Abstract
Asthma is a chronic obstructive lung disease characterized by recurrent episodes of bronchoconstriction and wheezing. Conventional asthma treatment involves the suppression of airway inflammation or improving airway flow. Rosmarinus officialis, also known as rosemary, is a Mediterranean plant that is used for the treatment of inflammatory diseases. Carnosol, a diterpenoid found in rosemary extracts, has been known to exhibit anti-inflammatory, anti-tumor, and anti-oxidant effects. The effect of carnosol on allergic responses has not been tested yet. The effect of carnosol on a murine allergic asthma model were investigated. Carnosol inhibited the degranulation of RBL-2H3 mast cells. Carnosol treatment inhibited the increase in the number of eosinophils in the bronchoalveolar lavage fluids (BALF) of mice treated with ovalbumin. Carnosol treatment also inhibited inflammatory responses and mucin production in histologic studies. Carnosol treatment inhibited the increases of IL-4 and IL-13 cytokines expression in both BALF and the lungs. These results suggest that carnosol may have a potential for allergic asthma therapy.
Keywords: Carnosol, Allergy, Asthma, Degranulation, Mast cell, Rosmarinus officialis
INTRODUCTION
Allergic asthma is a chronic inflammatory disease of the airways of the lungs and is characterized by chest tightness, coughing, shortness of breath, and wheezing with large heterogeneity of clinical phenotypes (Park and Im, 2019). More than 300 million people are patients of asthma worldwide (Park and Im, 2019). Environmental factors such as air pollution and allergens can cause asthma in addition to genetic factors (Aoki et al., 2008). No cure is available for asthma, but the use of inhaled corticosteroids, β-adrenergic receptor agonists or leukotriene D4 receptor antagonists could reduce the symptoms in addition to avoiding allergens (Lemanske and Busse, 2010; Averell et al., 2019; Trinh et al., 2019). Considering the side effects of long-term use of current therapeutics, identifying a new therapeutic strategy from natural resources is essential.
Rosmarinus officialis (also known as rosemary) is a Mediterranean plant, which is now widely spread in many countries (Kashyap et al., 2017). Rosemary extract is useful in curing inflammatory diseases (da Rosa et al., 2013; Kashyap et al., 2017). Carnosol, a phenolic diterpenoid found in rosemary extracts, has been shown to possess anti-inflammatory, anti-tumor, and anti-oxidant effects (Singletary, 1996; Johnson, 2011; Kashyap et al., 2017). However, the effect of carnosol in allergic responses has not been tested yet.
Previously, carnosol has been reported to have anti-inflammatory effects. Carnosol activated the anti-inflammatory peroxisome proliferator-activated receptor-γ at the transcriptional level (Rau et al., 2006) and inhibited NF-κB-mediated iNOS gene expression and NO production in macrophages (Chan et al., 1995; Lo et al., 2002; Mengoni et al., 2011). Carnosol also inhibited the formation of pro-inflammatory leukotrienes in intact human polymorphonuclear leukocytes (Poeckel et al., 2008), and inflammatory cytokines and chemokines production (Schwager et al., 2016). Therefore, the suppressive activity of carnosol in inflammatory responses could be useful in treating allergic asthma, as anti-inflammation in the lungs is one of the therapeutics for asthma. In the present report, the effects of carnosol were investigated on a murine allergic asthma model.
MATERIALS AND METHODS
Materials
Carnosol and other materials were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Assessment of degranulation
Rat RBL-2H3 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured at 37°C in a 5% CO2-humidified incubator as described previously (Huang et al., 2018). Degranulation from RBL-2H3 cells was evaluated by measuring β-hexosaminidase release (Lee et al., 2018). We used monoclonal anti-dinitrophenyl mouse immunoglobulin E and human dinitrophenyl albumin to induce degranulation (Kim and Im, 2019).
