Abstract
Objectives
Assess the impact of an electronic health record (EHR)‐embedded clinical pathway (ePATH) as compared to a paper‐based clinical decision support tool on outcomes for patients presenting to the emergency department (ED) with suspected acute coronary syndrome (ACS).
Methods
A retrospective, quasi‐experimental study using difference‐in‐differences and interrupted time series specifications to evaluate the impact of an EHR‐embedded clinical pathway between April 2013 and July 2017. The intervention was implemented in February 2016 at a large academic tertiary hospital and compared to a local community hospital without the intervention. Eligible patients included adults (>18 years) presenting to the ED with chest pain who had a troponin ordered within 2 hours of arrival and a chest pain‐related diagnosis. Patients with initial evidence of acute myocardial infarction were excluded. Primary outcomes included rates of admission and stress testing, hospital length of stay, and occurrence of major adverse cardiac events.
Results
On average, there were 170 chest pain visits per month at the intervention site. The frequency of hospital admission (unadjusted 28.2% to 20.9%, P < 0.001) and stress testing (unadjusted 15.8% to 12.7%, P < 0.001) significantly declined after ePATH implementation. After comparison with the comparator site, ePATH was still associated with a significant reduction in hospital admissions (‐10.79%, P < 0.001) and stress testing (‐6.05%, P < 0.001). Hospital length of stay and rates of major adverse cardiac events did not significantly change.
Conclusions
Implementation of ePATH for patients presenting to the ED with chest pain was associated with safe reductions in hospital admission and stress testing. ePATH appears to be an effective tool for implementing evidence‐based guidelines for ED patients with chest pain.
Keywords: acute coronary syndrome, chest pain, clinical, critical pathways, decision support systems, electronic health record, exercise test, myocardial infarction
1. INTRODUCTION
1.1. Background
Chest pain is a common ED chief complaint that is a leading cause for ED visits in the United States, with over 7 million visits per year and an annual economic burden of $10‐15 billion. 1 , 2 Only a small percentage of patients with chest pain are ultimately diagnosed with acute coronary syndrome (ACS) but significant resource utilization occurs to identify patients with this high consequence diagnosis.
A number of recent evidence‐based guidelines have been introduced to help improve risk stratification and accelerate diagnosis for patients with suspected ACS. 3 In both prospective and retrospective studies, these have demonstrated safe reductions in resource utilization (ie, reductions in admissions and cardiac testing without increasing morbidity or mortality). However, the prospective studies of chest pain‐related evidence‐based guidelines have involved implementation strategies that are unrealistic outside the typical ED workflow (eg, research staff prompting physicians with written evidence‐based guidelines when eligible patients presented to the ED). 4 Similarly, retrospective studies have not used computerized clinical decision support systems 4 , 5 or integration of evidence‐based decision logic into the electronic health record (EHR). 6 Thus, while the decision logic in these evidence‐based guidelines has been validated, 3 the optimal integration of guidelines into the electronic medical record warrants further study, particularly as it relates to guideline implementation and clinical outcomes. 7
Computerized clinical decision support systems have been promoted as an effective mechanism for improving adherence to evidence‐based guidelines, closing the evidence‐to‐practice gap. To that end, the Department of Health and Human Services has required integration of clinical decision support tools into EHRs in the Promoting Interoperability performance category within the Centers for Medicare & Medicaid Services's Quality Payment Program. 8 Yet, studies of these systems have yielded mixed results and adherence remains low. 9
1.2. Importance
Addressing this knowledge gap is particularly important for complex care environments such as the ED, where the frequency of high consequence, high cost decisions make clinical decision support systems critical to patient safety and quality.
1.3. Goals of this investigation
We conducted this study to determine the incremental impact on process and clinical outcomes of a workflow‐embedded clinical decision support system (ePATH) as compared to a paper‐based decision support tool for patients presenting to the ED with chest pain. We hypothesized that ePATH would be associated with reductions in length of stay and resource utilization, without affecting rates of major adverse cardiac events.
2. MATERIALS AND METHODS
2.1. Setting
The implementation of ePATH occurred in a large, urban academic medical center (intervention site) with ≈ 101,000 ED visits per year. The facility is staffed by board‐certified emergency physicians, residents in their post graduate training years (1‐4), and advanced practice providers (certified physician assistants or nurse practitioners). The site had a ED‐based clinical decision unit (CDU), for observation and care of short‐stay patients with specific diagnoses and/or treatment plans (eg, chest pain). The CDU is staffed by advanced practice providers with attending physician oversight. The external comparator site was a large urban community hospital with ≈102,000 ED visits per year. It is located in a similarly sized city in the same state, and is also within the same health system. Advance practice providers work at the community hospital but there is no ED CDU. Both sites offer percutaneous coronary intervention and cardiac surgery. Both also use the same commercially available EHR platform (Epic, Madison, WI).
2.2. Selection of participants
The study population was retrospectively identified and intended to include patients who would be appropriate for risk stratification using the chest pain pathway. We included patients ≥ 18 years old with a chief complaint of chest pain, an ED or hospital diagnosis within Clinical Classification System groupers 100–102 (see Table 4), and a troponin ordered within 2 hours of ED arrival (see Figure 1). Patient encounters were excluded if they had an ED diagnosis of ST‐segment elevation myocardial infarction (STEMI), an initial troponin value ≥ 0.5 ng/mL (normal range 0 to 0.499 ng/mL), or ED evaluation unrelated to cardiac chest pain as evidenced by advanced imaging tests or ED diagnosis (see Figure 1 and Table 5). For example, a patient with a chief complaint of chest pain who received a cardiac evaluation, but was ultimately diagnosed with cholecystitis after abdominal imaging, would be excluded. We also excluded patients with missing disposition data, missing level of care data, and those transferred to another acute care hospital or psychiatric facility. This study was approved by the Colorado Multiple Institutional Review Board.
