Abstract
Renal cell carcinoma (RCC) is likely to metastasize to other organs, and is often resistant to conventional chemotherapies. Thymoquinone (TQ), a phytochemical derived from the seeds of Nigella sativa, has been shown to inhibit migration and metastasis in various cancers. In this study, we assessed the effect of TQ on the migratory activity of human RCC Caki-1 cells. We found that treatment with TQ reduced the proteolytic activity of matrix metalloproteinase-9 (MMP-9) in Caki-1 cells. TQ significantly repressed prostaglandin E2 (PGE2) production, its EP2 receptor expression as well as the activation of Akt and p38, the well-known upstream signal proteins of MMP-9. In addition, treatment with butaprost, a PGE2 agonist, also induced MMP-9 activity and migration/invasion in Caki-1 cells. Moreover, pharmacological inhibitors of PI3K/Akt and p38 remarkably attenuated butaprost-induced Caki-1 cell migration and invasion, implying that activation of PI3K/Akt and p38 is a bridge between the PGE2-EP2 axis and MMP-9-dependent migration and invasion. Taken together, these data suggest that TQ is a promising anti-metastatic drug to treat advanced and metastatic RCC.
Keywords: Thymoquinone, Renal cell carcinoma (RCC), Prostaglandin E2 (PGE2), EP2 receptor, MMP-9
INTRODUCTION
Renal cell carcinoma (RCC) is the most common type of kidney cancer and accounts for approximately 3% of total malignancies (Cairns, 2010). If RCC is detected at the initial stage, it can be successfully removed by surgery, with a 5-year survival rate of nearly 70-80%. However, the early stage of RCC is usually asymptomatic, which makes it difficult to diagnose early. Thus, by the time it is discovered, RCC patients often present with metastasis to other organs, such as to the lymph, lung, and bone, which significantly lowers the survival rate (Bianchi et al., 2012). Even worse, advanced and metastatic RCC is particularly resistant to conventional chemotherapy and radiotherapy (Grimm et al., 2010). Therefore, it is crucial to inhibit and limit the metastatic activity of RCC for effective treatment.
Inflammation is a strong driving force for tumor metastasis (Wu and Zhou, 2009). During inflammatory responses, prostaglandin E2 (PGE2) is synthesized from arachidonic acids by cyclooxygenase-2 (COX-2) (Nakanishi and Rosenberg, 2013). PGE2 then binds to its four G protein-coupled receptors, EP1-EP4, stimulating cancer cell growth, invasion, and metastasis (Sugimoto and Narumiya, 2007). In addition to overproduction of PGE2, the aberrant expression of its receptors can also amplify PGE2 signaling, which can facilitate cancer promotion and metastasis. Previously, we found that EP2 and EP4 receptors were responsible for PGE2-dependent migration in a human RCC Caki-1 cell line (Woo et al., 2015). These results suggested that the PGE2-EP2/EP4 axis is a possible drug target for metastatic RCC.
The PGE2-EP2/EP4 axis can recruit matrix metalloproteinases (MMPs), which are pivotal for cancer metastasis (Yen et al., 2008; Jana et al., 2016). MMPs catalyze the proteolytic degradation of the extracellular matrix (ECM) in the tumor microenvironment, which is a physical barrier for cancer cell migration and invasion, thus leading to metastasis (Nabeshima et al., 2002). Consistent with this process, MMPs are overexpressed in various types of metastatic cancers, and serve as chemotherapeutic targets (Bergers et al., 2000; Stamenkovic, 2000).
Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone; TQ) is a monoterpene present in the seeds of black cumin, Nigella sativa (Kundu et al., 2014). TQ has been reported to exert various physiological functions, including anti-inflammatory, antiproliferative, and anticancer activities (Kundu et al., 2014). Hsu et al. (2017) have demonstrated that TQ inhibits human colon cancer cell migration by blocking the PGE2-EP2/EP4 axis. Moreover, TQ can inhibit metastasis through reduction of MMP-2 and MMP-9 in human and mouse brain cancers (Kolli-Bouhafs et al., 2012; Arumugam et al., 2016). TQ is believed to be nontoxic because it has long been used as a food additive, indicating that TQ can be a potential candidate for a nontoxic anti-metastatic drug to treat metastatic RCC. In the present study, we therefore investigated the effect of TQ on the metastatic properties of a human RCC Caki-1 cell line, and identified its underlying molecular mechanisms, particularly in the context of PGE2 signaling and MMP-9.
MATERIALS AND METHODS
Materials
TQ (purity 99%), gelatin, eosin Y, hematoxylin, and β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). EP2 antibody (#101750), PGE2, butaprost, and were purchased from Cayman Chemical (Ann Arbor, MI, USA). SB203580 was obtained from Calbiochem (San Diego, CA, USA). LY294002, Akt (#9272), p-Akt (#9271), p38 (#9212), and anti-rabbit IgG horseradish peroxidase-conjugated antibodies were obtained from Cell Signaling Technologies (Beverly, MA, USA). MMP-2 (ab92536) and MMP-9 (ab76003) antibodies were purchased from Abcam (Cambridge, UK). The p-p38 antibody (#sc-17852) was obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Cell culture
Human renal cancer Caki-1 cells were provided by Dr. T. K. Kwon (Keimyung University, Daegu, Korea) and were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotics (100 U/mL penicillin G and 100 µg/mL streptomycin) at 37°C under a humidified 95% air/5% CO2 mixture (v/v).
