Abstract
Objective
To determine the correlation between 3 lightweight portable pulse oximeter devices compared to a standard wall mount pulse oximetry device.
Methods
We performed a single‐center, prospective, observational study of 4 pulse oximetry devices, 3 of which are commercially available to the public. A convenience sample of 200 emergency department (ED) patients with chief complaints of cardiopulmonary origin or a peripheral capillary oxygen saturation ≤ 94 percent were enrolled. Analysis of variance was performed to compare SpO2s and test characteristics of the 3 devices compared to control.
Results
Although differences in measured SpO2s were observed (P < 0.001) across groups, the differences were small (mean differences ranged from 1.00% to 1.87%). The correlation between test devices and the control were high (r range 0.70–0.79). Although the test characteristics were not perfect, the devices did have good sensitivity using a cutoff value of 94% (sensitivity ranging from 90% to 92%), which improved with lower SpO2 cutoff values to 92% (sensitivity ranging from 96% to 97%).
Conclusion
The 3 commercially available devices were accurate enough to be clinically useful when compared to a hospital bedside monitor pulse oximeter. Consumer‐grade portable pulse oximeters may be useful if overwhelming numbers of patients require oxygen saturation monitoring, such as during the COVID‐19 pandemic.
Keywords: COVID‐19/novel coronavirus, hypoxia, oxygen, oxygenation status, pulse oximetry, respiratory physiological phenomena
1. INTRODUCTION
1.1. Background
Pulse oximetry (pulse ox) is an expedient and accurate tool to measure noninvasively the oxygenation status of any patient in whom this might be a clinical concern. It was developed during World War II by Glenn Allan Millikan, an American physiologist and mountaineer. 1 The term “oximetry” is attributable to him. Pulse oximeters measure peripheral capillary oxygen saturation (SpO2) by measuring the difference in absorption of oxygenated versus deoxygenated blood at 2 different wavelengths (typically red light at 660 nm and infrared light at 940 nm). Oxygenated hemoglobin absorbs more infrared light, and deoxygenated hemoglobin absorbs more red light. This difference is measured by the diodes on the device and is used to calculate the SpO2. 2 This tool can be placed on multiple spots on the body to obtain noninvasively an accurate measure of blood oxygenation and easily detect hypoxemia. 3 , 4 , 5 , 6 A finger probe is commonly used for measurements, but multiple other sites can be used with varying accuracy. 7 , 8 The use of pulse oximetry has been shown to reduce the need for more invasive measurements, such as an arterial blood gas. 9 More recently, portable finger probes have been developed, and these allow oxygen saturation measurements to be obtained in a variety of environments and situations. 10 , 11 Small, portable pulse ox devices allow measurement of oxygen saturation in resource‐limited environments.
1.2. Importance
The consensus in the literature is that portable pulse oximeters are acceptable for use but may experience inaccuracies as a patient becomes increasingly hypoxemic. 12 , 13 , 14 , 15 , 16 Pulse oximetry has several limitations that can cause inaccuracies in measurement: light‐emitting diode (LED) integrity, motion, hypotension, methemoglobinemia, carboxyhemoglobiemia, and anemia. 17 , 18 , 19 A variety of portable pulse oximetry devices exist and range from inexpensive, commercially available to more expensive, health‐care‐oriented models (ranging from ∼$25 to over $300). These devices may also be useful in austere conditions or when the supply of medical‐grade pulse oximeters is exhausted. Determining the accuracy of affordable, consumer pulse oximetry devices could allow for a cost‐effective addition to the wilderness medicine field kit for both the professional and layperson. We found in our previous study that a variety of iPhone apps that purported to measure pulse oximetry were inaccurate and should not be used—even in austere conditions. 20 Thus a true pulse oximeter is preferred.
The Bottom Line
The accuracy of consumer and medical‐grade home pulse oximetry devices has not been well studied. This study of 200 hypoxic emergency department patients demonstrated that three home pulse oximeters were accurate when compared to a standard emergency department monitor.
1.3. Goals of this investigation
This study assessed the accuracy of 2 consumer grade and 1 medical‐grade portable pulse oximetry devices as compared to a standard‐of‐care, wall‐mounted pulse oximetry device. We postulated that the portable devices would be equivalent and reliably measure oxygen saturation. We also postulated that these devices would provide accurate measurements in patients with some degree of hypoxia (ie, SpO2 ≤ 94%).
