Abstract
Objectives
Difficult intravenous access (DIVA) is common in the emergency department (ED). We investigated the extent to which DIVA is associated with care delay outcomes including time to first laboratory draw, therapies, imaging, and ED disposition.
Methods
An observational retrospective cohort analysis of patients with DIVA treated between 2018 and 2020 at 2 urban academic EDs was performed. DIVA was defined as patients requiring ultrasound‐guided intravenous access placed by physicians or advanced practice providers (APPs) as opposed to landmark‐based intravenous placement by nurses. ED throughput variables and disposition time were compared. We correlated DIVA with time to administration of intravenous pain medications, fluids, imaging contrast, laboratory results, and ED disposition.
Results
A total of 108,256 subjects with 161,122 total encounters were included. DIVA occurred in 4961 (3.1%) of ED visits. Patients with DIVA were more likely to be female (3.5% vs 2.6% for males, odds ratio [OR] 1.34, 95% confidence interval [CI]: 1.27–1.42), self‐identify as black (OR 1.78, 95% CI: 1.66–1.91), and have higher acuity of illness (P < 0.001). Among pediatric patients, DIVA occurred most often in the first year of life at a rate of 3.25%. In adults, DIVA occurred in 2 age peaks; at 35 years (4.02%), and at 63 years (3.44%). In all workflow metrics, the presence of DIVA was associated with significant delays in median time to completion: 50 minutes for pain medication administration, 36 minutes for intravenous fluid administration, 29 minutes for laboratory results, 57 minutes for intravenous contrast administration, and 87 minutes for discharge orders.
Conclusion
DIVA was associated with increased time to therapies, diagnostic studies, imaging completion, and ED disposition. A more expeditious approach to achieving intravenous access in patients with predicted DIVA could improve ED throughput and patient care overall.
Keywords: care delay, emergency department, intravenous access, length of stay, point‐of‐care ultrasound, ultrasound, ultrasound‐guided
1. INTRODUCTION
Timeliness and efficient throughput are critical components of emergency department care. Delay in diagnosis, failure to rapidly conduct diagnostic tests, and delay in treatment can cripple ED flow, adversely affect patient outcomes, 1 , 2 , 3 and contribute to poor patient satisfaction overall. 4 Delays in obtaining diagnostics such as blood cultures or serum lactate levels and delays in initiating therapies such as early antibiosis or fluid resuscitation are associated with increased risk‐adjusted in‐hospital mortality in specific populations. 1 , 2 , 3 Among the sickest patients, slowed ED care leading to delayed transfer to the ICU contributes to increased hospital length of stay and overall hospital mortality. 5 , 6 Identifying and addressing steps that consistently delay ED workups, therapies, and dispositions are crucial for improving care.
Intravenous access is often necessary in ED patients of all ages, with estimates suggesting as high as 150–200 million peripheral intravenous catheters are placed annually in North American EDs. 7 , 8 Key steps in ED workflow such as obtaining blood samples for laboratory testing, performing contrast‐enhanced diagnostic studies, and delivering therapies such as analgesics, antibiotics, blood products, or fluid resuscitation often rely on intravenous access. Although causes of ED care delays are often multifactorial, given the ubiquity of the need for intravenous access, difficulties in successful completion of this procedure likely represents a significant potential bottleneck that must be addressed.
Delays in obtaining intravenous access are often secondary to technical limitations of placement in patients considered to have difficult intravenous access (DIVA). These patients often require multiple intravenous placement attempts via traditional landmark‐based techniques, or, in many cases, may require advanced techniques such as intravenous placement under ultrasound guidance. In 1 case‐control study at an urban tertiary care center, need for advanced techniques for intravenous access beyond landmark‐based visual inspection and palpation of peripheral veins increased time from triage to first successful intravenous placement by an additional 118–135 minutes. 9 Similarly, another study estimated that intravenous access difficulty introduced care delays of 15–120 minutes for patients with DIVA. 10 It is reasonable to predict that these delays likely also contributed to increased ED length of stay and potentially even adverse patient outcomes, though these parameters were not measured in these prior studies.
Little is known about the impact of DIVA on delays in ED care and particularly among high‐risk populations. Data offering a quantitative analysis relating intravenous access delays to time‐sensitive treatments such as delivery of analgesics or antibiotics, ED throughput, and ED length of stay is currently lacking. Given the widespread ramifications of ED care delays, and the fact that a significant number of ED patients require intravenous placement for diagnostics and therapies, 7 , 8 we sought to quantify the delay in ED care attributable to DIVA. We aimed to identify associations between delayed intravenous access and patient care parameters such as time to initiation of diagnostic studies or delivery of therapeutics, ED throughput such as time to disposition, and overall ED length of stay. Additionally, we sought to identify specific patient factors are associated with DIVA.