Animals
Female five-week-old BALB/c mice were purchased from Daehan Biolink (Seoul, Korea). They were housed in the laboratory animal facility at Pusan National University and fed water and food ad libitum. The Pusan National University Institutional Animal Care Committee reviewed and approved the protocol with respect to ethical issues and scientific care (Approval Number, ED-PNU2019-0011)
Asthma induction in BALB/c mice
Six-week-old BALB/c mice (22 g) were randomly divided into three groups (n=5): vehicle-injected control group, ovalbumin (OVA)-injected asthma group, and carnosol (5 mg/kg)-treated plus OVA-injected group. Asthma was induced by intraperitoneal injection of OVA and aluminum hydroxide on D0 and D14. The mice were challenged by exposure to nebulized OVA for D28, D29, and D30 (Aoki et al., 2010). Carnosol was administrated via intraperitoneal injection 30 min before OVA challenge. Bronchoalveolar lavage fluid (BALF) was collected from the lungs on D32, and the cell population of BALF cells was analyzed after staining.
Cell counting and analysis in BALF
Using a Cellspin® centrifuge (Hanil Electric, Seoul, Korea), the immune cells in BALF were adhered to a glass slide and fixed in methanol for 30 s. Cells on slides were stained with May-Grünwald solution for 8 min and subsequently by Giemsa solution for 12 min.
Reverse transcription polymerase chain reaction (RT-PCR)
RT-PCR was performed to analyze the expression levels of Th2 and Th17 cells. Total RNA was isolated using TRIzol™ reagent (Invitrogen, Waltham, MA, USA), and used for cDNA synthesis. Promega GoTaq® DNA polymerase (Promega Corporation, Madison, WI, USA), primers for each gene, and the synthesized cDNA were reacted to amplify the specific genes. Primers sequences and PCR conditions for specific genes are described in a previous report (Park et al., 2018).
Histological examination of the lungs
Tissue sections of lungs from mice of each group were prepared. Periodic acid-Schiff (PAS) staining and hematoxylin and Eosin (H&E) staining were performed to identify mucus-secreting goblet cells and eosinophil infiltration, respectively. For PAS staining, the Schiff’s regent was used, and for H&E staining, hematoxylin and eosin regents were used (Heo and Im, 2019; Kim and Im, 2019).
The degree of lung inflammation was evaluated using a subjective scale of 0-3 by a treatment-blind observer (Heo and Im, 2019; Kim and Im, 2019). Mucin-secreting cells stained with PAS in the airways were counted from two lung sections per mouse. The length of the bronchi basal lamina using was also simultaneously measured ImageJ software (National Institute of Health, MD, USA). Mucous production was expressed by the number of PAS-positive cells per mm of bronchiole.
Measurement of cytokine levels
IL-4 levels in BALF were quantitated using an ELISA kit. A capture antibody and a biotinylated detection antibody specific for IL-4 were obtained from eBioscience (cat. 14-7041-68, and cat. 33-7042-68C, San Diego, CA, USA). Avidin-horseradish peroxidase was used and the absorbance was measured at 450 nm (Lee et al., 2018).
Statistical analysis
The results are expressed as means ± standard errors (SEs). For statistical significance, analysis of variance (ANOVA) was used, followed by Turkey’s post hoc test using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). p values<0.05 indicated statistical significance.
RESULTS
Carnosol inhibited degranulation of RBL-2H3 mast cells
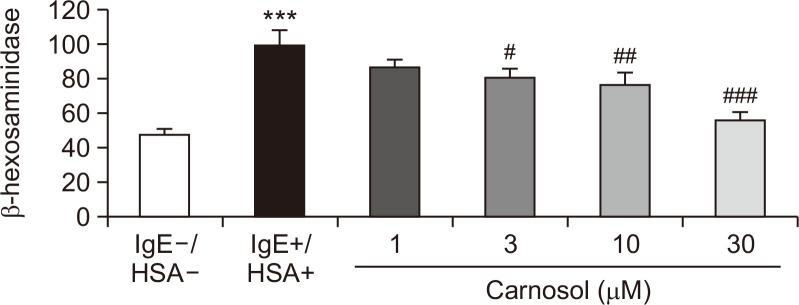
RBL-2H3 mast cells were used to ascertain if carnosol inhibits antigen-induced degranulation. β-Hexosaminidase activity was measured as degranulation activity in the medium after antigen exposure (Heo and Im, 2019; Kim and Im, 2019). Carnosol inhibited the release of β-hexosaminidase in a dose-dependent manner and also significantly inhibited degranulation at concentrations higher than 3 μM (Fig. 1).
