Abstract
Somatic embryogenesis (SE), which is in vitro regeneration of plant bodies from somatic cells, represents a useful means of clonal propagation and genetic engineering of forest trees. While protocols to obtain calluses and induce regeneration in somatic embryos have been reported for many tree species, the knowledge of molecular mechanisms of SE development is still insufficient to achieve an efficient supply of somatic embryos required for the industrial application. Cryptomeria japonica, a conifer species widely used for plantation forestry in Japan, is one of the tree species waiting for a secure SE protocol; the probability of normal embryo development appears to depend on genotype. To discriminate the embryogenic potential of embryonal masses (EMs) and efficiently obtain normal somatic embryos of C. japonica, we investigated the effects of genotype and transcriptome on the variation in embryogenic potential. Using an induction experiment with 12 EMs each from six genotypes, we showed that embryogenic potential differs between/within genotypes. Comparisons of gene expression profiles among EMs with different embryogenic potentials revealed that 742 differently expressed genes were mainly associated with pattern forming and metabolism. Thus, we suggest that not only genotype but also gene expression profiles can determine success in SE development. Consistent with previous findings for other conifer species, genes encoding leafy cotyledon, wuschel, germin-like proteins, and glutathione-S-transferases are likely to be involved in SE development in C. japonica and indeed highly expressed in EMs with high-embryogenic potential; therefore, these proteins represent candidate markers for distinguishing embryogenic potential.
Introduction
Somatic embryogenesis (SE), which involves in vitro development of the bipolar plant body from somatic cells, is an effective means of clonal propagation of forest trees and promote breeding in plantation forestry and the conservation of valuable tree species. The SE-based method has advantages over conventional methods of clonal propagation, such as cutting, grafting, and coppicing, in terms of efficiency and species preservation. Once a proper protocol for culturing is established, SE-based propagation enables indoor production of any number of clonal plants. As juvenile plants derived from somatic embryos can regenerate even after cryopreservation, the long-term preservation of genetic resources and stable supply of saplings is feasible with SE-based propagation. Currently, SE-based propagation is applied on a commercial scale for the forestry of limited number of conifer species in genera such as Abies, Larix, Picea, Pinus, and Pseudotsuga [1, 2].
The molecular mechanisms underlying SE development remain to be fully elucidated and thus, developing SE protocols has been difficult for many tree species. Based on the gene expression assays and recent genome-wide studies, some gene families that encode SE receptor kinase, leafy cotyledon (LEC), and wuschel (WUS) proteins likely have important functions in SE development [3, 4]. However, it is unclear whether the genetic mechanisms controlling SE development are common across species. Furthermore, the importance of epigenetic regulation, which can also modulate gene reprogramming and determine the embryogenic state of culture cells [5, 6], requires further investigation. Elucidating the molecular mechanisms underlying SE development will help improve current SE protocols, which have often been established through trial and error.
In Japan, Cryptomeria japonica D. Don (Cupressaceae) is one of the most important conifer species in the plantation forestry, comprising 4.5 million ha (~45%) of plantation stands [7]. Conventional breeding has depended on cutting of selected trees with superior traits; however, SE-based propagation could improve C. japonica breeding programs with, for example, genetic engineering. One of the promising applications of genetic engineering in C. japonica involves establishing male-sterile varieties, which would benefit the ~30% of the Japanese population who are allergic to pollens from C. japonica plantations [8]. Genetic engineering with SE is theoretically possible in C. japonica because protocols to induce and maintain somatic embryos have been developed [9–12] and transformation with somatic embryos has been reported [13]. Accumulated genetic data for C. japonica [e.g., 14–21], as well as a future genome assembly, will also support genetic engineering of varieties with desirable traits.
We recently found a substantial variation in the embryogenesis of calluses among C. japonica siblings (i.e., different genotypes originated from identical parents) even with the optimum protocols for the C. japonica SE system [10, 11]. Embryogenic potential cannot be distinguished until somatic embryos actually start developing; such unpredictable variability means that many different genotypes must be maintained and this hinders the industrial application of SE-based propagation for C. japonica. In the present study, to improve the SE protocols for C. japonica, we investigated (1) whether the variability in embryogenesis is genetically determined and (2) whether genetic expression profiles differ between embryonal masses with high-embryogenic and low-embryogenic potential. Our results indicate that not only genotypes but also gene expression profiles are associated with the variation in the embryogenic potential. We report some transcriptome markers to discriminate the embryogenic potential in the C. japonica SE system.
Materials and methods
Cell lines
We used six embryogenic C. japonica cell lines, namely SSD-18, 73, 100, 113, 182, and 352, which were obtained from zygotic embryos excised from immature seeds in July 2016. The seeds were collected from a hybrid offspring produced by ‘Shindai 3’ (maternal) and ‘Suzu 2’ (paternal). The detailed procedures used to obtain embryonal masses (EMs) were described in [22]. The EMs were maintained and proliferated every two weeks with EM medium (S1a Table). Embryogenesis was confirmed at least eight times with the maturation media (S1b Table) before this study began.
Maturation
In August 2019, 12 EMs (100 mg fresh weight each) per cell line were transferred to Petri dishes (three EMs per dish) with the maturation media (S1b Table). The Petri dishes were maintained in darkness at 25°C. At 5, 7, and 9 weeks after the maturation, the number of normal somatic embryos was recorded and compared between and within cell lines by Tukey’s multiple comparison test.