2. METHODS
2.1. Study design and setting
We performed a single‐center, open‐label, prospective observational study comparing the results of bedside pulse‐oximetry across 4 devices using a convenience sample. Two consumer‐grade and 1 medical‐grade portable pulse oximetry devices were compared to a standard emergency department non‐portable bedside unit (Figure 1). The 2 consumer‐grade models were Santa Medical SM‐165 (SM) and Walgreen's OxyWatch C20 (OxyWatch). The medical‐grade unit was a Nonin Onyx II 9550 (Onyx). The wall‐mounted hospital control unit used was an ED TRAM 451 pulse oximeter (General Electrics, New York City, NY). All portable units used in testing were obtained by University of Alabama at Birmingham (UAB) emergency and wilderness medicine faculty.
FIGURE 1.

The 3 portable pulse oximeters used in this study. From left to right: Santa Medical SM‐165 (SM), Walgreens’ OxyWatch C20 (OxyWatch), and Nonin Onyx II 9550 (Onyx)
2.2. Selection of participants
The study population was a convenience sample of 200 adults presenting to the UAB ED with either hypoxia (SpO2 ≤ 94%), an acute exacerbation of chronic obstructive pulmonary disease or a chief complaint of chest pain or dyspnea. Screening was performed via the ED electronic medical record. Exclusion criteria was the presence of peripheral artery disease, anemia, or inability to give consent. No compensation was offered. Authors obtained informed consent and performed enrollment. The authors recording and performing the documentation were not blinded to the devices being used. Data were collected from July 2018 to January 2019.
2.3. Measurements
We recorded the demographic data, SpO2 measurements, the use of supplemental O2, and the reason for inclusion for each participant. Then SpO2 measurements were taken consecutively from the same finger using the 3 experimental pulse oximetry devices adjacent to the control unit. The control measurement was obtained with the bedside TRAM 451 pulse oximeter. The 3 portable device measurements (ie, Onyx, OxyWatch, and SM) (Figure 1) were obtained sequentially in a predetermined random order without delay between readings. A single oximeter reading was recorded as soon as each device was applied and showed a good waveform.
2.4. Statistical analysis
Descriptive statistics were performed. Counts with percentages, means with SDs and medians with interquartile ranges were calculated and presented, as appropriate. Differences between control and test groups SpO2 were calculated and presented with 95% confidence intervals (CIs). Mean SpO2s were compared across groups using an analysis of variance test. Bland‐Altman plots were created to evaluate agreement between the 3 tested devices and control. Pearson's correlation was performed between each test group and the control. Subgroup analysis of hypoxic patients was performed and planned a priori. A cutoff value of ≤ 94% was chosen to define hypoxia a priori. Sensitivities, specificities, positive predictive values, and negative predictive values were calculated. Sensitivity analysis using an SpO2 of 92% as a cutoff value to hypoxia was performed post hoc and is provided as a supplemental table. McNemar's test was performed to evaluate the diagnostic characteristics for sensitivity of hypoxia. The sample size was chosen based on a convenience sample of patients and authors' availability to perform enrollment. All analysis was performed using SAS software, version 9.4 (SAS Institute). This study was approved by the UAB Institutional Review Board.
3. RESULTS
A total of 200 patients were evaluated. The characteristics of the enrolled patients are shown in Table 1. The group was evenly distributed between white and African American patients and sex. The majority were not receiving supplemental O2 at enrollment. The distribution of patient complaints used to include patients is also shown. Note that the total is >100% because many patients had more than one complaint, for example, chest pain and shortness of breath. The distribution of SpO2 is depicted in Figure 2. The mean SpO2 varied across devices, both in the entire population as well as those determined to be hypoxic based on a control SpO2 value of <94%, is shown in Tables 2 and 3. Correlation between test groups and control SpO2 was high (Table 2). The degree of correlation is also illustrated for all the devices by the Bland‐Altman plots (Figures 3–5).
TABLE 1.
