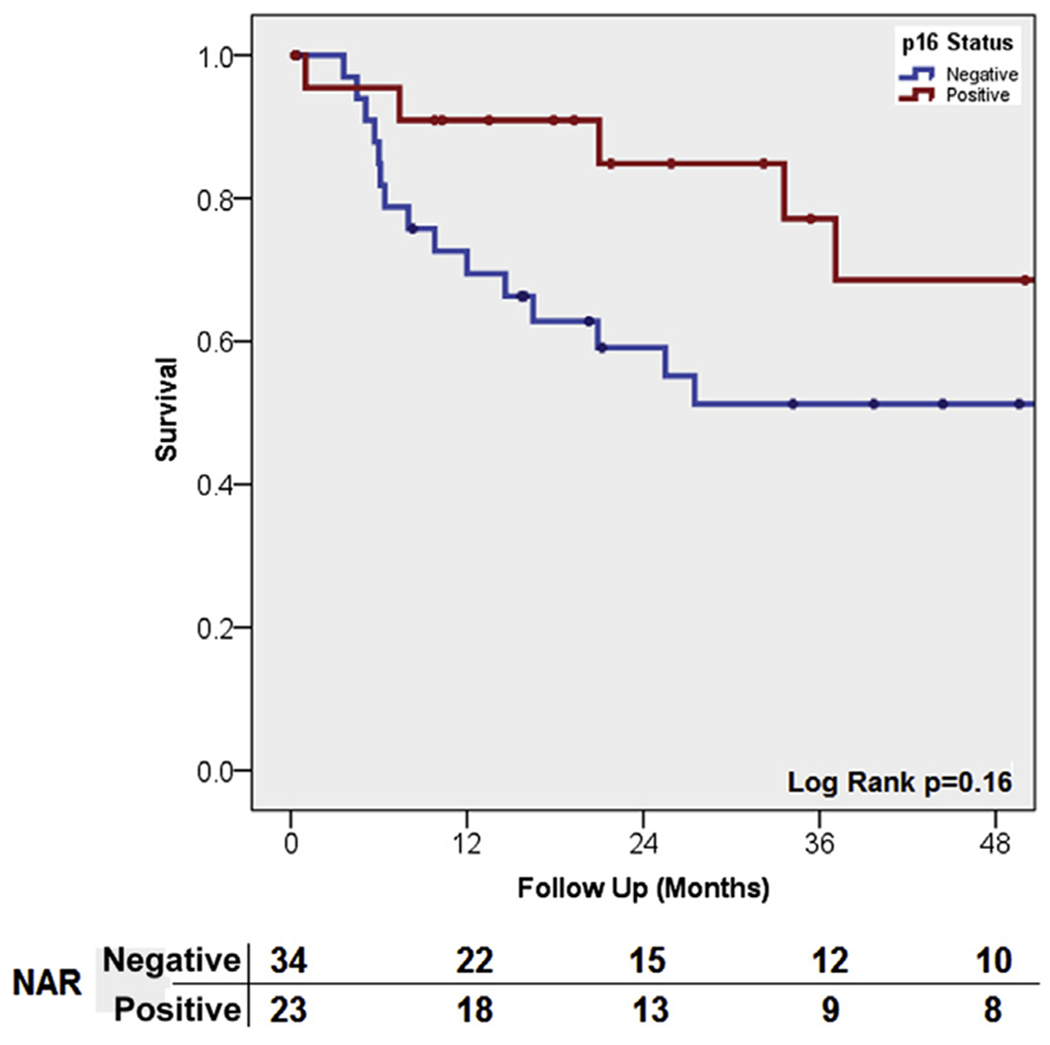
Abbreviation: NAR = Numbers at risk.
In pathologically node-positive patients, CSS was significantly worse in patients with negative p16 and p53 (H < 5) expression (8 vs. 34 months; P = .01; Figure 2).
Figure 2. Kaplan–Meier Curve for Cancer-Specific Survival in pN+ Patients Who Were Double p16 and p53-Negative Compared With Patients Who Were Not.