RNA isolation
We investigated the gene expression profiles of five EMs from SSD-100, in which we found marked differences in somatic embryos maturation. At 5, 7, and 9 weeks after maturation, 100 mg of tissue was preserved in 200 μl of CTAB buffer at −80°C until RNA extraction. As these EMs originated from an identical cell line (i.e., genotype), sequence polymorphisms in transcripts would not occur among samples. Therefore, the difference in the number of reads mapped on transcript sequences likely reflects differences in expression levels.
Immediately after the frozen tissue solutions had melted at room temperature, the tissues were macerated with a zirconium ball using TissueLyser II (Qiagen). After shaking at 30 Hz for 30 s, the sample tubes were placed on ice to avoid RNA degradation. This procedure was repeated four times. The sample tubes were then incubated at 65°C for 10 min with an additional 200 μl of CTAB buffer (containing 2% CTAB, 40 mM EDTA, 100 mM Tris-HCl (pH 8.0), 2% PVP, 1.4 M NaCl, and 2% 2-mercaptoethanol). Subsequently, the solutions were mixed with a half volume of chloroform/isoamyl alcohol and then centrifuged at 13,000 g for 15 min. After collection of approximately 300 μl of the aqueous phase in a new tube, the RNA molecules were precipitated with a quarter volume of 10M LiCl at −20°C for 2 h and subsequently centrifuged at 20,000 g and 4°C for 25 min. The pellet was purified using the SV Total RNA Isolation System (Promega) following the manufacturer’s protocol.
Transcriptome sequencing
The RNA-Seq libraries were prepared using TruSeq Stranded mRNA Library Prep (Illumina) and sequenced with 2 × 100 bp technology in the NovaSeq 6000 System (Illumina). The sequence quality of raw reads was controlled using Trimmomatic (ver. 0.39) [23]. The clean read pairs were aligned on the reference cDNA sequences CJ3006NRE (49,758 transcripts of 39,229 genes expressed in mature leaves, inner bark, and male flowers; [24]) using bowtie2 (ver. 2.3.4.1) [25]. The number of reads was counted for each gene using RSEM (ver. 1.3.2) [26].
We gathered unmapped read pairs, which constituted ~20% (range: 15.7%–26.0%) of input read pairs per sample, and created a new assembly using Trinity (ver. 2.8.4) [27]. Open reading frames (ORFs) in the new transcript assembly were identified using TransDecoder (ver. 5.5.0; https://github.com/TransDecoder/TransDecoder/wiki). Using NCBI BLAST-P (ver. 2.7.1), the homology sequences of the ORFs were searched against 45,644 isoform sequences for plants reposted in SwissProt (downloaded on March 30, 2020) [28]. Using hmmscan in HMMER (ver. 3.2.1; http://hmmer.org/), protein domains in the ORFs were identified by reference to 18,197 Pfam-A domains (ver. 33.0; downloaded on March 30, 2020 [29]). Based on these two annotations, the coding sequences in the new assembly were predicted using TransDecoder (ver. 5.5.0). Short transcript sequences (< 500 bp) were removed. The unmapped reads were aligned on the new transcript assembly using bowtie2 (ver. 2.3.4.1) and the read coverage per gene was counted using RSEM (ver. 1.3.2). The read count data for CJ3006NRE and the new transcript assembly were integrated into a single matrix.
Differentially expressed genes between EMs with high-embryogenic and low-embryogenic potential
Based on the observed number of embryos, we recognized two out of five EMs (namely, C1 and C5) to have high embryogenic (HE) potential and the remaining (namely, C3, C4, and C6) to have low-embryogenic (LE) potential. In C1 and C5, the number of embryos increased from the 5th to 7th week and decreased from the 7th to 9th week, indicating that the development of new somatic embryos was active at the 5th and 7th weeks but had decelerated by the 9th week. Therefore, we searched genes significantly expressed in HE samples (C1 and C5) at the 5th and 7th weeks relative to expression in the LE samples (C3, C4, and C6). We selected genes with more than one count per million reads in at least two samples. Read count data were normalized based on the trimmed mean of M values normalization method [30]. Differentially expressed genes between HE and LE samples were identified using the glmQLFit and glmQLFTest functions implemented in the R package edgeR (ver. 3.30.1) [30]. Genes with less than 0.05 false discovery rate and more than 2 log-fold-change were considered significant.
Gene functions enriched in the target genes were identified using the R package topGO with the “elim” algorithm (ver. 2.40.0) [31]. Gene Ontology (GO) terms with p < 0.01 were considered to be significant. For enrichment analysis, we annotated the reference cDNA sequences CJ3006NRE with the SwissProt plant isoform sequence data (the same data used to annotate the new assembly of unmapped reads; see above) and then obtained GO terms (S3 Table).
Validation of differentially expressed genes by quantitative PCR
Among the differentially expressed genes identified by RNA-Seq, the expression levels of six genes (S5 Table) were validated by quantitative PCR (qPCR). Alpha-tubulin (AT1G50010 in the Arabidopsis thaliana genome) was selected for a reference housekeeping gene, because it is suitable for qPCR assays during somatic embryo development in conifer species [32]. Based on the reciprocal best hits between CJ3006NRE and TAIR10, a C. japonica transcript CJt088185 was assumed to be the most homologous with AT1G50010 and used for the subsequent primer design.