Fig. 1.
Effect of carnosol on β-hexosaminidase release in RBL-2H3 cells. RBL-2H3 cells were incubated with anti-DNP IgE for 18 h, and challenged with DNP human serum albumin (HSA). Cells were treated with carnosol at the indicated concentrations 30 min before antigen challenge. Basal degranulation was calculated from the samples without IgE and HSA. The degranulation from samples treated with IgE and HSA was used as a positive control. The results are presented as means ± SEs of values from three independent experiments. ***p<0.001 vs. the non-HSA-treated group. #p<0.05, ##p<0.01, ###p<0.001 vs. the HSA-treated group.
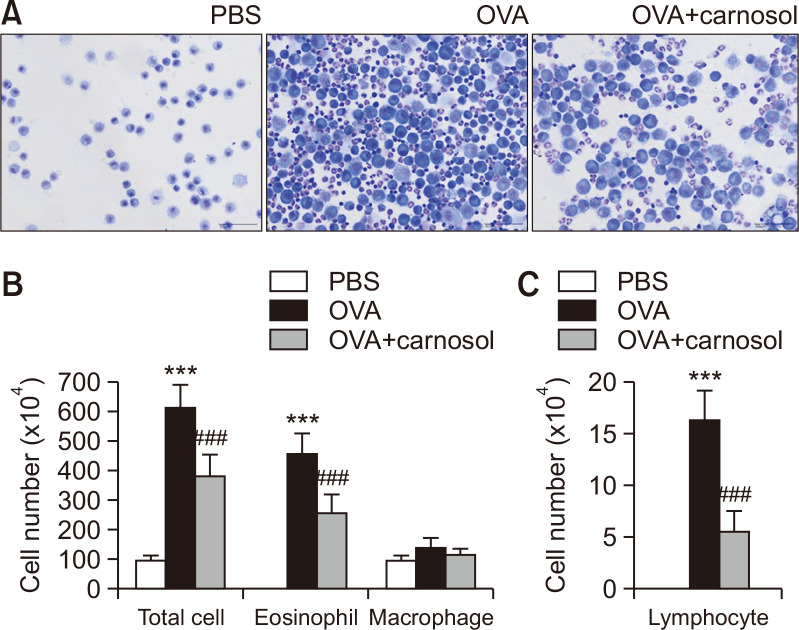
Carnosol inhibited immune cell accumulation in BALF
An OVA-induced allergic asthma model in female BALB/c mice was utilized to verify the inhibitory effects of carnosol in vivo. Total cell number and cell populations were analyzed in BALF (Fig. 2). The total cell number rose to 504.4% in the BALF of OVA-induced asthma mice compared to that in the vehicle-treated group (Fig. 2A, 2B). Carnosol inhibited OVA-induced rise in total cell number by 37.2% (Fig. 2A, 2B). The cell populations were analyzed from the BALF (Fig. 2B, 2C). Carnosol inhibited the significant increase in eosinophil numbers by 43.6% compared to the OVA-treated group (Fig. 2B). The macrophage population did not significantly increase by OVA treatment (Fig. 2B). The lymphocyte population was quite lower than the eosinophil population. OVA treatment increased the lymphocyte counts, and carnosol inhibited the increment by 65.7% (Fig. 2C).
Fig. 2.
Suppressive effects of carnosol on the increase in immune cells in BALF by OVA. (A) Mice were sensitized with OVA twice by i.p. injection on D0 and D14, and later challenged on D28, D29, and D30 with nebulized OVA. Carnosol was administrated intraperitoneally at the dose of 5 mg/kg, 30 min before OVA challenge. Cells in BALF were stained using May-Grünwald stain and counted. (B) Total cell counts, eosinophils, and macrophages in BALF. (C) Lymphocytes counts in the BALF. The results are presented the mean ± SE cell count values (n=5). ***p<0.001 vs. the PBS-treated group, ###p<0.001 vs. the OVA-treated group.