FIGURE 4.
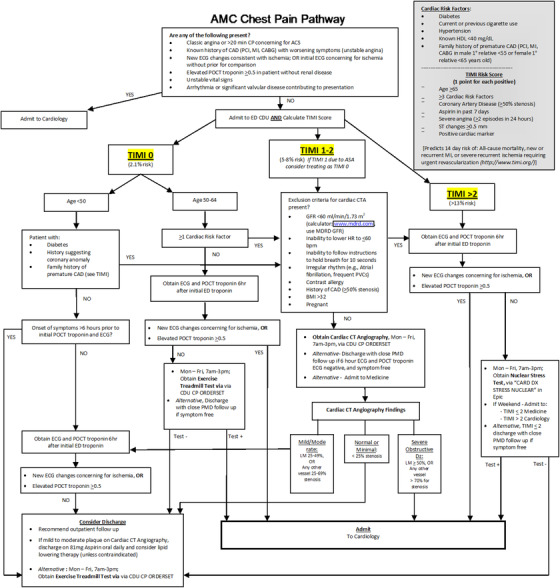
AMC (Intervention Site) Chest Pain Pathway Paper Tool
FIGURE 1.
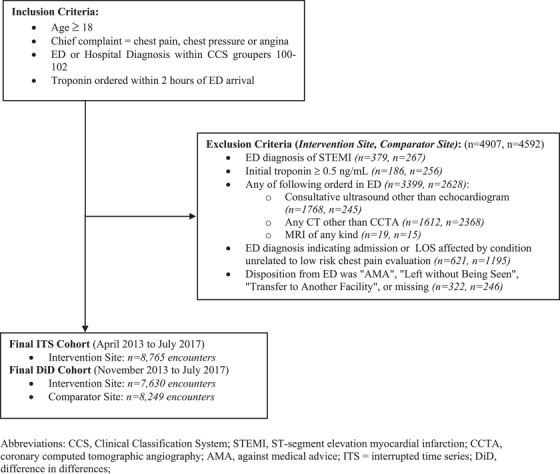
Enrollment flow diagram showing eligible encounters and manuscript cohort.AMA, against medical advice; CCS, Clinical Classification System; CCTA, coronary computed tomographic angiography; DiD, difference in differences; ITS, interrupted time series; STEMI, ST‐segment elevation myocardial infarction
2.3. Clinical pathway
The paper‐based clinical decision support tool at the intervention site was called the “Academic Medical Center Chest Pain Pathway” (AMC CPP; see Figure 4). It outlined the necessary steps and workflow in the evaluation of patients with suspected ACS including recommendations for immediate admission of patients with STEMI or non‐STEMI, serial biomarker testing, stress testing, and/or admission based on risk stratification. Content and decision logic for the AMC CPP were created using best available evidence 10 , 11 and, when not available, society consensus statements. It was then approved by a multidisciplinary and intraprofessional review committee that included representatives from cardiology, emergency medicine, and radiology. The AMC CPP was made available as a paper‐based clinical decision support tool starting in April 2013. It was presented at faculty and staff meetings in April 2013, then placed in all ED care areas in paper binders as well as digitally on a departmental server, accessible via hyperlink from a workstation desktop.
The Bottom Line
The potential benefit of computerized clinical decision support tools is huge, but there is still a lot of uncertainty about how to optimally design and implement these tools within an electronic medical record. This is especially true for fast‐paced, highly variable environments like the emergency department. We report here that embedding a computerized clinical decision support tool (ePATH) into normal medical record workflows was associated with reduced admissions (10.79%) and stress testing (6.05%) for patients presenting to the emergency department with chest pain. This occurred without negatively affecting the rate of major adverse cardiac events.
2.4. Intervention
On February 17th, 2016, the intervention site embedded their evidence‐based paper guideline, using a third‐party vendor (AgileMD, San Fransisco, CA), into the EHR (Figure 3) without altering its content or decision logic. We refer to this electronic implementation as ePATH. Hence the intervention, ePATH, occurred in February 2016. In contrast to the intervention site, the comparator site did not implement or embed any clinical decision support tools or guidelines for the care of patients with suspected ACS during the study period (ie, patients received usual care).
FIGURE 3.
