Abstract
Inactivating mutations of the ENPP1 gene are associated with generalized arterial calcification of infancy (GACI) and less often autosomal-recessive hypophosphatemic rickets type 2 (ARHR2). We aimed to investigate the spectrum of phenotypes in a family with monoallelic and biallelic mutations of ENPP1 after identification through whole exome sequencing of a 54-year-old female with biallelic mutation of ENPP1, c.323G > T; p.Cys108Phe and c.1441C > T; p.Arg481Trp. Including the proband, 2 subjects had biallelic mutations, 5 had monoallelic mutations, and 2 had no mutation of ENPP1. The maternal mutation, a known pathogenic variant associated with GACI, was found in 3 subjects with monoallelic mutations, while the paternal mutation, which was not previously reported, was present in 2 subjects with monoallelic mutations. Both subjects with biallelic mutations had bowing of bilateral femurs, periarticular mineral deposition, normocalcemic primary hyperparathyroidism with multigland parathyroidectomy, increased carotid intima-media thickness, and enthesopathy was also noted in one subject. Intact FGF23 was elevated in both subjects with biallelic mutations, while C-terminal FGF23 was only elevated in one and PPi was reduced in one. Subjects with monoallelic mutations did not have periarticular calcifications or bone deformities. To conclude, patients with biallelic GACI causing mutations can survive well into adulthood, and despite the same biallelic ENPP1 pathogenic variants, clinical and biochemical manifestations can significantly differ, and include enthesopathy and primary hyperparathyroidism, which have not been previously described. Although carriers of monoallelic ENPP1 variants appear unaffected by classic disease manifestations, there may be subtle biochemical and clinical findings that warrant further investigation. © 2019 American Society for Bone and Mineral Research.
Keywords: ENPP1, GENERALIZED ARTERIAL CALCIFICATION, GENETIC, HYPOPHOSPHATEMIA, RICKETS
Introduction
The ENPP1 gene encodes the enzyme ectonucleotide pyrophosphatase/phosphodiesterase 1, which generates pyro-phosphate(PPi) by degrading adenosine triphosphate(ATP). Normal levels of PPi prevent soft tissue and vascular calcification. Inactivating mutations of the ENPP1 gene lead to low or absent levels of the ENPP1 enzyme, which in turn leads to reduced levels of PPi in the blood, thus predisposing to precipitation of calcium and phosphorous to form amorphous calcium phosphate with pathological calcifications.(1) Inactivating mutations of the ENPP1 gene have also been associated with increased fibroblast growth factor 23 (FGF23), but the etiology behind the increase is unclear. This results in FGF23-mediated hypophosphatemia through decreased renal phosphate reabsorption and decreased 1-alpha hydroxylase activity, leading to inappropriately low or normal serum 1,25-dihydroxyvitamin D (1,25(OH)2D). Chronic hypophosphatemia is associated with abnormal bone mineralization and rickets.
Loss-of-function ENPP1 variants cause both generalized arterial calcification of infancy (GACI) type 1 (OMIN # 208000)(2,3) and autosomal-recessive hypophosphatemic rickets type 2 (ARHR2) (OMIN # 613312),(4) sometimes occurring in the same family but with heterogeneous expression. GACI type 1 is a devastating and often fatal disease affecting infants. It is characterized by calcification and narrowing of large and medium-sized arteries including coronary and intraparenchymal arteries, resulting in heart failure and death in about half of the patients within the first 6 months of life.(2,3) Other manifestations can include conductive deafness and angioid retinal streaks.(2,3)
ARHR2 is characterized by the effects of reduced/absent ENPP1 enzyme levels and increased FGF23. It manifests after infancy with early fusion of cranial sutures, rachitic skeletal deformities, lower limb deformities, weakened bones, repeated bone fractures, short stature (in some), bone and muscle pain, deafness, and periarticular calcifications (not found in ARHR type 1, caused by homozygous loss-of-function mutations in dentin matrix protein 1 [DMP1] gene), and hypophosphatemia due to decreased renal tubular phosphate reabsorption (mediated by FGF23).(4,5) We recently identified a 54-year-old female who presented with joint pain, bowed legs, periarticular calcifications, hypophosphatemia due to hyperphosphaturia, inappropriately normal 1,25(OH)2D, and concomitant normocalcemic primary hyperparathyroidism (PHPT). Gene sequencing identified two variants in the ENPP1 gene. This prompted us to investigate other family members for clinical, laboratory, and genetic abnormalities.
Materials and Methods
After Mayo Clinic Institutional Review Board study approval, written informed consent was provided by participating family members. The proband’s father only completed genetic testing, but his family confirmed the absence of a diagnosis of any bone or mineral abnormalities. The subjects were evaluated in the Clinical Research and Trials Unit at Mayo Clinic (Rochester, MN, USA), where medical histories were obtained, vitals were taken, and single fasting AM measures of the following were performed: tubular reabsorption of phosphorous (TRP); serum calcium, phosphorus, creatinine, albumin, bone alkaline phosphatase (BAP), creatinine, carboxyterminal cross-linking telopeptide of bone collagen (Beta-CTx), phosphorus, parathyroid hormone (PTH), and C-terminal FGF23 using procedures established in Mayo Clinic Laboratories. Serum 25-hydroxyvitamin D and 1,25(OH)2D were measured by mass spectrometry. Serum intact FGF23 was assayed using the method of Kainos Laboratories (Tokyo, Japan).
Blood plasma was prepared for the PPi assay, which was performed essentially as previously described(6,7) with minor variations. After plasma isolation and filtration to remove platelets, the plasma samples were diluted in 1:1 with 50 mM Tris-Acetate pH 8.0 buffer followed by filtration through a 30 kDa membrane (Amicon, Millipore Sigma, Burlington, MA, USA) via centrifugation and then frozen at −80°C. Background PPi in the samples and water was subtracted and normalized from the samples, and the values were interpolated using Prism (GraphPad 7, GraphPad, La Jolla, CA, USA).