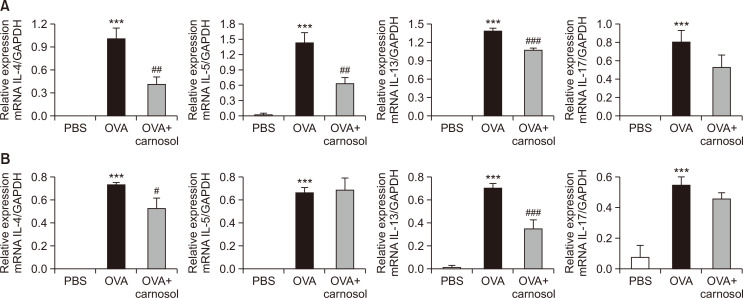
Carnosol suppressed the production of Th2 and Th17 cytokines in lungs and BALF
We measured the mRNA levels of Th2 cytokines in the lungs and immune cells of BALF. We also measured the mRNA levels of IL-17A, as Th17 cells were recently recognized as regulators of allergic asthma (Wakashin et al., 2009; Newcomb and Peebles, 2013). The mRNA levels of the four cytokines were increased by OVA treatment in the BALF and lungs (Fig. 3). Carnosol treatment significantly inhibited mRNA expression of IL-4, IL-5, and IL-13 by 58.9%, 55.7%, and 23.1%, respectively, but not that of the Th17 cytokine in the BALF (Fig. 3A). The mRNA levels of IL-4 and IL-13 were inhibited by carnosol in the lungs (IL-4 by 28.5% and IL-13 by 50.04%), but not the IL-5 and IL-17 levels (Fig. 3B).
Fig. 3.
Carnosol treatment inhibited the expression of cytokines at the mRNA level in the BALF cells and lungs. Analysis of mRNA expression of Th2 cytokines (IL-4, IL-5, and IL-13) and Th17 cytokine (IL-17A) from the BALF cells (A) or the lung tissues (B). Relative mRNA levels of cytokines were quantified as ratios to GAPDH transcript levels. Values represent the mean ± SE (n=5). ***p<0.001 vs. the PBS-treated group, #p<0.05, ##p<0.01, ###p<0.001 vs. the OVA-treated group.
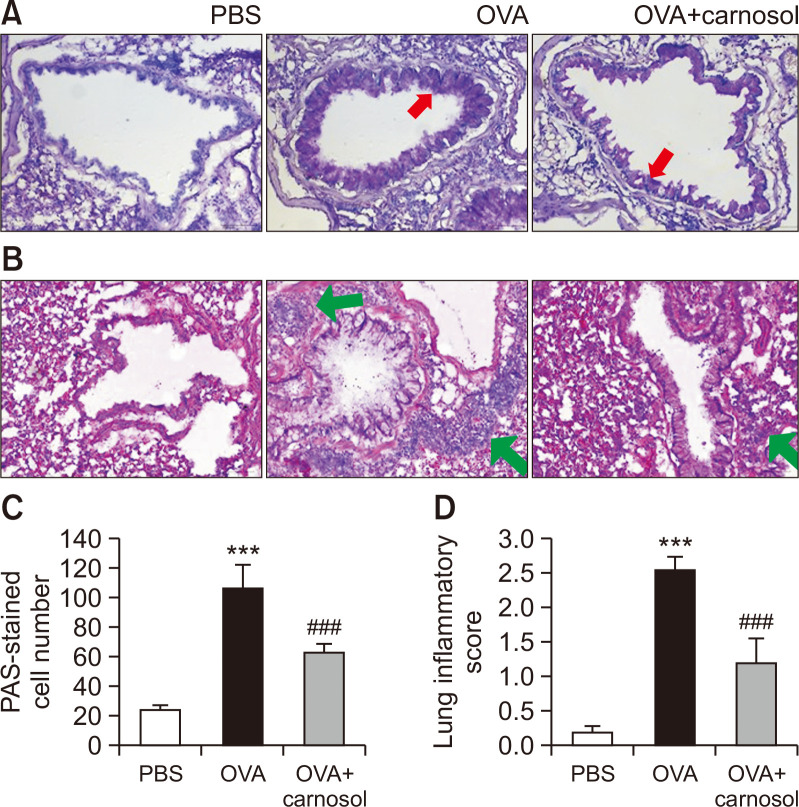
Carnosol inhibited mucin secretion and inflammation in the lungs
The lungs were histologically examined on D32. PAS staining was utilized to detect the presence of mucins. Stored or secreted mucins appear as violet in color in the PAS-stained sections. Dark purple-stained cells surrounded the bronchioles in the OVA-treated group, and carnosol treatment reduced mucin-stained cells (Fig. 4A). The eosinophils were rarely observed as small, navy blue dots in the control group, and in the OVA group, eosinophils were found to be densely surrounding the bronchioles by using H&E staining (Fig. 4B). Semi-quantitative analysis was performed by counting the PAS-stained cells per mm of the bronchioles (Fig. 4C) (Heo and Im, 2019; Kim and Im, 2019). PAS-stained cells were