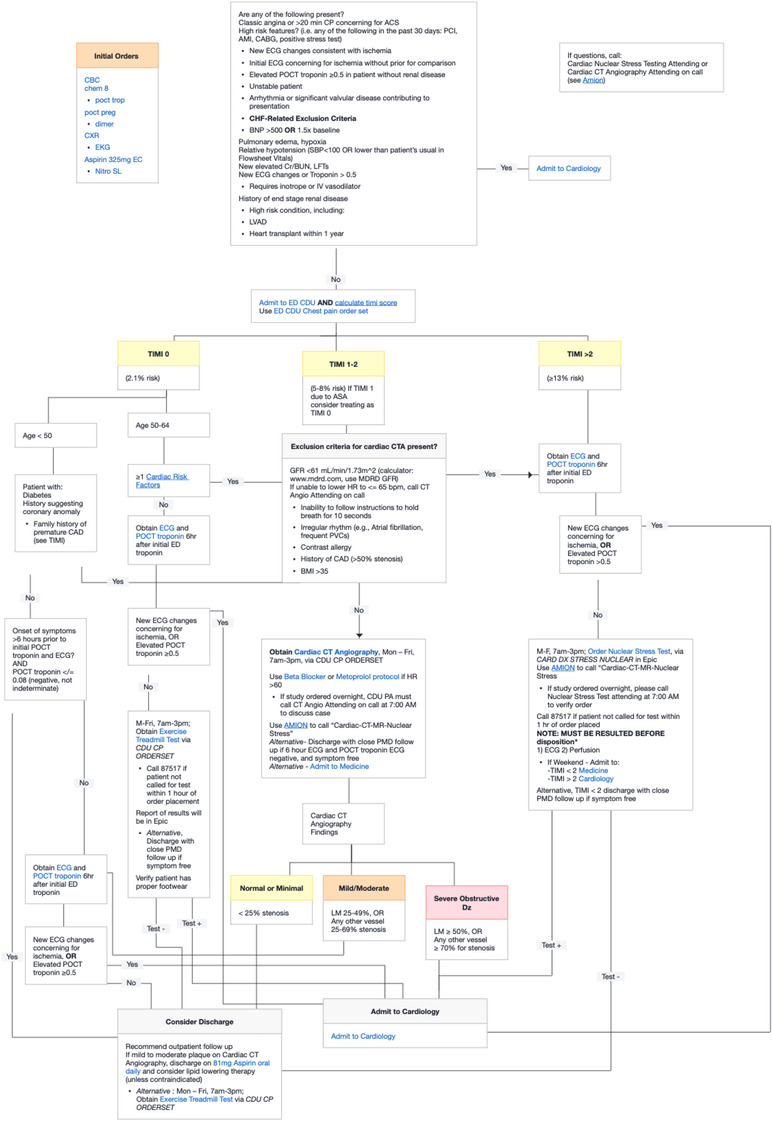
Example of chest pain pathway (ePATH) as shown in the electronic health record. ePATH, electronic health record embedded clinical pathway
The EHR integration process involved creating an activity button within the EHR to launch the clinical pathway from within a patient chart. Clicking on the activity button by the end‐user would open the clinical pathway within the same window as the patient chart. In addition to showing the decision logic, the clinician could also place orders from the pathway. An information technology analyst/local administrator created links to specific orders or order sets within the EHR. This would allow the clinician to click on a specific order from within the pathway and place orders for a medication, lab, or diagnostic test (eg, aspirin 324 mg, complete blood count, myocardial perfusion imaging stress test). Embedded clinical scoring tools (eg, thrombolysis in myocardial infarction score 12 ; history, ECG, age, risk factors, troponin [HEART] score 13 ) were also available from within the application to help risk stratify patients and guide management. Use of ePATH or the paper guideline was entirely voluntary and once ePATH was introduced in February 2016, paper copies of the AMC CPP were removed from the clinical space. Faculty and staff were introduced to ePATH as part of monthly faculty and staff meetings the month of implementation. Of note, because use of the embedded pathway was voluntary, physicians and advanced practice providers could adhere to the AMC CPP decision logic without opening the embedded pathway.
2.5. Design
In our study, the implementation of ePATH at the intervention site was leveraged using a quasi‐experimental design that compared the changes in outcomes occurring at the intervention site before ePATH was implemented (eg, only the paper clinical decision support tool was available) to those seen after ePATH (eg, when the clinical decision support tool was embedded into the EHR). To account for temporal trends at the intervention site that were unrelated to the launch of ePATH, an external comparator site was identified.
2.6. Data collection
Data were collected at the intervention site from April 2013 until July 2017. April 2013 corresponded to the time when the paper‐based clinical decision support tool was available, and July 2017 was chosen as the end of data collection because of the introduction of new cardiology admission guidelines at AMC in August 2017, which significantly changed disposition decisions for chest pain patients. The data collection period allowed for maximum pre‐intervention (34 months) and post‐intervention (18 months) trend analysis without incorporating significant operational confounders. Data from the comparator site were collected from November 2013 through July 2017. November 2013 was chosen as the beginning of data collection because the enterprise‐level EHR went live at the comparator site during that month. Thus, in analyses that required data from the comparator site, the data used were from November 2013 through July 2017. In analyses that did not require data from the comparator site, the data used were from April 2013 to July 2017 to maximize the data and trends observed. Data were also restricted to the common time period for the intervention site and comparator site (November 2013 through July 2017) to examine any potential differences in findings given the different data start times.
Patient demographics, clinical characteristics, length of stay, disposition, and stress testing results were collected from the EHR for each encounter. Because of variability in medical history data capture within the EHR over time and across sites, the presence of hypertension and diabetes at the index visit was determined by querying outpatient medication lists at the encounter level for antihyperglycemic and antihypertensive medications using Centers for Disease Control and Prevention Ambulatory Care Drug Database System categories. Data for 30‐day major adverse cardiac events (defined as percutaneous coronary intervention, coronary artery bypass grafting, acute myocardial infarction, and death from any cause) were obtained from a health system enterprise‐level data warehouse, the Colorado All Payer Claims Database (CO APCD, Center for Improving Value in Health Care), and the state death registry. Encounters were matched using patient demographics and date of service. If a patient had multiple ED encounters during the study period, each was coded and analyzed separately.
