Abstract
PURPOSE:
Understanding the role of transoral surgery in oropharyngeal cancer (OPC) requires prospective, randomized multi-institutional data. Meticulous evaluation of surgeon expertise and surgical quality assurance (QA) will be critical to the validity of such trials. We describe a novel surgeon credentialing and QA process developed to support the ECOG-ACRIN Cancer Research Group E3311 (E3311) and report outcomes related to QA.
PATIENTS AND METHODS:
E3311 was a phase II randomized clinical trial of transoral surgery followed by low- or standard-dose, risk-adjusted post-operative therapy with stage III-IVa (AJCC 7th edition) HPV-associated OPC. In order to be credentialed to accrue to this trial, surgeons were required to demonstrate active hospital credentials and technique-specific surgical expertise with ≥20 cases of transoral resection for OPC. In addition,10 paired operative and surgical pathology reports from the preceding 24 months were reviewed by an expert panel. Ongoing QA required <10% rate of positive margins, low oropharyngeal bleeding rates, and accrual of at least one patient per 12 months. Otherwise surgeons were placed on hold and not permitted to accrue until re-credentialed using a new series of transoral resections.
RESULTS:
120 surgeons trained in transoral minimally invasive surgery applied for credentialing for E3311 and after peer-review, 87 (73%) were approved from 59 centers. During QA on E3311, positive final pathologic margins were reported in 19 (3.8%) patients. Grade III/IV and grade V oropharyngeal bleeding was reported in 29 (5.9%) and 1 (0.2%) of patients.
CONCLUSIONS:
We provide proof of concept that a comprehensive credentialing process can support multicenter transoral head and neck surgical oncology trials, with low incidence of positive margins and *grade III/V oropharyngeal bleeding.
Keywords: Head and Neck cancer, HPV, transoral surgery, quality assurance
INTRODUCTION
Few prospective clinical trials have been undertaken to evaluate the efficacy of novel surgical therapies. In this regard, multicenter prospective trials are needed to understand the role of transoral endoscopic surgery in our treatment armamentarium. However, methodology to ensure consistent surgical quality and reliable accrual in large prospective, multicenter trials has not been developed. Without a credentialing or quality assurance (QA) process, surgical quality may be in question, endangering patients and jeopardizing study results involving de-intensified adjuvant therapy. Quality assurance in transoral surgery requires consistent complete primary tumor resection with negative margins, while minimizing operative morbidity to permit reduced-dose postoperative radiation with or without chemotherapy. Such a credentialing and quality assurance program in a multicenter, cooperative group trial has not been described, yet is essential to study of these emerging surgical techniques.
Human papillomavirus-associated (HPV+) oropharyngeal cancer (OPC) differs markedly from tobacco- and alcohol-related OPC.1,2 HPV+ OPC most commonly presents with a small primary tumor and cervical lymphadenopathy and is often amenable to surgical treatment.3 Transoral robotic surgery (TORS) or transoral laser microsurgery (TLM) may be used to address this malignancy in a minimally invasive manner.4 Retrospective reports suggest improved functional results with transoral surgery,4,5 yet its role in multidisciplinary management and adjuvant therapy deintensification remains uncertain. There is a risk of catastrophic bleeding after transoral surgery for cancer, and a well-recognized learning curve for both TORS and TLM6–8.
The ECOG-ACRIN Cancer Research Group E3311 (E3311) is a randomized phase II trial of transoral surgery, with adjuvant therapy based on pathologic risk assessment, for HPV+ OPC. This is the first prospective, multi-institutional randomized study incorporating transoral surgery into primary treatment. Application of minimally invasive transoral surgery, including either TORS or TLM, in E3311 required establishing the technical expertise of investigators before patient enrollment, to ensure consistent surgical quality. As an example applicable to other surgical clinical trials, we report our experience creating a novel surgeon credentialing and QA process and patient safety outcomes for this large (n=519), multicenter trial using transoral surgery, followed by adjuvant therapy as indicated. Our goal is: (1) to delineate a method to ensure consistency and quality in surgical oncology trials to understand the role of surgery within multi-disciplinary treatment; (2) to validate the safety of transoral surgery for HPV+ OPC in a large multi-institutional trial 9.
MATERIALS AND METHODS
E3311 (NCT 01898494) is a randomized phase II study of transoral surgery followed as indicated by reduced- or standard-dose radiation therapy with or without chemotherapy for stage III-IVA HPV+ OPC. The study protocol and eligibility criteria have been previously described.10 Briefly, patients with lateralized, resectable p16+ OPC (stage T1-T2 and N1-N2b) with no matted nodes provided written informed consent and underwent radiographic staging. Primary treatment was transoral surgical resection and neck dissection, followed by risk-based adjuvant therapy. Low-risk patients defined by T1-T2, N0-N1 cancer (using AJCC 7th edition) resected with negative or close (<3mm) margins and no extranodal extension (ENE), were observed (Arm A). Intermediate-risk patients were defined by T1-T2 cancers resected to negative margins; N1 with ENE >0 and ≤1 mm, -N2 disease without ENE or with ≤1mm ENE, or up to 4 positive nodes. These patients were randomized to 50Gy (Arm B) versus 60Gy (Arm C) of adjuvant radiation. Higher-risk patients (Arm D) were those with positive margins, >1mm of ENE or ≥5 metastatic lymph nodes. These patients received adjuvant radiation with concurrent weekly cisplatin at 40 mg/m2. N3 disease patients were removed from randomization and treated with 60Gy (Arm C). The Institutional Review Board (IRB) at each site approved the study protocol. All study subjects provided written informed consent. The trial enrolled 519 patients between January 2014 and July 2017.
