Abstract
Objective:
Older adults with type 2 diabetes (T2D) are at increased risk for depression, cognitive decline and dementia compared to those without T2D. Little is known about the association of simultaneous changes in depression symptoms and cognitive decline over time.
Methods:
Subjects [n=1021; mean age 71.6 (SD=4.6); 41.2% female] were initially cognitively normal participants of the Israel Diabetes and Cognitive Decline (IDCD) study who underwent evaluations of depression and cognition approximately every 18 months. Cognitive tests were summarized into four cognitive domains: episodic memory, attention/working memory, executive functions and semantic categorization. The average of the z-scores of the four domains defined global cognition. Depression symptoms were assessed using the Geriatric Depression Scale (GDS), 15-item version. We fit a random coefficients model of changes in depression and in cognitive functions, adjusting for baseline sociodemographic and cardiovascular variables.
Results:
“ Higher number of depression symptoms at baseline was significantly associated with lower baseline cognitive scores in global cognition (estimate=−0.1175, SE= 0.021, DF=1014, t=−5.59; p<0.001), executive functions (estimate=−0.186, SE=0.036, DF=1013, t=−5.15; p=<0.001), semantic categorization (estimate=−0.155, SE=0.029, DF=1008, t=−5.3; p<0.001) and episodic memory (estimate=−0.08165, SE=0.027, DF=1035, t=−2.92; p=0.0036), but not with rate of decline in any cognitive domain. During follow-up, a larger increase in number of depression symptoms, was associated with worse cognitive outcomes in global cognition (estimate=−0.1053, SE=0.027, DF=1612, t=−3.77; p=0.0002), semantic categorization (estimate=−0.123, SE=0,036, DF=1583, t=−3.36; p=0.0008) and in episodic memory (estimate=−0.165, SE=0.055, DF=1622, t=−3.02; p=0.003), but the size of this effect was constant over time. Conclusion: In elderly with T2D, increase in depression symptoms over time is associated with parallel cognitive decline, indicating that the natural course of the two conditions progresses concurrently and suggesting common underlying mechanisms”.
Keywords: trajectories, depression, cognition, older adults, type 2 diabetes
OBJECTIVE
Depression, especially in old age, has been consistently associated with increased dementia incidence (1, 2), though it is still unclear whether depression is a risk or causal factor for dementia or merely a presenting symptom (3). The evidence on the relationship of depression, and specifically its subtle presentations, with clinical entities that precede dementia, namely cognitive decline and mild cognitive impairment (MCI), is less consistent, with the majority of evidence indicating that depression and depression symptoms predict incident MCI (4);(5) and faster cognitive decline (6). However, several studies suggest no such associations (7);(8), an association only for certain depression-related symptoms (9) or only for some types of cognitive outcomes (e.g. amnestic versus non-amnestic MCI (9)). The simultaneous changes in depression and cognitive decline, which better reflect the natural course of both conditions, have rarely been studied. Clear understanding of the relationships between the course of depression and the course of cognitive decline may have important value for prediction of dementia and indication of optimal time points for administration of dementia- prevention strategies.
Patients with type 2 diabetes (T2D) are at increased risk for both impaired cognition (cognitive decline, MCI and dementia) and depression. The prevalence of depression in patients with T2D is 2–3 times higher compared to people without T2D (10). Depression in T2D, even if not fulfilling criteria for major depressive disorder (11), is associated with faster cognitive decline. The accelerating prevalence of T2D with age, combined with the increased risk for depression and dementia in T2D, the potential role of depression in poor cognitive outcomes (12), and the urgent need to develop dementia prevention strategies, stress the importance of examining whether depression and its trajectories can predict cognitive changes that precede dementia in patients with T2D.
In the present study, we examined the relationships between simultaneous changes over time in depression and cognition in >1000 initially cognitively normal older adults (≥65 years old) with T2D, participants of the Israel Diabetes and Cognitive Decline (IDCD) study (13). Participants underwent structured evaluations of depression symptoms and cognitive function every18- months for a median follow-up of 48 months.
METHODS:
The IDCD is a collaboration of the Icahn School of Medicine at Mount Sinai, NY, the Sheba Medical Center, Israel, and the Maccabi Healthcare Services (MHS), Israel. The study was approved by all three IRB committees and all participants signed informed consent.
Participants
Participants are patients with T2D aged ≥65, who are engaged in the longitudinal IDCD study, investigating the relationship of long-term T2D characteristics and cognitive decline. The IDCD methods have been described in detail elsewhere (13). Briefly, IDCD participants were selected randomly from the ~11,000 elderly with T2D listed in the diabetes registry of MHS, the second largest HMO in Israel. The diabetes registry was established in 1998 to facilitate T2D management and improve its treatment. Entry criteria to the registry are any of the following: (1)
HbA1c > 55.7s mmol/mol (7.25%), (2) Glucose >200 mg/dl on two exams more than three months apart, (3) purchase of anti-diabetic medication twice within 3 months supported by a HbA1c > 47.4 mmol/mol (6.5%) or Glucose > 125 mg/dl within half a year, (4) diagnosis of T2D (ICD9 code) by a general practitioner, internist, endocrinologist, ophthalmologist, or diabetes advisor, supported by a HbA1c > 47.4 mmol/mol (6.5%), or Glucose > 125 mg/dl within half a year.