We first identified exon-exon junctions on template transcript sequences based on the alignment with the homologous gene sequences in TAIR10 (S5 Table). Primer pairs over the exon-exon junctions were designed using Primer-BLAST [33], with 80–150 bp of product size, 57–63°C (optimal: 60°C) of annealing temperature, 17–25 bp (optimal: 20 bp) of primer length, and 45–60°C of primer GC content. Primer specificity was checked through in silico PCR with a total of 81,353 C. japonica transcript sequences used in this study (S3 and S4 Tables).
We performed qPCR for a total of 10 EM samples, which are composed of five EMs of SSD-100 collected at the 5th and 7th week after maturation and used for RNA-Seq. After removing genomic DNA from ca. 500 ng of total RNA using gDNA Remover, cDNA was synthesized using ReverTra Ace qPCR RT Master Mix (TOYOBO). Each of six target and one reference loci was amplified from the cDNA template in triplicate of 10 μl reaction solution including 5–10% of cDNA, 1×KOD SYBR qPCR Mix (TOYOBO), and 0.2 μM each of forward and reverse primers. Using LightCycler 480 (Roche), the intensity of SYBR Green I was measured during PCR, which was composed of an initial step at 98°C for 2 min and 40 cycles of denaturation at 98°C for 10 sec, annealing at 60°C for 10 sec, and extension at 68°C for 30 sec. After the amplification step, a denaturation at 95°C for 5 sec and an annealing at 65°C for 1 min followed, then a melting curve analysis was carried out from 65°C to 95°C with continuous fluorescent measurements at 5°C interval. The absence of primer dimers was confirmed by the reactions without cDNA (i.e., negative controls). The mean of cycle thresholds (Ct) was identified using 2nd derivative maximum method for each reaction, then relative expression level among the 10 samples was calculated for each locus with delta-delta Ct method, in which the expression level was normalized with that in C4 from the 7th week. The difference in gene expression level between EMs was examined using Tukey’s multiple comparison test.
Results
SE development
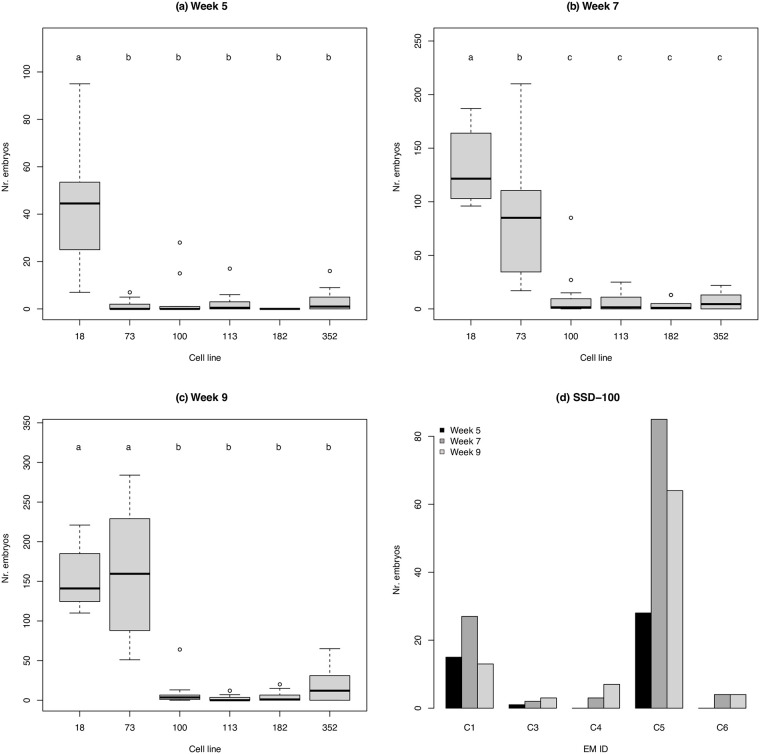
The number of embryos developed from the 5th to 9th week varied between cell lines. SSD-18 and 73 showed higher capacities for developing somatic embryos than the other four cell lines during 5–9 and 7–9 weeks after maturation, respectively (p < 0.01, Fig 1a–1c). In some cell lines, namely SSD-100 and 113, the mean number of embryos developed during the experiment period was significantly different among EMs within cell lines (p < 0.01, S1 Fig).
Fig 1.
(a–c) The number of embryos observed in six Cryptomeria japonica cell lines at (a) 5, (b) 7, and (c) 9 weeks after maturation. Twelve embryonal masses (EMs) were cultured per cell line. Means with different letters are significantly different (Tukey’s multiple comparison test, p < 0.01). (d) The number of embryos observed in five EMs of the cell line SSD-100 at 5, 7, and 9 weeks after maturation. These EMs were used for RNA-Seq.
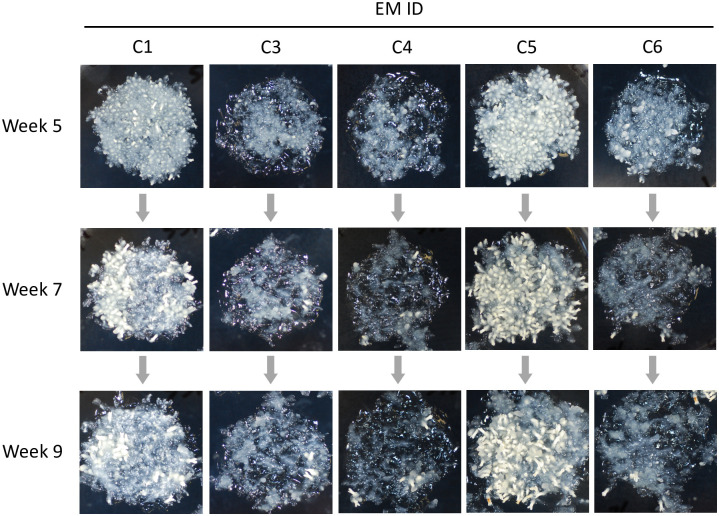
We found the five EMs from SSD-100 to be suitable to assay gene expressions because they showed a marked difference in the capacity to develop somatic embryos in spite of their shared genotype (Fig 2). C1 and C5 developed more than10 embryos throughout the experimental period, whereas C3, C4, and C6 did not (Fig 1d). Because we collected some normal somatic embryos at each time point, the number of embryos could subsequently decrease unless embryogenesis was actively maintained. In C1 and C5, new somatic embryos appeared at the 5th and 7th week, but did not appear at the 9th week. Based on these observations, we considered C1 and C5 at the 5th and 7th week to have high embryogenic potential.