Credentialing Committee
A committee of ten experienced head and neck surgeons formed the surgeon credentialing committee that determined applicant surgeon eligibility (Table 1). This committee was composed of leaders in head and neck and transoral surgery, including but not limited to experts in both TORS and TLM, and included members of the two National Cancer Trials Network (NCTN) head and neck committees. These surgeons met by teleconference every 2–3 weeks, and periodically in person, to review surgeon case submissions, to review on-hold status and to ensure ongoing QA. This committee was formed to provide oversight for E3311, as well as a now-closed trial of HPV-negative (HPV−) OPC, RTOG 1221 (Holsinger, PI). The latter trial, however, closed due the paucity of T1-T2 HPV-negative OPC and the lack of FDA approval for using robotics for T3 tumors.to failure to accrue.
Table 1:
Surgeon Credentialing Questionnaire
| Approved (n = 87) N (%) |
Not Approved (n = 22) N (%) |
Total (n = 109)* N (%) |
|
|---|---|---|---|
| Pre-treatment endoscopy routinely performed | |||
| Yes | 87 (100%) | 21 (95.5%) | 108 (99.1%) |
| Missing | 0 | 1 (4.5%) | 1 (0.9%) |
| Scope of Practice: | |||
| General Otolaryngology | |||
| Yes | 4 (4.6%) | 1 (4.5%) | 5 (4.6%) |
| No | 83 (95.4%) | 21 (95.5%) | 104 (95.4%) |
| Head and Neck with some endoscopic surgery | |||
| Yes | 45 (51.7%) | 9 (40.9%) | 54 (49.5%) |
| No | 42 (48.3%) | 13 (59.1%) | 55 (50.5%) |
| Head and Neck with focus on endoscopic surgery | |||
| Yes | 46 (52.9%) | 11 (50.0%) | 57 (52.3%) |
| No | 41 (47.1%) | 11 (50.0%) | 52 (47.7%) |
| Number of neck dissections per year |
Median (range) 75 (10–300) |
Median (range) 50 (10–150) |
Median (range) 70 (10–300) |
| Number of transoral endoscopic surgical procedures each year |
Median (range) 20 (0–200) |
Median (range) 20 (10–75) |
Median (range) 20 (0–200) |
| Minimum of 20 cases of transoral excision as primary surgeon | |||
| Yes | 87 (100%) | 21 (95.5%) | 108 (99.1%) |
| Missing | 0 | 1 (4.5%) | 1 (0.9%) |
| Minimum of 5–10 transoral resections in past 12 months | |||
| Yes | 87 (100%) | 21 (95.5%) | 108 (99.1%) |
| Missing | 0 | 1 (4.5%) | 1 (0.9%) |
| Other surgeons at same institution who have completed questionnaire | |||
| Yes | 42 (48.3%) | 12 (54.5%) | 54 (49.5%) |
| No | 45 (51.7%) | 9 (40.9%) | 54 (49.5%) |
| Missing | 0 | 1 (4.5%) | 1 (0.9%) |
109 surgeons (out of the 120 who applied) submitted a questionnaire, one of which was incomplete.
Surgeon Credentialing
Surgeons wishing to accrue were required to complete a multistep process to ensure expertise with transoral surgical techniques and quality. Only surgeons credentialed at institutions affiliated with an NCI cooperative group were eligible, and all were required to have valid hospital credentials and to attest to a personal experience of 20 or more cases of transoral surgery for OPC, prior to applying for trial participation. The latter requirement was intended to address a known learning curve effect with transoral resection.8 Credentialing was technique-specific and was granted for TORS or TLM. To ensure adequate volume and quality, surgeons needed to submit paired operative and pathology reports from 10 prior transoral surgical OPC cases, with at least one tonsil and one tongue base primary tumor case, within the preceding 24 months. Only resections of malignancies were included for evaluation, and HPV+ or HPV− squamous cell carcinoma (SCC) were preferred, although a minority of cases per surgeon could be from oropharyngeal salivary gland malignancies. Carcinoma of unknown primary cancer cases were considered acceptable if a primary tumor was identified on pathologic evaluation and margins were clear. It was possible for surgeons capable in both techniques to be credentialed in both TLM and TORS. However, this required 20 paired cases total (10 TLM and 10 TORS) to be submitted and reviewed. Pathological reports needed to show tumor within the specimen, adequate margin assessment, and no more than 10% of submitted cases with tumor cells <1mm from the cut specimen edge. Surgeons who met the above criteria were approved for participation and were made active through an online website (Medidata) used for trial data management. The most impactful technical details related to morbidity and mortality, as well as choice of adjuvant treatment, were selected for as objective measurements for use as surrogates of surgical quality. These included positive margin status and postoperative grade III/IV oropharyngeal bleeding.