Baseline population characteristics (N = 200)
| Characteristic | Median (IQR; Range) or N (%) |
|---|---|
| Age in Years | |
| Median (IQR; Range) | 58 (51–69; 21–97) |
| Race N (%) | |
| White | 96 (48.5%) |
| African American | 101 (50.5%) |
| Other | 2 (1%) |
| Missing | 1 (0.5%) |
| Sex N (%) | |
| Male | 108 (54%) |
| Female | 92 (46%) |
| Receiving supplemental oxygen N (%) | |
| Yes | 68 (34%) |
| No | 129 (64.5%) |
| Missing | 3 (1.5%) |
| Receiving CPAP N (%) | |
| Yes | 4 (2%) |
| No | 196 (98%) |
| Ventilator N (%) | |
| Yes | 0 (0%) |
| No | 200 (100%) |
| Oxygen flow rate (LPM) | |
| Median (IQR) | 0 (0–2.25) |
| Oxygen saturation below 94% N (%) | |
| Yes | 60 (30%) |
| No | 140 (70%) |
| Inclusion criteria | |
| Shortness of breath | 131(66%) |
| Chest pain | 78 (39%) |
| Exac COPD | 27(14%) |
| SpO2 ≤ 94% | 17(9%) |
CPAP, continuous positive airway pressure; Exac COPD, exacerbated chronic obstructive pulmonary disease; IQR, interquartile range.
FIGURE 2.
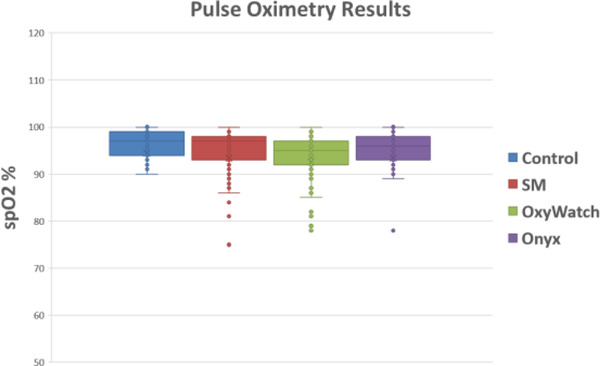
SpO2 measurements obtained by each respective device are shown here. Each data point on each device represents the oxygen reading for one patient. The oxygen percentage data shown includes outliers as well as mean lines and markers. SM, Santa Medical SM‐165; OxyWatch, Walgreens’ OxyWatch C20; Onxy, Nonin Onyx II 9550
TABLE 2.
Distribution of oxygen saturation and measured difference by instrument
| Measurement Instrument | ||||
|---|---|---|---|---|
| Measure | Control | OxyWatch | SM | Onyx |
| Total patients (N) | 200 | 198 | 200 | 200 |
| Oxygen saturation (%) | ||||
| Mean (SD)* | 96.08% (3.84) | 94.23% (4.70) | 95.02% (4.49) | 95.08% (3.90) |
| Min, Max SpO2 | 59%, 100% | 59%, 100% | 62%, 100% | 68%, 100% |
| Pearson Correlation Coefficient (P value) | Ref | 0.79 (< 0.001) | 0.76 (< 0.001) | 0.79 (0.001) |
| Difference in SpO2 (Control‐Measured; %) | ||||
| Mean (95% CI) | Ref | 1.87% (1.46–2.28) | 1.07% (0.65–1.48) | 1.00% (0.65–1.35) |
| Min, Max | Ref | −5%, 19% | −6%, 23% | −9%, 15% |
CI, confidence interval; SM, Santa Medical.
*Analysis of variance: P = 0 < 0.001; F value = 15.85.
TABLE 3.
Distribution of oxygen saturation and measured differences by instrument in patients with control SpO2 ≤ 94% (N = 60)
| Measurement Instrument | ||||
|---|---|---|---|---|
| Measure | Control | OxyWatch | SM | Onyx |
| Measured SpO2 | ||||
| Mean (SD) a | 92.15% (4.57) | 90.25% (5.76) | 91.37% (5.10) | 91.60% (4.68) |
| Min, Max SpO2 | 59%, 94% | 59%, 99% | 62%, 98% | 68%, 100% |
| Pearson's Correlation Coefficient (P value) | Ref | 0.75 (0.001) | 0.79 (0.001) | 0.70 (0.001) |
| Difference in measured SpO2 (Control‐Measured) | ||||
| Mean (95% CI) | Ref | 1.95% (0.96–2.94) | 0.78% (−0.04–1.60) | 0.55% (−0.37–1.47) |
| Min, Max | Ref | −5%, 13% | −6%, 10% | −9%, 15% |
CI, confidence interval; SM, Santa Medical.
