Supplemental Digital Content is available in the text.
Abstract
This single-center retrospective study evaluated a protocol for the intubation of patients with confirmed or suspected coronavirus disease 2019 (COVID-19). Twenty-one patients were intubated, 9 of whom were found to have COVID-19. Adherence to the airway management protocol was high. COVID-19 patients had lower peripheral capillary oxygen saturation by pulse oximetry (Spo2) nadirs during intubation (Spo2, 73% [72%–77%] vs 89% [86%–94%], P = .024), and a greater percentage experienced severe hypoxemia defined as Spo2 ≤80% (89% vs 25%, P = .008). The incidence of severe hypoxemia in COVID-19 patients should be considered in the development of guidelines that incorporate high-flow nasal cannula and noninvasive positive pressure ventilation.
Airway management in the coronavirus disease 2019 (COVID-19) era must incorporate both the risk of health care worker (HCW) exposure1,2 and the underlying pulmonary pathophysiology of patients with severe disease. Emergent intubations should be avoided to afford intubation teams sufficient time to properly don personal protective equipment (PPE), secure viral filters, and move patients to negative pressure rooms.1–4 Providers must also consider the profound hypoxemia and steep trajectory of respiratory failure that COVID-19 patients may experience. Even in the absence of COVID-19, tracheal intubation in the critically ill is a high-risk procedure: up to 20% of patients develop severe hypoxemia (peripheral capillary oxygen saturation by pulse oximetry [Spo2] <80%), and 2% suffer cardiac arrest.5,6 Avoidance of noninvasive positive pressure ventilation (NIPPV) and bag-valve-mask (BVM) ventilation to minimize aerosolization2,7 of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) places patients at even higher risk for critical oxygen desaturation during intubation.
Based on these considerations, a multidisciplinary task force developed consensus guidelines for when and how to intubate patients with confirmed or suspected COVID-19. The protocol was designed to maximize patient and HCW safety by avoiding emergent intubations and minimizing aerosolization. This descriptive analysis evaluates protocol adherence and incidence of peri-intubation complications in a cohort of patients under investigation (PUI) and confirmed COVID-19 patients.
METHODS
This retrospective cohort study took place at a single medical center with institutional review board approval (IRB No. 55954) and waiver of consent. All adults (≥18 years) with confirmed or suspected COVID-19 requiring tracheal intubation between March 10 and April 10, 2020 in the emergency department (ED), hospital ward, or intensive care unit (ICU) were included. PUI criteria at our institution are provided in Supplemental Digital Content 1 (http://links.lww.com/AACR/A394). Patients who arrived at the ED in cardiac arrest with cardiopulmonary resuscitation in progress were excluded. Two reintubations of COVID-19 patients were excluded because they occurred weeks following hospital admission and were prompted by airway issues (postextubation stridor and difficulty with secretion clearance).
Intubation criteria included (1) Pao2 <65 mm Hg or Spo2 <92% on 15 L/min nonrebreather (NRB) mask or (2) labored breathing with respiratory rate >35/min or Paco2 >50 mm Hg with pH <7.30. High-flow nasal cannula (HFNC) and NIPPV were avoided.
Intubations were performed in the ED by emergency medicine attendings or senior residents, while inpatient intubations were performed by a dedicated “COVID Airway Team” comprising attending anesthesiologists. Protocol adherence was assessed by (1) utilization of appropriate PPE (powered air-purifying respirator or N95 respirator with goggles or face shield) based on provider interview, (2) intubation in a negative pressure room, (3) videolaryngoscopy for first intubation attempt, and (4) rapid sequence induction with avoidance of BVM ventilation. Room entry during intubation was limited to 3 providers (nurse, respiratory therapist, and intubating physician) with a pharmacist, “runner,” and backup airway assistant outside the room. While BVM ventilation was avoided, a bag-mask device with a viral filter was available (Supplemental Digital Content 2, Intubation Checklist, http://links.lww.com/AACR/A395).