Surgeon Auditing and Surgical Quality Assurance
Once a surgeon was credentialed to participate in E3311, surgical quality was periodically audited throughout the study. Individual surgeons who were identified to have a high rate of positive margins defined a priori as a >10% rate of positive margins (≥1/5 cases after their first 5 accrued cases or ≥2/10 cases ongoing), were placed on hold. These surgeons could be re-credentialed if they repeated the credentialing process; i.e., = submitted a new series of cases, and were approved by the credentialing committee. A second criterion to ensure patient safety was a pre-specified trial stopping rule of >21% composite rate of grade III/IV oropharyngeal bleeding or positive margins reviewed periodically by the ECOG-ACRIN Data Safety Monitoring Committee. Postoperative oropharyngeal bleeding was selected as a primary QA outcome to measure perioperative morbidity, as it is a common and potentially fatal short-term complication.11–13 The incidence of bleeding was defined and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Additional surgical quality metrics analyzed included the number of lymph nodes removed during neck dissection.14,15 Lymph node yield of 15–20 lymph nodes was considered a minor quality deviation, and lymph node yield of <15 lymph nodes was considered a major quality deviation.
Statistical Analysis
The present analysis reports outcomes related to surgeon credentialing and surgical patient safety. We do not report oncological results of the randomized trial data as these are not mature. Basic statistics are reported.
RESULTS
Patients
The study opened in August 2013 and closed in July 2017. Investigators enrolled 519 patients, however 25 patients did not undergo the planned surgery, unrelated to surgical credentialing. The present analysis reports the findings of the 495 patients who underwent planned surgery, for whom full surgical QA data are available.
Pre-Trial Credentialing
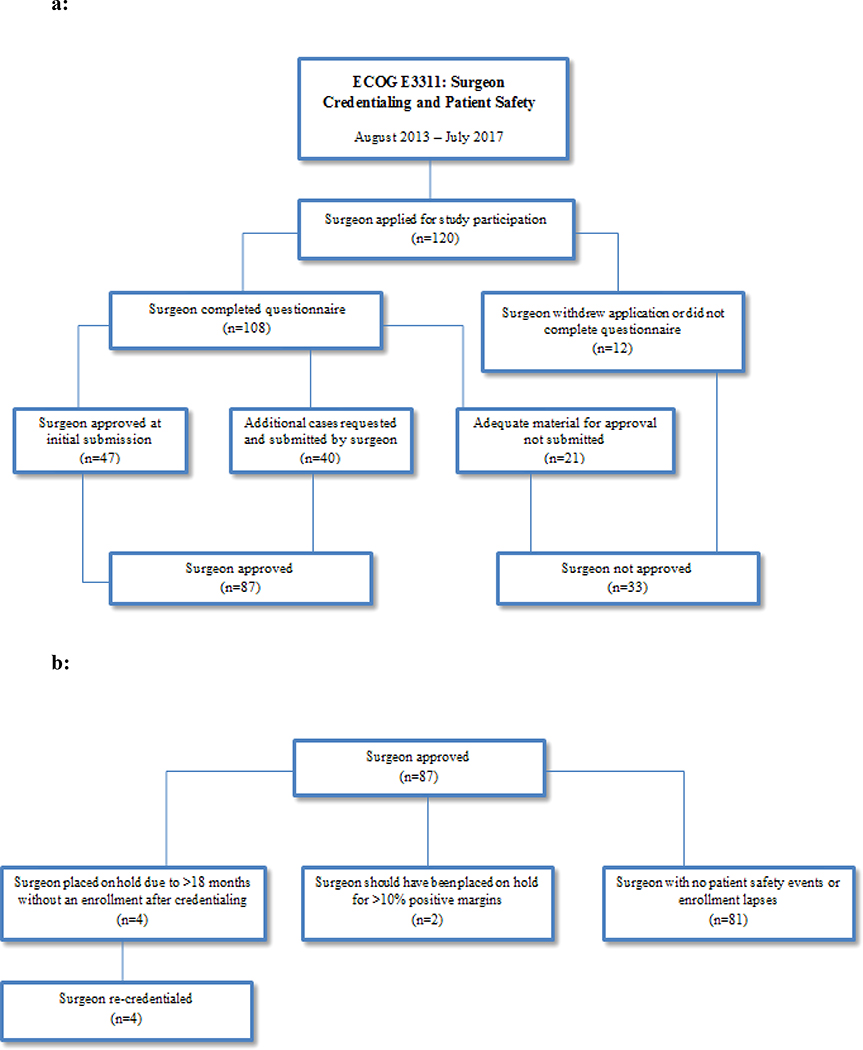
Of the 120 surgeons who applied for surgical credentialing, 87 (73%) were ultimately approved from 59 centers across North America. Accrual per surgeon ranged from 1–38 patients (median = 4). Fifty-one (n=51) surgeons were from academic medical centers and 8 were from community practice. Eighty-one surgeons completed TORS credentialing and 6 surgeons completed TLM credentialing. Fifty-nine (n=59) surgeons accrued >1, 30 surgeons accrued >5, 15 surgeons accrued >10, 4 surgeons accrued >20, and 2 surgeons accrued >30 patients. One non-credentialed surgeon (who was erroneously approved in his site’s data management system) accrued 3 patients. This surgeon was retrospectively evaluated using contemporaneously performed cases, once the deviation was discovered, and passed review by the credentialing committee.
The surgical credentialing committee did not approve 33 surgeons for participation in the trial (Figure 1a). Twelve surgeons withdrew their applications or did not complete forms fully or appropriately, and 12 surgeons did not provide adequate cases, based on submission of disallowable cases (non-cancer or non-OPC) or a large number (>5) cases with positive margin(s), and were immediately not approved. Another 9 surgeons with >1 positive margin case submitted initially, but did not re-apply with subsequent, satisfactory negative margin replacement cases, and were not approved.