Fig 2. The five Cryptomeria japonica embryonal masses (EMs) induced from an identical cell line SSD-100 at 5, 7, and 9 weeks after maturation.
The capacity to develop somatic embryos are different between EMs.
Transcriptome sequencing
We obtained 20–37 × 106 read pairs (4.1–7.5 × 109 bases) per sample (S2 Table). Raw data are deposited on DDBJ DRA (Accession number: DRA010541). After filtering, 3.98–7.33 × 109 bases remained per sample and 74–84% of these were properly mapped onto CJ3006NRE. De novo assembly of unmapped reads generated 204,943 transcript sequences. Based on a homology search against SwissProt and Pfam-A, 78,415 isoform sequences of 25,364 genes were predicted. After removing short (< 500 bp) sequences, 31,595 isoform sequences of 12,073 genes were used for subsequent analyses. This assembly is deposited in DDBJ (Accession number: ICQR01000001–ICQR01031595) and the annotation is shown in S4 Table.
Differentially expressed genes between EMs with high-embryogenic and low-embryogenic potential
At the 5th and 7th week, 742 genes were significantly upregulated in HE samples compared with LE samples. The 742 genes upregulated in HE samples included some candidate genes encoding such as leafy cotyledon (LEC; g28713; S3 Table), wuschel (WUS; g27218 and g34977; S3 Table), and germin-like protein (GLP; g31725 and g38183; S3 Table), as well as glutathione-S-transferase (GST; seven genes including g3323 and TRINITY_DN4815_c0_g1; S3 and S4 Tables). Consistent with the developmental stages in our experiment, we found ten genes encoding late embryogenesis abundant proteins. GO enrichment analysis showed that the 742 genes were significantly involved in the development of the plant body, especially specifications of polarity, and metabolic processes including the biosynthesis of flavonoids, gibberellins, and brassinosteroids (Table 1).
Table 1. Over-represented Gene Ontology (GO) terms in the 742 genes upregulated in high embryogenic Cryptomeria japonica embryonal masses.
| Category | GO ID | Term | p |
|---|---|---|---|
| Development | |||
| GO:0010158 | Abaxial cell fate specification | 2.E-05 | |
| GO:0080060 | Integument development | 3.E-04 | |
| GO:1902183 | Regulation of shoot apical meristem development | 8.E-04 | |
| GO:0009943 | Adaxial/abaxial axis specification | 3.E-03 | |
| GO:0010073 | Meristem maintenance | 4.E-03 | |
| GO:0009944 | Polarity specification of adaxial/abaxial axis | 4.E-03 | |
| GO:0099402 | Plant organ development | 7.E-03 | |
| GO:1901342 | Regulation of vasculature development | 8.E-03 | |
| Metabolic process | |||
| GO:0009813 | Flavonoid biosynthetic process | 3.E-09 | |
| GO:0009686 | Gibberellin biosynthetic process | 3.E-07 | |
| GO:0016131 | Brassinosteroid metabolic process | 5.E-07 | |
| GO:0016042 | Lipid catabolic process | 8.E-05 | |
| GO:0006110 | Regulation of glycolytic process | 2.E-04 | |
| GO:1901959 | Positive regulation of cutin biosynthetic process | 3.E-04 | |
| GO:0009699 | Phenylpropanoid biosynthetic process | 7.E-04 | |
| GO:0010345 | Suberin biosynthetic process | 8.E-04 | |
| GO:0033473 | Indoleacetic acid conjugate metabolic process | 9.E-04 | |
| GO:0042744 | Hydrogen peroxide catabolic process | 1.E-03 | |
| GO:0006694 | Steroid biosynthetic process | 1.E-03 | |
| GO:0016132 | Brassinosteroid biosynthetic process | 2.E-03 | |
| GO:2000762 | Regulation of phenylpropanoid metabolic process | 2.E-03 | |
| GO:0046463 | Acylglycerol biosynthetic process | 3.E-03 | |
| GO:0042761 | Very long-chain fatty acid biosynthetic process | 5.E-03 | |
| GO:0009690 | Cytokinin metabolic process | 7.E-03 | |
| GO:0046189 | Phenol-containing compound biosynthetic … | 7.E-03 | |