Eligibility criteria to the IDCD were: being listed in the MHS diabetes registry, T2D diagnosis, living in the central area of Israel, age ≥65 years, being identified as cognitively normal at baseline (based on a multidisciplinary weekly consensus conference), absence of major medical, psychiatric, or neurological diagnoses that may affect cognitive performance, having ≥3 HbA1c measurements in the diabetes registry, Hebrew fluency, and availability of an informant.
Participants’ recruitment process has been described in detail previously (13). In short, the MHS diabetes registry is thoroughly screened for identification of potential participants, excluding anyone with an ICD code for dementia, treatment with cholinesterase inhibitors, or with a major psychiatric or neurological condition (e.g., schizophrenia or Parkinson’s disease) that could affect cognitive performance. The MHS team contacts potential participants and asks for their participation after determining Hebrew fluency and availability of an informant. Consenting individuals undergo a thorough cognitive assessment (described below). Questionnaires for measurement of participants’ cognitive and functional performance, presence of depression and behavioral disturbances are also administered. All participants’ neuropsychological data, including the results of the cognitive battery administered, the score of the Clinical Dementia Rating (CDR) scale and results of the affective assessments, are discussed by a multidisciplinary consensus conference team in order to define cognitive status (i.e. cognitively normal, MCI, or dementia and their subtypes). If the participant is cognitively normal at baseline, follow up interviews, identical on procedures and content to the baseline assessment, are performed at 18 months intervals. Participants diagnosed as MCI or dementia at baseline, are not included in the study, however, those who convert from a status of normal cognition to MCI during follow up, continue their participation in the study until conversion to dementia.
Assessment of depressive symptoms:
Presence of depression symptoms is evaluated using the 15-item version of the Geriatric Depression Scale (GDS) (14), a self-reported, easily administered scale. This questionnaire is suitable for large scale studies of depression and advantageous in the context of elderly with T2D since it has little focus on somatic symptoms which could be confounded by diabetes symptoms such as neuropathy (14). GDS data is collected at every IDCD visit.
Cognitive Assessment:
A thorough neuropsychological battery is administered by experienced and certified interviewers which are blind to the diabetes related data (13). This battery covers four cognitive domains: (1) Memory [Alzheimer’s Disease Assessment Scale (ADAS) Word List Immediate Recall, Delayed Recall, and Recognition], (2) Attention/working memory [Diamond Cancellation, Digit Span forward, and backward], (3) Executive functions [Trails Making Test A and B, Digit Symbol Substitution Test], and (4) Semantic categorization [Similarities, Letter and Category Fluency]. Composite measures of the four cognitive domains are calculated at baseline by converting each test score to a z-score and then averaging the z-scores. An overall measure averaging the four cognitive domain scores is computed (global cognition). At each follow up, the same coefficients are used to calculate the follow up respective score.
Clinical Dementia Rating Scale (CDR)(15):
this scale is administered to participants and their informants. It assesses cognitive and functional abilities in the areas of memory, orientation, judgment and problem solving, social activities, home and time free and personal care. The scores of each of these areas are combined to obtain a composite score ranging from 0 to 3 (0=cognitively normal; 0.5= questionable dementia, ≥1=increasing degrees of severity of dementia).
Statistical analysis:
Descriptive data are reported as N (percent) or mean (standard deviation), as appropriate. Unadjusted bivariate analyses included t-test, one-way ANOVA or Chi-square tests to compare group differences on sociodemographic and clinical variables, as appropriate. The sociodemographic variables included baseline age, gender, and years of education. The cardiovascular variables included baseline BMI, hemoglobin A1c (HbA1c), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, triglycerides, and creatinine. Diabetes duration was also used as a variable.
The primary objective of the analysis is to assess the longitudinal association of number of depression symptoms as measured by the GDS with cognitive decline. We examined the effect of every additional one-point increase in number of depression symptoms on change of global cognition and each of the cognitive domains. We used a random coefficients model to describe the trend of cognitive z scores over the follow-up period. It was assumed that the trajectory of the cognitive data was linear and the coefficients of intercept and slope were unique to each participant. In this type of model, the participant term and the participant by time interaction term are both included as random effects. In addition, it has the flexibility to incorporate data that are measured at different time points for different participants. Conceptually, the model assumed that, after accounting for the baseline covariates, the cognitive z score at the time of assessment could be explained by a participant’s baseline GDS, the extent of change in GDS from baseline, time of the follow-up visit, and two 2-way interaction terms between baseline GDS and time and between change of GDS and time. The baseline GDS and time interaction allowed us to assess how much baseline GDS affects cognition over time. A significant negative interaction suggests that for participants who had high baseline GDS score (i.e. more depressive symptoms), the decline in cognition was greater over time than participants who had low baseline GDS score, controlling for the GDS change and other baseline covariates (age, gender, years of education, BMI, HbA1c, SBP, DBP, total cholesterol, triglycerides and creatinine). Similarly, the GDS change and time interaction allowed us to assess whether the effect of GDS change on cognition, changes over time. For example, if an increase in GDS (i.e. more depressive symptoms) during the initial follow-up was associated with decrease in cognitive function, then a significant negative interaction with time would suggest that this effect increased over time. Two sets of random coefficients models were fit. The first model adjusted for the sociodemographic variables (Model 1). The second model further adjusted for the cardiovascular covariates and diabetes duration (Model 2). Lastly, we assume that missing data and dropouts were at random, that is the unobserved data could be predicted using the covariates considered in the model through the EM algorithm. Model diagnostics for mixed models were applied. Studentized residuals appear random and normally distributed.