Analysis of variance: P < 0.0001, F value = 14.49.
FIGURE 3.
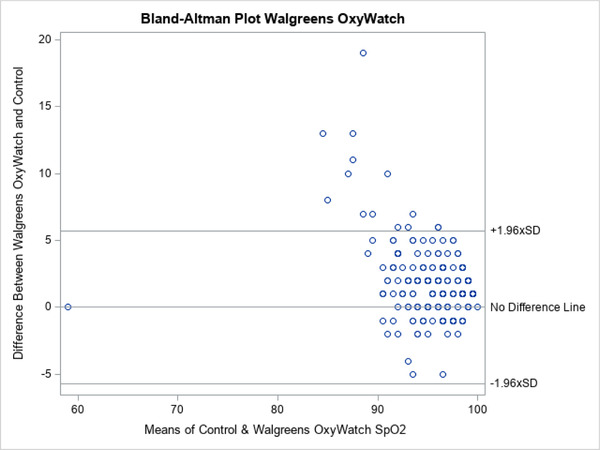
Bland‐Altman plots for Walgreens’ OxyWatch C20
FIGURE 5.
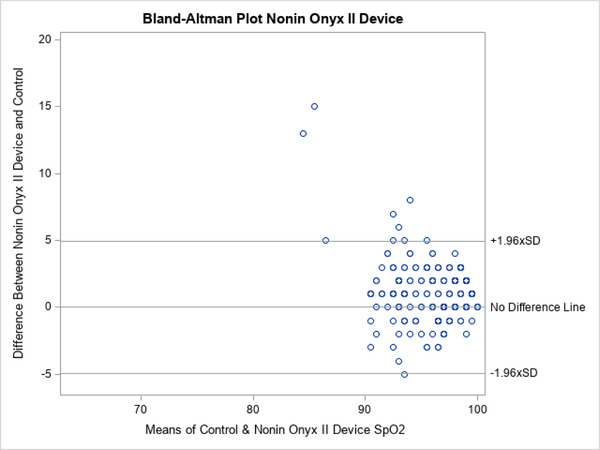
Bland‐Altman plots for Nonin Onyx II 9550
FIGURE 4.
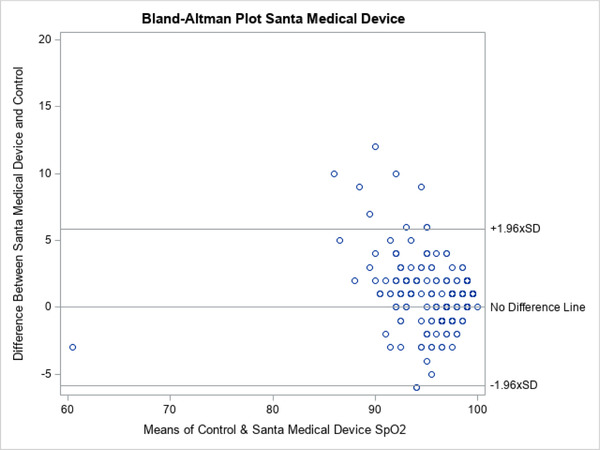
Bland‐Altman plots for Santa Medical SM‐165
The more expensive, medical‐grade Onyx device showed a narrower distribution of SpO2 readings that more closely mirrored the control values (Figure 2). The Bland‐Altman plots also show this visually with fewer outliers in SpO2 readings for Onyx (Figures 3–5). The mean difference between the device readings and controls for the entire population was narrow–between 1% and 1.87% for all 3 devices (Table 2). Note that there were 2 missing measurements in the OxyWatch group (N = 198). There were several outliers on individual patients, with SpO2 readings varying from control as much as 23%. In the subgroup of patients with hypoxia (SpO2 ≤94%), the distribution of measurements is also narrow. The mean difference from control readings were 1.95% for OxyWatch, 0.78% for SM, and 0.55% for the Onxy device (Table 3). Although, there were statistical differences across groups, the clinical difference was less than 2% SpO2.
To evaluate the ability of each device to detect hypoxia, we classified the test results as either no hypoxia or hypoxia (SpO2 ≤94). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of each device are shown in Table 4. All 3 devices had sensitivities over 90%. The negative predictive value of all 3 devices was ≥80%. It should be noted that there were often small ranges of device readings that affected this grouping. That is, a control reading of 94% (defined as hypoxia) and a device reading of 95% (defined as no hypoxia) would be considered a false negative. We also performed a sensitivity analysis evaluating 92% as a cutoff SpO2 for hypoxia (Table S1). Although agreement was lower in all 3 test groups with the lower cutoff values, sensitivity for hypoxia improved to 96%–97%.
