Abstract
Objective
At our emergency department (ED), opioid prescribing guidelines were implemented in September 2016. The opioid prescribing guidelines were adopted and revised from collective efforts and advocacy of the Michigan College of Emergency Physicians for ED‐led opioid stewardship. We performed a retrospective before and after study to determine if opioid prescribing guidelines would change the use of intravenous opioids per patient and the morphine equivalent units (MEU) per patient in a suburban academic ED.
Methods
A retrospective observational study was conducted at a tertiary care level 1 trauma center with an annual ED volume of ≈ 130,000 visits. All intravenous orders of fentanyl, morphine, and hydromorphone for adult patients from January 1, 2015, through December 31, 2017, were tabulated. A 3‐month (August 2016–October 2016) washout period was used. Poisson and ordinary linear regression analyses were employed to evaluate any difference in number of intravenous opioids ordered before and after adoption of the guidelines. Within our opioid prescribing guidelines was also guidance for oral opioid orders within the ED and oral opioid prescriptions for discharge, although these elements were not included in this investigation.
Results
A total of 108,327 intravenous opioid orders were included in the final analysis. After adoption of the opioid prescribing guidelines, the expected number of intravenous opioids ordered dropped by 3.1% (eβ, 0.969; 95% confidence interval [CI], 0.779–1.209), and there was an additional decrease of 0.1% per month (eβ, 0.999; 95% CI, 0.990–1.010). After the adoption of opioid prescribing guidelines, the average MEU dropped by 0.3 mg (95% CI, −0.47 to −0.13), and there was decrease of 0.01 mg per month (95% CI, ‐0.02 to ‐0.004).
Conclusion
After the adoption of opioid prescribing guidelines, our analysis suggests that opioid prescribing guidelines are associated with clinically small but statistically significant changes in MEU ordered in ED. We cannot determine if this represented a continued trend of decreased opioid use or associated with the opioid prescribing guidelines.
Keywords: emergency department, intravenous, morphine equivalents, opiate, opioid epidemic, opioid guideline
1. INTRODUCTION
1.1. Background
Although opioids are often effective for reducing pain, use of these medications is associated with risks of developing dependence, addiction, respiratory depression, and death. Presently, the United States is in the midst of an opioid epidemic. For the purposes of this article, the authors used the term opioids to describe opiates as well. The first widely recognized wave of this opioid epidemic, from about 1990 to 2010, was related to opioid prescriptions, in an era characterized by widespread, successful marketing. 1 , 2 The second wave began in 2010 with increased deaths involving heroin. The third wave, beginning in 2013, demonstrated increased deaths related to synthetic opioids, including fentanyl.
The prevalence of Americans with addiction to opioids may be underreported given its sensitive nature. From 1999 to 2017, almost 400,000 people in the United States died from overdoses involving opioids. Significantly increased overdose deaths involving opioids have occurred since 2013. 1 After 2014, there was a year‐over‐year decrease in life expectancy in the United States in which opioid‐related deaths were deemed contributory. 3 In the United States in 2018, the lifetime odds of dying of an opioid overdose were 1 in 98 and surpassed the odds of dying from a motor vehicle crash being 1 in 106. 4
Although the origins of the opioid epidemic have been multifactorial, the practices of the pharmaceutical industry have influenced opioid prescribing and addiction. This aggressive marketing exaggerating benefits and minimizing risks also led to pharma being targeted for litigation. 5 In 2001, the Joint Commission mandated assessment of pain in all patients and treatment of that pain, which resulted in popularization of pain as “the fifth vital sign.” 6 Both of the prior factors did have detrimental effects on opioid prescribing by clinicians that has also been implicated in the opioid epidemic. Surveyed emergency physicians felt “pressured to prescribe” even for patients exhibiting opioid‐addicted behaviors to avoid administrative complaints. 7 , 8
Given that this epidemic was preceded by an increase in physician‐prescribed opioids, it has been posited that more judicious prescribing is a necessary step in ameliorating the epidemic. One such approach has been the introduction of the “opioid‐free ED.” 9 Other centers have adopted guidelines that recommend non‐opioid strategies, such as the 2016 “Alternatives to Opioids (ALTO)” pathway. 8 , 10
There have been multiple studies of the ED population that examine the rates of oral (PO) opioid prescriptions that are provided to patients for home use. Several of these studies have found that the publication of policies and guidelines have led to decreased rates of prescriptions that emergency clinicians provided for outpatient use. 11 , 12 , 13 Ghobadi et al 14 analyzed intravenous opioid ordering in a large, multi‐ED, single health system before (2013) and after (2014) implementation of opioid prescribing guidelines and reported a 3.6% reduction. In addition, another study reported an intravenous morphine equivalent units (MEU) reduction with implementation of an opioid policy. 8 It is not clear if such guidelines affect the ordering patterns of clinicians or if they encourage more “appropriate” use of opioid medications.