2.7. Outcomes
We evaluated the association of ePATH on hospital length of stay (LOS), admission rate, frequency of stress testing, and occurrences of major adverse cardiac events within 30 days (30‐day MACE). MACE included acute myocardial infarction (MI), coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), and death from any cause. Hospital LOS was defined as the time from arrival in the ED until discharge from the ED, ED CDU, or inpatient hospital service. Admissions were defined as patients admitted to an inpatient hospital service. Patients admitted to the ED CDU were not coded as admissions but as observation stays. A stress test was defined as a provocative cardiac stress test (eg, chemical or exercise stress test) using any imaging modality or coronary computed tomography angiography (CCTA). Stress testing included those tests performed from the ED, ED CDU, and those performed after admission to an inpatient hospital service.
2.8. Statistical analysis
Baseline demographic and clinical characteristics of patients at the primary site were compared between the 2 time periods using a z‐test for categorical variables and student t‐test for continuous variables. Baseline characteristics were also compared between the intervention site and comparator site. Descriptive statistics were calculated for each outcome before and after the launch of ePATH at the intervention site, and a Wilcoxon rank sum test was calculated to determine if there were significant differences between the 2 time periods. A quasi‐experimental design was then used to assess the association between the intervention and each outcome, while controlling for demographic characteristics (eg, age, sex, and race), clinical history characteristics (hypertension, hypercholesterolemia, dyslipidemia, diabetes, non‐STEMI, acute coronary syndrome, and coronary artery disease), and temporal trends. For each outcome, we tested if pre‐period trends were parallel between the intervention and comparator site to inform our model specification of difference in differences (DiD) or interrupted time series (ITS). A DiD specification was the primary specification and was used when the comparator ED had parallel pre‐period trends relative to our intervention ED. This was the case for the following outcomes: percentage admitted, percentage receiving stress testing, and occurrence of major adverse cardiac events. Pre‐period trends were not parallel between the 2 sites for hospital LOS, and thus an ITS specification was used instead of a DiD. Statistical analysis was carried out using Stata/IC 13.1 (StataCorp, College Station, TX).
3. RESULTS
3.1. Sample
Of the 28,603 ED visits for chest pain at AMC during the study period, 13,672 (47.8%) met the inclusion criteria. A total of 4,907 ED visits (35.9%) that initially met the inclusion criteria were subsequently excluded for an ED diagnosis of STEMI (379), initial troponin value ≥ 0.5 ng/mL (186), non‐cardiac advanced imaging (3399), non‐cardiac ED diagnosis (621), or confounding disposition (322) (see Figure 1). Of the 24,265 ED visits for chest pain at the comparator site, 12,841 (52.9%) met the inclusion criteria. A total of 4592 ED visits (35.8%) that initially met the inclusion criteria were subsequently excluded for an ED diagnosis of STEMI (267), initial troponin value ≥ 0.5 ng/mL (256), non‐cardiac advanced imaging (2628), non‐cardiac ED diagnosis (1195), or confounding disposition (246) (Figure 1). Table 1 presents patient demographics and clinical characteristics among each cohort. Relative to the comparator site, patients at AMC were more likely to be male, African American, and have hypertension or diabetes.
TABLE 1.
Demographics and clinical characteristics of ED cohort
| Value | Intervention site (n = 8765) (%) | Comparator site (n = 8249) (%) | P a |
|---|---|---|---|
| Age, mean | 51.9 | 50 | 0.18 |
| Women | 4474 (51.0) | 4401 (53.3) | <0.001 |
| Race | |||
| African American | 2313 (26.3) | 1213 (14.7) | <0.001 |
| Caucasian | 4093 (46.7) | 5434 (65.9) | <0.001 |
| Comorbidities | |||
| Current Smoker | 3629 (41.4) | 3792 (46) | <0.001 |
| Hypertension | 2866 (32.7) | 1985 (24.1) | <0.001 |
| Diabetes | 1660 (18.9) | 1052 (12.8) | <0.001 |
| Systolic blood pressure, mean (SD), mmHg | 143.5 | 142 | <0.001 |
| Diastolic blood pressure, mean (SD), mmHg | 79.9 | 79.5 | 0.12 |
| Pulse, mean (SD), beats per minute | 81.7 | 79.6 | <0.001 |
| Oxygen saturation, mean (SD), % | 95.4 | 95.5 | 0.15 |
P values calculated using z‐tests of proportions for categorical variables and t tests for continuous variables. ED, emergency department.
3.2. Descriptive statistics
During the data collection period, ePATH was accessed for 27% (859 of 3185) of chest pain visits. The utilization rate of the preceding paper‐based tool could not be tracked due to its paper format and so cannot be compared to the ePATH rate. After application of inclusion and exclusion criteria, there was an average of 170 patient visits (SD: 15.4) per month at AMC. Before ePATH implementation, the hospital admission rate and proportion of patients receiving a stress test were 28.2% and 15.8% respectively. The median hospital LOS was 517 minutes. Following implementation of ePATH, the hospital admission rate, proportion receiving a stress test, and hospital LOS all decreased (28.2% vs 20.9%, P < 0.001; 15.8% vs 12.7%, P = 0.001; median 517 vs 431 minutes, P < 0.001;). In contrast, there were no significant differences observed for the unadjusted occurrences of MACE after ePATH implementation (Table 2).
TABLE 2.