Statistical analysis was conducted using SAS 9.4 (SAS Institute, Cary, NC). Two-sided p-value ≤ 0.01 was defined as the significance level in all statistical tests to adjust for five cognitive outcomes.
RESULTS:
Description of the sample:
The analyses included 1026 initially non-demented IDCD participants. Mean age at baseline was 71.6 (SD=4.7) years, 41.3% were female with an average of 13.2 (SD=3.6) years of education, mean MMSE score at baseline was 28.0 (SD=1.8) and the range was 24–30. All cases were determined to be cognitively normal at baseline by the multidisciplinary consensus conference team (see above). Thus, the MMSE scores are consistent with normal cognitive status. Mean GDS at baseline was 2.2 (SD=2.4) and the range was 0.0–14.0. Only 10.2% of participants had a score >5, suggesting that most participants did not suffer from depression symptoms of clinical significance. Other baseline characteristics of the sample are described in Table 1. Data from all participants and all assessments were used for analysis. The median follow-up time was 48 months [IQR: 24~54 months]. Over time, subjects declined in global cognitive function (annual slope of Z score change −0.106; SE 0.009; DF=854; t =−11.27; p<0.0001). Increase in number of GDS symptoms at the follow up visits ranged between 43–50% of participants.
Table 1:
Participant’s baseline characteristics
| Variable | n | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Age at b.l.* (years) | 1017 | 71.6 | 4.7 | 61.0 | 85.0 |
| Sex (% female) | 1025 | 41.3% | |||
| Education (years) | 1012 | 13.2 | 3.6 | 0.0 | 26.0 |
| T2D duration (years) | 1026 | 9.8 | 4.4 | 0.3 | 19.9 |
| MMSE* at b.l. | 1025 | 28.0 | 1.8 | 24.0 | 30.0 |
| GDS* score at b.l. | 1023 | 2.2 | 2.4 | 0.0 | 14.0 |
| % GDS> 5 at b.l. 1026 | 10.2 | ||||
| Cholesterol (mg/dl) | 1026 | 178.5 | 25.3 | 93.5 | 278.3 |
| Triglycerides (mg/dl) | 1026 | 156.8 | 61.5 | 41.4 | 707.4 |
| Creatinine (mg/dl) | 1022 | 1.0 | 0.2 | 0.3 | 3.2 |
| HbA1c* (%) | 1025 | 6.8 | 0.8 | 3.9 | 10.0 |
| SBP* (mm Hg) | 1024 | 134.4 | 9.7 | 99.9 | 171.4 |
| DBP* (mm Hg) | 1024 | 76.8 | 4.9 | 5.7 | 95.7 |
| BMI* (Kg/m2) | 995 | 28.3 | 4.3 | 7.9 | 50.0 |
b.l= baseline; GDS=geriatric depression scale; MMSE= mini mental state examination; HbA1c=hemoglobin A1c; SBP= systolic blood pressure; DBP= diastolic blood pressure; BMI= body mass index
We compared participants who dropped out from the study after baseline to those who remained in the study for at least one follow- up visit. The groups did not differ in the majority of baseline characteristics: age, sex, education, T2D duration, GDS score, percent participants with GDS>5, total cholesterol levels, triglycerides levels, creatinine levels, diastolic blood pressure and BMI. Those who dropped out, had minimally lower MMSE scores, slightly higher HbA1c and systolic blood pressure levels (supplemental table 1); these differences however, are not of clinical significance.
Cross sectional associations of number of depression symptoms with cognition:
As depicted in Table 2, at baseline, a higher number of depression symptoms was associated with lower cognitive scores in global cognition, executive functions, semantic categorization and episodic memory, and approached significance for working memory/attention (Table 2, model 1). These results remained essentially unchanged when adjusting for additional covariates (Table 2, model 2).