We hypothesized that, after the implementation of the opioid prescribing guidelines, there would be a change in intravenous opioids ordered in the ED. The primary goal of the study was to assess the number of intravenous opioid orders and whether the opioid prescribing guidelines had changed ordering practices. A subsequent goal was to further evaluate if the average MEU dose of intravenous opioids ordered in the ED changed before and after the adoption of the opioid prescribing guidelines.
2. METHODS
2.1. Study design and setting
We conducted a retrospective observational study at a hospital in Royal Oak, Michigan. This site is a tertiary care level 1 trauma center with an annual ED volume of ≈ 130,000 visits. Data were collected from electronic medical records from January 1, 2015, through December 31, 2017.
A set of opioid prescribing guidelines was adopted and revised from collective efforts and advocacy of the Michigan College of Emergency Physicians. Our final opioid prescribing guideline was created by clinical emergency physicians and displayed in our ED for patients to review in September 2016. The guidelines provided guidance regarding risk stratification of patients and encouraged clinicians to discuss the risks of opioids with patients, review the Michigan Automated Prescriptions System report to view patients’ previously filled prescriptions, maximize non‐opioid analgesia using multimodal pain control, use the lowest effective dose of opioids, and use PO rather than intravenous opioids when possible.
We evaluated the number of intravenous opioid orders and the average intravenous opioid MEU before and after the posting of the opioid prescribing guidelines. Because the opioid prescribing guidelines were implemented in September 2016, a 3‐month (August 2016 to October 2016) “washout” period was used for the purposes of our analysis. The “before” guideline period was defined as January 2015 to July 2016. The “after” guideline period was defined as November 2016 to December 2017. The study was approved by the institutional review board.
2.2. Selection of participants
Retrospective chart review was performed after L.Q. used the query condition "analgesics‐narcotic" pharm_class to identify all intravenous orders for fentanyl, morphine, or hydromorphone from the Epic Clarity database. To limit orders to those placed for patients who were primarily under the care of emergency physicians, we further excluded orders of intravenous opioids that met the following criteria: (1) placed after time of ED disposition and (2) incomplete dosage information. For admitted patients boarding in the ED, intravenous opioid orders may originate from an ED physician, physician assistant or nurse practitioner (with or without coordination with admitting physician), a transition of care physician assistant or nurse practitioner, or an admitting team physician. Given the ambiguity of who ordered the intravenous opioid when a patient was boarded, these were excluded.
Furthermore, orders placed for patients <18 years of age were excluded. The “washout” period that surrounded the formal introduction of the opioid prescribing guidelines was not included in the final analysis under the assumption that any change in behavior after the guidelines would require some time for the guidelines to be publicized and to influence behavior.
2.3. Intervention
The opioid prescribing guideline (Material Image S1) was displayed throughout the ED modules. The ordering physicians, residents, physician assistants and nurse practitioners were notified of its implementation via email and word of mouth, although no formal education pertinent to the opioid prescribing guidelines occurred. Patients were aware if they read the displayed opioid prescribing guidelines or if informed by treating staff. Copies of this opioid prescribing guideline were provided to patients on request. No metrics regarding opioid prescribing were tracked for research or administrative purposes during this study period. Within the opioid prescribing guidelines were also statements regarding in‐ED PO opioid orders and PO discharge prescriptions, although they were not included in this investigation.
2.4. Measurements
For each order in the Epic Clarity database meeting the inclusion and exclusion criteria, the date and time of the order was noted as well as the medication type and the dose of the medication. J.M. and D.B. reviewed all formulations and dosages of orders to ensure no patient‐controlled analgesia or intranasal or intramuscular doses were included; final decisions were adjudicated by S.M.