Descriptive comparison of outcomes at the intervention site only: Paper‐based decision support (pre period) versus ePATH (post period)
| Intervention site: Paper‐based tool (April 2013–February 2016) | Intervention site: ePath tool (March 2016–July 2017) | P a | |
|---|---|---|---|
| All ED visits | 332 minutes | 280 minutes | <0.001 |
| Median ED Length of Stay | |||
| ED visits for admitted patients | 246 minutes | 239 minutes | 0.17 |
| ED visits for discharged patients | 410 minutes | 321 minutes | <0.001 |
| Median Hospital Length of Stay | |||
| Hospital LOS b | 517 minutes | 431 minutes | <0.001 |
| Admission | |||
| Admit overall | 28.2% | 20.9% | <0.001 |
| Admit to floor | 22.9% | 17.8% | 0.002 |
| Admit to ICU | 5.2% | 3.2% | <0.001 |
| Proportion Receiving Stress Test | |||
| Stress Test | 15.8% | 12.7% | 0.001 |
| Major adverse cardiac events | |||
| MI | 5.7% | 6.1% | 0.30 |
| CABG | 0.58% | 0.72% | 0.56 |
| PCI | 2.3% | 2.5% | 0.69 |
| Death c | 0.33% | 0.34% | 0.96 |
CABG, coronary artery bypass grafting; CDS, clinical decision support; ED, emergency department; ePATH, electronic health record‐embedded clinical pathway; ICU, intensive care unit; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Wilcoxon rank sum test was calculated to determine if there were significant differences before and after the implementation of each decision support tool.
Defined as arrival in ED to discharge from hospital, inclusive of any time admitted to inpatient service.
Defined as death from any cause.
3.3. Quasi‐experimental results
An ITS specification was used when pre‐period trends were not parallel between the intervention site and comparator site. In the ITS specification, only data from the intervention site was used given the external comparator site did not pass the pre‐period trend assumption to be an appropriate comparator. Pre‐period trends were not parallel between the 2 sites for hospital LOS, and thus an ITS specification was used instead of a DiD. Using an ITS, at the intervention site, the average hospital LOS was 575 minutes at the beginning of the data collection window and was already declining over time by ≈4 minutes per month (P < 0.001) before the implementation of ePATH. ePATH was not significantly associated with any further reduction in hospital LOS relative to the pre‐intervention trend (immediate increase in LOS of 16.2 minutes per patient, P = 0.35; ongoing increase in LOS of 0.56 minutes per month per patient relative to pre‐intervention trend, P = 0.77) (Figure 2). None of the covariates controlled for were statistically significant, except for age, which was associated with a significantly longer length of stay (13.8 minutes per additional year of age, P = 0.02)
FIGURE 2.
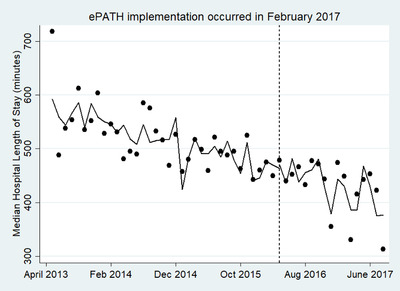
Interrupted time series analysis (intervention site data only) of association of ePATH on median hospital length of stay. The ITSA stata command was used to generate this figure and controlled for the following covariates: demographic characteristics (eg, age, sex, and race) and clinical history characteristics (hypertension, hypercholesterolemia, dyslipidemia, diabetes, non‐STEMI, acute coronary syndrome, and coronary artery disease). ePATH, electronic health record embedded clinical pathway; ITSA, interrupted time‐series analysis; non‐STEMI, non‐ST‐segment elevation myocardial infarction
A DiD specification was used when pre‐period trends were parallel between the intervention site and comparator site. In the DiD specification, data from both the intervention site and comparator site were used given the external comparator site passed the pre‐period trend assumption to be an appropriate comparator. Pre‐period trends were parallel between the 2 sites for the percentage admitted, percentage receiving stress testing, and occurrence of major adverse cardiac events. ePATH was associated with a 10.79% (P < 0.001) reduction in hospital admissions and a 6.05% reduction in the proportion of patients receiving stress tests (P < 0.001) at the intervention site as compared to the comparator site (Table 3). The reduction in hospital admissions was driven primarily by decreased admissions to hospital floor beds (relative reduction of 9.3%), rather than decreased admissions to intensive care beds (relative reduction of 1.4%). There were no significant differences in the occurrences of MACE within a 30 days (MI 0.7%, P = 0.33; CABG 0.33%, P = 0.30; PCI ‐0.26%, P = 0.65; death 0.05%, P = 0.82) between the intervention and comparator site.
TABLE 3.
Differences in differences analysis of ePATH, comparing changes over time in the intervention site to changes over time in the comparator site*
| ePATH Impact | P | |
|---|---|---|
| Proportion Receiving Stress Test | ||
| Receipt of Stress Test | −6.05% | <0.001 |
| Admissions | ||
| Admit Overall | −10.79% | <0.001 |
| Admit to Floor | −9.30% | <0.001 |
| Admit to ICU | −1.39% | 0.033 |
| Major adverse cardiac events | ||
| MI | 0.70% | 0.33 |
| CABG | 0.33% | 0.30 |
| Death | 0.05% | 0.82 |
| PCI | −0.26% | 0.65 |
ePath impact column represents the relative association of ePATH with outcome measures after difference‐in‐difference specification comparing the intervention site and comparator site pre and post‐intervention.
CABG, coronary artery bypass grafting; ePATH, electronic health record‐embedded clinical pathway; ICU, intensive care unit; MI, myocardial infarction; PCI, percutaneous coronary intervention.
TABLE 4.
Clinical classification system groupers 100–102
| AHRQ Clinical Grouper | Description |
|---|---|
| 100 | Acute myocardial infarction |
| 101 | Coronary atherosclerosis and other heart disease |
| 102 | Nonspecific chest pain |
AHRQ, Agency for Healthcare Research and Quality.
TABLE 5.