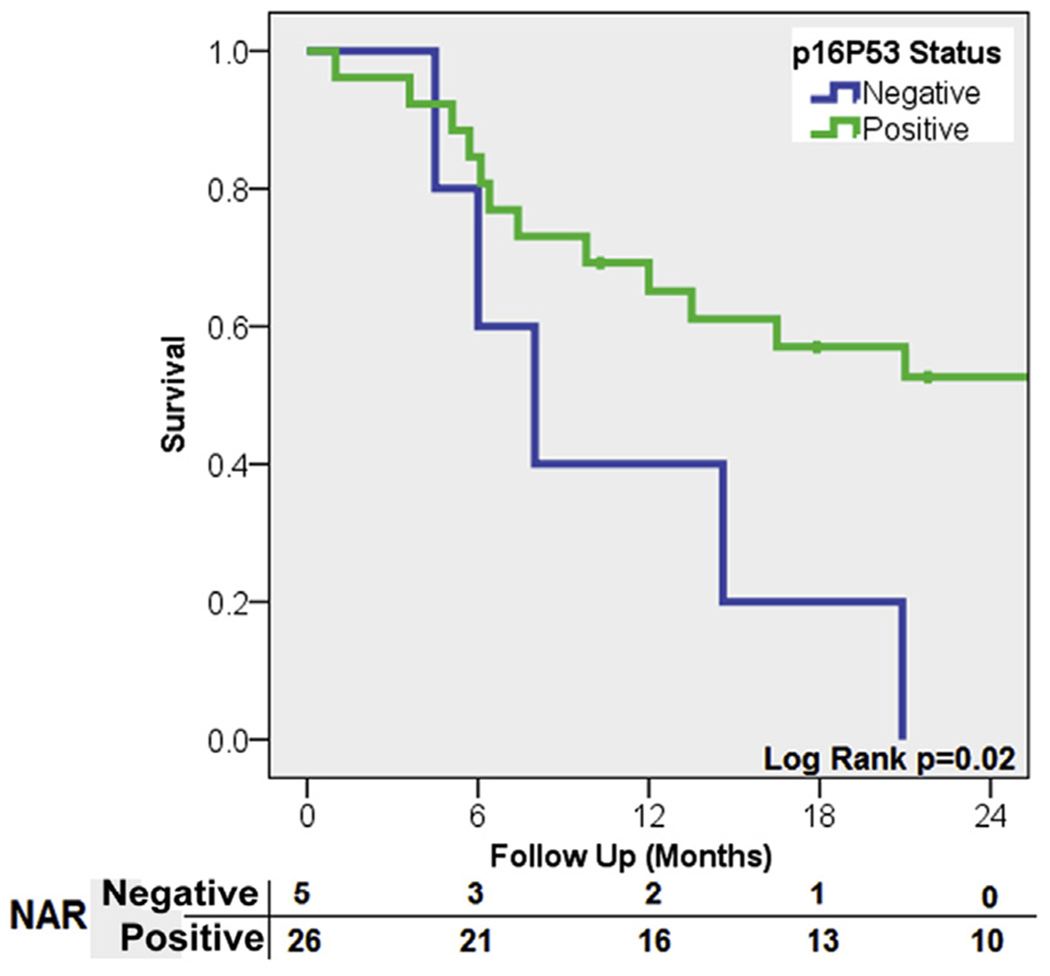
Abbreviation: NAR = Numbers at risk.
In a multivariable Cox proportional hazard model, when controlling for pathological nodal status and adjuvant chemotherapy, p16 status was a significant predictor for improved CSS (HR, 0.36; 95% CI, 0.13-0.99). In a similar model for OS, when age was also included, p16 status remained a predictor for better OS (HR, 0.33; 95% CI, 0.13-0.85; Table 3).
Table 3.