Cell viability assay
Caki-1 cells were seeded in 96-well plates at a density of 2×103 cells/well and were treated with 1, 5, and 10 μM TQ for 24, 48, and 72 h. Following treatment, the cells were incubated with fresh medium containing 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; AMRESCO, Solon, OH, USA) and incubated at 37°C for an additional 4 h. The medium was removed, and formazan was dissolved in 100 μL of dimethyl sulfoxide. The plates were then shaken for 5-10 min, and the absorbance at 570 nm was measured using a microplate reader (Tecan Trading, Mannedorf, Switzerland). The relative cell viability (%) was expressed as a percentage relative to the vehicle treated group.
Western blot analysis
The cells were harvested and lysed with RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) and the resulting protein samples were quantified using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein extracts were denatured by boiling at 100°C for 5 min in Laemmli sample buffer (Bio-Rad, Hercules. CA, USA). The proteins were separated by 8-12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 buffer (TBS-T) (10 mM Tris, 150 mM NaCl, pH 7.5, and 0.1% Tween 20) for 1 h at room temperature, and incubated with primary antibodies (diluted 1:1,000) overnight at 4°C. The membranes were then washed three times for 10 min each with TBS-T buffer and incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies. The membranes were then washed three times for 10 min each with TBS-T buffer, and incubated with Super-signal pico-chemiluminescent substrate or dura-luminol substrate (Thermo Fisher Scientific) according to the manufacturer’s instructions, and visualized using an ImageQuant LAS 4000 system (Fujifilm Life Science, Tokyo, Japan).
Wound-healing migration assay
The wound healing assay was conducted to determine the migratory ability of cells. Briefly, the cells were cultured at a concentration of 5×105 cells/mL in 6-well plates, and incubated until the cell density reached 90%. The cell monolayers were wounded by scratching with a 10-μL pipette tip, washed with phosphate-buffered saline (PBS) to remove detached cells, and then incubated in DMEM supplemented with 5% FBS containing appropriate reagents according to the experimental design. After 24 h at 37°C, the medium was replaced with PBS and washed twice. The gap-closure was then examined using a microscope (Olympus, Tokyo, Japan).
Cell invasion assay
The invasion capacity of Caki-1 cells was determined in vitro using Boyden Chambers (6.5-mm diameter filters, 8-mm pore size; Merck, Darmstadt, Germany). Briefly, Caki-1 cells (1×105 cells/200 µL serum-reduced medium) were placed in the upper chamber of Boyden Chambers (Merck). The appropriate reagents were added to the lower chamber (200 µL). The chambers were assembled and kept in an incubator for 24 h. At the desired time points, cells from the upper surface of the membranes (Millipore Sigma, Burlington, MA, USA) were removed with gentle swabbing and the migrating cells on the lower surface of membranes were fixed and stained with crystal violet. The membranes were then washed and mounted on glass slides, and the stained migrating cells were visualized using a microscope (Olympus).
Gelatin zymography assay
The gelatin zymography assay was performed to assess the proteolytic activities of MMPs by measuring their capability to degrade gelatin. To assess the enzymatic activity of MMPs, culture supernatants were collected, mixed with non-reducing sample buffer, and subjected to 10% SDS-PAGE containing 0.25% gelatin. The gel was washed three times with wash buffer (2.5% Triton X-100), and then incubated in incubating buffer (1 M Tris-HCl at pH 7.5, 5% NaN3 and 1 M CaCl2) overnight at 37°C. The gel was then stained with Coomassie Blue (Bio-Rad) and the MMP-9 enzymatic activity was quantified using the LAS 4000 imaging system (Fujifilm Life Science, Tokyo, Japan).
Statistical analysis
All data were obtained from more than three independent experiments. Statistical analysis was conducted using the paired Student’s t-test or analysis of variance. A value of p<0.05 was considered to be statistically significant.
RESULTS
TQ inhibits Caki-1 cell migration through suppression of MMP-9
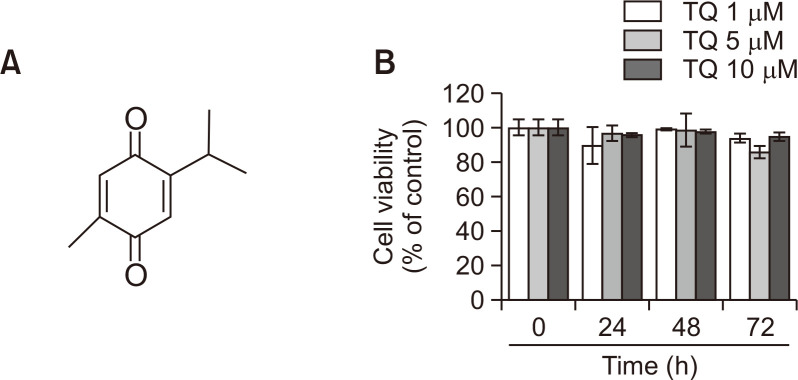
TQ is a phytochemical extracted from black cumin seeds and classified as a monoterpene based on its chemical structure as shown in Fig. 1A. To evaluate its effect on RCC migration, we utilized a human metastatic RCC Caki-1 cell line. Caki-1 cells were treated with various concentrations (1, 5, and 10 µM) of TQ for 24, 48, and 72 h. The MTT assay showed that TQ did not induce either cell death or cell proliferation, even after 72 h post-treatment (Fig. 1B). All the subsequent experiments were, therefore, performed at 24 h after treatment.