Whole-exome sequencing
Whole-exome sequencing (WES) was performed on the proband and her parents at a CLIA-certified laboratory (Mayo Clinic) following a standard procedure summarized as follows: Paired-end libraries were prepared following Agilent’s (Santa Clara, CA, USA) protocol. Whole-exon capture was carried out following the protocol for Agilent’s SureSelect Human All Exon v5 + UTRs 75 MB kit. The purified capture products were amplified using the SureSelect Post-Capture Indexing forward and Index PCR reverse primers (Agilent) for 12 cycles. The concentration and size distribution of the completed captured libraries were determined on Qubit (Invitrogen, Carlsbad, CA, USA) and Agilent Bioa-nalyzer DNA 1000 chip. Sequencing was performed following Illumina’s standard protocol in the Illumina (San Diego, CA, USA) cBot and HiSeq 3000/4000 PE Cluster Kit (average coverage ~80×). The flow cells were sequenced as 150 × 2 paired end reads on an Illumina HiSeq 4000 using HiSeq 3000/4000 sequencing kit and HCS v3.3.52 collection software. Base-calling was performed using Illumina’s RTA version 2.7.3.
ENPP1 targeted genotyping
Genomic DNA from peripheral blood from the proband’s siblings and children was amplified by polymerase chain reaction (PCR) using the Platinum Taq HiFi kit (Invitrogen) and the primers described in Table 1. Reaction took place for 34 cycles using 60°C for 20 seconds as the annealing conditions. PCR products were purified and cloned into Top 10 cells using the TOPO TA Cloning Kit for Sequencing (Invitrogen) as indicated by the manufacturer. Miniprep and DNA isolation was performed using a standard protocol described elsewhere, followed by Sanger sequencing.
Table 1.
Primers Used for Targeted Sequencing of ENPP1 Variants
| Variant | Oligonucleotide sequence | Orientation | Predicted size |
|---|---|---|---|
| c.323G > T p.Cys108Phe | 5′-GGGTTCCCTAAGGGTTGGAGATAAAAATCACAAACCAGGCACATAAGG-3′ | Forward | 277 bp |
| 5′- GTGCCAGCAAGATCCAATCTAGACATCACAGCGACAGTTCCCA-3′ | Reverse | ||
| c.1441C > T p.Arg481Trp | 5′-GGGTTCCCTAAGGGTTGGAACCAGGCACATAAGGTTACTTTCTG-3′ | Forward | 227 bp |
| 5′-GTGCCAGCAAGATCCAATCTAGAGATCTCTTGCCGTCAAAGAACCA-3′ | Reverse |
Protein modeling
Because there is no experimental structure for human ENPP1, we used homology-based methods(8) to construct a model of human ENPP1 from the experimental structure of murine ENPP1 (71% identical; PDB 4b56).(9) Domain annotations were obtained from UniProt. We used FoldX version 4 for computational mutagenesis and calculating the mean and standard deviation for △△Gfold using 20 replicates. Protein structure visualization and assessment was carried out using PyMOL(3) version 2.0.7.
Enzyme kinetic assay
Human polymorphisms were engineered into the hENPP1-Fc construct(10) using the Quikchange II-XL Site Directed Mutagenesis from Agilent Technologies. After sequence verification, constructs were transfected into CHO-K1 cells using Lipofectamine 2000 from Thermo Fisher Scientific (Waltham, MA, USA). Forty-eight hours after transfection, 10 μL of supernatant was mixed with 90 μL of assay buffer containing 250 mM Tris pH 8.0, 500 mM NaCl, 0.05% Triton X-100, and 1 mM Thymidine 5′-monophosphate p-nitrophenyl. The velocity of the p-nitrophenyl group liberated from the chromogenic substrate was reported as change at OD 405 nM/min, in replicates of at least 5 for each construct, and normalized to % WT.
Results
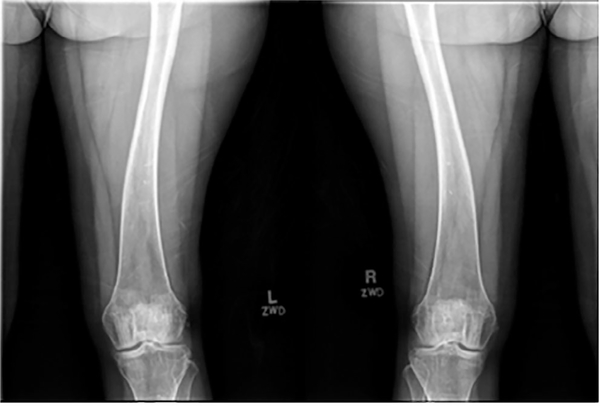
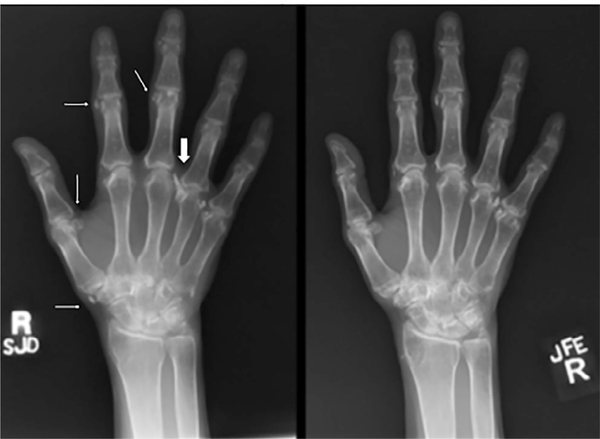
The subjects’ demographic, clinical, biochemical, and genetic analyses are presented in Table 2. The proband (Subject II-2) was a 54-year-old white female with a history of intermittent joint pains affecting her hands, feet, and elbows, which started in her twenties and were associated with periarticular growths that fluctuated over time. She was diagnosed with fibromyalgia and tophaceous gout but did not respond to therapies. A prior audiometry confirmed bilateral conductive hearing loss. At age 49 years, she had presented to Bone Clinic at Mayo Clinic Rochester due to electrolyte abnormalities and elevated PTH. Examination revealed normal mid-parental height, non-dysmorphic body habitus, Heberden’s and Bouchard’s nodes on hand exam, and hammertoe deformities of both feet. Laboratory workup was significant for a low serum phosphorous of 1.9 mg/dL (reference range 2.5–4.5), elevated PTH of 156 pg/mL (reference range 15–65), 1,25(OH)2D of 94 pg/mL (reference range 18–78), and reduced TRP of 76.6% (reference range >80). She did not have a history of fractures, renal dysfunction, nephrolithiasis, or hypercalcemia. Dual-energy X-ray