Cox Proportional Hazard Analysis for Survival
| Variable | Category | Overall Survival | Cancer-Specific Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | ≥60 vs. <60 | 3.21 (1.35-7.65) | .01 | – | – |
| Pathological Nodal Status | pN+ vs. pN0 | 6.07 (2.47-15.0) | <.01 | 25.4 (3.40-191) | <.01 |
| Adjuvant Chemotherapy | Yes vs. No | 2.50 (0.90-6.91) | .08 | 1.42 (0.47-4.25) | .53 |
| p16 Status | Pos vs. Neg | 0.33 (0.13-0.85) | .02 | 0.36 (0.13-0.99) | .05 |
Abbreviations: HR = hazard ratio; Neg = negative; Pos = positive.
Discussion
In this study, we assessed the incidence of HPV and p16 status in a group of 57 patients treated for invasive PC. In this cohort we observed a 42% and 40% HPV and p16 positive rate, respectively. Additionally, when controlling for important confounding variables, including pN state and adjuvant chemotherapy, p16 status was a significant predictor for OS and CSS. In p16-negative disease we observed an association with p53 status and nodal disease, and in pN+ patients double p16 and p53 negative status was associated with significantly worse CSS.
The reported rate of HPV in penile invasive carcinoma ranges from 23% to 50%, although higher rates of up to 83% have also been reported.6–9 Using polymerase chain reaction (PCR), the overall prevalence of HPV DNA in penile cancer is approximately 40% to 45%.10 Our reported rate of 40% is in line with most reports. We did observe discordance in 3 cases between p16 and HPV ISH.
It has been previously reported that HPV and subsequent p16-positive rates are higher in younger penile cancer patients. In our cohort, p16-positive patients were slightly older, although this difference was not significant. This absence of a specific age distribution difference between HPV-positive and -negative patients is also seen in series from The Netherlands where no age-related trend was seen when the patient population was stratified according to before and after 2001.11,12
We observed that when controlling for clinical and pathological confounders, CSS and OS were improved in p16-positive patients. These findings are similar to previous reports.5,12 We included important confounding variables, including adjuvant chemotherapy in the multivariate model, which has been shown to affect survival, especially in node-positive disease.13 In the analysis for OS, a nonsignificant trend suggestive of worse OS was seen for adjuvant chemotherapy, however, no such association was observed between adjuvant chemotherapy and CSS (HR, 1.42; 95% CI, 0.47-4.25; P = .53). Because CSS is not associated with adjuvant chemotherapy, the nonsignificant trend seen in the OS analysis is likely secondary to other patient- or therapy-related factors, and unrelated to tumor biology or marker status.
In the current cohort, most basaloid, warty, or warty basaloid subtypes were HPV-positive.14 Backes et al14 reported an HPV prevalence of 66% for basaloid and/or wart subtypes, with HPV being found more commonly in the basaloid warty subtype compared with keratinizing or other subtypes (OR, 3.5; 95% CI, 2.2-5.8). In our cohort, 92% of all warty basaloid subtypes were p16- (and HPV-) positive with 73% of keratinizing or other subtypes being p16-negative. The only p16-negative warty basaloid subtype was HPV-positive in ISH.
In this current study, using our p53 and p16 assessment procedures, we did not observe complete dissociation between p53 and p16 expression, with 12% significant (H > 150) and 4% strong p53 expression seen in p16-positive patients. Additionally, 17 men (30%) were negative for p53 and p16 expression. It is thought that binding of the HPV E6 protein to the oligomerization region of wild type p53 will promote ubiquitination and subsequent degradation by the 26S proteasome, in a process thought to be similar to p53 degradation by the MDM2 pathway.15 Therefore, E6 protein activation leads to a reduction in host cell p53 expression, hence, cells expressing only E7 might be positive for p53, whereas cells expressing E6 and E7 stain negative for p53.16 Mannweiler et al reported that in a cohort of 51 patients with invasive PC, lesions that were p16-negative (did not express intense and continuous p16 expression), had very low rates of HPV detection, with only 2 patients who exhibited high-risk HPV DNA in PCR analysis. Additionally, all p16-negative cases were positive for nuclear p53 expression. They also reported 72 patients with p16 overexpression, none of whom had p53 expression.1 The observed differences between our study and the latter report might relate to variation in interpretation of staining. In the study from Mannweiler et al, the authors did not consider single-cell staining or discontinuous staining as p16 overexpression and also did not define their criteria for p53 positivity. Lopes et al, using PCR analysis for HPV DNA, reported that 24% of their p53-positive patients were also HPV-positive and 65% of their p53-negative patients were also HPV-negative.3
Overall, the evidence of a link between HPV infection and p53 expression in PC is inconsistent.4 The mutation in the TP53 gene leads to overexpression of p53 protein, however, in 10% of cases this might also lead to the absence of p53.4 Additionally, although retinoblastoma protein is a target for the viral oncoprotein E7, Ferreux and colleagues have described other HPV independent pathways for p16ink4a/cyclin D/retinoblastoma disruption.17 Also, a number of other pathways such as p21, B-cell lymphoma 2-associated X protein and B-cell lymphoma 2, c-ras, and myc and others, with and without associated HPV infection, have been implicated.4 Therefore, several molecular and genetic pathways might play a role in events that lead to the disruption of the normal cellular regulatory mechanisms and resulting overexpression of p16 or p53 proteins. Therefore, as observed in this study, it is possible to detect p53 and p16 within the same cancer cell.