2. METHODS
2.1. Study design and setting
This was a retrospective observational cohort analysis of patients evaluated between 2018 and 2020 at EDs at the Massachusetts General Hospital (MGH) and Brigham and Women's Hospital (BWH) in Boston, Massachusetts. Patients with DIVA were defined as those requiring an ultrasound‐guided intravenous placement as opposed to those who underwent intravenous placement by a nurse via traditional vein visualization and palpation (RN‐intravenous). In our hospitals ultrasound‐guided intravenous are placed by a physician (MD) or advanced practice provider (APP) only, and each such attempt is supposed to be recorded in the electronic medical record (EMR) as a procedure note. At both sites, staff are required to watch a 20‐minute video on the correct strategies for ultrasound‐guided intravenous placement, receive training by fellowship‐trained emergency ultrasound faculty and fellows on placement, are required to be observed and certified placing a ultrasound‐guided intravenous before they can attempt such placements in patients. This study was approved by the local institutional review board.
The two hospitals queried have differences in volume, triaging, and patient populations. The hospitals were assessed together and individually. We expand upon these differences to allow others to extrapolate the findings presented here to their specific care settings. MGH and BWH EDs see 117,000 and 65,000 patients on an annual basis, respectively. Both facilities are urban, level I trauma centers, with large populations of patients with cancer. The MGH ED is divided into care areas designated by acuity, including care areas of Acute, Urgent, Eval, and Fast Track (in descending order of acuity). Of note, “Urgent” is not equivalent to an urgent care area but rather this pod typically sees the second highest acuity patients in the department. Nurses require a higher level of experience to work in Acute; the levels of experience between Urgent, Eval, and Fast Track are similar. Patients are triaged on arrival by perceived acuity by experienced triage nurses, an Emergency Severity Index (ESI) code is not routinely assigned. Patients in Acute and Urgent are kept on monitors, unlike Eval and Fast Track. The MGH ED also has a dedicated pediatric area. The BWH ED has 3 care areas: Alpha, Bravo, and Charlie. Alpha contains the trauma and resuscitation bays, but otherwise patients are not triaged to care areas strictly by acuity. In the BWH ED, an ESI code from 1 to 5 is assigned to all patients with 1 representing highest acuity patients and 5 the lowest. Experience levels of nursing staff between the 3 pods at BWH is roughly equivalent.
2.2. Subject selection and data abstraction
The subject cohort was generated by querying the Epic EMR (Epic Inc., Verona, WI) of MGH and BWH for all ED patients who had documented intravenous line placement between February 18, 2018 and February 20, 2020. This resulted in an initial data set of 118,296 patients and 175,525 encounters, as many subjects had multiple visits during the study period (Figure 1). Subjects were excluded if they had an unrecorded or ambiguous age, sex, care area, or disposition. Patients who were transferred to another hospital, expired in the ED, eloped, or left against medical advice were excluded because of the irregularity of their ED courses. Patients who were transferred from outside hospitals were also excluded as these patients often arrived with intravenous lines in place and had a large portion of their workup completed before arrival. Patients who did not have intravenous placement documented were excluded. This resulted in a final data set of 108,256 subjects with 156,161 total encounters.
FIGURE 1.
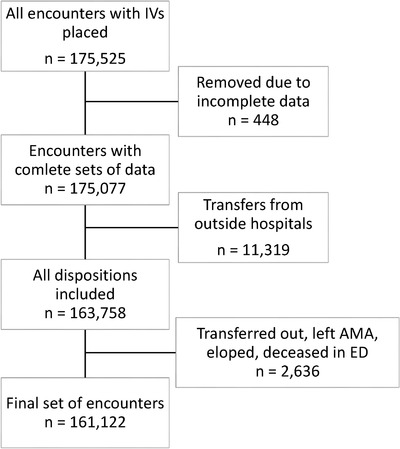
Inclusion flow chart showing the number of encounters generated by our query, those removed because of incompleteness, removed owing to being transferred from another hospital, and the final number of included encounters. AMA, against medical advice
Subject data abstracted from the EMR included patient age, sex, and race. Information pertaining to a specific encounter included the hospital and care area within the ED, ESI code (if recorded), disposition, documentation of an MD‐ or APP‐placed intravenous line, and time of multiple events including arrival to ED, rooming, disposition; first administration of intravenous pain medications, intravenous fluids, or imaging contrast; and resulting of first laboratory tests. Events were only included in the analysis if they were ordered and completed within the time frame of their ED course.
The Bottom Line
Many difficult IV patients come to the ED; is their care delayed by trouble getting IV access? In this study, 3% out of 108,000 patients turned out to be difficult intravenous sticks. For those patients, median delays to getting pain medications, intravenous fluids, lab results, intravenous contrast, and discharge orders were 50, 36, 29, 57, and 87 minutes respectively. This can add up over time and even result in worse care.