Fig. 1.
Effect of thymoquinone (TQ) on Caki-1 cell growth. (A) The chemical structure of TQ. (B) Caki-1 cells were treated with the indicated concentrations (1, 5, and 10 μM) of TQ for 24, 48, and 72 h. The cell viability was determined using the MTT assay as described in the Materials and Methods. The MTT formazan product was detected using a microplate reader. Data represent the mean ± standard deviation of three independent experiments (n=3).
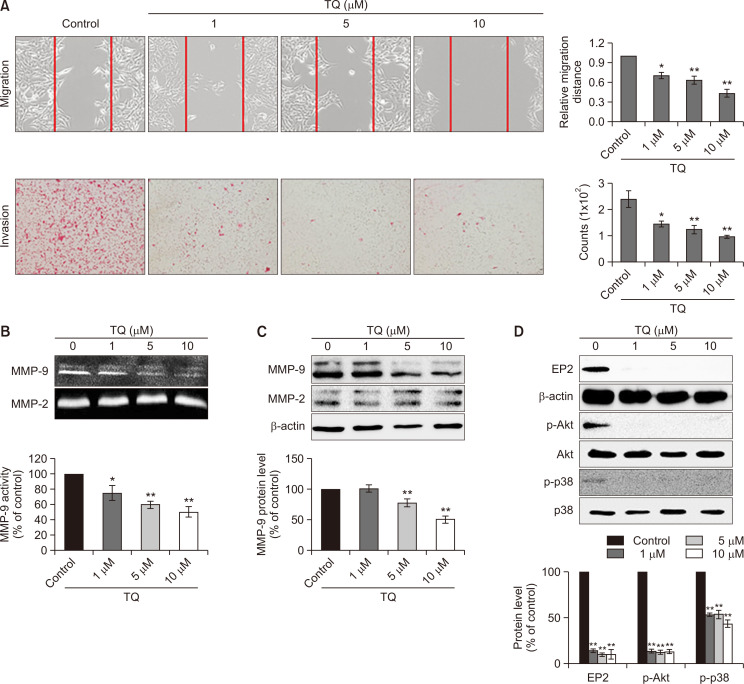
While TQ did not induce cancer cell death in Caki-1 cells, both wound-healing migration (Fig. 2A, upper panel) and invasion (Fig. 2A, bottom panel) assays showed that TQ significantly inhibited the migratory/invasive activity of Caki-1 cells with a high metastatic potential. Thus, we examined the effect of TQ on the activity and expression of MMP-9, a crucial enzyme for wound healing, cell spreading, and migration. Treatment with TQ reduced the proteolytic activity of MMP-9 in a concentration-dependent manner in Caki-1 cells (Fig. 2B). In addition to its activity, TQ also attenuated MMP-9 expression at the protein level (Fig. 2C). However, TQ was not able to modulate either the protein expression or enzymatic activity of MMP-2 (Fig. 2B, 2C). Taken together, these data suggested that TQ inhibited migration of human metastatic renal carcinoma Caki-1 cells by suppressing MMP-9.
Fig. 2.
The effect of thymoquinone (TQ) on Caki-1 cell migration. (A) Wound healing (upper panel) and invasion (bottom panel) assays were performed to determine the effect of TQ on the migration of Caki-1 cells. For the wound healing assay (upper panel), the cells were treated with dimethyl sulfoxide (control) or TQ (1, 5, and 10 µM) for 24 h after wounding. The gap closure was detected using a microscope (Olympus, Tokyo, Japan). The wound sizes were measured using a microscope (Olympus) and expressed as the mean of triplicates ± standard deviation (SD). *p<0.05, **p<0.005. For the migration assay (bottom panel), Caki-1 cells were seeded in the upper chamber of a Transwell and treated with TQ (1, 5, and 10 µM) for 24 h. The migrated cells were then stained and observed using a microscope (Olympus). The migrating cells per microscopic field were counted and represented on a bar graph as the mean of triplicates ± SD. *p<0.05, **p<0.005. (B) The proteolytic activities of MMP-9 and MMP-2 were determined in Caki-1 cells treated with 1, 5, and 10 µM of TQ for 24 h using the gelatin zymography assay. The proteolytic activity of MMP-9 is represented on a bar graph as the mean of triplicates ± SD. *p<0.05, **p<0.005. (C, D) Western blot analysis of the protein expression of MMP-9 and MMP-2 (C), EP2 and phosphorylated/non-phosphorylated forms of Akt and p38 (D) in Caki-1 cells treated with TQ (1, 5, and 10 µM) for 24 h. The β-actin was the protein loading control. The protein expression levels of EP2, p-Akt, and pp38 were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/) and then normalized to actin or the respective total forms. A bar graph represents the mean of triplicates ± SD. **p<0.005.