TABLE 4.
Comparison of test devices to standard bedside oximeter for detecting hypoxia (SpO2 ≤94%)
| Measurement Instrument | |||
|---|---|---|---|
| OxyWatch | SM | Onyx | |
| Hypoxia (≤94%) | |||
| Sensitivity (95% CI) | 92.17% (87.27–97.08) | 90.70% (85.69–95.71) | 92.06% (87.34–96.78) |
| Specificity (95% CI) | 60.00% (49.59–70.41) | 67.61% (56.72–78.49) | 67.57% (56.90–78.23) |
| Positive predictive value (95% CI) | 75.71% (68.61–82.82) | 83.57% (77.43–89.71) | 82.86% (76.61–89.10) |
| Negative predictive value (95% CI) | 85.00% (75.97–94.03) | 80.00% (69.88–90.12) | 83.33% (73.90–92.76) |
| McNemar's Test (Sensitivity)‐P (Chi2‐DOF) | 0.001 (14.53–1) | 0.063 (3.46–1) | 0.0165 (5.76–1) |
Chi2, chi square; CI, confidence interval; DOF, degree of freedom; SM, Santa Medical.
Lastly, we calculated correlation coefficients for all 3 devices. These r values showed strong correlation with control readings. The data are shown in Tables 3–4. The correlation of the medical‐grade Onyx was the highest at 0.79, but it did not statistically differ from the other 2 devices. In the subgroup of patients with hypoxia, the SM device had the highest correlation coefficient at 0.789. The ranges were from 0.756–0.790 for all patient readings and 0.703–0.789 for the subgroup of patients defined as hypoxic.
4. LIMITATIONS
Although the devices were tested on 200 patients, our sample was one of convenience. These were not sequential patients. We performed the study when the authors were available to spend time in the ED. The choice of a cutoff value of 94% for SpO2 is somewhat arbitrary, though consistent with our previous study 20 and the usual definition of normal SpO2 being between 95% and 100%. 21 We also calculated sensitivity analysis using a cutoff of 92% and provided this as a supplemental table. This post hoc analysis may better reflect the performance of these devices for home monitoring of coronavirus disease 2019 (COVID‐19) patients where a cutoff of 92% has been used. 22 The choice to allow our enrolling authors to screen patients with lower oxygenation was done to include a larger subset of these patients in order to test the device characteristics in this subset of patients. Hence, this was not an outcome variable but simply a cutoff level to allow subset analysis. Based on the limited focus of our data acquisition, it is difficult to determine the ultimate external validity of the study. There could have been moment‐to‐moment differences in pulse ox readings as the devices were placed sequentially on the patient's finger. To minimize this effect, we randomized the sequence of the device measurements. Nonetheless, an active treatment that was being instituted by the medical team caring for the patient might have improved the patient's saturation over the minute or 2 that it took to take 3 sequential measurements. We purposely included a significant subset of hypoxic patients, but these readings were taken under a controlled environment and by a trained observer, not under austere conditions or by a lay person. It is also possible that the utility of this device in austere conditions may be limited based on external factors (eg, excessive heat or cold). We did not explicitly exclude patients with hypotension, although patients who were critically ill did not represent a large subset of patients because they are often unable to provide consent, and screening/enrolling may have interfered with clinical care. Finally, this study was not blinded. The authors recording the data applied the devices in random order to the patient's finger and the data were collected by 2 separate authors (BM, JG). This minimizes but does not exclude observer bias.
5. DISCUSSION
Portable pulse oximeters bridge the gap between the hospital and out‐of‐hospital environment by providing a flexible and lightweight tool to both patient and health care practitioners. The oximeters tested provided an evaluation at 2 tiers of price. The SM and OxyWatch were the more affordable and commercially available devices. The medical‐grade Onyx device was the most expensive. Our study shows that although there are slight differences among the 3 devices tested, all performed relatively well compared to control in both patients with normal oxygen saturations and those with lower saturations. One third of our patient population had saturations at or below 94%. Our entire patient population were presenting to an ED with complaints that would make oxygen saturation an important vital sign. Unlike prior studies that relied on healthy volunteers, we analyzed these devices in a more clinically relevant population: ED patients at risk for hypoxia.