2.3. Statistical analysis
All statistical analyses were performed in the R statistical programming environment. 11 Continuous variables were reported as medians with the 25th and 75th percentiles because of the non‐normal distribution of the data and differences detected by the Wilcoxon rank‐sum test. Categorical data were reported as percentages with differences in proportions detected by Pearson chi‐square tests. We estimated the effect of age on the rate of DIVA with a generalized additive model with a cubic smoothing function. Association between encounter frequency and DIVA was assessed with a Spearman's rank correlation. Odds ratios were calculated and reported where possible. For subjects who had multiple encounters, we investigated how the previous encounter affected the odds of DIVA in subsequent encounters. The DIVA status of 2 consecutive encounters was tabulated into a two‐by‐two square consisting of all possible outcomes: DIVA‐to‐DIVA, DIVA‐to‐Non‐DIVA, Non‐DIVA‐to‐DIVA, and Non‐DIVA‐to‐Non‐DIVA. Where multiple testing was performed, P values were adjusted using the Holm‐Bonferroni method.
3. RESULTS
3.1. Characteristics of study subjects
A total of 108,256 unique subjects were included in our analysis, comprising 161,122 encounters. Of these, 68,315 subjects (100,118 encounters) were from MGH and 39,990 subjects (61,004 encounters) were from BWH; 4961 (3.1%) of patient visits were considered DIVA. The median age of all subjects was 56 (interquartile range [IQR] 37–70) with no significant difference between the DIVA and non‐DIVA groups. Those with DIVA were more likely to be female when compared to non‐DIVA patients (60.8% and 53.7% respectively, P value <0.001) and were more likely to be black (21.2% vs 13.1%, P < 0.001). DIVA patients were more likely to be triaged to a higher acuity area or get a more emergent ESI code designation (P < 0.001) and less likely to be discharged (16.9% vs 42.5%, P < 0.001). Results are summarized in Table 1.
TABLE 1.
Demographic and clinical course summary statistics for the overall cohort and stratified by their DIVA status
| Intravenous type | ||||
|---|---|---|---|---|
| Overall | Non‐DIVA | DIVA | P value | |
| N | 161,122 | 156,161 | 4961 | |
| Age | 56 [36, 70] | 56 [36, 70] | 55 [38, 69] | 0.655 |
| Sex (% female) | 86,812 (53.9) | 83,795 (53.7) | 3017 (60.8) | <0.001 |
| Race (%) | <0.001 | |||
| White | 105,001 (65.2) | 101,790 (65.2) | 3211 (64.7) | |
| Black | 21,561 (13.4) | 20,510 (13.1) | 1051 (21.2) | |
| Asian | 6596 (4.1) | 6466 (4.1) | 130 (2.6) | |
| Hispanic | 5780 (3.6) | 5681 (3.6) | 99 (2.0) | |
| Other/unknown | 22,184 (13.8) | 21,714 (13.9) | 470 (9.5) | |
| Department (% Hosp. A) | 100,118 (62.1) | 96,470 (61.8) | 3648 (73.5) | <0.001 |
| Care area (%) | <0.001 | |||
| Acute | 250,10 (25.0) | 24,097 (25.0) | 913 (25.0) | |
| Urgent | 18,342 (18.3) | 17,244 (17.9) | 1098 (30.1) | |
| Eval | 47,358 (47.3) | 45,962 (47.6) | 1396 (38.3) | |
| Fast track | 5474 (5.5) | 5295 (5.5) | 179 (4.9) | |
| Pedi | 3934 (3.9) | 3872 (4.0) | 62 (1.7) | |
| ESI code (%) | <0.001 | |||
| 1 | 982 (1.6) | 942 (1.6) | 40 (3.0) | |
| 2 | 27,873 (45.7) | 27,133 (45.5) | 740 (56.4) | |
| 3 | 31,179 (51.1) | 30,652 (51.4) | 527 (40.1) | |
| 4 | 923 (1.5) | 917 (1.5) | 6 (0.5) | |
| 5 | 47 (0.1) | 47 (0.1) | 0 (0.0) | |
| Discharged (%) | 67,244 (41.7) | 66,404 (42.5) | 840 (16.9) | <0.001 |
| DIVA (%) | 4961 (3.1) | 0 (0.0) | 4961 (100.0) | <0.001 |
DIVA, difficult intravenous access; ESI, Emergency Severity Index. The intraquartile range is displayed for continuous variables in brackets. Percentages are shown in parentheses for categorical variables.
3.2. Patient factors associated with DIVA
When stratified by age, the proportion of subjects with DIVA is highest in the mid‐30s with a second peak happening in the mid‐60s (Figure 2A). Because of the non‐linear nature of the relationship, we estimated the effect of age on the rate of DIVA with a generalized additive model. The first peak was modeled to occur at age 35 with 4.02% of encounters requiring an ultrasound‐intravenous. Though present at both sites, this effect was most prominent at MGH. The second peak occurred at age 63 with 3.44% of encounters having DIVA, after which the rate of DIVA continued to decline. The pediatric population had the highest rate of DIVA in the first year of life at 3.25%. There was large variability in the pediatric population owing to the low overall number of subjects and the low rate of DIVA as a whole in this population.