Previously, we reported that the PGE2-EP2/EP4 axis was crucial for the migratory activity in Caki-1 cells (Woo et al., 2015). Treatment with TQ significantly repressed the expression of EP2 and activation of Akt and p38, the well-known upstream signals of MMP-9 (Fig. 2D) (Ruhul Amin et al., 2003; Lee et al., 2009). These data indicated the possibility for reduction of the PGE2-EP2-MMP9 axis by TQ treatment in Caki-1 cells.
TQ suppresses the PGE2-EP2 axis-mediated migratory activity of Caki-1 cells
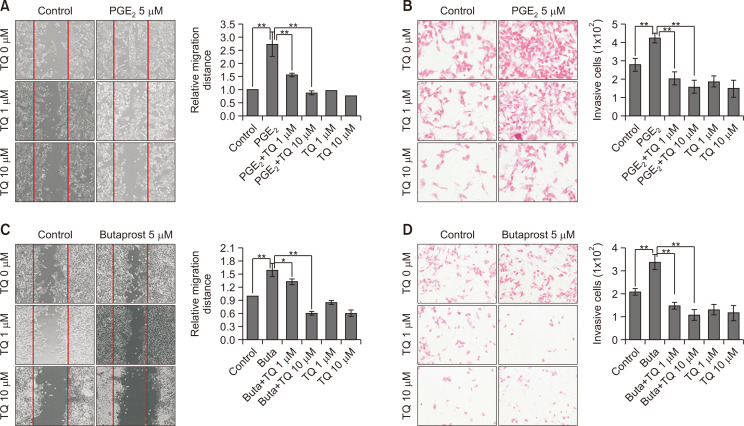
To verify the effect of TQ on PGE2-EP2 axis-induced migration, Caki-1 cells were co-treated with TQ plus either PGE2 or butaprost, an EP2 selective agonist. As previously reported, we confirmed that both PGE2 and butaprost remarkably accelerated wound closure (Fig. 3A, 3C) and invasion (Fig. 3B, 3D) of Caki-1 cells. In the presence of TQ, however, the PGE2- or butaprost-induced wound healing and invasion were markedly decreased (Fig. 3). These results showed that TQ suppressed the PGE2-EP2 axis-stimulated migration/invasion in Caki-1 cells.
Fig. 3.
The effect of thymoquinone (TQ) on prostaglandin E2 (PGE2)- or butaprost-induced Caki-1 cell migration. (A, C) Caki-1 cells were treated with TQ (1 and 10 µM) in the presence or absence of 5 µM PGE2 (A) or butaprost (C) after wounding. At 24 h post-treatment, microscopic examination was performed to determine the wound closure. Solid line, the gap space of controls. The wound sizes were measured using a microscope (Olympus, Tokyo, Japan) and expressed as the mean of triplicates ± standard deviation (SD). *p<0.05; **p<0.005. (B, D) Caki-1 cells were seeded in the upper chamber of the Transwell, then incubated in media containing TQ (1 and 10 µM) with or without 5 µM PGE2 (B) or butaprost (D) for 24 h. Crystal violet was used to stain and detect the migrated cells. The migrating cells per microscopic field were counted and represented on a bar graph as the mean ± SD. **p<0.005.
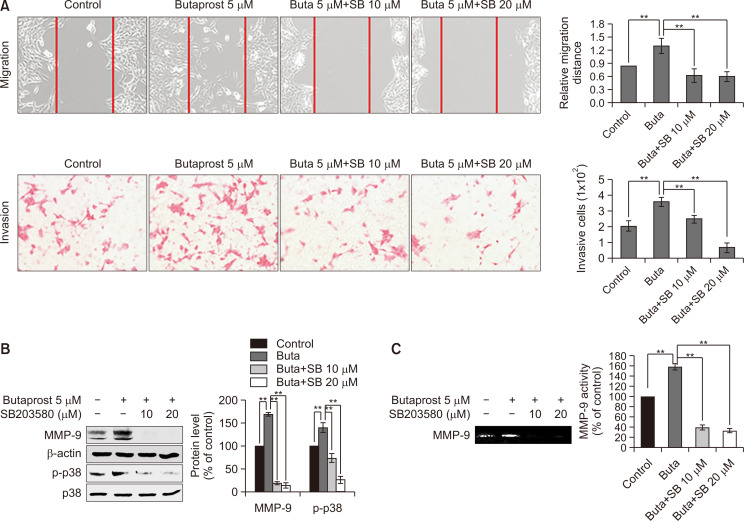
The EP2 agonist, butaprost, activates MMP-9, possibly via the Akt and p38 signaling pathways in Caki-1 cells
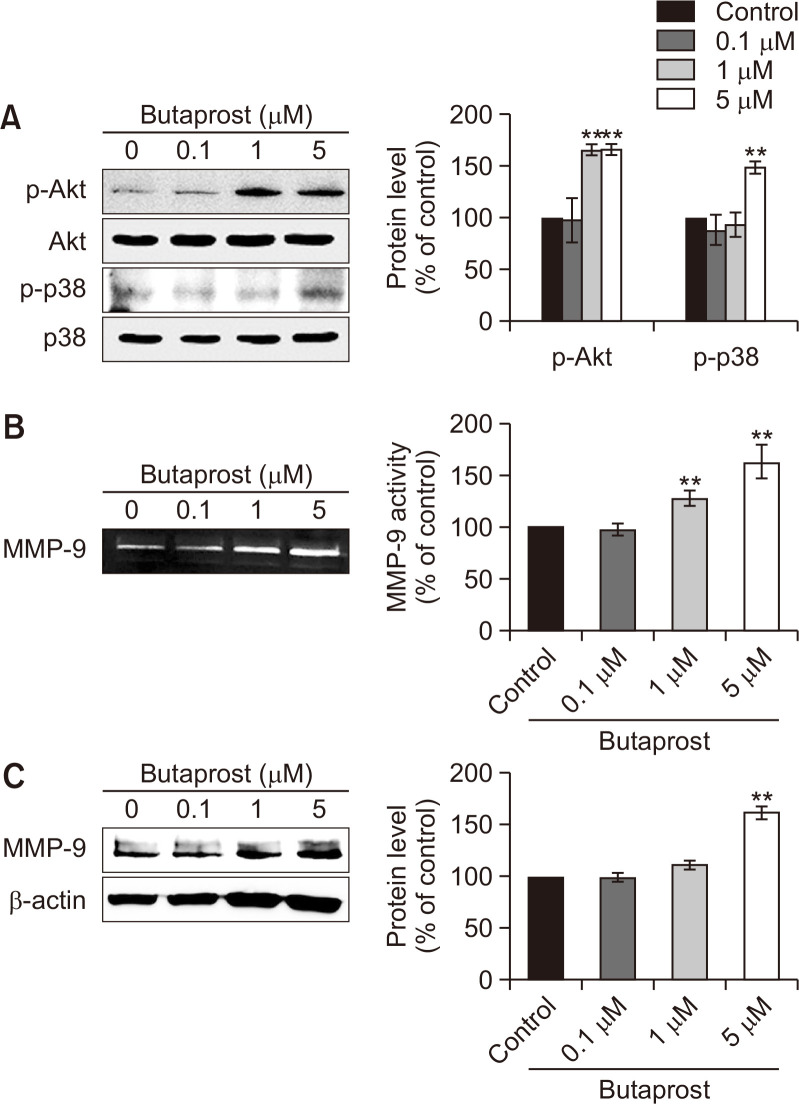