2.5. Outcomes
Our outcomes of interest were the number of intravenous opioid orders and the average MEU of intravenous opioids ordered in the ED across the study period. To be consistent with the institution's MEU, we defined 1 mg of intravenous hydromorphone to be equivalent to 7 mg of intravenous morphine and to 75 mcg of intravenous fentanyl. Our institution's MEU is based on the Institute for Safe Medical Practices and Shaheen et al “equianalgesic” Table 3. 15 During the study period, there was no preselected (default) dose for these 3 intravenous opioids during electronic ordering.
TABLE 3.
Results of segmented linear regression modeling on the impact of guidelines for dose of morphine equivalent order
| Parameter | Estimate (β) | 95% CI | P |
|---|---|---|---|
| Preguideline slope (presecular trend, per month) | −0.02 | −0.03 to −0.02 | <0.001 |
| Change in intercept (immediate effect) | −.30 | −0.47 to −0.13 | <0.001 |
| Change in slope (gradual effect, per month) | 0.01 | 0.004 to 0.02 | 0.002 |
| Postguideline slope (post‐secular trend, per month) | −0.01 | −0.02 to −0.004 | 0.001 |
CI, confidence interval.
The Bottom Line
This study examined the effect of the implementation of prescribing guidelines on the use of opiates in one emergency department. The guideline was shared via flyers and email. The expected number of opioids given dropped by 3%. The opioids ordered did decrease but was not statistically significant after implementation of the opioid prescribing guideline. However, the morphine equivalent units (MEU) decreased and was statistically significant.
2.6. Analysis
Descriptive analysis was used to summarize the demographic characteristics of subjects. A series of monthly intravenous opioid orders between January 2015 and December 2017 was used to assess the impact of the opioid prescribing guidelines. We calculated and plotted monthly changes on the number of intravenous opioid orders and intravenous opioid MEU in opioid ordering across the study time period before and after the adoption of opioid prescribing guidelines. We further examined immediate impact (level change) and gradual impact (slope change) to distinguish the effect of opioid prescribing guidelines from secular change through segmented trend analysis. For investigating monthly changes on the number of intravenous opioid orders, segmented Poisson regression analysis, accounting for the total visits of adults, was used. Subject volume data were obtained from internal metrics maintained by the ED administration team. Subsequently, to analyze monthly changes in intravenous opioid MEU, segmented linear regression analysis was used. All tests of statistical significance were 2‐sided with the P‐value < 0.05 considered to be statistically significant. Analyses were performed using R (version 3.6.2; R Foundation for Statistical Computing) and SAS (version 9.4; SAS Institute, Inc.).
3. RESULTS
3.1. Characteristics of study subjects
Of 139,357 intravenous opioid orders, 118,043 orders met selection criteria. After excluding orders during the washout period, 108,327 orders were included in the final analysis. Before the adoption of opioid prescribing guidelines, 65,917 intravenous opioid orders were placed for 39,547 ED visits; after the adoption of opioid prescribing guidelines, 42,410 intravenous opioids orders were placed for 25,495 ED visits (Figure 1). Characteristics of the study subjects are presented in Table 1.
FIGURE 1.
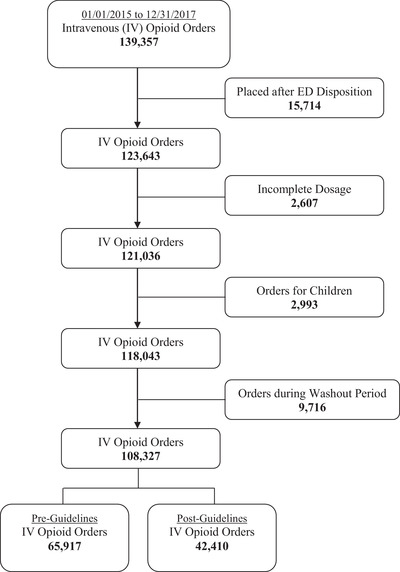
Flow diagram of study cohort. ED, emergency department
TABLE 1.