Table 2:
The associations of GDS score at baseline with baseline cognitive function and with cognitive decline
| model 1 | ||||||
|---|---|---|---|---|---|---|
| cognitive domain | effect | estimate | SE | DF | t value | p |
| Overall cognitive score | *GDS bl | −0.1175 | 0.02102 | 1014 | −5.59 | <.0001 |
| GDS bl · t yr | −0.00060 | 0.000348 | 1495 | −1.72 | 0.0854 | |
| Executive functions | GDS bl | −0.1863 | 0.03617 | 1013 | −5.15 | <.0001 |
| GDS bl · t yr | −0.00086 | 0.000676 | 729 | −1.28 | 0.2019 | |
| Working memory/ attention | GDS bl | −0.04562 | 0.02709 | 1028 | −1.68 | 0.0924 |
| GDS bl · t yr | −0.0005 | 0.000564 | 1495 | −0.89 | 0.3736 | |
| Semantic categorization | GDS bl | −0.1551 | 0.02927 | 1008 | −5.3 | <.0001 |
| GDS bl · t yr | −0.0001 | 0.000448 | 1479 | −0.23 | 0.8175 | |
| Episodic memory | GDS bl | −0.08165 | 0.02795 | 1035 | −2.92 | 0.0036 |
| GDS bl · t yr | −0.00141 | 0.000708 | 719 | −2 | 0.0462 | |
| model 2 | ||||||
| cognitive domain | effect | estimate | SE | DF | t value | p |
| Overall cognitive score | GDS bl | −0.1170 | 0.02142 | 971 | −5.46 | <.0001 |
| GDS bl · t yr | −0.00053 | 0.000358 | 693 | −1.48 | 0.1387 | |
| Executive functions | GDS bl | −0.1781 | 0.03647 | 971 | −4.88 | <.0001 |
| GDS bl · t yr | −0.00084 | 0.00069 | 714 | −1.21 | 0.2257 | |
| Working memory/ attention | GDS bl | −0.05381 | 0.0279 | 984 | −1.93 | 0.0541 |
| GDS bl · t yr | −0.00021 | 0.000573 | 1441 | −0.37 | 0.7146 | |
| Semantic categorization | GDS bl | −0.1512 | 0.02972 | 968 | −5.09 | <.0001 |
| GDS bl · t yr | −0.00008 | 0.000456 | 1429 | −0.18 | 0.8582 | |
| Episodic memory | GDS bl | −0.08547 | 0.02866 | 992 | −2.98 | 0.0029 |
| GDS bl · t yr | −0.00127 | 0.00073 | 701 | −1.74 | 0.829 | |
GDS bl is the GDS score at baseline and t is the follow-up time in year. GDS bl · t yr is the interaction term. A non-significant GDS bl · t yr interaction suggests that the effect of baseline GDS on cognition is constant over time. Because the coefficient for GDS bl is negative, a significant positive interaction indicates that the effect of baseline GDS on cognition diminishes over time, whereas a significant negative interaction indicates that the effect worsens over time. Controlling for other covariates and assuming no change in GDS during follow-up, every χ increase in baseline GDS is associated with B GDSbl · (χ) + B GDSbl · t yr · (χ t) change in cognitive z score. For example, for episodic memory domain, if subject A had a baseline GDS score 2 points higher than subject B, then the z score for subject A would be 0.082 ·2 + 0.001 · 2 ·1 = 0.162 (Model 1) lower than subject B at follow-up Year 1, and 0.082·2 + 0.001 · 2 ·3 = 0.170 at year 3.
Model 1: controlling for age, sex, education
Model 2: controlling for age, sex, education, diabetes duration, cholesterol, creatinine, HbA1c, triglycerides, systolic and diastolic blood pressure, BMI at baseline.
Longitudinal associations of number of depression symptoms with cognitive decline:
As depicted in Table 2, a higher GDS score at baseline was not associated with rate of decline in any of the cognitive domains or with global cognition (Table 2). However, increases in number of depression symptoms during follow-up were associated with poorer outcomes in global cognition, semantic categorization and in episodic memory, but did not reach statistical significance in executive functions and working memory/attention (Table 3, model 1). In the fully adjusted model (Table 3, model 2), the relationships of increases in number of depression symptoms with cognition in global cognition, semantic categorization and episodic memory remained significant. The association of increase in depression symptoms over time on cognition was constant (i.e., the interactions of time with change in depression score were not significant; table 3, figure 1) for all cognitive outcomes, except for semantic categorization, which approached significance after adjustment for multiple comparisons.
Table 3:
The effect of GDS change on changes in cognition over time
| model 1 | ||||||
|---|---|---|---|---|---|---|
| cognitive domain | effect | estimate | SE | DF | t value | p |
| overall | GDS Δ | −0.1053 | 0.02791 | 1612 | −3.77 | 0.0002 |
| GDS Δ · t | 0.000549 | 0.000650 | 1521 | 0.84 | 0.3988 | |
| executive functions | GDS Δ | −0.07939 | 0.05061 | 1354 | −1.57 | 0.1169 |
| GDS Δ · t | −0.00062 | 0.00123 | 1416 | −0.5 | 0.6146 | |
| working memory/ attention | GDS Δ | −0.07628 | 0.04542 | 1716 | −1.68 | 0.0932 |
| GDS Δ · t | 0.000658 | 0.001064 | 1545 | 0.62 | 0.5365 | |
| semantic categorization | GDS Δ | −0.1227 | 0.03649 | 1583 | −3.36 | 0.0008 |
| GDS Δ · t | 0.002018 | 0.000848 | 1512 | 2.38 | 0.0175 | |
| Episodic memory | GDS Δ | −0.1653 | 0.05481 | 1622 | −3.02 | 0.0026 |
| GDS Δ · t | 0.000122 | 0.001318 | 1340 | 0.09 | 0.9263 | |
| model 2 | ||||||
| cognitive domain | effect | estimate | SE | DF | t value | p |
| overall | GDS Δ | −0.1052 | 0.02839 | 1428 | −3.70 | 0.0002 |
| GDS Δ · t | 0.000580 | 0.000662 | 1210 | 0.88 | 0.3806 | |
| executive functions | GDS Δ | −0.07813 | 0.05131 | 1304 | −1.52 | 0.1281 |
| GDS Δ · t | −0.00073 | 0.001248 | 1377 | −0.59 | 0.5586 | |
| working memory/ attention | GDS Δ | −0.07605 | 0.0458 | 1645 | −1.66 | 0.097 |
| GDS Δ · t | 0.000712 | 0.001071 | 1488 | 0.66 | 0.5064 | |
| semantic categorization | GDS Δ | −0.1279 | 0.0368 | 1531 | −3.48 | 0.0005 |
| GDS Δ · t | 0.002173 | 0.000855 | 1461 | 2.54 | 0.0112 | |
| Episodic memory | GDS Δ | −0.1526 | 0.05542 | 1530 | −2.75 | 0.006 |
| GDS Δ · t | −0.00007 | 0.00134 | 1324 | −0.05 | 0.9581 |
GDS Δ is the change in GDS score from baseline ant tis the follow-up time in years. GDS Δ ·t yr is the interaction term. A non-significant GDS Δ · t yr interaction suggests that the effect of
GDS change on cognition is constant over time. Because the coefficient for GDS Δ is negative, a significant positive interaction indicates that the effect of GDS change on cognitive decline diminishes over time, whereas a significant negative interaction indicates that the effect of GDS change on cognitive decline worsens over time. Controlling for baseline GDS and other covariates, every χ increase in GDS at follow-up time t period is associated with B GDSΔ · (χ) + B GDSΔ · t yr · (χ t) change in cognitive z score. For example, let’s assume subject A had greater worsening in depression symptoms than subject B during follow-up (e.g., baseline scores were the same for subjects A and B, but the depression score was 2-point higher for subject A than for subject B at year 1 and also at year 3), then, in the semantic categorization domain, the z score at year 1 for subject A would be 0.252 (−0.128 ·2 + 0.002 · 2 ·1 = −0.252) lower than that of subject B, controlling for baseline GDS and covariates. At year 3, the difference is 0.244 (−0.128 ·2 + 0.002 · 2 ·3 = −0.244). The narrowing difference (0.252 vs 0.244) is the result of a positive GDS Δ · t yr interaction.