As shown in Fig. 2, TQ inhibited migration and MMP-9 activity, simultaneously suppressing the expression of EP2, phosphorylation of Akt, p38, and the MMP-9 upstream regulator. We thus assumed that Akt and p38 might be responsible for the PGE2/EP2 axis-induced MMP-9 activation and subsequent migration in Caki-1 cells. To test this hypothesis, we examined whether the EP2 selective agonist, butaprost, regulated the Akt and p38 signaling pathways in Caki-1 cells. Butaprost induced phosphorylation of both Akt and p38 in Caki-1 cells (Fig. 4A). Moreover, butaprost increased MMP-9 proteolytic activity (Fig. 4B) as well as protein expression levels (Fig. 4C) in a concentration-dependent manner. These data indicated that the PGE2-EP2 axis may stimulate migration through activation of Akt and p38 in Caki-1 cells.
Fig. 4.
The effect of butaprost on the activation and expression of MMP-9 in Caki-1 cells. (A) Western blot analysis of the phosphorylated levels of Akt and p38 in Caki-1 cells treated with butaprost (0.1, 1, and 5 µM) for 24 h. The protein expression levels were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA) and then normalized to the respective total forms. A bar graph represents the mean of triplicates ± standard deviation (SD). **p<0.005. (B, C) Under the same condition as in (A), the effects of butaprost on the proteolytic activity (B) and protein expression (C) of MMP-9 were determined by gelatin zymography (B) and western blotting (C), respectively. β-actin was used as a protein loading control for western blotting. Densitometry was performed using Image J software (National Institutes of Health). A bar graph represents the mean of triplicates ± SD. **p<0.005.
The PI3K/Akt inhibitor, LY294002, reduces the migratory activity of Caki-1 cells
To verify the involvement of the Akt and p38 signaling pathways, pharmacological inhibitors of Akt and p38, LY294002 and SB203580, respectively, were selected for subsequent studies. First, the effect of the PI3K/Akt inhibitor LY294002 on butaprost-induced Caki-1 cell migration was examined. The wound healing assay showed that treatment with LY294002 significantly reduced closure of open scratches stimulated by butaprost in Caki-1 cells (Fig. 5A, upper panel). In addition, LY294002 attenuated butaprost-induced invasion (Fig. 5A, bottom panel), further supporting the involvement of PI3K/Akt signaling in the PGE2-EP2 axis-promoted migration. Western blot analysis confirmed the inhibitory effect of LY294002 on Akt activation in Caki-1 cells (Fig. 5B). Together, the results showed that inhibition of the PI3K/Akt pathway by LY294002 treatment blocked butaprost-induced MMP-9 expression (Fig. 5B) as well as its enzymatic activation (Fig. 5C).