| GO:0010430 | Fatty acid omega-oxidation | 7.E-03 | |
| GO:0006571 | Tyrosine biosynthetic process | 8.E-03 | |
| GO:0009696 | Salicylic acid metabolic process | 8.E-03 | |
| GO:1900378 | Positive regulation of secondary metabolite biosynthetic process | 8.E-03 | |
| Response to stimulus | |||
| GO:0009744 | Response to sucrose | 5.E-04 | |
| GO:0043481 | Anthocyanin accumulation in tissues in response to UV light | 6.E-04 | |
| GO:0009725 | Response to hormone | 2.E-03 | |
| GO:1901700 | Response to oxygen-containing compound | 3.E-03 | |
| GO:0033993 | Response to lipid | 3.E-03 | |
| GO:0009958 | Positive gravitropism | 9.E-03 | |
| Transport | |||
| GO:0080170 | Hydrogen peroxide transmembrane transport | 6.E-06 | |
| GO:0015840 | Urea transport | 1.E-05 | |
| GO:0071577 | Zinc ion transmembrane transport | 3.E-04 | |
| GO:0008643 | Carbohydrate transport | 5.E-03 | |
| GO:0006833 | Water transport | 9.E-03 | |
| Others | |||
| GO:0010268 | Brassinosteroid homeostasis | 5.E-08 | |
| GO:0070207 | Protein homotrimerization | 2.E-06 | |
| GO:0010431 | Seed maturation | 3.E-05 | |
| GO:0036290 | Protein trans-autophosphorylation | 3.E-04 | |
Validation of differentially expressed genes by quantitative PCR
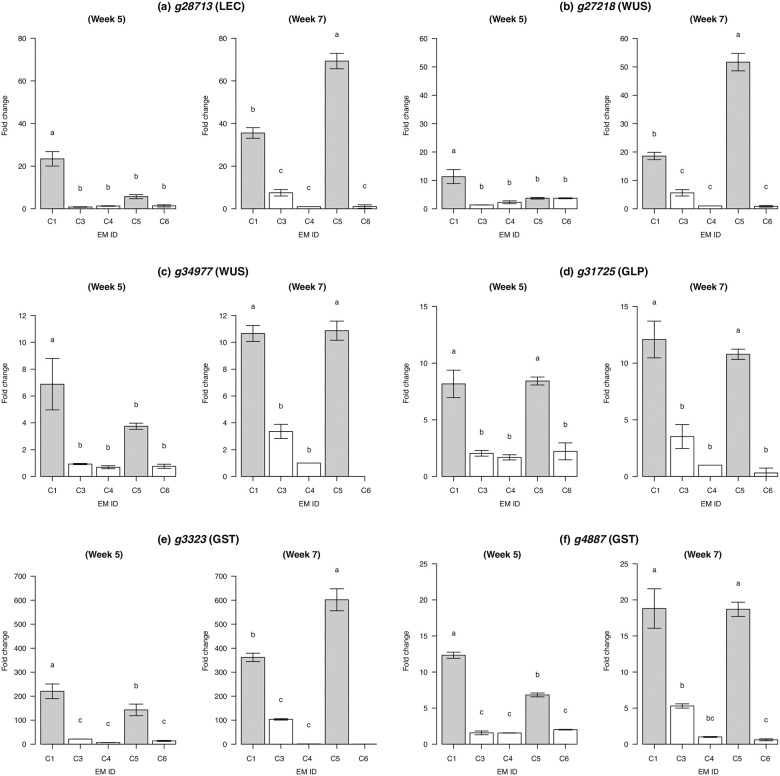
qPCR assays showed that HE samples (C1 and C5) more highly expressed six candidate genes compared to LE samples (C3, C4, and C6) except for the C5 at the 5th week, where the expression levels of three genes, namely g28713 (LEC), g27218 (WUS), and g34977 (WUS), were not significantly different from LE samples (Fig 3). Clear difference in expression levels between HE and LE samples was particularly found at the 7th week, consistent with the increased number of somatic embryos (Figs 1d and 2).
Fig 3. Relative expressions of putatively embryogenesis-related genes in Cryptomeria japonica.
Expressions of six genes encoding (a) leafy cotyledon (LEC), (b, c) wuschel (WUS), (d) germin-like protein (GLP), and (e, f) glutathione-S-transferase (GST) were quantitated. For each gene, expression levels were normalized with that in C4 from the 7th week. Means with different letters are significantly different (Tukey’s multiple comparison test, p < 0.01). Samples with high embryogenic potential (C1 and C5) were indicated in gray. In C6 from the 7th week, the expressions of (c) g34977 and (e) g3323 were not quantified due to the low expressions.
Discussion
Our results showed that the embryogenesis of C. japonica largely differs among cell lines (i.e., genotypes). Although we examined six cell lines with embryogenic potential, only two (SSD-18 and 73) showed stable embryogenesis (Fig 1a–1c). This is consistent with the varying potential of cell lines for forming embryos reported in other conifer species such as Pinus radiata [34]. Besides the variation among cell lines, we showed that embryogenesis differs within cell lines (S1 Fig). Given that all the EMs within cell lines share the same genomic background, embryogenic potential may also depend on gene expression regulation of relevant genes. Intrinsic factors, such as the totipotency of cells transplanted to maturation media, and extrinsic factors, including the concentration of the medium available for EMs, may affect the signaling pathways of gene expressions. The variation we observed in embryogenesis between and within cell lines confirms that a substantial number of cell lines as well as replicated EMs should be maintained to ensure a stable supply of C. japonica somatic embryos. These findings critically indicate the necessity of a means to identify embryogenic potential at certain developmental stages.