Model 1: controlling for age, sex, education
Model 2: controlling for age, sex, education, diabetes duration, cholesterol, creatinine, HbA1c, triglycerides, systolic and diastolic blood pressure, BMI at baseline.
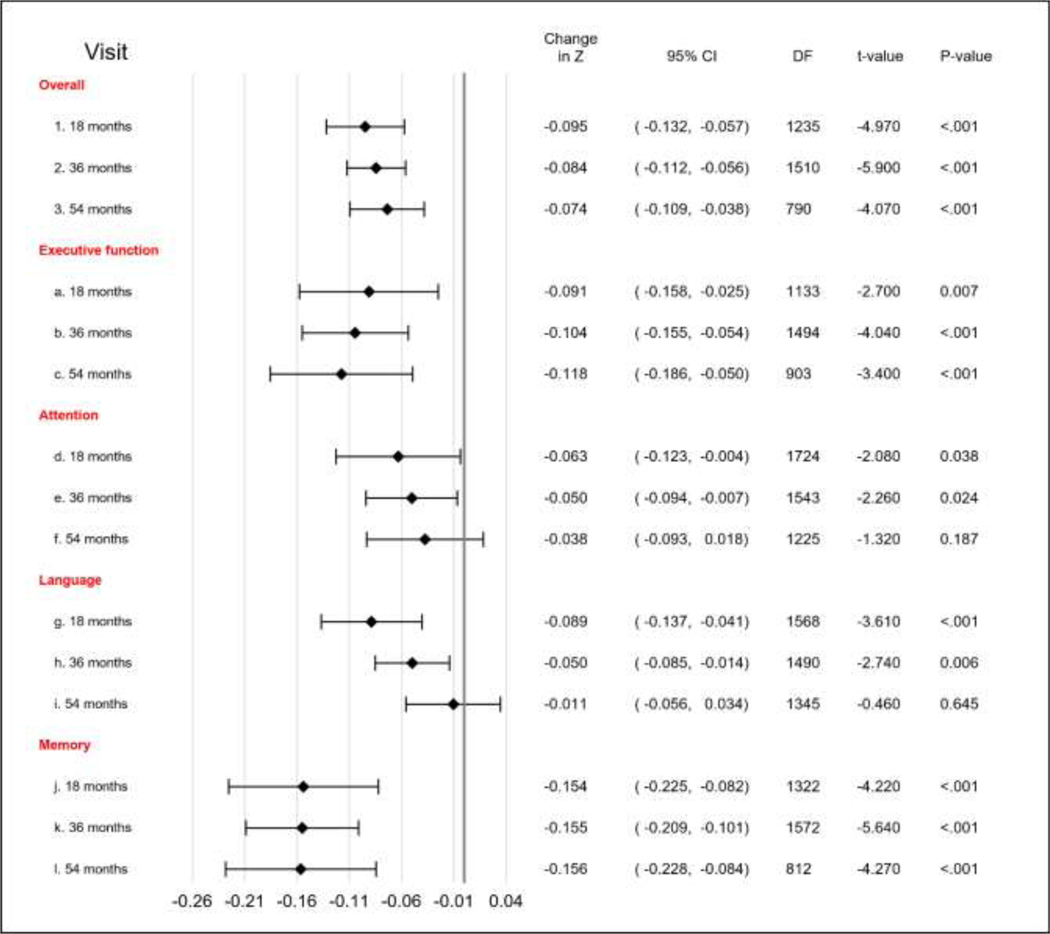
Figure 1: Associations of increase in depression symptoms with decrease in cognitive function over time.
* Visual representation of Table 3; Model 2. The bars represent the size of the effect of every additional one-point increase in number of depression symptoms on change of z score, by cognitive domain and by time (18, 36 and 54 months after baseline). P-values ≤0.01 in the figure, represent a significant change in z score from baseline due to the one-point increase in GDS from baseline. For example, for global cognition, one-point increase in GDS from baseline to 18 months was associated with a significant decline of 0.095 in the z-score, whereas one-point increase in GDS from baseline to 36 months was associated with a significant decline of 0.084 in z score, and so forth. This is interpreted as follows: increases in number of depression symptoms were associated with changes in global cognition, but the extent of these changes over time remained similar (p=0.381 of the change and time interaction – see table 3).