Data were obtained through chart review and additional direct follow-up with the intubating physician when sufficient documentation was not available in the electronic health record (EHR). Severity of illness was assessed by Sequential Organ Failure Assessment (SOFA) score during the first 24 hours postintubation. When an arterial blood gas was not available, the Pao2/fractional inspired oxygen (Fio2) ratio or P/F ratio was estimated based on the Spo2/Fio2 ratio as previously described.8 Descriptive statistics were used to assess protocol adherence and patient outcomes, and to compare confirmed COVID-19 patients with those who tested negative for SARS-CoV-2. Given the small sample size, median values were compared using Wilcoxon rank sum tests for continuous variables and Fisher exact tests for categorical variables. All analyses were performed using R (R Core Team, Version 1.2.5042, 2020, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org).
RESULTS
Twenty-one patients (median age, 66 years [58–73 years]; 76% men) were intubated during the study period, of whom 9 (43%) tested SARS-CoV-2 positive (Table 1). Compared to those who tested negative, COVID-19 patients had higher body mass index (BMI; 32 [28–33] vs 23 [22–27], P = .005) but were otherwise comparable in terms of age, sex, and comorbidities. Indications for intubation among PUI who tested negative were heterogeneous, whereas all COVID-19 patients were intubated for hypoxemia (P = .045). SOFA scores were similar between groups; however, COVID-19 patients had lower P/F ratios (100 [91–127] vs 161 [132–232], P = .017).
Table 1.
Demographic and Intubation Data
| All patients (n = 21) | SARS-CoV-2 positive (n = 9) | SARS-CoV-2 negative (n = 12) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 66 (58–73) | 66 (40–73) | 65 (60–74) | .89 |
| Male sex | 16 (76) | 6 (67) | 10 (83) | .61 |
| BMI, kg/m2 | 27 (22–31) | 32 (28–33) | 22 (22–27) | .005 |
| Preexisting lung disease | 10 (48) | 4 (44) | 6 (50) | >.99 |
| Preexisting cardiac disease | 14 (67) | 6 (67) | 8 (67) | >.99 |
| On home oxygen | 2 (9.5) | 0 | 2 (17) | .49 |
| SOFA score | 7 (6–9) | 7 (7–8) | 8 (6–10) | .54 |
| Lowest Pao2/Fio2 ratio | 132 (100–211) | 100 (91–127) | 161 (132–232) | .02 |
| Preinduction | ||||
| Intubation acuity | .34 | |||
| Emergent | 5 (24) | 1 (11) | 4 (33) | |
| Urgent | 16 (76) | 8 (89) | 8 (67) | |
| Intubation location | .002 | |||
| ED | 15 (71) | 3 (33) | 12 (100) | |
| ICU | 6 (29) | 6 (67) | 0 | |
| Indication for intubation | ||||
| Hypoxia | 16 (76) | 9 (100) | 7 (58) | .045 |
| Hypercarbia | 4 (19) | 0 | 4 (33) | .045 |
| Increased work of breathing | 11 (52) | 5 (56) | 6 (50) | >.99 |
| Altered mental status | 10 (48) | 0 | 10 (83) | <.001 |
| Spo2 before preoxygenation | 93 (85–95) | 93 (92–95) | 92 (83–96) | .69 |
| Respiratory rate | 30 (23–37) | 33 (29–44) | 26 (22–32) | .16 |
| Intubation | ||||
| Preoxygenation | .04 | |||
| Nonrebreather | 19 (91) | 9 (100) | 10 (83) | |
| CPAP/BiPAP | 1 (5)a | 0 | 1 (8) | |
| Bag-valve-mask ventilation | 1 (5)a | 0 | 1 (8) | |
| Bag-mask ventilation after induction | 1 (5)a | 0 | 1 (8) | >.99 |
| Highest Spo2 with preoxygenation | 98 (95–100) | 97 (96–98) | 99 (94–100) | .63 |
| Nadir Spo2 | 82 (72–90) | 73 (72–77) | 89 (86–94) | .02 |
| >1 attempt | 3 (14) | 2 (22) | 1 (8) | .55 |
| Vasoactive medication administered | ||||
| Yes, bolus | 10 (48) | 5 (56) | 5 (42) | .67 |
| Yes, infusion | 7 (33) | 2 (22) | 5 (42) | .64 |
| No | 10 (48) | 4 (44) | 6 (50) | >.99 |
| Complications | ||||
| None | 10 (48) | 1 (11) | 9 (75) | .008 |
| Severe hypoxia (Spo2 <80%) | 11 (48) | 8 (89) | 3 (25) | .008 |
| Cannot intubate, cannot ventilate | 0 | 0 | 0 | |
| Significant airway trauma | 0 | 0 | 0 | |
| Arrhythmia requiring intervention | 0 | 0 | 0 | |
| Cardiac arrest | 0 | 0 | 1 | >.99 |
| Death | 0 | 0 | 0 | |
All range data presented as median (interquartile range) or n (%).