Interobserver variability is also an important factor when comparing the results discussed earlier. Our data were interpreted by a single genitourinary pathologist, using predefined scoring criteria, to ensure consistency of the results. This might also highlight the limitation associated with immunohistochemical staining and scoring in this setting, because despite use of specified criteria, the results still depend on individual interpretation. Definitions for p53 staining are also varied. Some have defined positive when at least 20% of cells were stained in the nucleus.3,18 We used a combination of staining intensity as well as the extent of the staining to define p53 status and subsequently considered undetectable or < 5% of weak staining as negative.
In our p16-negative patients, p53 status showed a significant association with nodal disease. This has previously been shown in other studies.3,18 However, in our study this relationship was only seen in p16-negative patients. Additionally others have recently reported that the rate of recurrence is also higher in node-positive patients who were p16-negative.6
We observed a single patient in whom p16 was positive and HPV ISH was negative. One possibility is that this patient had other high-risk HPV subtypes, which were not detected using our ISH assays (54, 59, 68, or 69). However, as described earlier, HPV-independent p16ink4a/cyclin D/Rb pathways have also been described.17 Our 2 HPV ISH-positive and p16-negative patients also had very low p53 H scores (5 and 10). This combination represented more aggressive disease, because both patients had nodal involvement and died within a year of surgery. Last, there were 12 patients who were p53- and p16-negative. This combination was associated with significantly worse CSS in node-positive patients (8 vs. 34 months; P = .02), and might represent an important marker of more aggressive disease in this clinical situation.
Currently a standardized method has not been identified for HPV detection. Present methods include various PCR techniques, DNA ISH, and immunohistochemical detection of surrogate biomarkers such as p16 protein. ISH might have some practical advantages over other PCR-based techniques. ISH can be performed on formalin-fixed and paraffin-embedded tissues, in line with standard tissue processing procedures. Also, with use of the nonfluorescent chromogens, DNA hybridization can now be confirmed using a conventional light microscope. Additionally, sensitivity of ISH has been significantly improved with use of signal amplification steps with viral detection being possible at 1 viral copy per cell.19,20
Limitations of this study relate to the small sample size, retrospective nature, and heterogeneity of the patients with inclusion of node-positive and -negative patients for analysis. We used ISH for detection of HPV instead of PCR, and were not able to classify for HPV subtypes.
Conclusion
Our results show the clinical utility of p16 and HPV ISH. We observed a strong concordance between p16 and HPV ISH results. p16-negative patients had longer median survival and p16 status was an independent predictor of CSS and OS in our cohort. In p16-negative patients, p53 status might be a predictor of nodal disease. These additional tests can provide important information in determining risk of relapse and outcomes in patients with invasive PC. However, the interpretation and reporting of immunohistochemical findings must be standardized.
Clinical Practice Points.
The relationship between p16ink4a status, HPV infection, and PC has been previously studied.
In this study we found a very strong concordance between p16ink4a and HPV ISH.