Figure 1:
a: Flow Diagram of Surgeon Approval Process
b: Flow Diagram of Surgeon Quality Outcomes
Ongoing Surgical Quality Assurance
Ongoing QA was conducted to monitor surgical quality and patient safety using individual surgeon hold criteria that had been defined a priori. Of the 87 surgeons who were eventually approved, 4 surgeons were put on hold due a length of greater than 12 months after initial credentialing without an enrollment (Figure 1b). All 4 surgeons completed re-credentialing and were eligible to participate in the study again. Two surgeons should have been placed on hold based on positive margins within their first 5 accrued patients. However, surgical pathology data were not made available until after they had accrued additional patients. In two cases, the sites did not provide margin status information until several weeks or months later, after their first 5 patients’ data were reviewed. Another two cases were difficult to adjudicate since intraoperative margins were reported as clear, versus positive margins reported later on the final pathology report. Thus, the surgeons were not placed on hold, since they acted appropriately based on the intraoperative pathologic informative provided to them. In two cases, sites listed positive margin cases within their surgeon’s first 5 cases, but upon re-review by the study PI, the margin status was deemed sufficiently clear to be acceptable.
In September 2015, the DSMC reviewed the interim analyses results regarding surgical quality and the risk distribution. Based on the analyses results, the specified stopping rule of 21% combined grade III/IV oropharyngeal bleeding and positive margins was not met. No surgeon was placed on hold due to grade III/IV bleeding. After ligation of cervical vessels was made a “strong recommendation” in a trial amendment (activated January 13, 2016), the only grade V fatal oropharyngeal bleeding occurred (out of the 256 enrolled patients after amendment activation) with omission of recommended vessel ligation. The surgeon was placed on hold after deliberation by the credentialing committee. While 1 grade V bleed occurred before vessel ligation was made mandatory (September 21, 2016), no difference in grade III-V oral bleeding events was observed before and after amendment activation (6.1% vs 6.1%, p = 0.99). The final composite QA endpoint was an 8.9% composite rate with a 3.8% rate of positive margins and 5.9% rate of grade III/IV oral bleeding (Table 3), and 6.1% including the solitary grade V event.
Table 3:
Patient Demographics and Surgical Experience
| Grade III-V Bleeding | Positive Margins | Removal of 15–20 Nodes | Removal of <15 Nodes | Total | |
|---|---|---|---|---|---|
| (n = 30) N (row %) |
(n = 19) N (row %) |
(n = 41) N (row %) |
(n=9) N (row %) |
(n = 495) N (column %) |
|
| Age | Median (range) | Median (range) | Median (range) | Median (range) | Median (range) |
| 57.7 (43.6–70.4) | 57.9 (35.9–80.1) | 59.0 (44.3–78.7) | 68.1 (48.6–80.1) | 58.7 (35.9–80.1) | |
| Pathological T Stage | |||||
| T1 | 11 (4.6%) | 6 (2.5%) | 20 (8.3%) | 5 (2.1%) | 240 (48.5%) |
| T2 | 18 (7.8%) | 12 (5.2%) | 18 (7.8%) | 3 (1.3%) | 230 (46.5%) |
| T3 | 1 (4.7%) | 1 (4.4%) | 3 (13.0%) | 1 (4.4%) | 23 (4.7%) |
| T4a | 0 | 0 | 0 | 0 | 1 (0.2%) |
| Unevaluable | 0 | 0 | 0 | 0 | 1 (0.2%) |
| Oropharyngeal Subsite | |||||
| Base of Tongue | 12 (6.9%) | 7 (4.0%) | 16 (9.1%) | 5 (2.9%) | 175 (35.3%) |
| Tonsil | 18 (5.9%) | 12 (4.0%) | 22 (7.2%) | 4 (1.3%) | 304 (61.4%) |
| Glossopharyngeal Sulcus | 0 | 0 | 3 (18.8%) | 0 | 16 (3.2%) |
| Gender | |||||
| Male | 29 (6.5%) | 17 (3.8%) | 39 (8.8%) | 6 (1.4%) | 445 (89.9%) |
| Female | 1 (2.0%) | 2 (4.0%) | 2 (4.0%) | 3 (6.0%) | 50 (10.1%) |
| Arm* | |||||
| A | 3 (6.1%) | 0 | 7 (14.3%) | 1 (2.0%) | 49 (9.9%) |
| B | 7 (5.5%) | 1 (0.8%) | 8 (6.3%) | 1 (0.8%) | 127 (25.7%) |
| C | 7 (5.3%) | 1 (0.8%) | 14 (10.6%) | 3 (2.3%) | 132 (26.7%) |
| D | 8 (5.8%) | 15 (10.9%) | 10 (7.3%) | 3 (2.2%) | 138 (27.9%) |
| Surgery only (no arm assigment) | 5 (10.2%) | 2 (4.1%) | 2 (4.1%) | 1 (2.0%) | 49 (9.9%) |
| Surgical Technique | |||||
| TORS | 28 (6.3%) | 17 (3.8%) | 39 (8.8%) | 9 (2.0%) | 443 (89.5%) |
| TLM | 1 (2.4%) | 2 (4.9%) | 2 (4.9%) | 0 | 41 (8.3%) |
| Standard Equipment | 1 (9.1%) | 0 | 0 | 0 | 11 (2.2%) |
| Surgeon Enrollment** | |||||
| <5 patients | 6 (8.2%) | 4 (5.5%) | 6 (8.2%) | 1 (1.4%) | 73 (14.7%) |
| 5–10 patients | 8 (5.7%) | 7 (5.0%) | 8 (5.7%) | 2 (1.4%) | 141 (28.5%) |
| 11–20 patients | 12 (7.2%) | 6 (3.6%) | 19 (11.5%) | 3 (1.8%) | 166 (33.5%) |
| 21–30 patients | 2 (4.4%) | 0 | 5 (10.9%) | 1 (2.2%) | 46 (9.3%) |
| >30 patients | 2 (2.9%) | 2 (2.9%) | 3 (4.4%) | 2 (2.9%) | 69 (13.9%) |
Arm A: Low-risk patients defined by T1-T2, N0-N1 cancer resected with negative or close (<3mm) margins and no extranodal extension (ENE).