absorptiometry (DXA) bone mineral density (BMD) showed osteopenia (worst femur neck T-score – 2.3). A parathyroid sestamibi scan was non-localizing. Based on this information, she was diagnosed with normocalcemic primary hyperparathyroidism (PHPT) and PTH-mediated hyperphosphaturia and was managed with vitamin D and calcium supplementation. At age 50 years, she underwent left hemithyroidectomy with removal of left and right superior parathyroid glands at another institution, following which she had improvement in PTH but developed symptomatic hypocalcemia. She then returned to our clinic a year later with intermittent symptomatic hypocalcemia (8.3 mg/dL) and hypophosphatemia (2.1 mg/dL) but a normal PTH of 42 pg/mL and inappropriately normal 1,25(OH)2D of 32 pg/mL. At that time, due to hypophosphatemia despite normalization of PTH, laboratory testing was repeated including a C-terminal FGF23 of 103 RU/mL (reference range < 180) and TRP of 75.8% (Table 2). Detailed bone radiographs demonstrated bowing of bilateral femurs (Fig. 1) in addition to periarticular calcifications in hands (Fig. 2), feet, knees, and shoulders. Elemental CaCO3 600 mg twice daily and vitamin D3 2000 IU daily were continued and calcitriol 0.25 mcg daily was initiated and later increased to twice daily, which led to normalization of the calcium and phosphorus levels. Subsequent intact FGF23 measurement was elevated at 94.7 pg/mL (reference range 10–50).
Table 2.
Demographic, Clinical, Biochemical, and Genetic Data in a Family with ENPP1 Gene Mutations HET C.323G > T p.Cys108Phe and HET C.1441C > T p.Arg481Trp
| Subject no. | II–2 | I–1 | I–2 | II–3 | II–4 | II–5 | III–11 | III–2 | III–3 |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 54 | 78 | 78 | 55 | 53 | 52 | 29 | 28 | 25 |
| Sex | F | F | M | F | F | M | F | M | M |
| Height (cm) | 163 | 157.5 | — | 171.3 | 153 | 184 | 170.8 | 192 | 187 |
| Mutation present | c.323G > T/c.1441C > T | c.1441C > T | c.323G > T | None | c.323G>T/c.1441C>T | None | c.323G > T | c.1441C > T | c.1441 C> T |
| Normocalcemic PHPT with parathyroidectomy | Yes (2 glands) | No | No | No | Yes (3 glands) | No | No | No | No |
| Osteosarcoma | No | No | No | No | No | No | Yes | No | No |
| Melanoma | No | No | No | No | No | No | No | No | Yes |
| Schwannoma | No | Yes | No | No | No | No | No | No | No |
| Bowing of bilateral femurs | Yes | No | No | No | Yes | No | No | No | No |
| Hearlng loss | Yes3 | No | No | No | Yes2 | No | No | No | No |
| Congenital heart defect | No | No | No | No | Yes | No | No | No | No |
| Perlarticular mineral deposition | Yes | No | No | No | Yes | No | No | No | No |
| Enthesopathy | No | No | No | No | Yes | No | No | No | No |
| Coronary artery calclficatlon | Absent | — | — | — | Absent | — | — | — | — |
| Carotld artery plaque | Absent | — | — | — | Absent | — | — | — | — |
| Carotid intima-media thickness | >75% for age and sex | — | — | — | >75% for age and sex | — | — | — | — |
| Aortic calcificatlon | Absent | — | — | — | Absent | — | — | — | — |
| Tubular resorptlon of phosphate (>80%) | 76.64; 75.85 | 87 | — | 91.4 | 86.4 | 84.8 | 85.5 | 80.5 | 84.4 |
| Serum pyrophosphate (1600–2500 nM) | 2091 ± 14 | 1866 ± 12 | — | 1606 ±4 | 1275 ± 8 | 2057 ± 24 | 1131 ± 8 | 1124 ± 12 | 1189 ± 12 |
| Serum C-termlnal FGF23 (<180 RU/mL) | 103 | 153 | — | 68 | 190 | 78 | 70 | 65 | 61 |
| Serum Intact FGF23 (10–50 pg/mL) | 94.7 | 13.77 | — | 76.95 | 93.77 | 41.5 | 25.36 | 34.23 | 29 |
| 25-(OH)D (20–80 ng/mL) | 37 | 49 | — | 65 | 27 | 13 | 26 | 25 | 30 |
| 1,25-dlhydroxyvltamln D (18–64 pg/mL) | 944; 325 | 102 | — | 76 | 54 | 62 | 86 | 67 | 66 |
| Calclum (normal ranges, mg/dL) | 9.94; 8.65 (8.9–10.1) | 8.8 (8.8–10.2) | — | 9.8 (8.6–10) | 8.6 (8.6–10) | 9.4 (8.6–10) | 8.8 (8.6–10) | 10.2 (8.6–10) | 9.9 (8.6–10) |
| Albumin corrected calcium (mg/dL) | 8.04 | 8.72 | — | 9.16 | 8.68 | 9.08 | 8.64 | 9.32 | 9.1 |
| Phosphorus (2.5–4.5 mg/dL) | 1,94; 2.35 | 3.1 | — | 3.5 | 2.5 | 2.6 | 2.5 | 2.4 | 3.3 |
| PTH (15–65 pg/mL) | 1564; 475 | 79 | — | 87 | 83 | 88 | 21 | 30 | 32 |
| Creatinine (0.8–1.3 mg/dL) | 0.7 | 0.98 | — | 0.93 | 0.78 | 0.94 | 0.71 | 1.08 | 1.15 |
| eGFR (>90 ml/min) | > 90 | 55 | — | 69 | 87 | > 90 | > 90 | > 90 | 89 |
| Beta-CTx (pg/mL) Male: 120–946 (18–30 years); 93–630 (31–50 years); 35–836 (51–70 years); Female: 25–573 (premenopausal); 104–1008 (postmenopausal) | 4124 | 139 | — | 249 | 596 | 370 | 301 | 446 | 538 |
| Bone Alkaline Phosphatase (mcg/L) Male: ≤20 Female: ≤14 (premenopausal); ≤22 (postmenopausal) | 134 | 13 | — | 10 | 21 | 13 | 9.1 | 11 | 10 |
PHPT = primary hyperparathyroidism; PTH = parathyroid hormone.