Subject characteristics
| Variable a | Preguideline | Postguideline |
|---|---|---|
| N | 39,547 (60.8) | 25,495 (39.2) |
| Age, y | 51.7 ± 19.0 | 52.4 ± 18.8 |
| 18 to 39 | 11,935 (30.2) | 7296 (28.6) |
| 40 to 64 | 17,214 (43.5) | 11,124 (43.6) |
| 65 to 74 | 5061 (12.8) | 3530 (13.8) |
| ≥75 | 5337 (13.5) | 3545 (13.9) |
| Sex | ||
| Female | 24,382 (61.6) | 15,252 (59.8) |
| Male | 15,165 (38.4) | 10,243 (40.2) |
| Race | ||
| White | 25,349 (64.1) | 16,635 (65.2) |
| Black | 10,981 (27.8) | 7132 (28.0) |
| Other | 2294 (5.8) | 1502 (5.9) |
| Unknown | 923 (2.3) | 226 (0.9) |
For continuous variables, means ± SDs are presented. For categorical variables, frequencies and percentages are presented.
3.2. Main results
3.2.1. Opioid‐ordering change in relation to the implementation of guidelines
During the study period, monthly opioid orders in the ED were aggregated. Figure 2 presents actual data points and the fitted regression line that depicts changes in the number of intravenous opioid orders. Segmented Poisson regression modeling indicates that before the adoption of opioid prescribing guidelines, there was a month‐to‐month decrease of 0.6% in the expected number of orders (eβ, 0.994; 95% confidence interval [CI], 0.990–0.999); after the adoption of opioid prescribing guidelines, the expected number of orders dropped by 3.1% (eβ, 0.969; 95% CI, 0.779–1.209), and there was an additional decrease of 0.1% per month (eβ, 0.999; 95% CI, 0.990–1.010) (Table 2).
FIGURE 2.
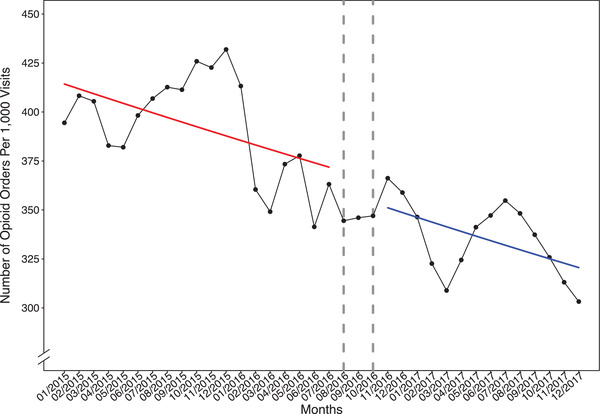
Emergency department disposition intravenous opioid orders during the study period. The fitted regression lines before and after the adoption of opioid prescribing guidelines were colored as red and blue solid lines, respectively
TABLE 2.
Results of segmented Poisson regression modeling on the impact of guidelines for number of opioid orders
| Parameter | Estimate (β) | 95% CI | P |
|---|---|---|---|
| Preguideline slope (presecular trend, per month) | −0.006 | −0.01 to −0.001 | 0.01 |
| Change in intercept (immediate effect) | −0.031 | −0.25 to 0.19 | 0.78 |
| Change in slope (gradual effect, per month) | −0.001 | −0.01 to 0.01 | 0.83 |
| Postguideline slope (postsecular trend, per month) | −0.007 | −0.01 to 0.001 | 0.08 |
CI, confidence interval.
3.2.2. Morphine equivalent dose for opioid prescriptions in relation to the implementation of guidelines
Segmented linear regression modeling shows that before the adoption of opioid prescribing guidelines, there was a month‐to‐month decrease of 0.02 mg in the average morphine equivalent dose (95% CI, −0.03 to −0.02); after the adoption of opioid prescribing guidelines, the average morphine equivalent dose dropped by 0.3 mg (95% CI, −0.47 to −0.13), and there was a decrease of 0.01 mg per month (95% CI, −0.02 to −0.004) (Table 3, Figure 3).
FIGURE 3.