Arm B: Intermediate-risk patients defined by T1-T2 cancers resected to negative margins, with N1-N2 disease with ≤1mm ENE, or up to 4 positive nodes.
Arm C: Intermediate-risk patients defined by N3 disease or T1-T2 cancers resected to negative margins, with N1-N2 disease with ≤1mm ENE, or up to 4 positive nodes.
Arm D: Higher-risk patients with positive margins, >1mm of ENE or ≥5 metastatic lymph nodes.
Surgeon enrollment categories determined post-hoc based on observed distribution.
Neck dissection lymph node yield of >20 lymph nodes was reported in 445 (89.9%) of patients. Neck dissection lymph node yield of 15–20 lymph nodes was considered a minor surgical quality deviation and was observed in 41 patients (8.3%). Neck dissection lymph node yield of <15 lymph nodes was considered a major surgical quality deviation and was observed in 9 patients (1.8%).
Subset Analysis:
TORS versus TLM
Comparing surgical technique, 3.8% of patients who underwent TORS (N=443) had positive margins, compared to 4.9% of patients who underwent TLM (N=41) (p = 0.89, adjusting for intra-surgeon correlation). Patients who underwent TORS experienced grade III-V oropharyngeal bleeding in 6.3%, compared to 2.4% of patients who underwent TLM (p = 0.24).
Surgeon Experience
Patients whose surgeons enrolled <5 patients had a 5.5% rate of positive margins, compared to a 3.6% rate for patients whose surgeons enrolled ≥5 patients (p = 0.56). The rate of grade III-V postoperative oropharyngeal bleeding was 8.2% for patients whose surgeons enrolled <5 and 5.7% for patients whose surgeons enrolled ≥5 patients (p = 0.42). There were also no statistically significant differences observed between patients whose surgeons enrolled <10 patients vs. ≥10 patients, with rates of 6.5% vs. 5.7% for grade III-V postoperative oropharyngeal bleeding (p = 0.75) and rates of 5.1% vs. 2.9% for positive margins (p = .29). Rates of positive margins and grade III-V postoperative oropharyngeal bleeding were compared across all enrollment groups. While there were observed trends with higher volume surgeons having lower rates of bleeding or positive margins, these trends were not statistically significant (p = 0.23 for positive margins, p = 0.19 for oropharyngeal bleeding). Comparisons were adjusted for intra-surgeon correlation.
Lateral Oropharyngectomy versus Base of Tongue Primary Site
The composite rate of positive margins or grade III-IV o oropharyngeal al bleeding was 9.5% for patients with a tonsillar primary site16, compared to 8.6% for base of tongue primary site (p = 0.77, adjusting for intra-surgeon correlation).
Prophylactic Transcervical Ligation of Arterial Feeding Vessels of Primary Tumor
Prophylactic transcervical ligation of arterial feeding vessels included external carotid branches: lingual and facial arteries at minimum, plus ascending pharyngeal branch for tonsil primary tumors, and proximal superior thyroid artery for BOT primary tumors. The rate of grade III-V oropharyngeal bleeding overall among those who underwent vessel ligation could not be determined, since the strong recommendation or ultimate requirement for vessel ligation were only implemented well into the trial enrollment, and thus CRF’s did not collect this information for approximately 70% of all patients.
DISCUSSION
Determining the role of transoral surgery in the multidisciplinary management for OPC requires multicenter prospective normative data. Such multicenter trials can be difficult to evaluate in the absence of rigorous processes to ensure consistency of technique as well as surgical quality and safety across multiple institutions. Given our ultimate goal of studying whether transoral surgery will permit treatment de-intensification in favorable risk HPV+ OPC, development and validation of such processes to ensure the interpretability of future phase III trials was an important goal of E3311. We report on the design and implementation of a novel surgeon credentialing and QA process for a large, multicenter phase II study investigating transoral surgery followed by reduced dose adjuvant therapy for HPV+ OPC. This is the most extensive credentialing process reported in head and neck surgical oncology and offers a prototype for future surgical trials. Further, this study validates the safety of transoral surgery in the treatment of HPV+ OPC.
Surgeon Certification Process
The surgeon credentialing process we developed was overseen by a standing credentialing committee. Based on successful surgeon certification processes from large multi-institutional group trials in other surgical fields,17,18 we designed a process to optimize consistent surgical quality on trial while avoiding undue credentialing burdens which would hamper trial accrual. First, parameters for the required volume of operations per surgeons needed to be established to ensure that the well-demonstrated phenomenon of a surgical learning curve did not affect results. An inflection point in the learning curve for transoral robotic surgery, in terms of decreasing the rate of positive margins and length of time of resection is reported to occur after 20 cases.8–19 Thus, surgeons were required to attest to an experience of >20 transoral surgical cases for OPC. Clinical trial certification was supplemented with paired review of the operative record and pathology results, as has been done in other trials.20 Alternately other studies have required surgeons to submit operative videos,21 or be observed by a proctor to confirm surgical competence.22 However for E3311, these were considered too cumbersome and subjective for an NCI cooperative group. In order to implement clinically relevant, objective measures as surrogates for surgical quality, we selected positive margin status and grade III/IV bleeding, based on the available literature supporting these metrics4.