Estimated 7 to 8 weeks pregnant.
Maternal measles during pregnancy.
Conductive hearing loss.
Tested before parathyroidectomy.
Tested after parathyroidectomy.
Fig. 1.
Bowing deformity of both femurs in subject 1 with biallelic ENPP1 mutations.
Fig. 2.
Right-hand radiographs in subject 1 with biallelic ENPP1 mutations demonstrating areas of soft tissue calcinosis (thin arrows) in the hand and wrist from 4 years prior (left panel) compared with current evaluation (right panel). A prominent area of calcinosis along the radial aspect of the right 4th MCP joint (thick arrow) has nearly completely resolved, while other areas of calcinosis have increased slightly.
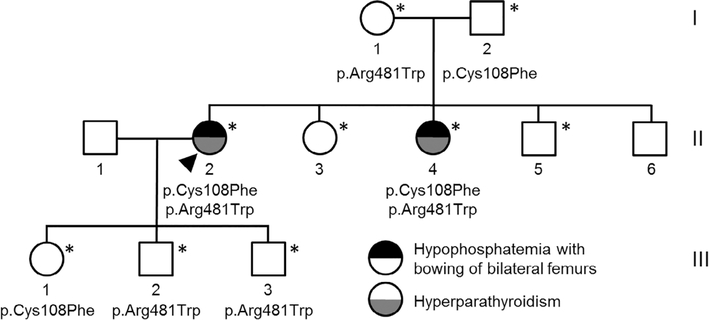
She was evaluated by Department of Mayo Medical Genetics and underwent WES, which identified two variants in the ENPP1 gene, c.323G > T; p.Cys108Phe and c.1441C > T; p.Arg481Trp. The maternally inherited variant (p.Arg481Trp) was a pathogenic variant reported to cause GACI in compound heterozygosity with other pathogenic variants.(11,12) Thus, vascular health screening was performed, which was unrevealing other than increased carotid intima-media thickness (CIMT). The paternally inherited variant (p.Cys108Phe) has not been previously reported in the literature or variant databases (ClinVar and HGMD) by other groups. This variant is rare in healthy populations,(13) with only a single heterozygous allele described in gnomAD. In silico predictors of pathogenicity (SIFT, Polyphen2, MutationTaster, and M-CAP) agree that this change is deleterious. Sequencing of the rest of the family indicated that the proband’s similarly affected sister (II-4) also carried both genetic variants, indicating segregation with the phenotype in an autosomal recessive manner. We also searched the WES data from the proband and her parents for other genetic alterations that could be traced to other phenotypes observed in the family but no additional mutations were found.
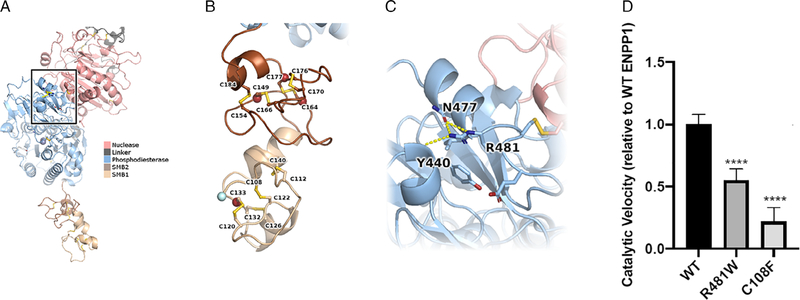
The cysteine coded by this codon (Cys108) is highly conserved across species and it is located in the somatomedin B domain 1 (SMB1), described to mediate homodimerization needed for the correct function of the protein. In silico modeling predictions indicate that this cysteine forms a disulfide bond with Cys122 of ENPP1 that would be lost due to this change. Structure-based calculations indicated significant destabilization with △△Gfold = 26.7 ± 0.5 kcal/mol; losing disulfide bonds is energeti-cally costly and suggest impairment of ENPP1 activity. To confirm that the p.Cys108Phe mutation results in loss of function of ENPP1 catalytic activity, we compared the velocity of the enzymatic reactions of wild-type (WT), p.Cys108Phe, and p.Arg481Trp ENPP1 using a standard colorimetric substrate as previously described.(14) We found the p.Cys108Phe mutation to be severe, exhibiting only 20% of the activity present in the WT enzyme. In comparison, the p.Arg481Trp mutation, previously observed as a compound heterozygous mutation in GACI, was relatively mild, retaining 55% of the activity in the native enzyme (Fig. 3D). Using the evidence described above, we classified the p.Cys108Phe variant as “Patho-genic” according to ACMG guidelines(15) (PP1 + PM1 + PM2 +PM3 + PP3 + PP4). No PHEX, FGF23, DMP1 mutations were iden-tified. Hence, she was diagnosed with ARHR2.
Fig. 3.
ENPP1 structure providing insights into potential mechanism of dysregulation. (A) ENPP1 model colored by domain. Disulfide bonds between cysteine residues are shown in sticks representation. The region around p.Arg481 is boxed. (B) Zoom-in on the two SMB domains. Each has four disulfide bonds. Residues with AD variants are marked by red spheres. The N-terminal residue is marked by a cyan sphere. The transmembrane domain is further N-terminal than our model. P.Cys108 is the first residue involved in a disulfide bond, after the transmembrane domain. (C) Zoom-in on the region around p.Arg481. Polar contacts are represented by dashed lines. P.Arg481 makes multiple interactions within the phosphodiesterase domain and is nearby the nuclease domain. (D) Comparison of the enzymatic velocity rates of WT, p.Arg481Trp, and p.Cys108Phe ENPP1 variants sequenced in the patients. The data are scaled relative to the WT velocity rate. The p.Arg481Trp mutation was relatively mild, reducing the enzymatic velocity by 45%, and the p. Cys108Phe mutation was more severe, reducing the enzymatic velocity rate by 80%.