We found that gene expression profiles clearly differed between the HE and LE samples at the 5th and 7th weeks after maturation, during which somatic embryos developed in abundance. GO enrichment analysis suggested that genes upregulated in HE samples were involved in the pattern forming and metabolism required to develop somatic embryos (Table 1). In the 742 upregulated genes, we found some candidate genes: leafy cotyledon (LEC), wuschel (WUS), germin-like protein (GLP), and glutathione-S-transferase (GST) genes. LEC genes are required for the specification of cotyledon identity and the completion of embryo maturation [35]. The WUS gene family acts to maintain the stem cell population in shoot apical meristems [36, 37], root apical meristems [38], and cambial meristems [39, 40]. Both LEC and WUS proteins play central roles in the auxin signaling pathway and regulate somatic cells for embryogenic development [41]. In Arabidopsis thaliana, LEC genes are activated by WUS genes to promote SE [42]. The high expression of LEC and WUS genes during SE development has also been confirmed in other conifer species (Picea and Pinus species; [34, 43, 44]). The GLP gene family is thought to mediate the initiation of embryo germination in wheat [45], Arabidopsis [46], and cotton [47]. In Pinus and Picea, GLPs likely function during the early stages of SE induction, as suggested by proteomic and transcriptomic studies [48–50]. GST enzymes are known to act under stress conditions and to minimize oxidative damage on cells. The transcripts have been detected in somatic embryos in Chicorium [51] and wheat [52].
According to a review by Mahdavi-Darvari et al. [3], LEC, WUS, GLP, and GST genes are good candidate markers for embryogenic potential. As our qPCR results validated the different expressions between HE and LE samples (Fig 3), the primers (S5 Table) can be used as appropriate markers to discriminate embryogenic potential in C. japonica. Identifying the expression profiles of these genes in the early stages of SE development will further inform the effectiveness of these genes as markers. Besides the focus on gene expression, studies on epigenetic [53] and proteome processes [54] could provide additional insights to improve the efficient collection of quality somatic embryos.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Means with different letters are significantly different (Tukey’s multiple comparison test, p < 0.01).
(TIF)
Acknowledgments
We thank Shizue Tanaka for her assistance with laboratory work and Satoko Hirayama for providing seeds used for SE induction.
Data Availability
Raw sequence data are available from DDBJ (accession number: DRA010541). De novo cDNA assembly obtained in this study is deposited in DDBJ (accession number: ICQR01000001–ICQR01031595).
Funding Statement
This work was supported in part by grants from the Ministry of Agriculture, Forestry and Fisheries of Japan and NARO Bio-oriented Technology Research Advancement Institution to Y. M. (No. 28013B and No. 28013BC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klimaszewska K, Trontin J-F, Becwar MR, Devillard C, Park Y-S, Lelu-Walter M-A. Recent progress in somatic embryogenesis of four Pinus spp. Tree and Forest Science and Biotechnology. 2007;1: 11–25. [Google Scholar]
- 2.Lelu-Walter M-A, Thompson D, Harvengt L, Sanchez L, Toribio M, Pâques LE. Somatic embryogenesis in forestry with a focus on Europe: State-of-the-art, benefits, challenges and future direction. 2013;9: 883–899. 10.1007/s11295-013-0620-1 [DOI] [Google Scholar]
- 3.Mahdavi-Darvari F, Noor NM, Ismanizan I. Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tiss Organ Cult. 2015. 10.1007/s11240-014-0615-0 [DOI] [Google Scholar]
- 4.Plomion C, Bastien C, Bogeat-Triboulot M-B, Bouffier L, Déjardin A, Duplessis S, et al. Forest tree genomics: 10 achievements from the past 10 years and future prospects Annals of Forest Science. 2nd ed 2016;73: 77–103. 10.1007/s13595-015-0488-3 [DOI] [Google Scholar]
- 5.Noceda C, Salaj T, Pérez M, Viejo M, Cañal MJ, Salaj J, et al. DNA demethylation and decrease on free polyamines is associated with the embryogenic capacity of Pinus nigra Arn. cell culture. Trees. 2009;23: 1285. [Google Scholar]
- 6.Zhang S, Zhou J, Han S, Yang W, Li W, Wei H, et al. Four abiotic stress-induced miRNA families differentially regulated in the embryogenic and non-embryogenic callus tissues of Larix leptolepis. Biochem Biophys Res Commun. 2010;398: 355–360. 10.1016/j.bbrc.2010.06.056 [DOI] [PubMed] [Google Scholar]
- 7.Forestry Agency. Statistical handbook of forest and forestry. Ministry of Agriculture, Forestry and Fisheries; 2014. pp. 8–9. [Google Scholar]
- 8.Baba K, Nakae K. The national epidemiological survey of allergic rhinitis in 2008—comparison between 1998 and 2008. Prog Med. 2008;28: 2001–2012. [Google Scholar]
- 9.Ogita S, ishikawa H, Kubo T, Sasamoto H. Somatic embryogenesis from immature and mature zygotic embryos of Cryptomeria japonica I: Embryogenic cell induction and its morphological characteristics. Journal of wood science. Dordrecht: Springer, Dordrecht; 1999;45: 87–91. [Google Scholar]
- 10.Maruyama E, Tanaka T, Hosoi Y, Ishii K, Morohoshi N. Embryogenic Cell Culture, Protoplast Regeneration, Cryopreservation, Biolistic Gene Transfer and Plant Regeneration in Japanese Cedar (Cryptomeria japonica D. Don). Plant Biotechnology. Japanese Society for Plant Cell and Molecular Biology; 2000;17: 281–296. 10.5511/plantbiotechnology.17.281 [DOI] [Google Scholar]