Surgical Quality Assurance
After surgeons begin participating in a study, the PI and investigators must monitor surgical results, analogous to stopping rules for drug studies. If a surgeon is unable to meet the technical requirements to ensure patient safety, there must be rules to allow removal of the surgeon from the study both for patient safety as well as to achieve a valid and generalizable trial result. To ensure quality, hold criteria for individual surgeons of >10% rate of positive margins were created a priori, based on twice the rate of large published retrospective series.4,23 Only 2 surgeons (2.3%) met the criteria to be placed on hold for this criterion, demonstrating that our credentialing process accurately selected capable surgeons. In two cases, the sites did not provide margin status information until several weeks or months later, after their first 5 patients’ data were reviewed. Another two cases were difficult to adjudicate since intraoperative margins were reported as clear, versus positive margins reported later on the final pathology report. Thus, the surgeons were not placed on hold since they acted appropriately based on the intraoperative pathologic informative provided to them. Both of these situations highlight important “real world” experience with surgeon credentialing and QA during clinical trials and should be considered in the design of future trials.
Postoperative oropharyngeal bleeding is a common and potentially life-threatening complication of transoral resection of tonsillar and base of tongue tumors,11–13 and was included along with positive margins in a composite QA outcome. Our composite rate of positive margins (3.8%) or grade III/IV oropharyngeal bleeding (5.9%) of 8.9% compares favorably with previously reported retrospective case series.4,11,24,25 As a result of emerging retrospective data regarding the reduction in severe (life-threatening) oropharyngeal bleeding after ligation of cervical arteries, an investigators’ meeting was held at the 2016 annual meeting of the American Head and Neck Society to review the literature on risk of TORS/TLM for OPC11,12. A single postoperative death (grade V oropharyngeal bleeding) occurred after a surgical procedure that omitted vessel ligation, prior to this amendment making it mandatory. The surgeon was counseled on trial protocol adherence and placed on hold during this period and did not subsequently enroll any further patients. A trial amendment activated on January 13, 2016 included a “strong recommendation” to ligate feeding arterial vessels to the OPC primary site. Thus, although the risk of death from postoperative bleeding is extremely rare in reports of the transoral resection of tonsillar and base of tongue tumors, given that this safety endpoint has to date not been prospectively evaluated, it was included along with positive margins in the composite QA endpoint.
Early prospective series of TORS reported a 3.1–13.1% rate of severe bleeding as a potential complication.4,11,13 Severe postoperative oropharyngeal hemorrhage can cause airway compromise, aspiration and death. We observed a 5.9% rate of grade III/IV oropharyngeal bleeding; however, the majority of this was grade III and the rate of life-threatening grade IV/V bleeding was only 0.8%, with a single death (0.2%) due to this complication. We also recommended that neck dissection be performed prior to or concomitantly with the transoral surgical resection, to ensure that vessel ligation could occur prior to the risk of postoperative oropharyngeal bleeding.
Prior multicenter studies have reported rates of positive margins of 4.3%4 for TORS and 7% for TLM.25 However, outside of experienced centers with high volume radiologists and surgeons, it was feared that the rate of positive could be higher. Validating this concern, a recent study of the National Cancer Database identified a 10–20% rate of positive margins with TORS;24 this may vary by oropharyngeal subsite.23 On E3311 -with the use of prospective and ongoing surgical credentialing and QI - we observed a low rate of positive margins of 3.8% (CI 2.5%−5.9%). Our results show that with rigorous review of surgical experience and monitoring of patient safety with transoral surgery can yield low rates of positive margins, confirming the safety of this technique in experienced hands.
Both of these outcomes above assess the quality of transoral surgery while they are not reflective of the quality of the neck dissection. Thus, neck dissection lymph node yield of >20 was chosen as a supplemental quality outcome. Robust data show that higher lymph node yield during neck dissection is associated with improved survival in head and neck cancer.15,26 However the individual cutoff identified varies between studies. We acknowledge that this may differ in impact based on HPV status, given the lower prognostic impact of nodal metastasis in HPV+ OPC patients. Thus, a two-tier system of a minor protocol violation for a lymph node yield of 15–20 lymph nodes and a major protocol violation for a lymph node yield <15 was employed in this study. Using these cutoffs, 8.3% of patients had a minor protocol violation and 1.8% had a major violation (10.3% with <20 lymph nodes examined). This compares favorably with the literature: in the National Cancer Data base only 73.1% of patients undergoing neck dissection for head and neck cancer have a lymph node yield of ≥18.27
Limitations
Our study has several limitations. First, to ensure surgical quality, we excluded low-volume surgeons from participation. This initial requirement for experience with ≥ 20 cases is not a stringent threshold, which may limit the generalizability of our findings to inexperienced surgeons and centers. However, 56.5% of E3311 credentialed and accruing surgeons enrolled ≤5 cases, suggesting that not all E3311 surgeons were “high volume.” Indeed, 14.5% of accruing surgeons enrolled only one patient and 21.7% enrolled only two. The large number of mid- to high-volume surgeons that participated in this study should ensure that study conclusions are generalizable to the broader head and neck oncology community. Our endpoints also provide potentially useful surgical quality metrics for standard clinical practice, as this emerging field has a paucity of prospectively validated measures. We identified a challenging issue regarding use of intraoperative margins, versus final pathologic margins, to determine surgical QA for putting surgeons “on hold.” Four (n=4) surgeons were deemed to have encountered positive margin(s) in their first 5 cases, but upon surgical PI review., these were called “negative” intraoperatively, then became positive on the final pathology report, due to deeper sectioning and greater sampling or immunohistochemical analysis. Due to the lack of a plan or infrastructure to perform real-time review, these surgeons continued to accrue. Future trials should consider modifications to our procedures to incorporate this experience into surgeon credentialing and ongoing QA. Second, participating surgeons may have selected to enroll patients who were more easily resectable in order to prevent trial violations from positive margins. Thus, the trial results may not apply to patients with borderline resectable HPV+ OPC. Regarding the attempt to associate surgeon volume with margin status and bleeding events (Table 3), we note that this is a retrospective and likely underpowered analysis, and that we did not collect data on the surgeon’s off-trial volumes for these procedures. Any trend should be viewed with caution and investigated prospectively.