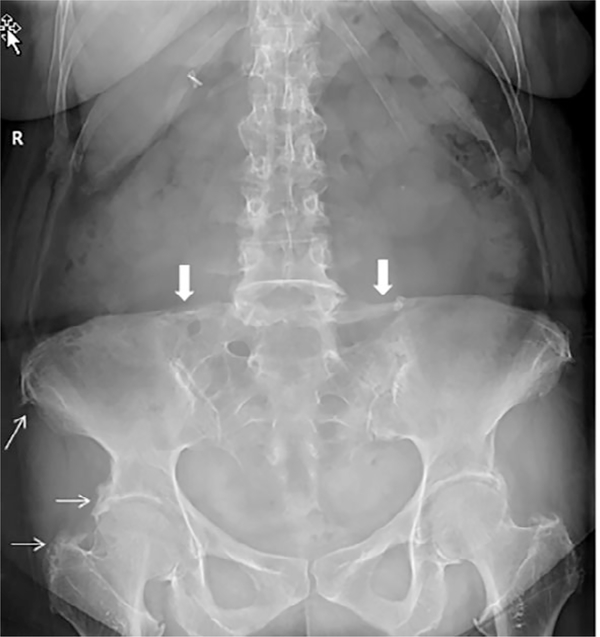
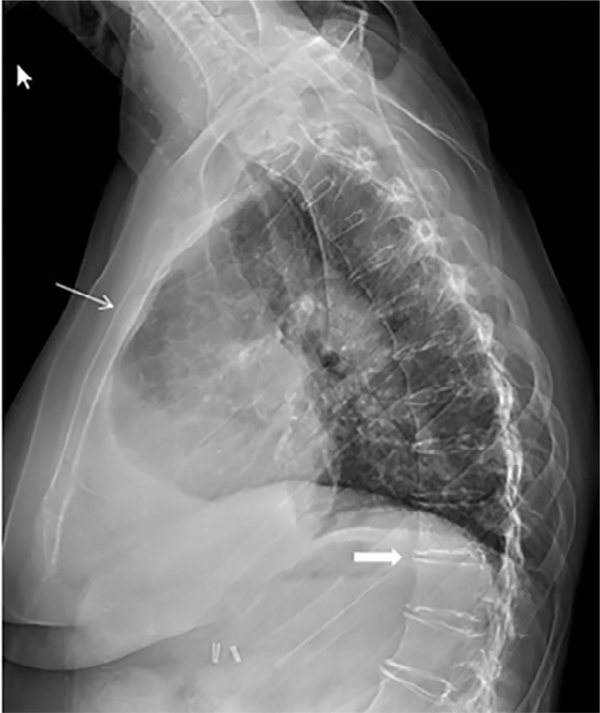
The family pedigree is outlined in Fig. 4. The proband’s 53-year-old sister (II-4) had more severe clinical manifestations, which started at a younger age. She was deaf at birth, but her mother had measles during pregnancy. At 3 years of age, she had cardiac surgery for a “hole in her heart.” She also developed intermittent periarticular “inflammation” episodes beginning around age 17 years of her wrists, knees, elbows, ankles, and spine (Figs. 5 and 6). Because of progressive back pain, she developed a stooped gait, including difficulty with ambulation. She also had normocalcemic PHPT with PTH measures ranging from 80 to 126 pg/mL, serum calcium levels of 8.9 to 9.1 mg/dL, and intermittently low serum phosphorus values (nadir of 2.1 mg/dL), resulting in a three-gland parathyroidectomy a year before evaluation in the Bone Clinic at Mayo Clinic Rochester. Her physical examination was significant for hearing loss, Heberden’s and Bouchard’s nodes with joint contractures, and limited range of motion of her fingers bilaterally with firm nodules to palpation on both her fingers and toes without associated warmth or erythema. She also had a stooped posture. Her skeletal radiographs demonstrated bowing of bilateral femurs, periarticular calcifications, and periarticular enthesopathy. DXA BMD showed osteopenia (worst femur neck T-score –1.5). She did have a history of a right wrist fracture after missing a step and falling as well as radiographic nephrolithiasis. Laboratory values are outlined in Table 2 and were significant for a PTH of 83 pg/mL, serum calcium of 8.6 mg/dL, and phosphorus of 2.5 mg/dL after her parathyroid surgery 1 year earlier. Her vascular health screening was also unremarkable other than increased CIMT.
Fig. 4.
ENPP1 mutation family pedigree. Arrowhead indicates the proband. *Subjects genetically tested.
Fig. 5.
Anteroposterior radiograph of the lumbar spine, pelvis, and hips demonstrates enthesopathy of both innominate bones and proximal femurs (thin arrows). In addition, there is ossification of the iliolumbar ligaments at L5 (thick arrows).
Fig. 6.
Lateral radiograph of the thoracic spine demonstrates hypertrophic changes at all of the thoracic and lumbar interspaces (thick arrow) and ankylosis of the sternomanubrial joint (thin arrow).
In addition to the above two subjects with biallelic mutations (II-2, II-4), five subjects had monoallelic mutations (I-1, mother of proband; I-2, father of proband; III-1, daughter of proband; III-2, son of proband; III-3, son of proband), while two individuals had no mutations identified (II-3, sister of proband; II-5, brother of proband). Of the four individuals with monoallelic mutations, the maternal mutation associated with GACI was identified in three subjects (I-1, III-2, III-3), while two subjects had the paternal mutation (I-2, III-1). As opposed to biallelic mutations, subjects with monoallelic mutations did not have a history suggestive of periarticular calcifications or bowing of femurs. However, the proband’s daughter with the paternal monoallelic mutation (III-1) did have an osteosarcoma of her right distal femur diagnosed at 15 years of age. In addition, a melanoma was diagnosed in one individual (III-3) with a monoallelic maternal mutation at 17 years of age, and the proband’s mother (I-1) had surgical removal of a cranial nerve schwannoma at 56 years of age. Biochemically, in subjects with monoallelic mutations, albumin-corrected calcium was low in two (I-1, III-1) and phosphorus was low in one (III-2). Both intact and C-terminal FGF23 as well as TRP were normal in all subjects with monoallelic mutations. When compared with siblings without ENPP1 variants (II-3, II-5), three individuals with monoallelic ENPP1 variants exhibited reduced plasma PPi (III-1, III-2, III-3), as did one with biallelic ENPP1 variants (II-4).