Table of excluded ED diagnoses
| ICD‐10 Code | Diagnosis |
|---|---|
| I48.x | Atrial Fibrillation and Flutter |
| I46.x | Cardiac arrest |
| I50.43 | Acute on chronic systolic and diastolic heart failure |
| I50.33 | Acute on chronic systolic and diastolic heart failure |
| I50.23 | Acute on chronic systolic heart failure |
| I50.813 | Acute on chronic right heart failure |
| I16.0 | Hypertensive Urgency |
| I16.1 | Hypertensive Emergency |
| I16.9 | Hypertensive Crisis, unspecified |
| T82.198x | Other mechanical complication of other cardiac electronic device |
| I30.x | Acute Pericarditis |
| I31.x | Other diseases of pericardium |
| I32 | Pericarditis in diseases classified elsewhere |
| I40.x | Acute Myocarditis |
| I41 | Myocarditis in diseases classified elsewhere |
| I47.1 | Supraventricular Tachycardia |
| R55 | Syncope and Collapse |
| I47.2 | Ventricular Tachycardia |
| E13.1x | DM with Ketoacidosis with or without coma |
| E11.1x | DM2 with Ketoacidosis |
| E11.69 | DM2 with other specified complication |
| K81.x | Cholecystitis |
| K80.0x | Calculus of GB with acute cholecystitis |
| K80.1x | Calculus of GB with other cholecystitis |
| K80.4x | Calculus of bile duct with cholecystitis |
| K80.6x | Calculus of gallbladder and bile duct with cholecystitis |
| K80.21 | Calculus of gallbladder without cholecystitis |
| K80.3x | Calculus of bile duct with cholangitis |
| K80.51 | Calculus of bile duct without cholangitis or cholecystitis, with obstruction |
| K80.71 | Calculus of gallbladder and bile duct without cholecystitis, with obstruction |
| K80.81 | Other cholelithiasis with obstruction |
| I85.01 | Esophageal varices with bleeding |
| I85.11 | Secondary esophageal varices with bleeding |
| K25.0 | Acute gastric ulcer with hemorrhage |
| K25.2 | Acute gastric ulcer with hemorrhage and perforation |
| K25.4 | Chronic or unspecified gastric ulcer with hemorrhage |
| K25.6 | Chronic or unspecified gastric ulcer with hem and perf |
| K26.0 | Acute duodenal ulcer with hemorrhage |
| K26.2 | Acute duodenal ulcer with hemorrhage and perforation |
| K26.4 | Chronic or unspecified duodenal ulcer with hemorrhage |
| K26.6 | Chronic or unspecified duodenal ulcer with hemorrhage and perforation |
| K27.0 | Acute peptic ulcer with hemorrhage |
| K27.2 | Acute peptic ulcer with hemorrhage and perf |
| K27.4 | Chronic unspecified peptic ulcer with hemorrhage |
| K27.6 | Chronic unspecified peptic ulcer with hemorrhage and perforation |
| K28.0 | Acute gastrojejunal ulcer with hemorrhage |
| K28.2 | Acute gastrojejunal ulcer with hemorrhage |
| K28.4 | Chronic unspecified gastrojejunal ulcer with hemorrhage |
| K28.6 | Chronic unspecified gastrojejunal ulcer with hemorrhage and perforation |
| K62.5 | Hemorrhage of anus and rectum |
| K92.0 | Hematemesis |
| K92.1 | Melena |
| K92.2 | Gastrointestinal hemorrhage, unspecified |
| K85.9 | Acute Pancreatitis, unspecified |
| K86.0 | Alcohol‐induced chronic pancreatitis |
| K86.1 | Other chronic pancreatitis |
| D70.x | Neutropenia |
| D57.0x | Hb‐SS disease with crisis |
| D57.21x | Sickle‐cell/Hb‐C with crisis |
| D57.41x | Sickle cell thalassemia with crisis |
| D57.81x | Other sickle cell disorders with crisis |
| N10 | Acute pyelonephritis |
| N12 | Tubulo‐interstitial nephritis, not acute or chronic |
| A41.x | Other Sepsis |
| A40.x | Streptococcal sepsis |
| R65.21 | Severe sepsis with septic shock |
| R65.20 | Severe sepsis |
| E87.5 | Hyperkalemia |
| E87.1 | Hypo‐osmolality and hyponatremia |
| S22.3 | Fracture of one rib |
| S22.4 | Multiple fractures of ribs |
| F10.129 | Alcohol abuse with intoxication |
| F10.239 | Alcohol dependence with withdrawal |
| R45.85x | Suicidal and homicidal ideations |
| J44.1 | COPD with acute exacerbation |
| J89.6 | Pyothorax without fistula |
| 786.0 | Pyothorax with fistula |
| J91.8 | Pleural effusion |
| J13 | Pneumococcal pneumonia |
| J14 | Pneumonia due to Hemophilus influenzae |
| J15.x | Bacterial pneumonia |
| J16.x | Pneumonia due to other infectious organism |
| J17 | Pneumonia in diseases classified elsewhere |
| J18.x | Pneumonia, unspecified organism |
| J93.83 | Other pneumothorax |
| J93.9 | Pneumothorax, unspecified |
| J93.11 | Primary spontaneous pneumothorax |
| I26.0x | PE with acute cor pulmonale |
| I26.9x | PE without acute cor pulmonale |
| N18.6 | End Stage Renal Disease |
| Z99.2 | Dependence on renal dialysis |
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ED, emergency department; GB, gallbladder; PE, pulmonary embolism.