While the speculative statements above are a cautionary warning to surgeons not to operate on borderline resectable candidates, the data in this study do not support that patient selection for earlier stage, more easily resectable primary tumors played a major role in this trial or its low rate of positive margins. Indeed, almost half of the patients enrolled had T2 tumors (2–4 cm in greatest dimension) and approximately 5% were pathologically T3 (Table 3). We do recognize that since 95.0% of the patients in this study were roughly equally split between pT1 and pT2 cancers, results from this trial do not apply to T3 and T4 oropharyngeal cancers, for which TORS is not FDA approved. Despite these limitations, the prospective and multicenter nature of the data offer the promising notion that large, prospective studies of new surgical techniques can be successfully conducted. Others have shown a high rate of triple-modality therapy in the NCDB and emphasize the point that transoral surgery may be suitable for study of deintensification when judicious patient selection is employed 28.
CONCLUSION
Large multicenter randomized trials involving new surgical techniques need to incorporate rigorous credentialing protocols. We describe a surgical credentialing and QA process for a large, multicenter phase II trial involving transoral surgery. Initial surgeon credentialing and ongoing QA resulted in low rates of positive margins or grade III/IV oropharyngeal bleeding providing proof of concept that such trials can be successfully completed in head and neck surgical oncology with favorable patient safety outcomes.
Supplementary Material
Table 2:
Surgical Quality and Safety Outcomes
| Study Cohort (n = 495) | |
|---|---|
| Grade III/IV Oropharyngeal Bleeding or Positive Margins | |
| Yes | 44 (8.9%) |
| No | 451 (91.1%) |
| Transoral Surgical Margins | |
| Positive | 19 (3.8%) |
| Negative | 476 (96.2%) |
| Grade III-V Oropharyngeal Bleeding | |
| Yes | 30 (6.1%) |
| No | 465 (93.9%) |
| Lymph Nodes Removed During Neck Dissection | |
| <15 | 9 (1.8%) |
| 15–20 | 41 (8.3%) |
| ≥20 | 445 (89.9%) |
Highlights.
Transoral surgery is an FDA approved method for oncologic removal of selected oropharynx cancer
Surgical credentialing and quality assurance are important to standardize transoral surgery on clinical trials
Clinical trials of surgical techniques can benefit from credentialing procedures that are common between different surgical techniques
Our results may have applicability to other surgical disciplines and prospective clinical trials
Credentialing process:
Surgeon requests CTEP ID and pre-review of surgical cases from Co-Chairs.
Surgeon certifies hospital credentialing for each transoral resection technique, as well as surgical experience with >20 cases or transoral surgery for OPSCC.
10 paired operative notes and pathology reports are uploaded to Rave/Medidata website.
Surgical Credentialing Committee teleconference reviews cases.
Surgeon is either 1) approved, 2) additional/replacement cases are requested, or 3) disapproved.
After surgeon approval, margin status and bleeding are monitored every 5 cases.
Acknowledgments
The authors acknowledge the following conflicts of interest:
Dr. Ferris reports serving on the advisory board for Amgen, Astra-Zeneca/MedImmune, Bristol-Myers Squibb, EMD Serono, GlaxoSmithKline, Lilly, MacroGenics, Merck, Numab Therapeutics AG, Oncorus, Inc, Pfizer, PPD, Regeneron Pharmaceuticals, Inc., Tesaro; Consulting Efforts for Aduro Biotech, Bain Capital Life Sciences, Iovance Therapeutics, Inc, Nanobiotix, Ono Pharmaceutical Co. Ltd, Torque Therapeutics Inc, and TTMS, Clinical Trial/Research Funding from Astra-Zeneca/MedImmune, Bristol-Myers Squibb, Merck, Tesaro, TTMS and VentiRx Pharmaceuticals.
Dr. Hinni’s employer receives royalties for his laryngoscope design.
Dr. Patel is an instructor for Intuitive Surgical.
Dr. O’Malley receives royalties from Penn Medicine through a licensing agreement with Olympus Corporation.