Discussion
Loss-of-function variants of the ENPP1 gene, which encodes the enzyme ENPP1, can cause both GACI and ARHR2. Variable disease severity has been reported, with infants presenting with severe GACI, whereas post-infancy children and adults often present with ARHR2. However, such pathogenic variants are extremely rare, and to date, only 7 adults with ages ranging from 19 to 35 years are reported as surviving with GACI, causing ENPP1 variants.(4,16,17) Additionally, ARHR2 caused by an inactivating ENPP1 variant has been described in 6 adults ranging from 20 to 62 years.(4,5,18) Hence, these two individuals with inactivating compound heterozygous ENPP1 pathogenic variants, one of which has been associated with GACI (p.Arg481Trp), in addition to a novel ENPP1 variant not previously identified (p.Cys108Phe), add to the limited knowledge of these rare conditions.
Plasma PPi is not a clinically measured analyte and assays to measure plasma PPi are available in only a limited number of research laboratories. A limitation of our study is therefore the lack of a clinically approved CLIA assay to reliably and reproducibly quantitate plasma PPi in patients. However, PPi has long been recognized as a strong endogenous inhibitor of mineralization(19) and vascular calcification.(20) Patients with biallelic ABCC6 mutations associated with GACI type 2(21) exhibit plasma PPi levels of approximately 0.5 μM,(6) and assays performed on a limited number of infants with biallelic ENPP1 deficiency and extensive vascular calcifications (overt symptoms of GACI type 1) returned levels between 70 and 250 nM (KZ and DTB, unpublished observations). Finally, PPi assays performed on ENPP 1-deficient mice in our laboratory routinely return levels between 250 and 500 nM.(10) However, plasma PPi has not been systematically studied in asymptomatic patients, and therefore normal levels of plasma PPi are less well established. In a detailed study on the relationship between calcification, plasma PPi, and ESRD, the plasma PPi in 22 normal patients was reported to be 2.63 ± 0.47 μM,(20) and vascular calcification in ESRD patients who were on hemodialysis or peritoneal dialysis was significantly increased after 1 year in the patients with a mean plasma PPi of 1.49 μM. It therefore appears that levels of plasma PPi in asymptomatic patients are around 2.5 μM and that mineralization problems begin to develop in patients with ESRD when their plasma PPi drops to 1.5 μM, suggesting a lower limit of normal for plasma PPi in healthy adults.
To confirm that the proband’s variants in the ENPP1 sequence reduced catalytic activity, we performed in vitro measurements comparing the enzymatic velocity of WT ENPP1 with the p. Cys108Phe and p.Arg481Trp variants present in the proband. Both variants demonstrated reduced enzymatic velocity using a synthetic colorimetric substrate for ENPP1, and the p. Cys108Phe was severe, exhibiting 20% of WT ENPP1 catalytic velocity, while p.Arg481Trp was not, retaining 55% of WT activity. These findings are consistent with clinical reports that heterozygous carriers of p.Arg481Trp do not exhibit decreased plasma PPi.(11,12) These findings should translate to low plasma PPi in the proband, but instead the proband exhibited a plasma PPi of 1.8 μM, which is higher than expected in a patient manifesting symptoms of ectopic mineralization. There is currently no clinically validated assay for measurement of plasma PPi, a limitation of our study. However, the plasma PPi levels were measured in triplicate and exhibited standard deviations of approximately 1%. In addition, the plasma concentrations measured were within the linear range of our standard curve (Supplemental Fig. S1), suggesting that the values obtained were reliably measured. Nevertheless, we are unable to exclude the possibility of error in the assay, especially in light of the fact that platelet-dense granules contain large concentrations of PPi and therefore any platelet degranulation during sample collection and processing could introduce significant error. Other plausible explanations for the higher than expected plasma PPi levels in the proband include genetic factors compensating for reduced plasma PPi levels. For example, bone alkaline phosphatase in the proband is approximately half that of her affected sister, who also exhibits half the concentration of plasma PPi as the proband. In summary, plasma PPi in the proband was greater than expected, given our characterization of the ENPP1 mutations present and her clinical symptoms, and may be related to inherent uncertainty in the assay.