All three devices showed a narrow mean difference from control readings and had high sensitivity, for detecting hypoxia (SpO2 ≤ 94%). Caution must be used as based on the sensitivity alone as many as 10% (or 15% if the lowest 95% CI is used) of patients with hypoxia might be missed. However, using a lower and more likely clinically relevant cut‐off of 92% saturation, the sensitivity of all 3 devices was even higher—all 3 essentially 97% (99.6‐94.2). This is reassuring because these portable devices would likely be used as screening tools in the field where supplies may be more limited or as we postulate a tool to detect hypoxia in patients with possible COVID‐19.
We show that the 3 portable oximeters tested demonstrated a strong correlation to control for SpO2 readings.
All 3 devices had rare outliers of mean differences from control being >5%. These occurred less often with the Onyx device and had the lowest mean difference from control of 1%. However, whether this possible advantage outweighs its almost 10‐fold cost difference is questionable.
We undertook this study after our previous work showed that iPhone applications proved to be inaccurate. 20 We were looking for tools for the clinician that were small, portable, and accurate and could be easily packed. An accurate device to measure oxygen saturation could be helpful as a fifth vital sign in an austere environment to assess a patient or victim having problems with respiration because of trauma, infection, or altitude.
We did not envision our results having applicability to a pandemic of a novel coronavirus (severe acute respiratory syndrome coronavirus 2) that has infected more than 30 million persons and killed nearly 1 million people worldwide as of this writing. 23 The rapidity of spread of the virus and its lethality have overwhelmed healthcare systems worldwide. 24 , 25 , 26 , 27 This has resulted in shortages of medical materials like ventilators for patients in respiratory failure 28 and personal protective equipment (masks, gloves, gowns) to protect health care practitioners. 29 In its more severe form, the disease affects the lungs by causing viral pneumonia, hypoxia, respiratory failure, and death. The ability to measure oxygen saturation in hundreds or thousands of patients is an important determinant to guide such clinical decisions as the need for supplemental oxygen and, ultimately, mechanical ventilation. 30
Hospital systems have been overrun, and many areas have been or are expected to have more seriously ill patients than they can care for under usual practice. Many health systems have used atypical locations to care for these patients. China built several new hospital facilities within days. 31 Hospitals across the United States have erected temporary spaces in ball fields or have used other spaces, like hotels, to care for the surge of patients. 30 The ability to monitor patients in makeshift facilities is and will be important. Pulse oximetry plays a key role. Some patients have been given a small, portable pulse oximeter, like the ones we studied, to take home to monitor themselves so that they know when to seek a higher level of care. 22 , 33 , 34
Although we did not study the entire spectrum of portable pulse oximeters, the devices selected were sensitive in detecting hypoxia using a 92% cutoff and had a strong correlation with standard ED oximetry in an ED population at risk for hypoxia. Further, the less‐expensive consumer grade models compare favorably to a costlier, medical‐grade device. This makes the inclusion of a pulse ox at home, in a medical bag, or a non‐traditional health care location, doable for most people.
Overall, there were statistically significant differences between the 3 devices tested; however, the differences were minor and unlikely to be clinically significant. All had sufficient degrees of correlation with the control device, >90% sensitivity, and greater than 80% NPV for hypoxia. Although these devices were not tested in an austere environment or during a global pandemic, we expect that they would perform well under such conditions. Our study provides evidence that these devices can accurately detect hypoxia and may be a useful tool for health care practitioners or patients during this pandemic. Futures studies should analyze whether these findings are applicable to a wider range of ED patients with features not present in our population (hypotension, mechanical ventilation, anemia). Further study in specific environments, such as extreme cold or altitude, might be needed to further elucidate their utility in austere environments. A study of these consumer grade devices in patients suffering with COVID‐19 infection would also be useful.
CONFLICTS OF INTEREST
None
AUTHOR CONTRIBUTIONS
Each author takes public responsibility for appropriate portions of the content and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.All authors made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data.All authors were involved in drafting the manuscript or revising it critically for important intellectual content. WS gave final approval of the version to be published.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Jill W. Roberts, M.S., for editing assistance in the preparation of this manuscript.