CONCLUSIONS:
In a cohort of initially cognitively normal older adults with T2D, higher number of depression symptoms at baseline was associated cross-sectionally, with lower performance in global cognition and in the specific domains of executive function, semantic categorization and episodic memory. Number of depression symptoms at baseline was not associated with decline in global cognition or in any of the specific cognitive domains. However, increasing number of depression symptoms over time was consistently associated with worse cognitive outcomes in global cognition and in the domains of episodic memory and semantic categorization; there was no interaction with time, i.e. the extent of decrease in cognitive function paralleled the increase in GDS score, but did not accelerate over time.
In accordance with the present results, previous studies on the relationship of depression with cognition in general populations (not necessarily with T2D), have quite consistently demonstrated a cross-sectional relationship of depression, with worse cognitive functioning- either global or in specific cognitive domains (6);(16);(17);(18). Evidence on the longitudinal relationships of depression with cognitive outcomes have been more complex. In contrast to our results, depression at baseline, even at sub-syndromal levels (19), was associated in some studies with worse cognitive outcomes over time (6);(18);(20). Others however, demonstrated that only moderate to severe (21), or persistent (16);(17) depression predict greater cognitive decline at follow up. In accordance with our results, in the Cache County study, baseline depression was not associated with worsening over time in cognitive performance (7). In this study, depression was measured with the NPI-depression scale, with a mean baseline score of 0.09 (SD=0.57), which, similarly to the IDCD baseline depression score, is lower than the score considered to be of potential clinical significance.
In the context of T2D, participants who have T2D combined with depression at baseline, had faster cognitive decline (22) and increased risk for dementia (23) compared to those with only one of these conditions or neither. In our study, mean GDS score at baseline was below the score considered to be of clinical significance, with only 10.2% of participants above the threshold of clinical depression (GDS>5), and follow duration was relatively short (48 months), potentially explaining discrepancies from studies of longer duration or more severe forms of depression at baseline (24);(25). We found significant associations between the increase in depressive symptoms during follow up, rather than their number at baseline, with worse cognitive outcomes. Since increase in depression symptoms was associated with a parallel (rather than accelerated) rate of concomitant cognitive decline, we speculate that depression or its underlying neuropathology do not necessarily exacerbate the pathology underlying cognitive decline, but, rather, that depression and cognitive impairment result from similar underlying mechanisms, or that the pathologies underlying each condition, tend to evolve co-temporaneously.
We are not aware of studies examining the simultaneous relationship of changes in depression with changes in cognition in T2D. In community dwelling elderly, not necessarily with T2D, trajectories of depression were better predictors of longitudinal changes in cognition than depression measured at baseline (26). Importantly, these relationships have been demonstrated over the full range of depression scores and specifically, in those with a low and moderate rather than high grade depression trajectories (26), stressing the value of sub-syndromal depression.
Several mechanisms may explain the relationship of depression and cognition in old age. Similar to its association with cognitive function, Alzheimer’s disease (AD) pathology has been shown to predict incident mood disorders (27). This explanation may be less relevant in T2D patients who do not have greater extent of AD neuropathology compared to non-T2D individuals (28). Brain atrophy, not necessarily due to AD neuropathology, has consistently been demonstrated in T2D (29), even in dementia free middle-aged adults (30). Brain atrophy is also associated with depression, even at subsyndromal levels (31), possibly suggesting that atrophy may underlie depression in T2D. The atrophy observed in T2D has also been associated with worse cognitive functioning in most studies (32), potentially pointing to a common underlying neuropathological mechanism. According to the vascular depression hypothesis (33), cerebrovascular risk factors (including T2D), lead to cerebrovascular pathology, subsequently resulting in late life depression. In line with this notion, cerebrovascular risk factors during midlife predicted depressive symptomatology in later life and, in older adults, presence of depression symptoms was associated with volume of white matter hyperintensities (34). In T2D, cerebrovascular disease was the complication most strongly associated with incident depression symptoms (35) and with cognitive impairment and dementia (36) suggesting an additional common pathology for the two conditions and possibly explaining our results of simultaneous increases over time of depression symptoms with cognitive decline.
In an era of lack of disease modifying treatments for dementia, and a very modest symptomatic effect of approved anti-dementia medications, the role of dementia prevention is increasingly recognized. This has led the field to examine risk factors for dementia and its preceding states. Depression has quite consistently been demonstrated to be a risk factor for dementia (37), but the role of more subtle forms of depression has been inconsistent. Moreover, the effect of antidepressant treatments on dementia prevention has not been consistently demonstrated (38). Our results and those of others highlight the importance of trajectories of depression symptoms over time in predicting cognitive decline in non-demented individuals with T2D and stress the importance of looking at decline in specific cognitive domains rather than global cognitive function as candidates for dementia prevention interventions. Future studies should examine whether different depression phenotypes (in terms of severity and specific symptoms) should be treated differently in order to prevent cognitive decline and dementia.