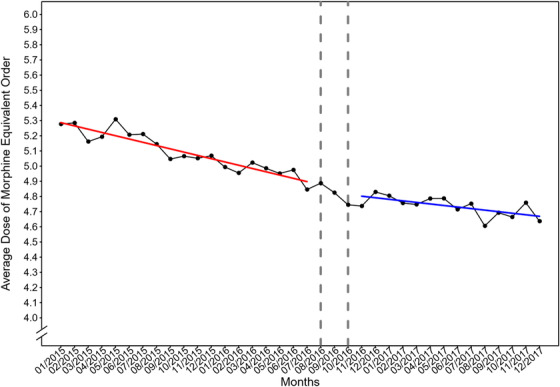
Average dose of morphine equivalent units (per patient) of intravenous opioid orders during the study period. The fitted regression lines before and after the adoption of opioid prescribing guidelines were colored as red and blue solid lines, respectively
4. DISCUSSION
Presently, the United States is in the midst of an opioid epidemic. In September 2016, our ED implemented opioid prescribing guidelines. After adoption of the opioid prescribing guidelines, the number of intravenous opioids ordered for pain during the ED encounter decreased, but was not statistically significant. However, the MEU was statistically significantly decreased during the study period. The power of the study benefited from use of the Epic Clarity database that, after inclusions and exclusions, yielded 108,327 intravenous opioid orders for our analysis.
A retrospective before/after study analyzed intravenous opioid ordering for adults in a large, multi‐ED, single health system in California, and the authors reported a statistically significant decrease of 3.6%. 14 There are several similarities between the investigation by Ghodabi et al 14 and our investigation, including means for age and percentage of women as well as the influence of our respective statewide ACEP chapters. There were key differences including that our cohort had a less diverse racial distribution and did not evaluate physician‐specific data, and we excluded intravenous opioid orders after a patient was admitted. Statistically, they used change in proportion of ED encounters where an opioid was ordered pre/post opioid prescribing guidelines, whereas our study reported intravenous opioids controlled for ED volumes (per 1000 visits). In contrast to our pre‐intervention slope downtrending before our opioid prescribing guidelines, Ghodabi et al 14 had an increasing slope in the pre‐intervention cohort. This slope increase may be related to their study duration (2013–2014), which predates national reporting of year‐over‐year life expectancy data decrease, and therefore must be interpreted in context.
Similar to our investigation, Duncan et al 8 analyzed intravenous MEU at a single‐site level 1 trauma center in 2015 and 2016. Their ED used an ALTO‐first approach with a mean age that was lower, although this may be reflective of the inclusion of pediatric patients. They reported a predicted mean MEU reduction of 0.25 MEU per visit, whereas our MEU reduction was 0.3 MEU per intravenous opioid order, thus although similar in value are different measurements. Of note, although their individual month comparisons of intravenous MEU per visit did reduce with statistical significance, their combined 3‐month pre‐intervention versus 3‐month postintervention did not (P = 0.7).
Although this study has limitations, intradepartmental prescribing guidelines may be an effective tool to empower clinicians and may lead to more judicious prescribing of opioid medications. Given the retrospective nature of our study, we recommend prospective studies to validate the efficacy of such guidelines.
5. LIMITATIONS
This was a study of a single‐site, high‐volume, high‐acuity ED in the state of Michigan. The US opioid epidemic has disproportionately impacted some geographic areas more than others, with significant variation in opioid deaths between the states. This is further complicated by variation in the quality of reporting between the states. In Michigan, there were 21.2 opioid‐related deaths per 100,000 persons in 2017, which was greater than the national average of 14.9 per 100,000. 16 , 17 Given these differences and others, the results of this study may be variably generalizable to other states, emergency medical settings, and other patient populations.
One potential limitation is the exclusion of orders written after a patient had a disposition selected within the electronic medical record. Although this likely caused some orders written in the ED to be excluded, it was necessary to exclude these orders that may have been written by the admitting or observation team rather than in the ED. This exclusion was applied to both “pre” and “post” populations. Therefore, we believe the effect of these exclusions on our results is negligible.
Another limitation is excluding patients <18 years old, as we recognize that this opioid epidemic is not an adult‐only disease. This exclusion occurred for 2 reasons. First, patients <18 years old are managed in our dedicated pediatric ED (most often by pediatric emergency physicians). Furthermore, pediatric emergency physicians often use weight‐based intravenous opioid dosing and intranasal opioids, both of which are not conventionally used in adult ED patients at our facility.