Biography
Walter A. Schrading, MD, is the Director of the Office of Wilderness Medicine and is supervising the Wilderness Medicine Track within the EM residency program at the University of Alabama at Birmingham.

Schrading WA, McCafferty B, Grove J, Page DB. Portable, consumer‐grade pulse oximeters are accurate for home and medical use: Implications for use in the COVID‐19 pandemic and other resource‐limited environments. JACEP Open. 2020;1:1450–1458. 10.1002/emp2.12292
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Faheem W. Guirgis, MD
REFERENCES
- 1. Severinghaus JW, Astrup PB. History of blood gas analysis. VI. Oximetry. J Clin Monitor Comput. 1986;2:270. [DOI] [PubMed] [Google Scholar]
- 2. Pinsky MR. Assessing the Circulation: Oximetry, Indicator Dilution, and Pulse Contour Analysis In: Hall JB, Schmidt GA, Kress JP. eds. Principles of Critical Care, 4e NY: McGaw‐Hill, 2014. [Google Scholar]
- 3. Sohila S, Alireza K, Gholamreza M, et al. Accuracy of pulse oximetry in detection of oxygen saturation in patients admitted to the intensive care unit of heart surgery: comparison of finger, toe, forehead, and earlobe probes. BMC Nurs. 2018;17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilin N, Behbahan AG, Abdinia B, et al. Validity of pulse oximetry in detention of hypoxemia in children: comparison of ear, thumb, and toe probe placements. East Mediterr Health J. 2010;16(2):218‐222. [PubMed] [Google Scholar]
- 5. Sinex JE. Pulse oximetry: principles and limitations. Am J Emerg Med. 1999;17(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 6. Wilson BJ, Cowan HJ, Lord JA, et al. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective cohort study. BMC Emerg Med. 2010;10(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blaylock V, Brinkman M, Carver S, et al. Comparison of finger and forehead oximetry sensor in post anesthesia care patients. J Perianesth Nurs. 2008;23(6):379‐386. [DOI] [PubMed] [Google Scholar]
- 8. Berkenbosch JW, Tobias JD. Comparison of new forehead reflectance pulse oximeter with a conventional digit sensor in pediatric patients. Respir Care. 2006;51(7):726‐731. [PubMed] [Google Scholar]
- 9. Durbin CG Jr, Rostow SK. More reliable pulse oximetry reduces the frequency of arterial blood gas analyses and hastens oxygen weaning after cardiac surgery, a prospective randomized trial of the clinical impact of new technology. Crit Care Med. 2002;30(8):1735‐1740. [DOI] [PubMed] [Google Scholar]
- 10. Pupim D, Iwaki filho L, Takeshita WM, et al. Evaluation of accuracy of portable fingertip pulse oximeter, as compared to that of a hospital oximeter with digital sensor. Indian J Dent Res. 2013;24(5):542‐546. [DOI] [PubMed] [Google Scholar]
- 11. Costa JC, Faustino P, Lima R, et al. Research: comparison of the accuracy of a pocket versus standard pulse oximeter. Biomed Instrum Tech. 2016;50(3):190‐193. [DOI] [PubMed] [Google Scholar]
- 12. Lipnick MS, Feiner JR, Au P, et al. The accuracy of 6 inexpensive pulse oximeters not cleared by the food and drug administration: the possible global public health implications. Anesth Analg. 2016;123(2):338‐345. [DOI] [PubMed] [Google Scholar]
- 13. Louie A, Feiner JR, Bickler PE, et al. Four types of pulse oximeters accurately detect hypoxia during low perfusion and motion. Anesth. 2018;128(3):520‐530. [DOI] [PubMed] [Google Scholar]
- 14. Smith RN, Hofmeyr R. Perioperative comparison of the agreement between a portable fingertip pulse oximeter v. a conventional bedside pulse oximeter in adult patients (COMFORT trial). S Afr Med J. 2019;109(3):154‐158. [DOI] [PubMed] [Google Scholar]
- 15. Ross EM, Matteucci MJ, Shepherd M, et al. Measuring arterial oxygenation in a high altitude field environment: comparing portable pulse oximetry with blood gas analysis. Wilderness Environ Med. 2013;24(2):112‐117. [DOI] [PubMed] [Google Scholar]
- 16. Mandolesi G, Avancini G, Bartesaghi M, et al. Long‐term monitoring of oxygen saturation at altitude can be useful in predicting the subsequent development of moderate‐to‐severe acute mountain sickness. Wilderness Environ Med. 2014;25(4):384‐391. [DOI] [PubMed] [Google Scholar]