rarely found in the control group. Approximately 110 PAS-stained cells were counted per mm in the OVA group, and carnosol treatment significantly inhibited the PAS-stained cells (Fig. 4C). Also semi-quantitative evaluation using a subjective scale of 0-3 was applied to assess lung inflammation from H&E staining (Heo and Im, 2019; Kim and Im, 2019). The inflammation score in the OVA group was approximately 2.5, while carnosol treatment significantly inhibited the score (Fig. 4D).
Fig. 4.
Protective effect of carnosol against airway inflammation and mucin production. (A) Panels show H&E-stained sections of lung tissues from the PBS group, OVA group, and carnosol plus OVA group. Sections from mice in the OVA-induced and carnosol-treated OVA-induced groups showed an accumulation of eosinophils (small navy blue dots, green arrows). (B) Panels show PAS-stained sections of lung tissues. Mucin is stained in purple color (red arrows). (C) Lung inflammation was semi-quantitatively evaluated. Histological findings were scored as described in the materials and methods section. (D) Mucous production was evaluated by counting the number of PAS-positive cells per mm of bronchiole (n=5 per group). Bar=50 μm. Graphs represent the mean ± SE values (n=5). ***p<0.001 vs. the PBS-treated group, ###p<0.001 vs. the OVA-treated group.
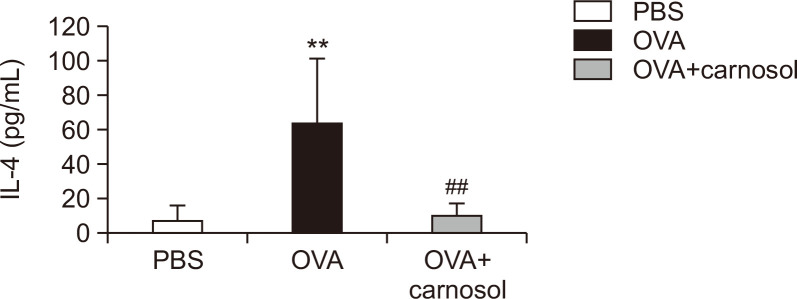
Carnosol suppressed the IL-4 protein levels in the BALF
The protein levels of IL-4 and IL-13 in BALF were measured by using ELISA to verify the effect of carnosol on Th2 cytokine expression. The IL-4 levels were increased in the OVA-induced group compared to the vehicle-treated control group, and the increase in IL-4 levels was significantly suppressed by carnosol treatment (Fig. 5). IL-13 levels in the samples were out of detectable ranges.
Fig. 5.
Effect of carnosol treatment on IL-4 protein levels in BALF. ELISA was used to measure the protein levels of IL-4 in BALF (n=5 per group). The results represent the mean ± SE of protein levels (n=5). **p<0.01 vs. the PBS-treated group, ##p<0.01 vs. the OVA-treated group.