The ages of diagnosis in our patients, 54 and 53 years, represent two of the older subjects reported to date, and the oldest patients with GACI-causing mutations. Despite these pathogenic variants, we found no evidence of vascular calcification but did find intima-media proliferation of the vascular wall, a finding previously observed in GACI patients(22) and recently associated with inhibition of ENPP1-generated adenosine monophosphate (AMP).(23) The fact that the biallelic variants were compound heterozygous possibly contributed to the prolonged survival in these patients, especially in light of the relatively mild nature of the p.Arg481Trp mutation, which retains 55% of the WT ENPP1 catalytic activity when measured in our in vitro assay. Homozygous biallelic mutations known to cause GACI have been associated with death in early childhood, whereas compound heterozygous mutations, perhaps with mild loss-of-function mutations, have demonstrated survival beyond the critical early childhood period in some families.(24) These findings suggest improved survival and protection from severe arterial calcification in individuals with compound heterozygous mutations such as our two subjects (II-2, II-4). Hypophosphatemia has also been postulated to be protective in patients with ENPP1 genetic variants.(3,24) Specifically, phosphorus is a major component of hydroxyapatite, and therefore, deficiency via renal phosphate loss due to increased FGF23 may mitigate the risk of arterial calcification due to the lack of PPi. In addition, phosphate-poor diet and crossbreeding with PHEX knockout mice inducing hypophosphatemia in GACI mice models have been shown to significantly reduce arterial and periarticular calcification.(25)
The clinical features of ARHR2 in adults can include periarticular calcifications with a waxing and waning clinical course over years, history of bone deformities, and a conductive hearing loss. Loss-of-function ENPP1 variants have been associated with periarticular calcifications in ARHR2 due to PPi deficiency, which inhibits hydroxyapatite crystal deposition and calcification of the media of large and medium-sized arteries and intimal proliferation in GACI.(24) Although both of our patients with biallelic pathogenic variants had periarticular calcifications, one (II-4) also had prominent periarticular enthesopathy that has not been previously described in ARHR2 to our knowledge. Enthesopathy is commonly found in X-linked hypophosphatemic rickets (XLH), the most common form of heritable rickets, and is associated with increased body mass index, older age, and male sex.(26) The cause is unclear, but in mouse models of XLH that overex-press FGF23, enthesopathy is found.(27) ENPP1 knockout rodent models have also observed mineralization of tendons, cartilage, and ligaments with severe osteoarthritis and ankyloses.(28)
Biochemically, individuals with ENPP1 pathogenic variants may also be difficult to characterize based on the subtle and variable nature of the laboratory results. PTH, vitamin D, and FGF23 are key regulatory hormones in mineral homeostasis.(29) Reduced 1,25(OH)2 D due to FGF23-mediated downregulation of 1-alpha hydroxylase activity can lead to a compensatory increase in PTH to maintain eucalcemia. It is conceivable that over time chronic parathyroid stimulation could lead to the development of PHPT.(30) In addition, it is notable that a patient with increased alpha-klotho production and increased FGF23 levels due to a translocation with the development of hypophosphatemia and hyperparathyroidism has been described.(31) PHPT has not been previously noted in patients with ENPP1 variants, and a pathogenic variant associated with familial causes of PHPT was not identified in our WES. There have been two reports of secondary hyperparathyroidism that improved after correction of mineral abnormalities.(5,32) However, neither of our patients with normocalcemic PHPT had previously received phosphate supplementation.
Our patients confirm the observation that C-terminal FGF23 can be normal with biallelic ENPP1 genetic variants, as it was in one of our subjects (II-2). Intact FGF23, however, was elevated in both patients carrying both ENPP1 pathogenic variants described above. This is similar to the report by Lorenze-Depiereux and colleagues, where C-terminal FGF 23 levels were normal compared with intact FGF23 levels that were elevated or in the upper normal range.(4) Tubular reabsorption of phosphate was low in only one subject (II-2) but normal in the other subject with biallelic ENPP1 pathogenic variants. Although extracellular PPi generation has been shown to be reduced in ENPP1 mutations, we noted lower levels in only 4 of 6 subjects with ENPP1 mutations. Furthermore, the reduction in PPi does not seem to correlate with the mutation status, as evidenced by the proband with normal PPi levels, suggesting that there may be a compensatory mechanism involved.
Given the challenges of diagnosing ARHR2 based on clinical and biochemical findings, targeted sequencing of the ENPP1 gene should be considered in individuals when it is suspected.
This family study suggests that biallelic compound heterozygous variants lead to clinical and biochemical disease, whereas carriers of monoallelic variants appear clinically unaffected, consistent with the autosomal recessive nature of this disorder. However, hypophosphatemia was present in one subject with monoallelic ENPP1 deficiency (III-2), who also exhibited normal FGF23 and TRP, and calcium was low in two subjects with monoallelic ENPP1 variants (I-1, III-1). It is notable that two subjects with monoallelic ENPP1 variants had malignancies at a young age— osteosarcoma (III-1) and melanoma (III-3)—but we cannot discard other possible contributing causes. Also, WES did not identify a pathogenic variant associated with osteosarcoma. Although malignancy has not been previously described with ENPP1 variants, 1,25(OH)2D receptors have been demonstrated in malignant melanomas, and treatment with 1,25(OH)2D has shown inhibitory effects on human melanoma cells in vitro.(33) It is possible that chronic reductions in 1,25(OH)2D due to 2 increased FGF23 levels could contribute to malignancy risk, but this needs further investigation.
Identification of individuals with ENPP1 gene alterations causing GACI or ARHR2 has increased clinical relevance given the development of ENPP1 enzyme replacement therapy (INZ-701). In preclinical studies, replacement therapy has shown potential to restore PPi to normal levels, preventing the complications associated with ENPP1 deficiency.(10,34) Oral administration of PPi has also been reported to inhibit connective tissue calcification in mouse models of GACI.(35) Finally, bisphosphonate treatment has been shown to improve survival beyond infancy in 65% of treated patients.(3)
There are several limitations to this study. In addition to the small cohort, the patients only had a single measure of bone, calcium, and plasma PPi biomarkers. The latter is important because of the inherent difficulty in the plasma PPi assay and the lack of a clinically viable assay. Finally, we lack longitudinal follow-up of these subjects to better understand the natural history of this disorder. However, the findings are relevant due to the extremely rare nature of the disease and limited reports of adults with ENPP1 genetic variants.
In summary, patients with biallelic ENPP1 mutations can survive well into older adulthood without vascular calcification, including those with GACI-causing mutations. Clinical and biochemical manifestations of biallelic ENPP1 mutations are variable, even in individuals with identical mutations, but include bone deformities associated with renal phosphate wasting and hypophosphatemia with high intact FGF23, waxing and waning periarticular calcifications, and hearing loss. Periarticular enthesopathy and multi-gland PHPT, which have not been previously described, also appear to be potential sequelae of such mutations. Vascular intima-media proliferation appears to be present in older adults. Finally, although carriers with monoallelic ENPP1 mutations appear unaffected by clinical manifestations, there may be subtle biochemical abnormalities that warrant further investigation to better understand the fundamental role of ENPP1 in health and disease.
Supplementary Material
Acknowledgments
This study was made possible using the resources of CTSA grant no. UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS) and internal funding from the Center for Individualized Medicine (Mayo Clinic).