- 17. Sinex JE. Pulse oximetry: principles and limitations. Am J Emergency Med. 1999;17(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 18. Milner QJ, Mathews GR. An assessment of the accuracy of pulse oximeters. Anaesthesia. 2012;67(4):396‐401. [DOI] [PubMed] [Google Scholar]
- 19. Louie A, Feiner JR, Bickler PE, et al. Four types of pulse oximeters accurately detect hypoxia during low perfusion and motion. Anesthesiology. 2018;128(3):520‐530. [DOI] [PubMed] [Google Scholar]
- 20. Jordan T, Meyers C, Schrading W, et al. The utility of iphone oximetry apps: a comparison with standard pulse oximetry measurement in the emergency department. Am J Emerg Med. 2019;38(5):925‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mecham C, Pulse Oximetry Now. UptoDate. https://www.uptodate.com/contents/pulse-oximetry?search=pulse%20oximetry&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 Accessed Aug 19, 2020
- 22. Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID‐19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med. 2020. https://onlinelibrary.wiley.com/doi/full/10.1111/acem.14053. Accessed Aug 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. COVID‐19 Dashboard. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed Aug 21, 2020.
- 24. Mareiniss DP. The impending storm: cOVID‐19, pandemics and our overwhelmed emergency departments. Am J Emerg Med. 2020;38(6):1293‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID‐19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 26. Doce N, Allen N. They just sedate them; coronavirus overwhelms Spain's care homes. Reuters. https://www.reuters.com/article/us-health-coronavirus-spain-homes/they-just-sedate-them-coronavirus-overwhelms-spains-care-homes-idUSKBN21L2TP. Accessed April 12, 2020.
- 27. Feuer A. Coronavirus in N.Y.: Toll Soars to Nearly 3,000 as State Pleads for Aid. The New York Times. 2020. https://www.nytimes.com/2020/04/03/nyregion/coronavirus-new-york-death-toll.html. Published April 3, 2020. Accessed April 12, 2020. [Google Scholar]
- 28. Cha AE, McGinley L. Who gets a shot at life if hospitals run short of ventilators? Washington Post. 2020. https://www.washingtonpost.com/health/2020/04/07/ventilators-rationing-coronavirus-hospitals/. Published April 7, 2020. Accessed April 12, 2020. [Google Scholar]
- 29. Jacobs A, Richtel M, Baker M, ‘At war with no ammo’: doctors say shortage of protective gear is dire. The New York Times. https://www.nytimes.com/2020/03/19/health/coronavirus-masks-shortage.html. Published March 19, 2020. Accessed April 12, 2020. [Google Scholar]
- 30. Jessica Mason, Mel Herbert, Novel Coronavirus 2019 (COVID‐19). In: Mattu A. and Swadron S, ed. Burbank, CA: CorePendium, LLC; 2020. https://www.emrap.org/corependium/chapter/rec906m1mD6SRH9np/Novel-Coronavirus-2019-COVID-19?SearchType=%22text%22. Accessed April 12, 2020. [Google Scholar]
- 31. Butler K. China constructed new hospitals in days, and other lessons from their response to the coronavirus. Mother Jones. https://www.motherjones.com/politics/2020/03/who-report-china-constructed-new-hospitals-in-days-and-other-lessons-from-their-response-to-the-coronavirus/. Accessed April 12, 2020. [Google Scholar]
- 32. Rowland C, Cha AE. Surge in coronavirus patients threatens to swamp U.S. hospitals. Washington Post. https://www.washingtonpost.com/business/2020/03/14/hospital-doctors-patients-coronavirus/. Accessed April 12, 2020. [Google Scholar]
- 33. Care model for COVID‐19 patients isolated at home foreshadows design for future health care delivery | Coronavirus (COVID‐19) Information for Employees and Patients. https://www.vumc.org/coronavirus/latest-news/care-model-covid-19-patients-isolated-home-foreshadows-design-future-health-care. Accessed April 12, 2020.
- 34. News ABC . Should pulse oximeters be used at home to track coronavirus symptoms? ABCNews. https://abcnews.go.com/Health/pulse-oximeters-home-track-coronavirus-symptoms/story?id=69939772. Accessed April 12, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