FIGURE 2.
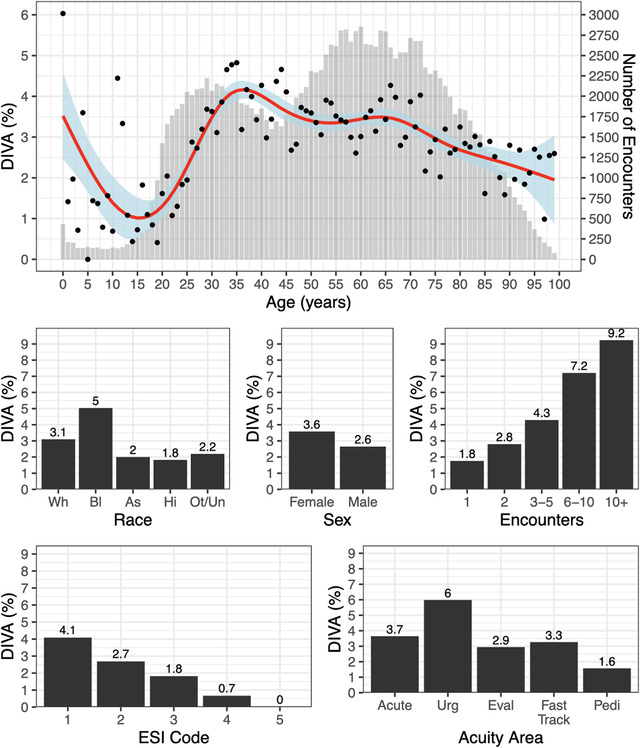
Demographic and clinical features associated with difficult intravenous access (DIVA). (A) Percentage of DIVA by Age. The red line shows fitted generalized additive model depicting the relationship between the percentage of DIVA and age with blue shading representing the 95% confidence intervals. Black dots are the point average per year of life. In gray bars are the number of encounters that were recorded for each year of life. (B‐F) Shows the percentage of DIVA stratified by B) race (Wh = white, Bl = black, As = Asian, Ot/Un = Other/Unknown), (C) sex, (D) number of encounters, (E) ESI code, and (F) acuity area. Labels for race are abbreviated to first 2 letters. Labels represent the exact percentage
In addition to age, several demographic factors were found to have strong associations with the rate of DIVA. The rate of DIVA rate was highest in black subjects (black 4.9%, white 3.1%, Asian 2.0%, Hispanic 1.7%, and 2.1% for unidentified or other races, Figure 2B) and nearly twice the rate for other races with an odds ratio (OR) of 1.8 (95% confidence interval [CI]: 1.66–1.91). Females were more likely to have DIVA than males (3.5% vs 2.6%) with an estimated OR of 1.3 (95% CI: 1.27–1.42) (Figure 2C). Frequency of ED encounters correlated positively with DIVA rates (rho = 0.019, r2 = 0.035, P < 0.001) (Figure 2D). Moreover, subjects were much more likely to have DIVA if they had DIVA on their previous encounter (29.5%) than if they had not (4.2%), (OR 9.5, CI 8.5–10.5).
3.3. Hospital factors associated with DIVA
There was a difference in the rates of DIVA between our sites with 3.6% and 2.2% in MGH and BWH, respectively. In BWH, where the ED makes use of the ESI system, we found a strong association between DIVA and acuity (Figure 2E). Encounters with an ESI score of 1 (highest acuity) had a DIVA rate of 4.1% and decreased ≈1 point per acuity level (ESI 2%–2.7%, ESI 3%–1.7%, ESI 4%–0.7%, and ESI 5 0.0%, P < 0.001). The other clinical site does not make use of the ESI system and instead assigns patients to different care areas based on their acuity and chief complaint. In this case, the highest acuity area, Acute, did not have the highest rate of DIVA (Figure 2F). The care area with the highest rates of DIVA was the Urgent area (6.0%, P < 0.001), followed by Acute (3.7%), Fast Track (3.3%), Eval (2.9%), and Pedi (1.6%). The numbers of encounters with DIVA stratified by ESI code and acuity area are tabulated in Table 1.
3.4. DIVA impact on ED throughput
We compared the time it took to accomplish various measures of ED throughput for those with and without DIVA, including: time to administer (1) pain medications, (2) intravenous fluids, and (3) intravenous contrast; (4) time for lab tests to result; and (5) time to admission order or (6) discharge order (Figure 3). In all metrics, the presence of DIVA was associated with significant delays in median time to completion: 50 minutes for pain medication administration, 36 minutes for intravenous fluid administration, 29 minutes for laboratory results, 57 minutes for intravenous contrast administration, 37 minutes for admission orders, and 87 minutes for discharge orders (Table 2). All metrics were significant with a P value <0.001.