Study design: AK and RAW. Study conduct: AK, AF, and RAW. Data collection: AK, RAW, and KZ. Data analysis: AK, AF, RAW, RK, and KZ. Data interpretation: AK, RAW, AF, RK, RJS, YW, DTB, KZ, and VM. Drafting manuscript: AK, DTB, RAW, RK, AF, and VM. Revising manuscript content: AK, AF, RAW, DTB, and VM. Approving final version of manuscript: AK, RAW, AF, and KZ take responsibility for the integrity of the data analysis.
Disclosures
AK, AF, RK, RJS, LS-R, MZ, BL, KZ, PRS, EK, and RAW state that they have no conflicts of interest. DTB is an inventor of patents owned by Yale University that describe therapeutics for ENPP1 deficiency and is an equity holder and receives research and consulting support from Inozyme Pharma, Inc. VM serves as a consultant on the scientific advisory board of Encepheal Therapeutics, Inc., which is not related to subject matter of this study.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Caswell AM, Whyte MP, Russell RG. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: clinical and laboratory aspects. Crit Rev Clin Lab Sci. 1991;28(3):175–232. [DOI] [PubMed] [Google Scholar]
- 2.Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P, Rutsch F. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI). Hum Mutat. 2005;25(1):98. [DOI] [PubMed] [Google Scholar]
- 3.Rutsch F, Boyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1(2):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86(2):267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito T, Shimizu Y, Hori M, et al. A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone. 2011;49(4): 913–6. [DOI] [PubMed] [Google Scholar]
- 6.Jansen RS, Duijst S, Mahakena S, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arter-ioscler Thromb Vasc Biol. 2014;34(9):1985–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen RS, Kucukosmanoglu A, de Haas M, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci U S A. 2013;110(50): 20206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eswar N, Webb B, Marti-Renom MA, et al. Comparative protein structure modeling using modeller Curr Protoc Bioinformatics. 2006. chapter 5:unit 5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen S, Perrakis A, Ulens C, et al. Structure of NPP1, an ectonucleotide pyrophosphatase/phosphodiesterase involved in tissue calcification. Structure. 2012;20(11):1948–59. [DOI] [PubMed] [Google Scholar]
- 10.Albright RA, Stabach P, Cao W, et al. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat Commun. 2015;6:10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutsch F, Ruf N, Vaingankar S, et al. Mutations in ENPP1 are associated with ’idiopathic’ infantile arterial calcification. Nat Genet. 2003; 34(4):379–81. [DOI] [PubMed] [Google Scholar]
- 12.Thumbigere-Math V, Alqadi A, Chalmers NI, et al. Hypercementosis associated with ENPP1 mutations and GACI. J Dent Res. 2018;97(4): 432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders LP, Ouellette A, Bandle R, et al. Identification of small-molecule inhibitors of autotaxin that inhibit melanoma cell migration and invasion. Mol Cancer Ther. 2008;7(10):3352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for molecular pathology. Genet Med. 2015;17(5): 405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrott PK, Newcombe KD, Becroft DM, Friedlander DH. Idiopathic infantile arterial calcification with survival to adult life. Pediatr Car-diol. 1984;5(2):119–22. [DOI] [PubMed] [Google Scholar]
- 17.van der Sluis IM, Boot AM, Vernooij M, Meradji M, Kroon AA. Idiopathic infantile arterial calcification: clinical presentation, therapy and long-term follow-up. Eur J Pediatr. 2006;165(9):590–3. [DOI] [PubMed] [Google Scholar]
- 18.Levy-Litan V, Hershkovitz E, Avizov L, et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86(2):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231(1):1–8. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant. 2010;25(1):187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90(1): 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran JJ. Idiopathic arterial calcification of infancy: a clinicopathologic study. Pathol Annu. 1975;10:393–417. [PubMed] [Google Scholar]
- 23.Nitschke Y, Yan Y, Buers I, Kintziger K, Askew K, Rutsch F. ENPP1-Fc prevents neointima formation in generalized arterial calcification of infancy through the generation of AMP. Exp Mol Med. 2018;50 (10):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stella J, Buers I, van de Wetering K, Hohne W, Rutsch F, Nitschke Y. Effects of different variants in the ENPP1 gene on the functional properties of ectonucleotide pyrophosphatase/phosphodiesterase family member 1. Hum Mutat. 2016;37(11):1190–201. [DOI] [PubMed] [Google Scholar]
- 25.Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19(9):1093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor J, Olear EA, Insogna KL, et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metabol. 2015;100(10):3625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karaplis AC, Bai X, Falet JP, Macica CM. Mineralizing enthesopathy is a common feature of renal phosphate-wasting disorders attributed to FGF23 and is exacerbated by standard therapy in hyp mice. Endocrinology. 2012;153(12):5906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie NC, Huesa C, Rutsch F, MacRae VE. New insights into NPP1 function: Lessons from clinical and animal studies. Bone. 2012;51(5): 961–8. [DOI] [PubMed] [Google Scholar]
- 29.Blau JE, Collins MT. The PTH-vitamin D-FGF23 axis. Rev Endocr Metab Disord. 2015;16(2):165–74. [DOI] [PubMed] [Google Scholar]
- 30.Arnold A, Brown MF, Urena P, Gaz RD, Sarfati E, Drueke TB. Monoclonality of parathyroid tumors in chronic-renal-failure and in primary parathyroid hyperplasia. J Clin Invest. 1995;95(5):2047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brownstein CA, Adler F, Nelson-Williams C, et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105(9): 3455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta P, Mitchell A, Tysoe C, Caswell R, Owens M, Vincent T. Novel compound heterozygous mutations in ENPP1 cause hypophospha-taemic rickets with anterior spinal ligament ossification. Rheumatology. 2012;51(10):1919–21. [DOI] [PubMed] [Google Scholar]
- 33.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108(3):1083–6. [DOI] [PubMed] [Google Scholar]
- 34.Khan T, Sinkevicius KW, Vong S, et al. ENPP1 enzyme replacement therapy improves blood pressure and cardiovascular function in a mouse model of generalized arterial calcification of infancy. Dis Models Mech. 2018;11(10):dmm035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dedinszki D, Szeri F, Kozak E, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med. 2017; 9(11):1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.