Abbreviations: BiPAP, bilevel positive airway pressure; BMI, body mass index; CPAP, continuous positive airway pressure; ED, emergency department; Fio2, fractional inspired oxygen; ICU, intensive care unit; NIPPV, noninvasive positive pressure ventilation; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SOFA, Sequential Organ Failure Assessment; Spo2, peripheral capillary oxygen saturation by pulse oximetry.
aTwo patients arrived to the ED in extremis, receiving NIPPV en route, which was continued for preoxygenation before rapid intubation by ED providers. Only one additional patient had a protocol deviation by receiving bag-valve-mask ventilation after an intubation attempt due to suspicion of esophageal intubation necessitating repeat laryngoscopy. All 3 of these patients subsequently tested negative for SARS-CoV-2.
Protocol Adherence
Appropriate PPE was used during every intubation, and 90% of intubations occurred in a negative pressure room (Table 2). Videolaryngoscopy was utilized for all first intubation attempts, though 1 PUI required a fiberoptic rescue intubation. Two patients arrived at the ED in extremis, with prehospital NIPPV continued for preoxygenation before immediate intubation. One ED patient received BVM ventilation before a second intubation attempt. All 3 of these patients subsequently tested negative for SARS-CoV-2. No inpatients required emergent intubation.
Table 2.
COVID-19 Intubation Protocol Adherence
| All N, (%) | |
|---|---|
| Intubated in negative pressure room | 18 (90)a |
| PPE worn by provider | 21 (100) |
| N95 + face shield | 1 (4.8) |
| PAPR + face shield | 16 (76.2) |
| PAPR + hood | 4 (19.0) |
| NIPPV postinduction | 1 (4.8) |
| Videolaryngoscopy for first attempt | 21 (100) |
Abbreviations: COVID-19, coronavirus disease 2019; NIPPV, noninvasive positive pressure ventilation; PAPR, powered air purifying respirator; PPE, personal protective equipment.
aType of intubation room unknown for 1 patient.
Peri-intubation Outcomes
Intubation was successful on the first attempt in 86% of cases. Despite similar oxygen saturation levels before and after preoxygenation, confirmed COVID-19 patients had significantly lower nadirs during intubation compared to SARS-CoV-2–negative patients (73% [72%–77%] vs 89% [86%–94%], P = .024), as well as a trend toward lower nadirs compared to the subset of SARS-CoV-2–negative patients intubated for hypoxemia (88% [86%–90%], P = .059). COVID-19 patients were also more likely to experience severe hypoxemia, defined as Spo2 <80% (89% vs 25%, P = .008) (Figure). Comparable numbers of patients required vasopressor boluses (56% vs 42%, P = .67) and infusions (22% vs 42%, P = .64) peri-intubation.
Figure.