FIGURE 3.
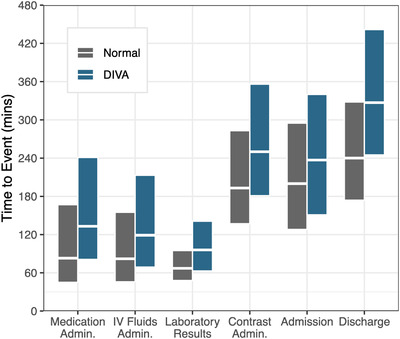
Box plot depicting various measures of ED throughput stratified by difficult intravenous access (DIVA) status. The y‐axis shows the time in minutes to completion of the specified throughput measure on the x‐axis. The boxes show the 25th and 75th percentiles, with the middle line showing the 50th percentile or median. Gray boxes represent normal encounters and blue boxes represent DIVA
TABLE 2.
Association of difficult intravenous access (DIVA) and emergency department throughput measures
| Non‐DIVA | DIVA | ||||||
|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 25% | 50% | 75% | P value | |
| Pain med. admin. | 45 | 83 | 167 | 81 | 133 | 241 | <0.001 |
| Intravenous fluids admin. | 46 | 82 | 155 | 69 | 119 | 213 | <0.001 |
| Laboratory results | 48 | 67 | 95 | 63 | 96 | 141 | <0.001 |
| Intravenous contrast admin. | 137 | 193 | 283 | 181 | 250 | 356 | <0.001 |
| Admission order | 128 | 200 | 295 | 151 | 237 | 340 | <0.001 |
| Discharge order | 174 | 240 | 328 | 245 | 327 | 442 | <0.001 |
DIVA, difficult intravenous access. Reported is the median along with the 25th and 75th percentiles of times to completion in minutes.
4. DISCUSSION
In this study we investigated the extent to which delays in obtaining intravenous access affected time to diagnostics and therapeutics in the ED, as well as overall ED length of stay. Our data demonstrate DIVA is strongly associated with delays in median time to administration of pain medication, intravenous fluids, and intravenous contrast, as well as delays in serum laboratory results. We also demonstrate DIVA is clearly associated with an increased ED length of stay. This raises questions as to whether strategies can be implemented to mitigate the effect of DIVA thereby potentially improving lab turnaround time, time to treatment, and overall length of stay.
Using the definition for DIVA as intravenous placement requiring ultrasound‐guidance by an MD or APP, we identified 3.1% of our population who had DIVA. In prior works, patients with DIVA range from 3.2% to nearly 13% of the ED population. 7 , 9 , 12 , 13 , 14 The percentage of patients with DIVA in our population overall seems to be at the low end of the range compared to prior studies. This could be explained by either increased abilities to obtain intravenous access by our nursing staff, 15 or, more likely, by variable definitions of DIVA in the literature that may include any intravenous access that requires >1 attempt but not necessarily ultrasound guidance. Our data did not capture patients for whom intravenous access required multiple attempts by nursing staff as the number of attempts is rarely documented in the EMR. Thus, our data may actually underreport the occurrence of DIVA in our EDs.
DIVA is more likely to occur in female compared to male patients, as well as patients who self‐identify as black compared to other ethnicities. In prior works, patient sex has been inconsistently identified as a risk factor for DIVA. However, in 1 study, being female has been associated with up to a 3‐fold increase in likelihood of DIVA in “highly complex” patients. 16 Patients who self‐identified as black were more likely to present with DIVA and also had the highest rates of DIVA compared to other races. Prior work has inconsistently identified dark skin as a risk factor for failed initial attempt at intravenous placement through visualization and palpation methods; however, several studies identify poor vein visibility as an independent risk factor for DIVA. 17 , 18
Among adults, DIVA was distributed in 2 age peaks, 1 peak for patients in their mid‐30s, and another peak for patients in their mid‐60s. A history of intravenous drug use has been consistently correlated with DIVA in prior works, 7 , 10 and this may explain the peak we observe for patients in their mid‐30s. 19 For DIVA patients in their mid‐60s, it is reasonable to speculate many patients in this age range have chronic comorbidities such as diabetes, end‐stage renal disease, cancer requiring prior peripheral chemotherapy, which have all been associated with DIVA previously. 7 , 17 , 20 Unfortunately we were unable to reliably extract data on comorbidities for our patient pool; however, we could expand our work in this space further in subsequent studies.
Among all care areas at MGH, rates of DIVA were found to be lowest in the pediatric ED overall, with the highest rates of DIVA seen in patients under 1 year old in this population. This age trend is consistent with prior studies. 21 The volume of pediatric patients is typically quite low at MGH, and among pediatric patients not all require intravenous access. BWH does not see pediatric patients; thus compared to the volume of our adult data our pediatric patient sample is fairly low. Future studies could be aimed at collecting a larger number of pediatric‐specific data to further delineate associations with DIVA in this population.