- 11.Igasaki T, Sato T, Akashi N, Mohri T, Maruyama E, Kinoshita I, et al. Somatic embryogenesis and plant regeneration from immature zygotic embryos of Cryptomeria japonica D. Don. Plant Cell Reports. 2003;22: 239–243. 10.1007/s00299-003-0687-5 [DOI] [PubMed] [Google Scholar]
- 12.Maruyama E, Hosoi Y. Polyethylene glycol enhance somatic embryo production in Japanese cedar (Cryptomeria japonica D. Don). Propagation of Ornamental Plants. 2007;7: 57–61. [Google Scholar]
- 13.Taniguchi T, Ohmiya Y, Kurita M, Tsubomura M, Kondo T. Regeneration of transgenic Cryptomeria japonica D. Don after Agrobacterium tumefaciens-mediated transformation of embryogenic tissue. Plant Cell Reports. 2008;27: 1461–1466. 10.1007/s00299-008-0569-y [DOI] [PubMed] [Google Scholar]
- 14.Futamura N, Ujino-Ihara T, Nishiguchi M, Kanamori H, Yoshimura K, Sakaguchi M, et al. Analysis of expressed sequence tags from Cryptomeria japonica pollen reveals novel pollen-specific transcripts. Tree Physiology. 2006;26: 1517–1528. 10.1093/treephys/26.12.1517 [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi Y, Totsuka S, Iwai J, Matsumoto A, Ueno S, Tsumura Y. Pyramiding of male-sterile genes in Cryptomeria japonica D. Don with the aid of closely linked markers. Tree Genetics & Genomes. 2017;13: 61. [Google Scholar]
- 16.Moriguchi Y, Uchiyama K, Ueno S, Ujino-Ihara T, Matsumoto A, Iwai J, et al. A high-density linkage map with 2560 markers and its application for the localization of the male-sterile genes ms3 and ms4 in Cryptomeria japonica D. Don. Tree Genetics & Genomes. 2016;12: 57. [Google Scholar]
- 17.Moriguchi Y, Ujino-Ihara T, Uchiyama K, Futamura N, Saito M, Ueno S, et al. The construction of a high-density linkage map for identifying SNP markers that are tightly linked to a nuclear-recessive major gene for male sterility in Cryptomeria japonica D. Don. BMC Genomics. 2012;13: 95 10.1186/1471-2164-13-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno S, Moriguchi Y, Uchiyama K, Ujino-Ihara T, Futamura N, Sakurai T, et al. A second generation framework for the analysis of microsatellites in expressed sequence tags and the development of EST-SSR markers for a conifer, Cryptomeria japonica. BMC Genomics. 2012;13: 136 10.1186/1471-2164-13-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchiyama K, Ujino-Ihara T, Ueno S, Taguchi Y, Futamura N, Shinohara K, et al. Single nucleotide polymorphisms in Cryptomeria japonica: their discovery and validation for genome mapping and diversity studies. Tree Genetics & Genomes. 2012;8: 1213–1222. [Google Scholar]
- 20.Mishima K, Hirao T, Tsubomura M, Tamura M, Kurita M, Nose M, et al. Identification of novel putative causative genes and genetic marker for male sterility in Japanese cedar (Cryptomeria japonica D.Don). BMC Genomics. 2018;19: 277 10.1186/s12864-018-4581-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa Y, Ueno S, Matsumoto A, Ujino-Ihara T, Uchiyama K, Totsuka S, et al. Fine mapping of the male-sterile genes (MS1, MS2, MS3, and MS4) and development of SNP markers for marker-assisted selection in Japanese cedar (Cryptomeria japonica D. Don). Chen C, editor. PLoS ONE. Public Library of Science; 2018;13: e0206695 10.1371/journal.pone.0206695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama E, Hosoi Y, Ueno S, Oonishi N, Totsuka S, Iwai J, et al. Somatic embryogenic cell induction from seeds of pollen-free sugi (Cryptomeria japonica) produced at the Niigata prefecture. Kanto Shinrin Kenkyu. 2017;68: 41–44. [Google Scholar]
- 23.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. Oxford University Press; 2014;30: 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei F-J, Ueno S, Ujino-Ihara T, Saito M, Tsumura Y, Higuchi Y, et al. Inspecting abundantly expressed genes in male strobili in sugi (Cryptomeria japonica D. Don) via a highly accurate cDNA assembly. bioRxiv. 2020;: 2020.04.21.054320. [Google Scholar]
- 25.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. SN; 9: 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12: 323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8: 1494–1512. 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucl Acids Res. 2017;45: D158–D169. 10.1093/nar/gkw1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Research. 2016;44: D279–D285. 10.1093/nar/gkv1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26: 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexa A, Rahnenfuhrer J. topGO: Enrichment Analysis for Gene Ontology. 2nd ed. [Google Scholar]
- 32.de Vega-Bartol JJ, Santos RR, Simões M, Miguel CM. Normalizing gene expression by quantitative PCR during somatic embryogenesis in two representative conifer species: Pinus pinaster and Picea abies. Plant Cell Reports. 2013;32: 715–729. 10.1007/s00299-013-1407-4 [DOI] [PubMed] [Google Scholar]
- 33.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13: 134 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bravo S, Bertin A, Turner A, Sepúlveda F, Jopia P, Parra MJ, et al. Differences in DNA methylation, DNA structure and embryogenesis-related gene expression between embryogenic and non embryogenic lines of Pinus radiata D. don. Plant Cell Tiss Organ Cult. Springer Netherlands; 2017;130: 521–529. 10.1007/s11240-017-1242-3 [DOI] [Google Scholar]