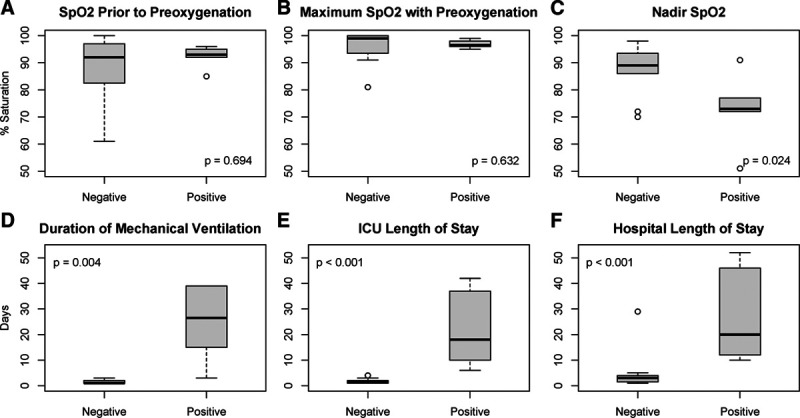
Oxygenation and outcomes. Oxygen levels during the peri-intubation period (A–C) and outcomes (D–F) for confirmed SARS-CoV-2–positive versus SARS-CoV-2–negative patients. Duration of mechanical ventilation (D) shown for patients who survived to extubation (n = 13). Statistical comparisons performed using Wilcoxon rank sum test. Data displayed as box plot indicating median ± interquartile range with outliers displayed. ICU indicates intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; Spo2, peripheral capillary oxygen saturation by pulse oximetry.
Patient Outcomes
Three of the 9 COVID-19 patients died of multisystem organ failure, while 6 were discharged. COVID-19 patients had significantly longer duration of ventilation and length of stay in the ICU and hospital (Figure).
DISCUSSION
While several recommendations have been published, the optimal timing and method of intubation in COVID-19 patients is unknown.1–4 The intubation algorithm described in this study was designed to identify patients at risk for respiratory collapse early, affording intubation teams time to don PPE and minimize HCW exposure. This is one of the first studies to assess the feasibility and safety of such a protocol.
Our study revealed strong adherence to an airway management protocol, proving the feasibility of a consistent practice model at our hospital. No inpatients required emergent intubation, suggesting that the intubation criteria facilitated controlled intubations and allowed for recommended precautions to be taken. Because HCW outcomes were not formally tracked, conclusions about HCW safety cannot be made. To our knowledge, however, there have been no cases of COVID-19 among intubating providers at our institution.
One striking observation in our cohort was the severity of peri-intubation hypoxemia among COVID-19 patients. In a study of 202 patients in Wuhan, China, a majority of COVID-19 patients experienced Spo2 <90%, but no details of the degree of hypoxemia were reported.9 In our cohort, hypoxemia was more profound among COVID-19 patients than PUI, despite using the same intubation technique and having similar preintubation oxygen saturation levels. This observation likely reflects the significant lung injury seen with SARS-CoV-2 infection; however, direct comparisons with the PUI group are limited given the small sample size and differences between groups. Specifically, the PUI group had lower BMI and more varied indications for intubation beyond hypoxemia; and 3 received peri-intubation positive pressure ventilation. Even so, this study confirms the potential for profound peri-intubation hypoxemia with our airway protocol in the COVID-19 population. Despite marked hypoxemia, however, no COVID-19 patients suffered any other major peri-intubation complication.
This single-center study is limited by its small sample size and retrospective nature, as well as the aforementioned differences between the COVID-19 and PUI groups. Furthermore, because some procedure notes did not document the nadir Spo2, study authors verbally obtained these values from the intubating provider at a later time, introducing potential recall bias. On the other hand, most providers intubated only one patient in our cohort and reported no difficulty recalling events.
In our study, patients were intubated after failing oxygen therapy using an NRB mask at 15 L/min flow. This approach was taken due to the perceived risk of aerosolization with HFNC and concern that HFNC might mask progressive respiratory failure leading to higher-risk intubations. However, HFNC has previously been shown to improve 90-day mortality in nonhypercapnic hypoxemic respiratory failure.10 Based on the Surviving Sepsis Campaign guidelines released in April 2020,11 our oxygen therapy strategy was amended after completion of the study period to allow for the use of HFNC (Supplemental Digital Content 3, Intubation Guidelines, http://links.lww.com/AACR/A396). We anticipate that HFNC will (1) diminish the degree of hypoxemia observed in our nonintubated COVID-19 patients and (2) help some patients avoid intubation altogether. It is also possible, however, that peri-intubation hypoxemia will worsen when intubating from HFNC, because these patients may have more severe respiratory failure at the time of intubation.