4. LIMITATIONS
Our study has several limitations. Though pre‐intervention trends at the study sites were parallel for admission rate, stress testing, and MACE, there were demographic and clinical differences that may have confounded results. In order to mitigate this effect, we added covariates to each model. Moreover, because ePATH was not mandatory and had decision logic that could be used without accessing ePATH, we could not accurately assess physician or advanced practice provider compliance. The ePATH tool was only accessed for 27% of studied patient visits, but the proportion of visits that adhered to AMC CPP decision logic is likely much greater. Accordingly, though we suspect that ePATH affected outcome measures as a consequence of improved guideline compliance, the non‐mandatory design of our ePATH precluded us from collecting data to confirm this. Lastly, we could not evaluate the influence of ePATH as compared to “usual care” at the intervention site because our evidence‐based care guideline was available as a paper‐based tool for nearly 3 years before implementation of the ePATH.
5. DISCUSSION
The implementation of ePATH was associated with a reduction in hospital admissions (10.79%) and stress testing (6.05%) without increases in 30‐day MACE for patients presenting to the ED with chest pain. ePATH was also associated with a decrease in unadjusted hospital LOS, but this was not significant after accounting for pre‐intervention trends. Our results are similar to those seen in a recent study 14 which showed a 12% decrease in hospital admission rate after implementation of an evidence‐based decision support tool for ED patients presenting with chest pain. Unlike the prior study, we did not simultaneously introduce new decision logic or educational efforts at the same time as ePATH implementation. Our institutional chest pain guideline existed in paper form before ePATH. Thus, the decrease in admission rate we observed can likely be attributed to the ePATH tool, as opposed other processes or clinical interventions. Importantly, reductions in admission rates occurred without negative impacts on safety outcomes (eg, MACE). Our safety data is particularly robust as we obtained 30‐day MACE data from our state death registry and all payer claims database, allowing capture of MACE outcome events that occurred outside our health system EHR. The use of a comparator site also adds to the strength of our results. We chose to use a comparator site within the same health system and with similar characteristics (large urban trauma center with 101,000 yearly ED visits). Together, our data offer evidence supporting the positive impact of ePATH on the care of ED patients with suspected ACS.
Our results are particularly timely given that the Physician‐Focused Payment Model Technical Advisory Committee (PTAC) recently supported a new bundled payment model aimed at reducing inappropriate inpatient admissions from the ED. 15 As hospitals and emergency departments plan for participation in this new care delivery model, they will need to reduce variability across providers and facilities. Effective use of health information technology and clinical decision support tools like ePATH will be a cornerstone for these efforts. This is particularly true for ED diagnoses with the greatest variability in admission rates, such as chest pain. 16
Operationalizing evidence‐based guidelines is challenging, particularly in complex clinical environments like the ED. 9 One of the primary cited reasons for the limited impact of computerized decision support tools in published studies is a limited focus on usability and workflow integration. 17 , 18 , 19 Human factors research has shown that physician decisions are influenced by the structure of their practice environment, namely that the path of least resistance is the path most often taken. 20 Clinical decision support tools designed for use in the ED must account for unpredictable clinical conditions, frequent interruptions, time pressures, incomplete information, and vast, non‐linear decision space. 9 , 17 , 21 , 22 , 23 In our study, we assessed the impact of a clinical decision support tool that was embedded into the EHR with specific design elements addressing 3 important barriers to success: integration into usual workflows, ease of use, and recognition of physician and advanced practice provider expertise. 18 , 24 , 25 , 26 , 27 Moreover, the ePATH interface espouses many of the optimal design heuristics established by Nielson and expert consensus (eg, match between system and real world, user control, and freedom, flexibility, and efficiency of use, minimalist design). 19 Accordingly, the positive impact of clinical decision support at our institution is likely a consequence of its design.
ePATH was associated with a reduction in hospital admissions and stress testing without significantly increasing major adverse cardiac events. Our results suggest that well‐designed clinical decision support tools can catalyze the use of evidence‐based guidelines. Future work will examine the cost implications and potential efficiency gains associated with ePATH.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
JSD, MDW, PMH, and JW contributed to conception and design of the study, as well as data analysis and interpretation. JSD, MDW, and FG were involved with drafting the manuscript. KB, RZ, and JW contributed to critical revisions of the manuscript. All authors have given approval for manuscript submission and agree to be accountable for the work with respect to accuracy and integrity.
ACKNOWLEDGMENTS
We thank Manisha Parmar, analytics developer at UCHealth, for her work to query multiple data sources and curate our datasets for the study. Her contribution was invaluable to the project. We would also like to thank the staff at Health Data Compass for their assistance obtaining data from our state death registry and all‐payer claims database. We would like to thank Manisha Parmar for her assistance in collating data for this study.
Biography
Jasmeet Singh Dhaliwal, MD, MPH, is an emergency physician in the Department of Emergency Medicine, University of Colorado School of Medicine.

Dhaliwal JS, Goss F, Whittington MD, et al. Reduced admission rates and resource utilization for chest pain patients using an electronic health record‐embedded clinical pathway in the emergency department. JACEP Open. 2020;1:1602–1613. 10.1002/emp2.12308
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Marna Rayl Greenberg, DO, MPH
Contributor Information
Jasmeet S. Dhaliwal, Email: dhaliw@gmail.com.
Jennifer Wiler, Email: Jennifer.Wiler@cuanschutz.edu.
REFERENCES
- 1. Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2015 Emergency Department Summary Tables. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf. Published 2015. Accessed January 15, 2019.