Furthermore, it is limited through its retrospective design, and it is difficult to exclude other confounding variables with certainty. Notably, the opioid epidemic and its relationship to physician prescribing has become better known to the general public. Aside from the intradepartmental opioid prescribing guidelines evaluated in this study, several other initiatives have been underway in recent years in an effort to curb opioid prescribing. For example, the ALTO program aimed to limit the use of opioids when possible and recommended several alternative protocols for pain management. Beyond the walls of our ED, the Department of Health and Human Services declared the opioid crisis a public health emergency in October 2017 as advised by our president, and a year later the SUPPORT for Patients and Communities Act became law. 18 , 19 In addition, the state of Michigan passed a series of laws in December 2017 introducing new regulations intended to decrease opioid prescribing and encouraged more safe prescribing. 20 The study period was intentionally designed to preempt the period of time surrounding these laws being enacted by several months.
The authors also have recognized the potential bias for studies that evaluate human behavior. In this regard, we readily acknowledge that our opioid prescribing guidelines did not have a formal education component before implementation and did not evaluate orderer‐specific data. We cannot exclude the Hawthorne effect, including fear of intravenous opioid ordering even in opioid‐appropriate instances, if physicians, physician assistants and nurse practitioners believed opioid metrics were being actively tracked or indifference to an initiative encouraged by administration when felt “pressured to prescribe” to avoid patient complaints. 7 Our washout period duration was an ad hoc decision to combat the Hawthorne effect, although the impact of our chosen washout duration is unknown.
From a patient perspective, the population that was prescribed intravenous opioids and that was not prescribed intravenous opioids may also be dissimilar in the time periods before and after the opioid prescribing guidelines. We suspect that by implementing the opioid prescribing guidelines, people with addictions to opioids could have been less likely to return to our institution once aware of the change in ordering behavior. We acknowledge that this raised the likelihood of selection bias, as we did not quantify the patients who were denied intravenous opioids. This bias may be further compounded by the lack of data about patients who refused intravenous opioids that were offered by ED prescribers or the change in use in non‐narcotic analgesia. Lastly, the authors acknowledged the limitation of not prospectively knowing attitudes about or compliance with the opioid prescribing guidelines and how this may have varied by job type (attending physician, resident physician, physician assistant or nurse practitioner).
6. CONCLUSION
After the adoption of opioid prescribing guidelines, this analysis suggests that opioid prescribing guidelines are associated with clinically small but statistically significant changes in MEU ordered in ED. We cannot determine if this represented a continued trend of decreased opioid use or is associated with the opioid prescribing guidelines.
CONFLICTS OF INTEREST
This research did not receive any funding or grant support. After our study, author Sheena J. Merwine left our institution and is now employed by Chiesi. Her contribution is not related to work performed as a Chiesi employee, and Chiesi did not fund or support the article or publication.
AUTHOR CONTRIBUTIONS
All listed authors have contributed to this article process, with specific expertise as noted. David A. Berger takes responsibility for the article as a whole. James D. Maloy, Sheena J. Merwine, James Ziadeh, and David A. Berger conceived the study. James D. Maloy, Nai‐Wei Chen, and David A. Berger designed the trial. Nai‐Wei Chen, Sheena J. Merwine, and Lihua Qu performed the data curation. Nai‐Wei Chen performed the formal analysis. James D. Maloy, Nai‐Wei Chen, and David A. Berger conducted the investigation/methodology. James D. Maloy and David A. Berger conducted the project administration. Sheena J. Merwine provided resources. Nai‐Wei Chen and Lihua Qu provided software. David A. Berger provided supervision. James D. Maloy provided the original draft. All authors conducted the reviewing/editing.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
We graciously acknowledge that our opioid prescribing guidelines were built on prior work by the Michigan College of Emergency Physicians and their advocacy for emergency department (ED)–led opioid stewardship and personally thank Dr. Todd Anderson and Dr. James Ziadeh at our study site for finalizing our opioid prescribing guidelines. We would like to thank all of our ED colleagues who have expertly adjusted to the opioid prescribing guidelines. We also acknowledge the many victims and families affected by the opioid epidemic. This research did not receive any specific grant funding from agencies in the public, commercial, or not‐for‐profit sectors.
Biography
James Maloy, MD, is an emergency physician, former chief resident in emergency medicine at Beaumont Health, and current fellow in health policy at the George Washington University Department of Emergency Medicine.

Maloy JD, Chen N‐W, Qu L, Merwine SJ, Ziadeh J, Berger DA. Opioid ordering habits in the acute emergency department visit: before and after implementation of departmental prescribing guidelines. JACEP Open. 2020;1:1472–1479. 10.1002/emp2.12320
Meetings: This article has never been previously published in any form except as an abstract at the October 2019 American College of Emergency Physicians Research Forum (Denver, Colorado)
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Karl A. Sporer, MD.