None of the other authors have anything to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine 2007;356:1944–56. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. The Lancet Oncology 2016;17:440–51. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein GS, O’Malley BW Jr., Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. The Laryngoscope 2012;122:1701–7. [DOI] [PubMed] [Google Scholar]
- 5.Albergotti WG, Jordan J, Anthony K, et al. A prospective evaluation of short-term dysphagia after transoral robotic surgery for squamous cell carcinoma of the oropharynx. Cancer 2017;123:3132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollei TR, Hinni ML, Moore EJ, et al. Analysis of postoperative bleeding and risk factors in transoral surgery of the oropharynx. JAMA otolaryngology-- head & neck surgery 2013;139:1212–8. [DOI] [PubMed] [Google Scholar]
- 7.Chia SH, Gross ND, Richmon JD . Surgeon experience and complications with Transoral Robotic Surgery (TORS). Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2013;149:885–92. [DOI] [PubMed] [Google Scholar]
- 8.Albergotti WG, Gooding WE, Kubik MW, et al. Assessment of Surgical Learning Curves in Transoral Robotic Surgery for Squamous Cell Carcinoma of the Oropharynx. JAMA otolaryngology-- head & neck surgery 2017;143:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holsinger FC, Ferris RL. Transoral Endoscopic Head and Neck Surgery and Its Role Within the Multidisciplinary Treatment Paradigm of Oropharynx Cancer: Robotics, Lasers, and Clinical Trials. J Clin Oncol 2015;33:3285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Transoral Surgery Followed By Low-Dose or Standard-Dose Radiation Therapy With or Without Chemotherapy in Treating Patients With HPV Positive Stage III-IVA Oropharyngeal Cancer. 2013. (Accessed December 6, 2017, at https://clinicaltrials.gov/ct2/show/NCT01898494.)
- 11.Kubik M, Mandal R, Albergotti W, Duvvuri U, Ferris RL, Kim S. Effect of transcervical arterial ligation on the severity of postoperative hemorrhage after transoral robotic surgery. Head & neck 2017;39:1510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleysteen J, Troob S, Light T, et al. The impact of prophylactic external carotid artery ligation on postoperative bleeding after transoral robotic surgery (TORS) for oropharyngeal squamous cell carcinoma. Oral oncology 2017;70:1–6. [DOI] [PubMed] [Google Scholar]
- 13.Mandal R, Duvvuri U, Ferris RL, Kaffenberger TM, Choby GW, Kim S. Analysis of post-transoral robotic-assisted surgery hemorrhage: Frequency, outcomes, and prevention. Head & neck 2016;38 Suppl 1:E776–82. [DOI] [PubMed] [Google Scholar]
- 14.Divi V, Harris J, Harari PM, et al. Establishing quality indicators for neck dissection: Correlating the number of lymph nodes with oncologic outcomes (NRG Oncology RTOG 9501 and RTOG 0234). Cancer 2016;122:3464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemieux A, Kedarisetty S, Raju S, Orosco R, Coffey C. Lymph Node Yield as a Predictor of Survival in Pathologically Node Negative Oral Cavity Carcinoma. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2016;154:465–72. [DOI] [PubMed] [Google Scholar]
- 16.Isaacson B, Telian SA, El-Kashlan HK. Facial nerve outcomes in middle cranial fossa vs translabyrinthine approaches. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2005;133:906–10. [DOI] [PubMed] [Google Scholar]
- 17.Edelman MJ, Hu C, Le QT, et al. Randomized Phase II Study of Preoperative Chemoradiotherapy +/− Panitumumab Followed by Consolidation Chemotherapy in Potentially Operable Locally Advanced (Stage IIIa, N2+) Non-Small Cell Lung Cancer: NRG Oncology RTOG 0839. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2017;12:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. Jama 2015;314:1346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White HN, Frederick J, Zimmerman T, Carroll WR, Magnuson JS. Learning curve for transoral robotic surgery: a 4-year analysis. JAMA otolaryngology-- head & neck surgery 2013;139:564–7. [DOI] [PubMed] [Google Scholar]
- 20.Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery 2012;144:S35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. The New England journal of medicine 2013;369:1434–42. [DOI] [PubMed] [Google Scholar]
- 22.Bonati LH, Jongen LM, Haller S, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). The Lancet Neurology 2010;9:353–62. [DOI] [PubMed] [Google Scholar]
- 23.Persky MJ, Albergotti WG, Rath TJ, et al. Positive Margins by Oropharyngeal Subsite in Transoral Robotic Surgery for T1/T2 Squamous Cell Carcinoma. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2017:194599817742852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zevallos JP, Mitra N, Swisher-McClure S. Patterns of care and perioperative outcomes in transoral endoscopic surgery for oropharyngeal squamous cell carcinoma. Head & neck 2016;38:402–9. [DOI] [PubMed] [Google Scholar]
- 25.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head & neck 2011;33:1683–94. [DOI] [PubMed] [Google Scholar]
- 26.Divi V, Chen MM, Nussenbaum B, et al. Lymph Node Count From Neck Dissection Predicts Mortality in Head and Neck Cancer. J Clin Oncol 2016. [DOI] [PubMed] [Google Scholar]
- 27.Cramer JD, Speedy SE, Ferris RL, Rademaker AW, Patel UA, Samant S. National evaluation of multidisciplinary quality metrics for head and neck cancer. Cancer 2017;123:4372–81. [DOI] [PubMed] [Google Scholar]
- 28.Kelly JR, Park HS, An Y, et al. Upfront surgery versus definitive chemoradiotherapy in patients with human Papillomavirus-associated oropharyngeal squamous cell cancer. Oral oncology 2018;79:64–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.