CONCLUSIONS
In this single-center cohort study, implementation of a COVID-19 intubation protocol was associated with a high rate of protocol adherence and complete avoidance of “crash” intubations among inpatients with suspected or confirmed COVID-19. Our findings also highlight the severity of peri-intubation hypoxemia in COVID-19 patients. Institutional flexibility, interdisciplinary collaboration, and an iterative approach to treatment algorithms for COVID-19 respiratory failure are necessary to optimize both HCW and patient safety.
ACKNOWLEDGMENTS
The authors would like to thank all health care providers and hospital staff who are caring for our patients during this pandemic. We specifically want to acknowledge Dr Keir Warner (Emergency Medicine Resident, Department of Emergency Medicine, Stanford University Medical Center, Stanford, CA) for his assistance tracking emergency department (ED) intubations, Dr Ana Crawford (Clinical Assistant Professor, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University Medical Center, Stanford, CA) as the head of the “COVID Airway Team,” Dr Rebecca Aslakson (Associate Professor, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University Medical Center, Stanford, CA) for sharing her infographics that are in the supplementary materials and the multidisciplinary COVID-19 critical care task force that is constantly reviewing and updating practice guidelines in the face of rapidly evolving evidence.
DISCLOSURES
Name: Jai Madhok, MD, MSE.
Contribution: This author helped in formulating study design, data extraction, manuscript preparation, critical revisions, and final approval for submission.
Name: Melissa A. Vogelsong, MD.
Contribution: This author helped in formulating study design, data extraction, data analysis, manuscript preparation, critical revisions, and final approval for submission.
Name: Tiffany C. Lee, MD.
Contribution: This author helped in formulating study design, manuscript preparation, critical revisions, and final approval for submission.
Name: Jennifer G. Wilson, MD, MS.
Contribution: This author helped in formulating study design, data extraction, critical revisions, and final approval for submission.
Name: Frederick Mihm, MD.
Contribution: This author helped in formulating study design, data extraction, critical revisions, and final approval for submission.
This manuscript was handled by: Mark C. Phillips, MD.
Supplementary Material
GLOSSARY
- BiPAP =
- bilevel positive airway pressure
- BMI =
- body mass index
- BVM =
- bag valve mask
- COVID- 19 =
- coronavirus disease 2019
- CPAP =
- continuous positive airway pressure
- ED =
- emergency department
- EHR =
- electronic health record
- Fio2 =
- fractional inspired oxygen
- HCW =
- health care worker
- HFNC =
- high-flow nasal cannula
- ICU =
- intensive care unit
- NIPPV =
- noninvasive positive pressure ventilation
- NRB =
- nonrebreather
- P/F =
- Pao2/Fio2
- PPE =
- personal protective equipment
- PUI =
- patient under investigation
- SARS-CoV-2 =
- severe acute respiratory syndrome coronavirus-2
- SOFA =
- Sequential Organ Failure Assessment
- Spo2 =
- peripheral capillary oxygen saturation by pulse oximetry
Funding: None.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website.
J. Madhok and M. A. Vogelsong contributed equally to this study.
REFERENCES
- 1.Greenland JR, Michelow MD, Wang L, London MJ. COVID-19 infection: implications for perioperative and critical care physicians. Anesthesiology. 2020; 132:1346–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020; 67:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orser BA. Recommendations for endotracheal intubation of COVID-19 patients. Anesth Analg. 2020; 130:1109–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020; 75:785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan JP, Kelly FE. Airway challenges in critical care. Anaesthesia. 2011; 66suppl 281–92 [DOI] [PubMed] [Google Scholar]
- 6.Casey JD, Janz DR, Russell DW, et al. ; PreVent Investigators and the Pragmatic Critical Care Research Group PreVent Investigators and the Pragmatic Critical Care Research Group. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019; 380:811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012; 7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilan N, Dastranji A, Ghalehgolab Behbahani A. Comparison of the Spo2/Fio2 ratio and the Pao2/Fio2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J Cardiovasc Thorac Res. 2015; 7:28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao W, Wang T, Jang B, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020; 125:e28–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015; 372:2185–2196 [DOI] [PubMed] [Google Scholar]
- 11.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020; 48:e440–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