Fig. 5.
The effect of the PI3K inhibitor, LY294002, on cell migration and MMP-9 expression in Caki-1 cells. (A) Wound healing (upper panel) and invasion (bottom panel) assays were conducted to assess the effect of LY294002 (LY) on butaprost-induced cell migration. Caki-1 cells were treated with butaprost (5 µM) plus or minus LY294002 (10 and 20 µM) for 24 h, followed by obtaining microphotographs of the gap closure (for the wound healing assay, upper panel) and Crystal violet-stained migrated cells (for the migration assay, bottom panel). Bar graphs represent the relative gap distance (upper panel) and the migrating cells per field (lower panel); *p<0.05; **p<0.005. (B, C) Caki-1 cells were treated in the same manner as described in (A). At 24 h after treatment, western blotting analyzed Akt phosphorylation and MMP-9 expression (B), and gelatin zymography revealed MMP-9 proteolytic activity (C). β-actin was used as a protein loading control for western blotting. Densitometry was performed using Image J software (National Institutes of Health, Bethesda, MD, USA). A bar graph represents the mean of triplicates ± standard deviation. **p<0.005.
Inhibition of p38 inhibition by treatment with SB203580 suppresses Caki-1 cell migration
We also investigated the effects of the p38 inhibitor, SB203580, in the same manner as shown in Fig. 5. SB203580 also had similar inhibitory effects on butaprost-induced Caki-1 cell migration/invasion (Fig. 6A). SB203580 treatment inactivated p38 in a concentration-dependent manner, which led to the attenuation of MMP-9 induction by butaprost (Fig. 6B, 6C).
Fig. 6.
The effect of the p38 inhibitor, SB203580 , on cell migration and MMP-9 expression in Caki-1 cells. (A) Wound healing (upper panel) and invasion (bottom panel) assays were performed to assess the effect of SB203580 (SB) on butaprost-induced cell migration. Caki-1 cells were treated with butaprost (5 µM) plus or minus SB (10 and 20 µM) for 24 h, followed by obtaining microphotographs of the gap closure (for the wound healing assay, upper panel) and Crystal violet-stained migrated cells (for the migration assay, bottom panel). For the control and butaprost-treated groups, the same representative images were used as shown in Fig. 5A, because the experiments for Fig. 5A and 6A were conducted simultaneously. Bar graphs represent the relative gap distance (upper panel) and the migrating cells per field (lower panel). **p<0.005. (B, C) Caki-1 cells were treated in the same manner as described in (A). At 24 h after treatment, western blotting determined p38 phosphorylation and MMP-9 expression (B) and gelatin zymography revealed the MMP-9 proteolytic activity (C). β-actin was used as a protein loading control for western blotting. Densitometry was performed using Image J software (National Institutes of Health, Bethesda, MD, USA). A bar graph represents the mean of triplicates ± standard deviation. **p<0.005.
DISCUSSION
Although cancer treatment strategies have been evolving rapidly, including patient-derived xenograft and immunotherapies, the treatment of metastatic RCC using cancer therapies has not undergone similar advances. At the time of diagnosis, metastasis is found in more than half of RCC patients, who often cannot be treated using conventional chemotherapy or radiotherapy (Grimm et al., 2010). Thus, the most efficient strategy to effectively treat metastatic RCC is to prevent metastatic spread from the primary tumor site.
The metastatic process can be initiated by cancer cell migration, requiring proteolytic degradation of the ECM that acts as a physical barrier against cell migration (Nabeshima et al., 2002). MMPs, the main ECM enzymes, can cleave the connection between ECM components, leading to the disruption of cellular physical scaffolds as well as facilitation of cell invasion and migration. MMP-2 and MMP-9 have been reported to play major roles in the tumor microenvironment. Wang et al. (2010) have shown that MMP-9 gene silencing significantly inhibits metastasis in a tail vein injection model. In addition, inhibition of either MMP-2 or MMP-9 by using small molecules can reduce the metastatic potential of retinoblastoma, as determined by wound healing and Boyden chamber assays (Webb et al., 2017). MMP-9 is strongly associated with tumor invasion and metastasis in RCC (Sun et al., 2014). Moreover, reduction of TIMP1 expression induces the activity of MMP-9 and promotes migration and invasion of RCC cells (Guo et al., 2019). Notably, consistent with our results, silencing of MMP-9, but not MMP-2, abolished migration induced by the treatment of the G-protein coupled estrogen receptor agonist in RCC cells (Sun et al., 2014). In the present study, we tested whether MMP-2 and MMP-9 served as crucial molecular targets for metastatic RCC treatment by using TQ.