DISCUSSION
Although the anti-inflammatory, anti-oxidant, and anti-tumor effects of carnosol have been previously reported (Singletary, 1996; Johnson, 2011; Kashyap et al., 2017), its anti-asthma effect has not been tested yet. In this report, the effect of carnosol, a constituent of rosemary extract, on allergic asthma was investigated for the first time. Mast cells are a key player in the initiation and development of allergic responses. FcεRI is equipped with circulating IgEs on the plasma membranes of mast cells. Cross-linking of IgEs by specific antigens induces degranulation of mast cells (Lane and Lee, 1996). Allergic mediators such as histamine and leukotrienes are released from granules of mast cells by degranulation, which is a key step in the induction of allergic responses. Carnosol was found to inhibit degranulation of mast cells in the RBL-2H3 cells.
Carnosol treatment significantly inhibited the allergic features in BALF and the lungs of mice in the OVA-induced murine allergic asthma model. The responses of Th2 cytokines such as IL-4 and IL-13 are very important in the development of asthma (Fish et al., 2005; Locksley, 2010). Those cytokines induce mucus hypersecretion, metaplasia of goblet cells, proliferation of smooth muscle cells, and recruitment of eosinophils (Fish et al., 2005; Locksley, 2010). Production of Th2 cytokines was suppressed by carnosol treatment. Furthermore, the inflammatory scores were reduced and mucin production was suppressed by carnosol treatment in the lung tissues. The level of Th17 cytokine was increased by OVA treatment, but was not inhibited by carnosol in both BALF and lungs, in contrast to the suppressive effects of carnosol on Th17A levels in cargeenan-induced pleurity and experimental autoimmune encephalomyelitis (da Rosa et al., 2013; Li et al., 2018). Carnosol suppressed OVA-induced increase of IL-5 in BALF, though not in the lungs. In experimental autoimmune encephalomyelitis, mRNA levels of IL-5 were increased in the splenocytes from carnosol-treated mice (Li et al., 2018). These differential and contrasting effects of carnosol on cytokines IL-17A and IL-5 may be attributed to different mice models and different stages of carnosol treatment. Carnosol treatment inhibited differentiation of CD4+ T cells to Th17 cells and suppressed development of experimental autoimmune encephalomyelitis (Li et al., 2018). In the present OVA-induced allergic asthma model, carnosol was administration during the OVA challenge stage and not during the sensitization stage. This may minimize the effect of carnosol on Th17 differentiation. The effects of carnosol treatment during the sensitization stage and on differentiation of CD4+ T cells to IFNγ+ (Th1), IL4+ (Th2), IL17+ (Th17), and Foxp3+ (Treg) cells need to be further investigated in the near future.
The anti-allergic effect of carnosol has also been observed in other Th2 cell-related diseases. In skin pharmacology, topical application of carnosol has been reported in PMA-induced ear edema, phthalic anhydride-induced atopic dermatitis, and UVB-induced inflammation (Mengoni et al., 2011; Lee et al., 2017; Yeo et al., 2019). Carnosol reduced the expression of IL-1β and TNF-α and inhibited COX-2 in PMA-induced ear inflammation in vivo (Mengoni et al., 2011). Carnosol treatment decreased TNF-α and IL-1β in phthalic anhydride-induced atopic dermatitis via STAT-3 inhibition (Park et al., 2014; Lee et al., 2017).
Based on literature, the anti-asthma effects of carnosol could result from several mechanistic modulations in inflammation-related proteins: (i) carnosol activation of anti-inflammatory peroxisome proliferator-activated receptor-γ transcriptionally (Rau et al., 2006), (ii) carnosol inhibition of pro-inflammatory leukotrienes formation through antagonizing intracellular Ca2+ response induced by a chemotactic stimulus and attenuating ROS formation in intact human polymorphonuclear leukocytes (Poeckel et al., 2008), (iii) carnosol suppression of iNOS gene expression and NO production by NF-κB inhibition in macrophages (Chan et al., 1995; Lo et al., 2002; Mengoni et al., 2011), (iv) carnosol inhibition of inflammatory cytokines and chemokines production (Schwager et al., 2016). All the mechanistic observations could be linked to each other at transcriptional levels such as peroxisome proliferator-activated receptor-γ activation and NF-κB inhibition. Carnosol anti-inflammation may contribute to the present anti-allergic effect, as allergic asthma is a kind of chronic inflammatory disease.