[Correction added on 19 November 2020, after first online publication: the value under the column “Estimate (β)” and row “Change in intercept (immediate effect)” in Table 3 is corrected from “0.30” to “−.30”.]
REFERENCES
- 1. Center for Disease Control and Prevention, National Center for Injury Prevention and Control. U.S Department of Health and Human Services. https://www.cdc.gov/drugoverdose/epidemic/index.html#combatting-the-epidemic. Accessed November 6, 2020.
- 2. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 2019;322(20):1996‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Safety Council, Injury Facts. National Safety Council. https://www.nsc.org/membership/member-resources/injury-facts. Accessed November 6, 2020.
- 5. Lopez G, The thousands of lawsuits against opioid companies, explained. Vox. https://www.vox.com/policy-and-politics/2017/6/7/15724054/opioid-epidemic-lawsuits-purdue-oxycontin. Published 2019. Accessed November 6, 2020.
- 6. Phillips DM. JCAHO pain management standards are unveiled. Joint Commission on Accreditation of Healthcare Organizations. JAMA. 2000;284:428‐429. [DOI] [PubMed] [Google Scholar]
- 7. Kelly S, Johnson GT, Harbison RD. Pressured to prescribe” the impact of economic and regulatory factors on South‐Eastern ED physicians when managing the drug seeking patient. J Emerg Trauma Shock. 2016;9(2):58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duncan RW, Smith KL, Maguire M. Alternatives to opioids for pain management in the emergency department decreases opioid usage and maintains patient satisfaction. Am J Emerg Med. 2019;37(1):38‐44. [DOI] [PubMed] [Google Scholar]
- 9. Weingart S. Opioid‐Free ED with Sergey Motov. EMCrit Blog. 2014. Accessed November 6, 2020. [Google Scholar]
- 10. LaPietra AM. St. Joseph's regional medical center aims to avoid opioid use in emergency department. ACEP Now. 2016;35(8):1‐3. [Google Scholar]
- 11. Osborn SR, Yu J, Williams B, Vasilyadis M, Blackmore CC. Changes in provider prescribing patterns after implementation of an emergency department prescription opioid policy. J Emerg Med. 2017;52(4):538‐546. [DOI] [PubMed] [Google Scholar]
- 12. del Portal DA, Healy ME, Satz WA, McNamara RM. Impact of an opioid prescribing guideline in the acute care setting. J Emerg Med. 2016;50(1):21‐27. [DOI] [PubMed] [Google Scholar]
- 13. Chacko J, Greenstein J, Ardolic B, Berwald N. Effect of an emergency department opioid prescription policy on prescribing patterns. Am J Emerg Med. 2017;35(9):1327‐1329. [DOI] [PubMed] [Google Scholar]
- 14. Ghobadi A, Van Winkle PJ, Menchine M, Chen Q, Huang BZ, Sharp AL. Reduction of parenteral opioid use in community emergency departments following implementation of treatment guidelines. Acad Emerg Med. 2018;25(8):901‐910. [DOI] [PubMed] [Google Scholar]
- 15. Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous?. J Pain Symptom Manage. 2009;38(3):409‐417. [DOI] [PubMed] [Google Scholar]
- 16. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid‐involved overdose deaths—United States 2013–2017. Morb Mortal Wkly Rep 2017:1419‐1427. [DOI] [PMC free article] [PubMed]
- 17. National Institute of Drug Abuse. Michigan opioid summary. U.S Department of Health and Human Services. https://www.drugabuse.gov/drug-topics/opioids/opioid-summaries-by-state/michigan-opioid-involved-deaths-related-harms. Accessed November 6, 2020. 2018:1–7.
- 18.U.S. Department of Health and Human Services. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html#. Accessed November 6, 2020.
- 19. Davis CS. The SUPPORT for patients and communities act—what will it mean for the opioid‐overdose crisis? N Engl J Med. 2019;380(1):3‐5. [DOI] [PubMed] [Google Scholar]
- 20. Opioid and Prescription Drug Epidemic. Michigan Health & Hospital Association. https://www.mha.org/Issues-Advocacy/Opioid-Epidemic#Legislation. Accessed November 6, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