TQ is a phytochemical derived from the seeds of Nigella sativa, classified as a monoterpene based on its chemical structure (Kundu et al., 2014). Numerous investigators have reported that TQ exerts various beneficial effects, such as antitumorigenic, antioxidative, and anti-inflammatory activities (Kundu et al., 2014). In addition, clinical interventions also recommend consumption of TQ or Nigella sativa to improve the health or prevent and/or treat clinical symptoms without severe adverse effects in patients (Tavakkoli et al., 2017; Gholamnezhad et al., 2019; Yimer et al., 2019). We thus aimed to validate TQ as a potential candidate for metastatic RCC treatment. In the present study, we found that TQ treatment significantly inhibited Caki-1 cell migration. Moreover, TQ reduced MMP-9 protein expression, as well as its proteolytic activity, in Caki-1 cells. However, as shown in the Fig. 2B and 2C, TQ was unable to modulate both expression and enzymatic activity of MMP-2 (Fig. 2B, 2C), suggesting that the inhibitory effect of TQ on Caki-1 cell migration specifically inactivated MMP-9. Consistent with our results, multiple studies have reported that MMPs play important roles in cellular interactions with the matrix in different organ systems (Johnson and Galis, 2004; Giannandrea and Parks, 2014). While MMP-2 is constitutively expressed, MMP-9 levels are usually low and enzyme expression is induced by various cytokines (Sternlicht and Werb, 2001; Lynch and Matrisian, 2002). Because the matrix-degrading activity of MMP-9 is stronger than that of MMP-2, MMP-9 may be a more important target for metastasis in carcinomas (Yamagata et al., 1989; Yasumitsu et al., 1992). Furthermore, MMP-9 expression has been found to be significantly correlated with poor prognoses in RCC patients (Kallakury et al., 2001).
In response to inflammatory stimuli, COX-2 induces eicosanoid synthesis from arachidonic acids, which can modulate cell proliferation, inflammation, and immune responses (Nakanishi and Rosenberg, 2013). Among the eicosanoids produced by COX-2 activation, PGE2 is the most potent protumorigenic eicosanoid that promotes cell proliferation, proinflammatory cytokine stimulation, and cancer cell migration (Greenhough et al., 2009). PGE2 exerts its cellular functions via binding to its G protein-coupled receptors, EP1-EP4. EP2 is mostly responsible for the protumorigenic effects of PGE2. EP2 knockout mice showed reduced tumorigenesis in a carcinogen-induced skin cancer model, while EP2 overexpressing transgenic mice exhibited increased numbers of tumors when compared with wild-type mice (Sung et al., 2006; Asting et al., 2017). Moreover, the PGE2-EP2/EP4 signaling axis can induce MMP-9 expression as well as its activation, resulting in the invasion and migration of human endometrial epithelial and stromal cells (Lee et al., 2011). Importantly, increased levels of PGE2 in RCC indicate poor prognoses and a reduced survival time (Wang and Dubois, 2010). In agreement with our findings, Li et al. (2013) reported that PGE2 treatment promoted EP2-mediated Akt activation in human renal cell carcinoma SN12C cells. In a similar manner, we have previously shown that the PGE2-EP2 signaling is crucial for Caki-1 cell migration (Woo et al., 2015). It is thus worthwhile identifying the function of EP2 signaling that regulates the migration and/or invasion in RCC. In the present study, TQ treatment simultaneously inhibited EP2 expression and MMP-9 activation, which strongly suggested that TQ inhibited Caki-1 cell migration via suppression of the PGE2-EP2-MMP-9 axis. Previously, we have reported that activation of the EP4 receptor induces Caki-1 cell migration (Woo et al., 2015). Given all these results, we assumed that Caki-1 cells utilize either EP2 or EP4 as a driving force for migration, depending on their surrounding microenvironment. This will provide a distinct direction for further investigation of regulatory mechanisms, to identify the antimigratory and antimetastatic effects of TQ in metastatic RCCs.
Here, we found that activation of PI3K/Akt and p38 was a missing link between the PGE2-EP2 axis and MMP-9 in Caki-1 cells. It has been reported that proinflammatory cytokines, such as IL-1β and TNFα, promote the secretion of MMP-9 via upregulation of PI3K/Akt and mitogen-activated protein kinases (Ruhul Amin et al., 2003; Lee et al., 2009). Inflammation is a pivotal driving force for MMP-9-dependent metastasis because it produces either PGE2 or cytokines. Thus, inflammation could also facilitate combination of the regulatory circuits, such as EP2 activation, Akt and p38 phosphorylation, and MMP-9 induction. In a previous report, Chun et al. showed that EP2 overexpression led to Src and β-arrestin binding, and induced the phosphorylation/activation of p38 in the human embryonic kidney HEK293 cells (Chun and Shim, 2015). The elucidation of the relationship between EP2 and β-arrestin in Caki-1 cells, together with TQ, would, therefore, be an important objective for future studies.