In summary, we report that carnosol induces suppressive effects on degranulation of mast cells in vitro and anti-allergic effects in vivo in an OVA-induced murine model, suggesting the potential therapeutic efficacy of carnosol from Rosmarinus officialis.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program of the Korean National Research Foundation, funded by the Korean Ministry of Education, Science and Technology (NRF-2019R1A2C1005523).
Footnotes
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
REFERENCES
- Aoki H., Hisada T., Ishizuka T., Utsugi M., Kawata T., Shimizu Y., Okajima F., Dobashi K., Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Aoki H., Hisada T., Ishizuka T., Utsugi M., Ono A., Koga Y., Sunaga N., Nakakura T., Okajima F., Dobashi K., Mori M. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem. Biophys. Res. Commun. 2010;400:128–133. doi: 10.1016/j.bbrc.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Averell C. M., Laliberte F., Duh M. S., Wu J. W., Germain G., Faison S. Characterizing real-world use of tiotropium in asthma in the USA. J. Asthma Allergy. 2019;12:309–321. doi: 10.2147/JAA.S216932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. M., Ho C. T., Huang H. I. Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett. 1995;96:23–29. doi: 10.1016/0304-3835(95)03913-H. [DOI] [PubMed] [Google Scholar]
- da Rosa J. S., Facchin B. M., Bastos J., Siqueira M. A., Micke G. A., Dalmarco E. M., Pizzolatti M. G., Frode T. S. Systemic administration of Rosmarinus officinalis attenuates the inflammatory response induced by carrageenan in the mouse model of pleurisy. Planta Med. 2013;79:1605–1614. doi: 10.1055/s-0033-1351018. [DOI] [PubMed] [Google Scholar]
- Fish S. C., Donaldson D. D., Goldman S. J., Williams C. M., Kasaian M. T. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J. Immunol. 2005;174:7716–7724. doi: 10.4049/jimmunol.174.12.7716. [DOI] [PubMed] [Google Scholar]
- Heo J. Y., Im D. S. Anti-allergic effects of salvianolic acid A and tanshinone IIA from Salvia miltiorrhiza determined using in vivo and in vitro experiments. Int. Immunopharmacol. 2019;67:69–77. doi: 10.1016/j.intimp.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Huang J., Su M., Lee B. K., Kim M. J., Jung J. H., Im D. S. Suppressive effect of 4-hydroxy-2-(4-hydroxyphenethyl) isoindoline-1,3-dione on ovalbumin-induced allergic asthma. Biomol. Ther. (Seoul) 2018;26:539–545. doi: 10.4062/biomolther.2018.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. J. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011;305:1–7. doi: 10.1016/j.canlet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap D., Kumar G., Sharma A., Sak K., Tuli H. S., Mukherjee T. K. Mechanistic insight into carnosol-mediated pharmacological effects: recent trends and advancements. Life Sci. 2017;169:27–36. doi: 10.1016/j.lfs.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Kim M. J., Im D. S. Suppressive effects of type I angiotensin receptor antagonists, candesartan and irbesartan on allergic asthma. Eur. J. Pharmacol. 2019;852:25–33. doi: 10.1016/j.ejphar.2019.02.035. [DOI] [PubMed] [Google Scholar]
- Lane S. J., Lee T. H. Mast cell effector mechanisms. J. Allergy Clin. Immunol. 1996;98:S67–S71. doi: 10.1016/S0091-6749(96)70019-2. [DOI] [PubMed] [Google Scholar]