The main limitation of the study is definition of depression based on the GDS score rather than clinical diagnosis. Nevertheless, this tool enabled measurement of very subtle symptoms which may have been missed in a clinical assessment (which is usually directed towards detection of depression with clinical significance) and has reliably been associated with major depression (39). The GDS has been commonly used as a screening tool for depression, even at sub- syndromal levels (40) in older adults. The results of this study may not necessarily be generalizable to all patients with T2D, as IDCD participants are relatively well educated, cognitively normal, mostly without clinically significant affective symptoms, with a fairly narrow range of GDS and MMSE scores and with well-controlled diabetes [(mean HbA1c value of 6.8% (SD=0.8%)]. Also, the effect sizes detected are small. Nevertheless, they are consistent, withstand in most cases multiple comparisons, and should be discussed in the context of the population studied: if the longitudinal relationships between depression and cognition were detected in this population, the findings could potentially be more pronounced in another sample with poorly-controlled diabetes, higher degrees of depression and cognitive impairment. It is plausible that the discrepancies between cross- sectional and longitudinal relationships of depression symptoms and cognitive outcomes may result from confounders that were not measured in the present study, such as life history of depression (age of onset, number, duration and severity of symptoms), comorbid anxiety or other medical comorbidities. Nevertheless, the relationships found withstood adjustment for a large number of demographic, T2D and health- related factors as well as adjustment for multiple comparisons.
The present study adds to the results of previous studies in several ways. First, we examined the relationship of subtle depression symptoms with cognition specifically in a sample of T2D patients, which are prone to cognitive decline but differ from other populations in terms of the underlying mechanisms. By administering a comprehensive cognitive assessment battery, we were able to show that different cognitive domains are not homogenously associated with depressive symptoms. We measured depression symptoms longitudinally and showed that the extent of depression symptoms over time, which reflects the natural course of depression, compared to a single time measurement, was associated with accelerated parallel cognitive decline. Additional strengths of the study include the large cohort of patients with a well- validated diagnosis of T2D and availability of long-term information on numerous potential confounders.
Supplementary Material
HIGHLIGHTS.
1). What is the primary question addressed by this study?
The present study examined the relationships between simultaneous changes over time in depression and cognition in >1000 initially cognitively normal older adults (≥65 years old) with T2D.
2). What is the main finding of this study?
In a cohort of initially cognitively normal older adults with T2D, higher number of depression symptoms at baseline was associated cross-sectionally, with lower performance in global cognition and in the specific domains of executive function, semantic categorization and episodic memory, but not with cognitive decline at follow up.
Increasing number of depression symptoms over time was consistently associated with worse cognitive outcomes in global cognition and in the domains of episodic memory and semantic categorization.
3). What is the meaning of the finding?
Our results highlight the importance of trajectories of depression symptoms over time in predicting cognitive decline in non-demented individuals with T2D and stress the importance of looking at decline in specific cognitive domains rather than global cognitive function as candidates for dementia prevention interventions.
Acknowledgements:
This study was supported by the National Institute of Aging (grants R01 AG034087 to MS, P50 AG05138 to Mary Sano), and the Helen Bader Foundation and the Leroy Schecter Foundation Award (to MSB).
Previous presentation: Poster presented at the Alzheimer’s Association International conference (AAIC), Los Angeles, California, 2019 14-18 July.
Footnotes
DISCLOSURE/ CONFLICTS OF INTEREST: The authors report no conflicts with any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Ismail Z, Gatchel J, Bateman DR, et al. : Affective and emotional dysregulation as pre-dementia risk markers: exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. Int Psychogeriatr 2018; 30:185–196 [DOI] [PubMed] [Google Scholar]
- 2.Kaup AR, Byers AL, Falvey C, et al. : Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia. JAMA Psychiatry 2016; 73:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byers AL, Yaffe K: Depression and risk of developing dementia. Nat Rev Neurol 2011; 7:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RS, Capuano AW, Boyle PA, et al. : Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology 2014; 83:702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher D, Kiss A, Lanctot KL, et al. : Toward Prevention of Mild Cognitive Impairment in Older Adults With Depression: An Observational Study of Potentially Modifiable Risk Factors. J Clin Psychiatry 2018; 80: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddle M, Potter GG, McQuoid DR, et al. : Longitudinal Cognitive Outcomes of Clinical Phenotypes of Late-Life Depression. Am J Geriatr Psychiatry 2017; 25:1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burhanullah MH, Tschanz JT, Peters ME, et al. : Neuropsychiatric Symptoms as Risk Factors for Cognitive Decline in Clinically Normal Older Adults: The Cache County Study. Am J Geriatr Psychiatry 2020; 28:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dlugaj M, Winkler A, Dragano N, et al. : Depression and mild cognitive impairment in the general population: results of the Heinz Nixdorf recall study. J Alzheimers Dis 2015; 45:159–174 [DOI] [PubMed] [Google Scholar]