At BWH where patients are assigned ESI scores at triage but acuity is roughly evenly distributed to care areas, DIVA was positively correlated with patient acuity. At MGH however where patients are triaged to care areas by acuity, the second highest acuity unit (Urgent) demonstrated higher rates of DIVA than the highest acuity unit (Acute). Although patients triaged in Urgent are not typically the sickest in the department, they often are the more medically complex and have the highest number of chronic comorbidities such as diabetes, end‐stage renal disease, sickle cell disease, and cancer, all of which have been positively correlated with DIVA. 7 , 17 , 20 Similarly, patients with a history of intravenous drug use are often triaged to urgent depending on their medical needs, which may also increase the rates of DIVA in this pod.
Ultrasound guidance leads to increased frequency and rapidity of first‐pass intravenous attempts among adult and pediatric patients with DIVA and improves patient satisfaction. 22 , 23 , 24 , 25 , 26 For both adult and pediatric patients, prior works have demonstrated the benefit of specially trained nursing‐ or technician‐based ultrasound‐intravenous teams that can be deployed as needed to address DIVA. 12 , 13 , 14 , 29 , 30 , 31 Our own data as well as prior works identified factors such as sex, race, medical comorbidities, and level of acuity that correlate with high likelihood of DIVA. We as well as others also demonstrated that patients who had DIVA in the past are significantly more likely to demonstrate DIVA in subsequent visits. 7 , 9 , 12 Although these categories remain broad currently, these data are a helpful start to promote early identification of cases with potential DIVA through screening in triage. This screening as well as validated scoring protocols 29 , 30 , 31 , 32 could expedite successful intravenous access in patients with DIVA potentially via deployment of a dedicated ultrasound‐intravenous team. This strategy could help to minimize the significant ED care delays caused by DIVA.
5. LIMITATIONS
This study had several limitations. First, multiple patient populations were excluded from the analysis including patients who had unrecorded or ambiguous age, sex, care area, or disposition as they were unable to be appropriately placed in a cohort in our data set. Patients who expired in the ED, eloped, left against medical advice, or were transferred to an outside hospital were also excluded as the irregularity and often incompleteness of their ED courses was predicted to reflect inaccurate time measurements. Similarly, patients transferred from outside hospitals were also excluded as these patients often already have intravenous linesin place and arrive with much of their workup complete. This led to a roughly 10% decrease in the number of encounters analyzed overall; however, exclusion of these patients likely led to a more accurate reflection of a typical ED course.
In this study, the need for use of ultrasound‐guidance for successful intravenous placement was used as a surrogate for DIVA. In reality, there are likely other circumstances that also reflect DIVA such as intravenous placements requiring multiple attempts by nursing, intravenous lines requiring placement by an MD or APP but without ultrasound‐guidance, or the need to obtain venous access via intraosseous line or at alternative sites such as at the external jugular vein. These events are unreliably captured in the EMR and thus were not able to be included in our dataset. Although all ultrasound‐intravenous placement is supposed to be documented in the EMR, it is likely that not every procedure over our study period was documents, which would decrease the accuracy of our dataset. Thus, there are several confounding variables to defining DIVA that unfortunately could not be measured using a retrospective design. Given that at our hospitals ultrasound‐intravenous are attempted almost exclusively after attempts by traditional palpation have failed, and during the study period only MDs and APPs were credentialled to place ultrasound‐intravenous, we ultimately felt this was the most appropriate surrogate for DIVA that could be reliably captured. Our analysis did not incorporate patients who underwent central venous catheter placement initially without attempts at peripheral catheterization. Although these events may also represent patients with DIVA, we chose to focus on DIVA as defined by patients requiring ultrasound‐intravenous placement.
In the pediatric ED at MGH, the unit is typically staffed by pediatric residents who are not trained in placement of ultrasound‐intravenous. Thus, for pediatric patients it is common that a pediatric ICU nurse may be called to the ED to establish intravenous access when other attempts have failed. These scenarios likely also introduce care delays similar to those seen with our DIVA population that, however, may also not be accurately reflected in our data. This is particularly important in the pediatric population where our dataset is limited.
Patients with cancer who have received chemotherapy via peripheral intravenous lineshave been previously identified as a group with high rates of DIVA. 17 Although both MGH and BWH have large populations of patients with cancer, many of these patients already have indwelling central access when they present to the ED and do not require additional intravenous access. Thus, our population of cancer patients may not be reflective of patient populations nationally or globally and may skew our data toward decreased magnitude of care delays.
Both MGH and BWH host a robust residency training program and APP cohort who are well trained in ultrasound‐intravenous placement. Although non‐emergency medicine rotators who may be less familiar with ultrasound‐intravenous placement do rotate through both EDs, there is typically sufficient skilled staffing to assist with such placement as necessary. This rich personnel resource may falsely decrease the magnitude of ED delays associated with DIVA as compared to facilities where providers are less familiar with use of ultrasound for obtaining peripheral intravenous access and may instead need to resort to alternative strategies such as central venous catheter placement.