- Lee B. K., Park S. J., Nam S. Y., Kang S., Hwang J., Lee S. J., Im D. S. Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J. Ethnopharmacol. 2018;213:256–261. doi: 10.1016/j.jep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Hwang C. J., Choi J. Y., Park M. H., Song M. J., Oh K. W., Son D. J., Lee S. H., Han S. B., Hong J. T. Inhibitory effect of carnosol on phthalic anhydride-induced atopic dermatitis via inhibition of STAT3. Biomol. Ther. (Seoul) 2017;25:535–544. doi: 10.4062/biomolther.2017.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanske R. F., Jr., Busse W. W. Asthma: clinical expression and molecular mechanisms. J. Allergy Clin. Immunol. 2010;125:S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhao L., Han J. J., Zhang F., Liu S., Zhu L., Wang Z. Z., Zhang G.X., Zhang Y. Carnosol modulates Th17 cell differentiation and microglial switch in experimental autoimmune encephalomyelitis. Front. Immunol. 2018;9:1807. doi: 10.3389/fimmu.2018.01807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A. H., Liang Y. C., Lin-Shiau S. Y., Ho C. T., Lin J. K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- Locksley R. M. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengoni E. S., Vichera G., Rigano L. A., Rodriguez-Puebla M. L., Galliano S. R., Cafferata E. E., Pivetta O. H., Moreno S., Vojnov A. A. Suppression of COX-2, IL-1b and TNF-a expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia. 2011;82:414–421. doi: 10.1016/j.fitote.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Newcomb D. C., Peebles R. S., Jr. Th17-mediated inflammation in asthma. Curr. Opin. Immunol. 2013;25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. W., Kundu J., Chae I. G., Kim D. H., Yu M. H., Kundu J. K., Chun K. S. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int. J. Oncol. 2014;44:1309–1315. doi: 10.3892/ijo.2014.2281. [DOI] [PubMed] [Google Scholar]
- Park S. J., Im D. S. Blockage of sphingosine-1-phosphate receptor 2 attenuates allergic asthma in mice. Br. J. Pharmacol. 2019;176:938–949. doi: 10.1111/bph.14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J., Lee S. J., Nam S. Y., Im D. S. GPR35 mediates lodoxamide-induced migration inhibitory response but not CXCL17-induced migration stimulatory response in THP-1 cells; is GPR35 a receptor for CXCL17? Br. J. Pharmacol. 2018;175:154–161. doi: 10.1111/bph.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeckel D., Greiner C., Verhoff M., Rau O., Tausch L., Hornig C., Steinhilber D., Schubert-Zsilavecz M., Werz O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008;76:91–97. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Rau O., Wurglics M., Paulke A., Zitzkowski J., Meindl N., Bock A., Dingermann T., Abdel-Tawab M., Schubert-Zsilavecz M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006;72:881–887. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- Schwager J., Richard N., Fowler A., Seifert N., Raederstorff D. Carnosol and related substances modulate chemokine and cytokine production in macrophages and chondrocytes. Molecules. 2016;21:465. doi: 10.3390/molecules21040465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singletary K. W. Rosemary extract and carnosol stimulate rat liver glutathione-S-transferase and quinone reductase activities. Cancer Lett. 1996;100:139–144. doi: 10.1016/0304-3835(95)04082-X. [DOI] [PubMed] [Google Scholar]
- Trinh H. K. T., Lee S. H., Cao T. B. T., Park H. S. Asthma pharmacotherapy: an update on leukotriene treatments. Expert Rev. Respir. Med. 2019;13:1169–1178. doi: 10.1080/17476348.2019.1670640. [DOI] [PubMed] [Google Scholar]
- Wakashin H., Hirose K., Iwamoto I., Nakajima H. Role of IL-23-Th17 cell axis in allergic airway inflammation. Int. Arch. Allergy Immunol. 2009;149 Suppl 1:108–112. doi: 10.1159/000211382. [DOI] [PubMed] [Google Scholar]
- Yeo I. J., Park J. H., Jang J. S., Lee D. Y., Park J. E., Choi Y. E., Joo J. H., Song J. K., Jeon H. O., Hong J. T. Inhibitory effect of Carnosol on UVB-induced inflammation via inhibition of STAT3. Arch. Pharm. Res. 2019;42:274–283. doi: 10.1007/s12272-018-1088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]