- 2. Yau AA, Nguyendo LT, Lockett LKL, Michaud E. The HEART pathway and hospital cost savings. Crit Pathw Cardiol. 2017; 16(4): 126‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang AM, Fischman DL, Hollander JE. Evaluation of chest pain and acute coronary syndromes. Cardiol Clin. 2018; 36(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 4. Than MP, Pickering JW, Aldous SJ, et al. Effectiveness of EDACS versus ADAPT accelerated diagnostic pathways for chest pain: a pragmatic randomized controlled trial embedded within practice. Ann Emerg Med. 2016; 68(1): 93‐102. [DOI] [PubMed] [Google Scholar]
- 5. Gafni‐Pappas G, DeMeester SD, Boyd MA, et al. The HAS‐Choice study: utilizing the HEART score, an ADP, and shared decision‐making to decrease admissions in chest pain patients. Am J Emerg Med. 2018; 36(10): 1825‐1831. [DOI] [PubMed] [Google Scholar]
- 6. Allen BR, Simpson GG, Zeinali I, et al. Incorporation of the HEART score into a low‐risk chest pain pathway to safely decrease admissions. Crit Pathw Cardiol. 2018; 17(4): 184‐190. [DOI] [PubMed] [Google Scholar]
- 7. Gesell SB, Golden SL, Limkakeng AT, et al. Implementation of the HEART Pathway. Crit Pathw Cardiol. 2018; 17(4): 191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Medicare and Medicaid Services. 2018 MIPS Cost Performance Category Fact Sheet. https://qpp-cm-prod-content.s3.amazonaws.com/uploads/154/2018%20Cost%20Performance%20Category%20Fact%20Sheet.pdf. Published 2018. Accessed December 12, 2018.
- 9. Gaddis GM, Greenwald P, Huckson S. Toward improved implementation of evidence‐based clinical algorithms: clinical practice guidelines, clinical decision rules, and clinical pathways. Acad Emerg Med. 2007; 14(11): 1015‐1022. [DOI] [PubMed] [Google Scholar]
- 10. Than M, Cullen L, Aldous S, et al. 2‐Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091–2098. [DOI] [PubMed] [Google Scholar]
- 11. Aldous SJ, Richards M, Cullen L, Troughton R, Than M. A 2‐hour thrombolysis in myocardial infarction score outperforms other risk stratification tools in patients presenting with possible acute coronary syndromes: comparison of chest pain risk stratification tools. Am Heart J. 2012; 164(4): 516‐523. [DOI] [PubMed] [Google Scholar]
- 12. Pollack CV, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non‐ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006; 13(1): 13‐18. [DOI] [PubMed] [Google Scholar]
- 13. Backus B, Six A, Kelder J, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013; 168(3): 2153‐2158. [DOI] [PubMed] [Google Scholar]
- 14. Smulowitz P, Dizitzer Y, Tadiri S, Thibodeau L, Jagminas L, Novack V. Impact of implementation of the HEART pathway using an electronic clinical decision support tool in a community hospital setting. Am J Emerg Med. 2018; 36(3): 408‐413. [DOI] [PubMed] [Google Scholar]
- 15. Firth S. PTAC backs new payment models for emergency medicine. MedPage Today; 2018. https://www.medpagetoday.com/publichealthpolicy/medicare/75025
- 16. Venkatesh AK, Dai Y, Ross JS, Schuur JD, Capp R, Krumholz HM. Variation in US hospital emergency department admission rates by clinical condition. Med Care. 2015; 53(3): 237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutenstein M, Pickering JW, Than M. Development of a digital clinical pathway for emergency medicine: lessons from usability testing and implementation failure. Health Informatics J. 2018. X. [DOI] [PubMed] [Google Scholar]
- 18. Chung P, Scandlyn J, Dayan PS, Mistry RD. Working at the intersection of context, culture, and technology: provider perspectives on antimicrobial stewardship in the emergency department using electronic health record clinical decision support. Am J Infect Control. 2017; 45(11): 1198‐1202. [DOI] [PubMed] [Google Scholar]
- 19. Miller K, Capan M, Weldon D, et al. The design of decisions: matching clinical decision support recommendations to Nielsen's design heuristics. Int J Med Inform. 2018; 117(May): 19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clack L, Sax H. Inpatient notes: human factors engineering and inpatient care ‐ New ways to solve old problems. Ann Intern Med. 2017; 166(8): H02‐H03. [DOI] [PubMed] [Google Scholar]
- 21. Patel VL, Zhang J, Yoskowitz NA, Green R, Sayan OR. Translational cognition for decision support in critical care environments: a review. J Biomed Inform. 2008; 41(3): 413‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chisholm CD, Weaver CS, Whenmouth L, Giles B. A task analysis of emergency physician activities in academic and community settings. Ann Emerg Med. 2011; 58(2): 117‐122. [DOI] [PubMed] [Google Scholar]
- 23. Raja AS, Venkatesh A, Mick N, et al. "Choosing Wisely” imaging recommendations: initial implementation in New England Emergency Departments. West J Emerg Med. 2017; 18(3): 454‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shortliffe EH, Informatics B. Clinical decision support in the era of artificial intelligence. JAMA. 2018; 10025: 5–6. [DOI] [PubMed] [Google Scholar]
- 25. Kawamoto K. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. Bmj. 2005; 330(7494): 765‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rand CS, Powe NR, Wu AW, Wilson MH. Why don ’ t physicians follow a framework for improvement. Jama. 1999; 282(15): 1458‐1465. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Medicare and Medicaid Services. Clinical Decision Support: More Than Just “Alerts” Tipsheet. https://www.cms.gov/regulations-and-guidance/legislation/EHRincentiveprograms/downloads/clinicaldecisionsupport_tipsheet-.pdf. Published 2014. Accessed January 15, 2019.