- 9.Lee JR, Suh SW, Han JW, et al. : Anhedonia and Dysphoria Are Differentially Associated with the Risk of Dementia in the Cognitively Normal Elderly Individuals: A Prospective Cohort Study. Psychiatry Investig 2019; 16:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nefs G, Hendrieckx C, Reddy P, et al. : Comorbid elevated symptoms of anxiety and depression in adults with type 1 or type 2 diabetes: Results from the International Diabetes MILES Study. J Diabetes Complications 2019; 33:523–529 [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Berroa E, Ravona-Springer R, Schmeidler J, et al. : Depressive Symptoms Are Associated with Cognitive Function in the Elderly with Type 2 Diabetes. J Alzheimers Dis 2018; 65:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaMonica HM, Hickie IB, Ip J, et al. : Disability in older adults across the continuum of cognitive decline: unique contributions of depression, sleep disturbance, cognitive deficits and medical burden. Int Psychogeriatr 2019; 31:1611–1625 [DOI] [PubMed] [Google Scholar]
- 13.Beeri MS, Ravona-Springer R, Moshier E, et al. : The Israel Diabetes and Cognitive Decline (IDCD) study: Design and baseline characteristics. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2014; 10:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh JL YJA: Geriatric Depression Scale (GDS): a recent evidence and development of a shorter version. Clin. Gerontol 1986; 5:165–173 [Google Scholar]
- 15.Morris JC: The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 16.Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. : Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci 2014; 69:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goveas JS, Espeland MA, Hogan PE, et al. : Depressive Symptoms and Longitudinal Changes in Cognition: Women’s Health Initiative Study of Cognitive Aging. J Geriatr Psychiatry Neurol 2014; 27:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dotson VM, Resnick SM,Zonderman AB: Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry 2008; 16:318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson L, Guerra M, Prince M, et al. : Associations between Depression, Depressive Symptoms, and Incidence of Dementia in Latin America: A 10/66 Dementia Research Group Study. J Alzheimers Dis 2019; 69:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaninotto P, Batty GD, Allerhand M, et al. : Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J Epidemiol Community Health 2018; 72:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defrancesco M, Marksteiner J, Kemmler G, et al. : Severity of Depression Impacts Imminent Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. J Alzheimers Dis 2017; 59:1439–1448 [DOI] [PubMed] [Google Scholar]
- 22.Sullivan MD, Katon WJ, Lovato LC, et al. : Association of depression with accelerated cognitive decline among patients with type 2 diabetes in the ACCORD-MIND trial. JAMA Psychiatry 2013; 70:1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katon W, Pedersen HS, Ribe AR, et al. : Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry 2015; 72:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demakakos P, Muniz-Terrera G,Nouwen A: Type 2 diabetes, depressive symptoms and trajectories of cognitive decline in a national sample of community-dwellers: A prospective cohort study. PLoS One 2017; 12:e0175827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Downer B, Vickers BN, Al Snih S, et al. : Effects of Comorbid Depression and Diabetes Mellitus on Cognitive Decline in Older Mexican Americans. J Am Geriatr Soc 2016; 64:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graziane JA, Beer JC, Snitz BE, et al. : Dual Trajectories of Depression and Cognition: A Longitudinal Population-Based Study. Am J Geriatr Psychiatry 2016; 24:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan NJ, Locascio JJ, Marshall GA, et al. : Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am J Psychiatry 2018; 175:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dos Santos Matioli MNP, Suemoto CK, Rodriguez RD, et al. : Diabetes is Not Associated with Alzheimer’s Disease Neuropathology. J Alzheimers Dis 2017; 60:1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardlaw JM, Smith EE, Biessels GJ, et al. : Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang F, Zhan YF, Zhuo YY, et al. : Brain atrophy in middle-aged subjects with Type 2 diabetes mellitus, with and without microvascular complications. J Diabetes 2018; 10:625–632 [DOI] [PubMed] [Google Scholar]
- 31.Brendel M, Reinisch V, Kalinowski E, et al. : Hypometabolism in Brain of Cognitively Normal Patients with Depressive Symptoms is Accompanied by Atrophy-Related Partial Volume Effects. Curr Alzheimer Res 2016; 13:475–486 [DOI] [PubMed] [Google Scholar]
- 32.Manschot SM, Brands AM, van der Grond J, et al. : Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006; 55:1106–1113 [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos GS, Meyers BS, Young RC, et al. : ‘Vascular depression’ hypothesis. Arch Gen Psychiatry 1997; 54:915–922 [DOI] [PubMed] [Google Scholar]
- 34.Jorm AF, Anstey KJ, Christensen H, et al. : MRI hyperintensities and depressive symptoms in a community sample of individuals 60–64 years old. Am J Psychiatry 2005; 162:699–705 [DOI] [PubMed] [Google Scholar]
- 35.Deschenes SS, Burns RJ, Pouwer F, et al. : Diabetes Complications and Depressive Symptoms: Prospective Results From the Montreal Diabetes Health and Well-Being Study. Psychosom Med 2017; 79:603–612 [DOI] [PubMed] [Google Scholar]
- 36.Moran C, Beare R, Phan T, et al. : Neuroimaging and its Relevance to Understanding Pathways Linking Diabetes and Cognitive Dysfunction. J Alzheimers Dis 2017; 59:405–419 [DOI] [PubMed] [Google Scholar]
- 37.Norton S, Matthews FE, Barnes DE, et al. : Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. The Lancet. Neurology 2014; 13:788–794 [DOI] [PubMed] [Google Scholar]
- 38.Zhang DA, Lam V, Chu V, et al. : Type 2 Diabetes with Comorbid Depression in Relation to Cognitive Impairment: an Opportunity for Prevention? Mol Neurobiol 2018; 55:85–89 [DOI] [PubMed] [Google Scholar]
- 39.Almeida OP, Almeida SA: Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999; 14:858–865 [DOI] [PubMed] [Google Scholar]
- 40.Mackin RS, Insel P, Tosun D, et al. : The effect of subsyndromal symptoms of depression and white matter lesions on disability for individuals with mild cognitive impairment. Am J Geriatr Psychiatry 2013; 21:906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.