As discussed previously, DIVA is likely multifactorial, and although our data demonstrate strong correlations of DIVA to particular populations, causation cannot be implied from a retrospective analysis. Other influences such as nurse‐specific factors (level of experience, comfort with asking a provider for assistance), or circumstantial factors such as the volume of patients in the department on any given day likely also contribute to delays in ED care and represent potential major confounders to our data. Our analysis is based on records in the EMR; thus, it was not possible in this study to control for or measure the effect of all possible confounders. Furthermore, given the retrospective nature of our study, only correlations and not causal relationships between DIVA as defined by need for ultrasound‐intravenous placement and outcomes can be identified. Future prospective studies aimed at defining a potential causal relationship between DIVA and ED care delays is needed.
6. CONCLUSIONS
In summary, this is the first large‐scale analysis to demonstrate a relationship between DIVA and delayed ED care. We show that delays in intravenous access are associated with increased time to therapy, diagnostics, and imaging completion, and our data suggest DIVA may predict an increased ED length of stay. Risk factors associated with DIVA include female sex, black race, higher acuity of illness, prior history of DIVA, and age range in the mid‐30s, and mid‐60s among adult patients. Future research should attempt to identify a causal relationship between DIVA and delayed care parameters and also should focus on strategies to identify patients with DIVA early and implement initiatives to optimize obtaining rapid intravenous access to minimize delays in care.
CONFLICT OF INTERESTS
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Hamid Shokoohi conceived the study. Sayon Dutta and Dustin McEvoy assisted with acquisition of data. Michael A. Loesche performed statistical analysis and interpretation of data and drafted the figures and results of the manuscript. Nicole M. Duggan and Hamid Shokoohi planned on data presentation and drafted the manuscript. Andrew S. Liteplo, Calvin Huang, Ahad A. Al Saud, and Shan W. Liu, critically reviewed the study proposal, provided mentorship, and commented on the manuscript draft.
Biography
Hamid Shokoohi, MD, MPH, is an Associate Professor of Emergency Medicine at Harvard Medical School who practices at the Department of Emergency Medicine at Massachusetts General Hospital.

Shokoohi H, Loesche MA, Duggan NM, et al. Difficult intravenous access as an independent predictor of delayed care and prolonged length of stay in the emergency department. JACEP Open. 2020;1:1660–1668. 10.1002/emp2.12222
Presentations: The abstract of the study is accepted for presentation at 2020 SAEM Annual Meeting in Denver, CO, in May 2020.
Funding and support: This is a non‐funded study, with no compensation or honoraria for participation, consulting or conducting the study. Resources required for this project were provided by institutional departmental funds at the Massachusetts General Hospital, Department of Emergency Medicine, with no particular budgeting allocated to this project.
Supervising Editor: Michael Blaivas, MD, MBA.
REFERENCES
- 1. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304‐377. [DOI] [PubMed] [Google Scholar]
- 2. Seymour CW. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235‐2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seymour CW, Kahn JM, Martin‐Gill C, et al. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med. 2017;45(5):759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pitrou I, Lecourt AC, Bailly L, Brousse B, Dauchet L, Ladner J. Waiting time and assessment of patient satisfaction in a large reference emergency department: a prospective cohort study, France. Eur J Emerg Med. 2009;16(4):177‐82. [DOI] [PubMed] [Google Scholar]
- 5. Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP, DELAY‐ED study group . Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477‐83. [DOI] [PubMed] [Google Scholar]
- 6. Handel D, Epstein S, Khare R, et al. Interventions to improve the timeliness of emergency care. Acad Emerg Med. 2011;18(12):1295‐302. [DOI] [PubMed] [Google Scholar]
- 7. Fields JM, Piela NE, Au AK, et al. Risk factors associated with difficult venous access in adult ED patients. Am J Emerg Med. 2014;32(10):1179‐82. [DOI] [PubMed] [Google Scholar]
- 8. Alexandrou E. The One Million Global Catheters PIVC worldwide prevalence study. Br J Nurs. 2014;23(8):S16‐S17. [DOI] [PubMed] [Google Scholar]
- 9. Witting MD. IV access difficulty: incidence and delays in an urban emergency department. J Emerg Med. 2012;42(4);483‐487. [DOI] [PubMed] [Google Scholar]
- 10. Witting MD, Moayedi S, Brown LA, Ismail A. Predictors and delays associated with the need for advanced techniques for intravenous access. J Emerg Med. 2017;53(2):172‐177. [DOI] [PubMed] [Google Scholar]