- 35.Gaj MD, Zhang S, Harada JJ, Lemaux PG. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta. 2005;222: 977–988. 10.1007/s00425-005-0041-y [DOI] [PubMed] [Google Scholar]
- 36.Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. Role of WUSCHEL in Regulating Stem Cell Fate in the Arabidopsis Shoot Meristem. Cell. Elsevier; 1998;95: 805–815. 10.1016/s0092-8674(00)81703-1 [DOI] [PubMed] [Google Scholar]
- 37.Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T. The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes. Cell. 2000;100: 635–644. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446: 811–814. 10.1038/nature05703 [DOI] [PubMed] [Google Scholar]
- 39.Hirakawa Y, Kondo Y, Fukuda H. TDIF Peptide Signaling Regulates Vascular Stem Cell Proliferation via the WOX4 Homeobox Gene in Arabidopsis. Plant Cell. 2010;22: 2618 10.1105/tpc.110.076083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji J, Strable J, Shimizu R, Koenig D, Sinha N, Scanlon MJ. WOX4 Promotes Procambial Development. Plant Physiol. 2010;152: 1346 10.1104/pp.109.149641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wójcik AM, Wójcikowska B, Gaj MD. Current Perspectives on the Auxin-Mediated Genetic Network that Controls the Induction of Somatic Embryogenesis in Plants. IJMS. 2020;21: 1333–19. 10.3390/ijms21041333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Niu Q-W, Teng C, Li C, Mu J, Chua N-H, et al. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Research. 2009;19: 224–235. 10.1038/cr.2008.276 [DOI] [PubMed] [Google Scholar]
- 43.Hedman H, Zhu T, Arnold von S, Sohlberg JJ. Analysis of the WUSCHEL-RELATED HOMEOBOX gene family in the conifer picea abies reveals extensive conservation as well as dynamic patterns. 2013;13: 89 10.1186/1471-2229-13-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Zhang S, Wang J. Transcriptome analysis of callus from Picea balfouriana. BMC Genomics. 2014;15: 553 10.1186/1471-2164-15-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson EW, Lane BG. Relation of protein synthesis in imbibing wheat embryos to the cell-free translational capacities of bulk mRNA from dry and imbibing embryos. J Biol Chem. 1980;255: 5965–5970. [PubMed] [Google Scholar]
- 46.Membré N, Berna A, Neutelings G, David A, David H, Staiger D, et al. cDNA sequence, genomic organization and differential expression of three Arabidopsis genes for germin/oxalate oxidase-like proteins. Plant Molecular Biology. 1997;35: 459–469. 10.1023/a:1005833028582 [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Triplett BA. Cotton fiber germin-like protein. I. Molecular cloning and gene expression. Planta. 2004;218: 516–524. 10.1007/s00425-003-1133-1 [DOI] [PubMed] [Google Scholar]
- 48.Domon JM, Dumas B, Lainé E, Meyer Y, David A, David H. Three glycosylated polypeptides secreted by several embryogenic cell cultures of pine show highly specific serological affinity to antibodies directed against the wheat germin apoprotein monomer. Plant Physiol. 1995;108: 141–148. 10.1104/pp.108.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neutelings G, Domon JM, Membré N, Bernier F, Meyer Y, David A, et al. Characterization of a germin-like protein gene expressed in somatic and zygotic embryos of pine (Pinus caribaea Morelet). Plant Molecular Biology. 1998;38: 1179–1190. 10.1023/a:1006033622928 [DOI] [PubMed] [Google Scholar]
- 50.Lippert D, Zhuang J, Ralph S, Ellis DE, Gilbert M, Olafson R, et al. Proteome analysis of early somatic embryogenesis in Picea glauca. Proteomics. 2005;5: 461–473. 10.1002/pmic.200400986 [DOI] [PubMed] [Google Scholar]
- 51.Galland R, Blervacq A-S, Blassiau C, Smagghe B, Decottignies J-P, Hilbert J-L. Glutathione-S-Transferase is Detected During Somatic Embryogenesis in Chicory. Plant Signal Behav. Landes Bioscience; 2007;2: 343–348. 10.4161/psb.2.5.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singla B, Tyagi AK, Khurana JP, Khurana P. Analysis of expression profile of selected genes expressed during auxin-induced somatic embryogenesis in leaf base system of wheat (Triticum aestivum) and their possible interactions. Plant Molecular Biology. 2007;65: 677–692. 10.1007/s11103-007-9234-z [DOI] [PubMed] [Google Scholar]
- 53.Uddenberg D, Valladares S, Abrahamsson M, Sundström JF, Sundås-Larsson A, Arnold von S. Embryogenic potential and expression of embryogenesis-related genes in conifers are affected by treatment with a histone deacetylase inhibitor. Planta. 2011;234: 527–539. 10.1007/s00425-011-1418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morel A, Teyssier C, Trontin J-F, Eliášová K, Pešek B, Beaufour M, et al. Early molecular events involved in Pinus pinaster Ait. somatic embryo development under reduced water availability: transcriptomic and proteomic analyses. Physiol Plant. 2014;152: 184–201. 10.1111/ppl.12158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
Means with different letters are significantly different (Tukey’s multiple comparison test, p < 0.01).
(TIF)
Data Availability Statement
Raw sequence data are available from DDBJ (accession number: DRA010541). De novo cDNA assembly obtained in this study is deposited in DDBJ (accession number: ICQR01000001–ICQR01031595).