We have also seen that in p16-negative patients, positive p53 status is associated with nodal metastasis and a novel finding of this study was that worse CSS was seen in men with negative p16ink4a and p53 expression.
This latter finding requires further confirmation.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol 2013; 69:73–81. [DOI] [PubMed] [Google Scholar]
- 2.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–9. [DOI] [PubMed] [Google Scholar]
- 3.Lopes A, Bezerra AL, Pinto CA, et al. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol 2002; 168:81–6. [PubMed] [Google Scholar]
- 4.Kayes O, Ahmed HU, Arya M, et al. Molecular and genetic pathways in penile cancer. Lancet Oncol 2007; 8:420–9. [DOI] [PubMed] [Google Scholar]
- 5.Gunia S, Erbersdobler A, Hakenberg OW, et al. p16(INK4a) is a marker of good prognosis for primary invasive penile squamous cell carcinoma: a multi-institutional study. J Urol 2012; 187:899–907. [DOI] [PubMed] [Google Scholar]
- 6.Tang DH, Clark PE, Giannico G, et al. Lack of P16ink4a over expression in penile squamous cell carcinoma is associated with recurrence after lymph node dissection. J Urol 2015; 193:519–25. [DOI] [PubMed] [Google Scholar]
- 7.Kirrander P, Kolaric A, Helenius G, et al. Human papillomavirus prevalence, distribution and correlation to histopathological parameters in a large Swedish cohort of men with penile carcinoma. BJU Int 2011; 108:355–9. [DOI] [PubMed] [Google Scholar]
- 8.Ferrandiz-Pulido C, Masferrer E, de Torres I, et al. Identification and genotyping of human papillomavirus in a Spanish cohort of penile squamous cell carcinomas: correlation with pathologic subtypes, p16(INK4a) expression, and prognosis. J Am Acad Dermatol 2013; 68:73–82. [DOI] [PubMed] [Google Scholar]
- 9.Do HT, Koriyama C, Khan NA, et al. The etiologic role of human papillomavirus in penile cancers: a study in Vietnam. Br J Cancer 2013; 108:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol 2004; 193:35–44. [DOI] [PubMed] [Google Scholar]
- 11.Djajadiningrat RS, Graafland NM, van Werkhoven E, et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J Urol 2014; 191:68–73. [DOI] [PubMed] [Google Scholar]
- 12.Djajadiningrat RS, Jordanova ES, Kroon BK, et al. Human papillomavirus prevalence in invasive penile cancer and association with clinical outcome. J Urol 2015; 193:526–31. [DOI] [PubMed] [Google Scholar]
- 13.Hakenberg OW, Comperat EM, Minhas S, et al. EAU Guidelines on penile cancer: 2014 update. Eur Urol 2015; 67:142–50. [DOI] [PubMed] [Google Scholar]
- 14.Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009; 20: 449–57. [DOI] [PubMed] [Google Scholar]
- 15.Camus S, Menendez S, Cheok CF, et al. Ubiquitin-independent degradation of p53 mediated by high-risk human papillomavirus protein E6. Oncogene 2007; 26:4059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanton RA, Coltrera MD, Gown AM, et al. Expression of the HPV16 E7 gene generates proliferation in stratified squamous cell cultures which is independent of endogenous p53 levels. Cell Growth Differ 1992; 3:791–802. [PubMed] [Google Scholar]
- 17.Ferreux E, Lont AP, Horenblas S, et al. Evidence for at least three alternative mechanisms targeting the p16INK4A/cyclin D/Rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J Pathol 2003; 201:109–18. [DOI] [PubMed] [Google Scholar]
- 18.Liu JY, Li YH, Zhang ZL, et al. The risk factors for the presence of pelvic lymph node metastasis in penile squamous cell carcinoma patients with inguinal lymph node dissection. World J Urol 2013; 31:1519–24. [DOI] [PubMed] [Google Scholar]
- 19.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010; 116:2166–73. [DOI] [PubMed] [Google Scholar]
- 20.Huang CC, Qiu JT, Kashima ML, et al. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization method. Mod Pathol 1998; 11:971–7. [PubMed] [Google Scholar]