- 11. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2018. Available at: https://www.R-project.org/
- 12. Witting MD, Moayedi S, Beverly SK, Stover BJ, Miller AC. Incidence of advanced intravenous access in 2 urban EDs. Am J Emerg Med. 2015 May;33(5):705‐7. [DOI] [PubMed] [Google Scholar]
- 13. Civetta G, Cortesi S, Mancardi M, et al. EA‐DIVA score (Enhanced Adult DIVA score): a new scale to predict difficult preoperative venous cannulation in adult surgical patients. J Vasc Access. 2019;20(3):281‐289. [DOI] [PubMed] [Google Scholar]
- 14. Lee SU, Jung JY, Ham EM, et al. Factors associated with difficult intravenous access in the emergency department. J Vasc Access. 2010;21(2):180‐185. [DOI] [PubMed] [Google Scholar]
- 15. Yalcinli S, Akarca FK, Can Ö, Şener A, Akbinar C. Factors affecting the first‐attempt success rate of intravenous cannulation in older people. J Clin Nurs. 2019;28(11‐12):2206‐2213. [DOI] [PubMed] [Google Scholar]
- 16. Armenteros‐Yeguas V, Gárate‐Echenique L, Tomás‐López MA, et al. Prevalence of difficult venous access and associated risk factors in highly complex hospitalized patients. J Clin Nurs. 2017;26(23‐24):4267‐4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piredda M, Biagioli V, Barrella B, et al. Factors affecting difficult peripheral intravenous cannulation in adults: a prospective observational study. J Clin Nurs. 2017;26(7‐8):1074‐1084. [DOI] [PubMed] [Google Scholar]
- 18. Jacobson AF, Winslow EH. Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005;34(5):345‐59. [DOI] [PubMed] [Google Scholar]
- 19. Arreola S, et al. Characteristics of people who initiate injection drug use later in life. Drug Alcohol Depend. 2014;138:244‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scoppettuolo G, Pittiruti M, Pitoni S, et al. Ultrasound‐guided “short” midline catheters for difficult venous access in the emergency department: a retrospective analysis. Int J Emerg Med. 2016;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuper NJ, de Graaff JC, van Dijk AT, Verdaasdonk RM, van der Werff DB, Kalkman CJ. Predictive factors for difficult intravenous cannulation in pediatric patients at a tertiary pediatric hospital. Paediatr Anaesth. 2012;22(3):223‐229. [DOI] [PubMed] [Google Scholar]
- 22. Bahl A, Pandurangadu AV, Tucker J, Bagan M. A randomized controlled trial assessing the use of ultrasound for nurse performed IV placement in difficult access ED patients. Am J Emerg Med. 2016;34(10):1950‐1954. [DOI] [PubMed] [Google Scholar]
- 23. McCarthy ML, Shokoohi H, Boniface KS. Ultrasonography versus landmark for peripheral intravenous cannulation: a randomized controlled trial. Ann Emerg Med. 2016;68(1):10‐18. [DOI] [PubMed] [Google Scholar]
- 24. Schoenfeld E, Boniface K, Shokoohi H. ED technicians can successfully place ultrasound‐guided intravenous catheters in patients with poor vascular access. Am J Emerg Med. 2011;29(5):496‐501. [DOI] [PubMed] [Google Scholar]
- 25. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound‐guided peripheral intravenous access in the emergency department: patient‐centered survey. West J Emerg Med. 2011;12(4):475‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first‐attempt success in children with predicted difficult intravenous access in the emergency department. Ann Emerg Med. 2019;74(1):19‐27. [DOI] [PubMed] [Google Scholar]
- 27. Bauman M, Braude D, Crandall C. Ultrasound‐guidance vs. standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135‐140. [DOI] [PubMed] [Google Scholar]
- 28. Doniger SJ, Ishimine P, Fox JC, Kanegaye JT. Randomized controlled trial of ultrasound‐guided peripheral intravenous catheter placement versus traditional techniques in difficult access pediatric patients. Pediatr Emerg Care. 2009;25(3):154‐159. [DOI] [PubMed] [Google Scholar]
- 29. O'Neill MB, Dillaine M, Hanipah NF. Validating the difficult intravenous access clinical prediction rule. Pediatr Emerg Care. 2012;28(12):1314‐6. [DOI] [PubMed] [Google Scholar]
- 30. Van Loon FH, Pujin LA, Houterman S, Bouwman AR. Development of the A‐DIVA scale: a clinical predictive scale to identify difficult intravenous access in adult patients based on clinical observations. Medicine (Baltimore) 2016;95(16):e3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yen K, Riegert A, Gorelick MH. Derivation of the DIVA score: a clinical prediction rule for the identification of children with difficult intravenous access. Pediatr Emerg Care. 2008;24:143‐147 [DOI] [PubMed] [Google Scholar]
- 32. Carr PJ, Rippey JCR, Cooke ML, et al. Development of a clinical prediction rule to improve peripheral intravenous cannula first attempt success in the emergency department and reduce post insertion failure rates: the Vascular Access Decisions in the Emergency Room (VADER) study protocol. BMJ Open 2016;6:e009196. [DOI] [PMC free article] [PubMed] [Google Scholar]