As described in Fig. 7, TQ inhibits the migration of RCC cells via inactivation of Akt and p38, as well as decreased MMP-9 activity, by decreasing PGE2-EP2 expression in RCC cells. However, more investigations are needed to determine whether Akt and p38 occur continuously or independently in regulating the activity of MMP-9 in RCC. Taken together, these findings suggested that TQ is a promising antimetastatic drug to treat human RCCs. The phytochemical TQ has long been used as a food additive, ensuring its nontoxicity, which should benefit patients during long-term therapy. Moreover, it can be utilized as an adjuvant for combination therapy or immunotherapy, which would be helpful, at least in part, in the establishment of the most effective treatment strategy in clinical settings.
Fig. 7.
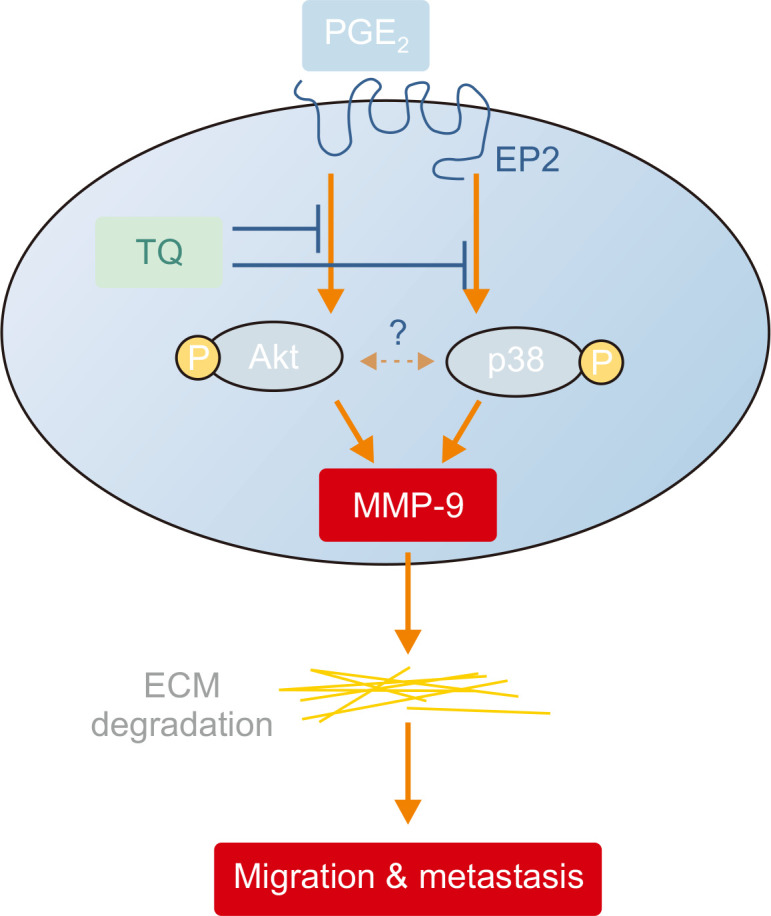
A proposed mechanism underlying the inhibition of EP2-Akt/p38-MMP-9 by thymoquinone (TQ) in Caki-1 cells. TQ inhibits renal carcinoma cell migration via the inactivation of Akt and p38 as well as decreasing MMP-9 activity by decreasing the expression of prostaglandin E2-EP2.
ACKNOWLEDGMENTS
This research was supported by the Basic Research Grant of Keimyung University in 2019.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- Arumugam P., Subramanian R., Priyadharsini J. V., Gopalswamy J. Thymoquinone inhibits the migration of mouse neuroblastoma (Neuro-2a) cells by down-regulating MMP-2 and MMP-9. Chin. J. Nat. Med. 2016;14:904–912. doi: 10.1016/S1875-5364(17)30015-8. [DOI] [PubMed] [Google Scholar]
- Asting A. G., Iresjo B. M., Nilsberth C., Smedh U., Lundholm K. Host knockout of E-prostanoid 2 receptors reduces tumor growth and causes major alterations of gene expression in prostaglandin E2-producing tumors. Oncol. Lett. 2017;13:476–482. doi: 10.3892/ol.2016.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Brekken R., McMahon G., Vu T. H., Itoh T., Tamaki K., Tanzawa K., Thorpe P., Itohara S., Werb Z., Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M., Sun M., Jeldres C., Shariat S. F., Trinh Q. D., Briganti A., Tian Z., Schmitges J., Graefen M., Perrotte P., Menon M., Montorsi F., Karakiewicz P. I. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann. Oncol. 2012;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9:461–473. doi: 10.3233/CBM-160615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun K. S., Shim M. EP2 induces p38 phosphorylation via the activation of Src in HEK-293 cells. Biomol. Ther. (Seoul) 2015;23:539–548. doi: 10.4062/biomolther.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamnezhad Z., Shakeri F., Saadat S., Ghorani V., Boskabady M. H. Clinical and experimental effects of Nigella sativa and its constituents on respiratory and allergic disorders. Avicenna. J. Phytomed. 2019;9:195–212. [PMC free article] [PubMed] [Google Scholar]
- Giannandrea M., Parks W. C. Diverse functions of matrix metalloproteinases during fibrosis. Dis. Model. Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhough A., Smartt H. J., Moore A. E., Roberts H. R., Williams A. C., Paraskeva C., Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- Grimm M. O., Wolff I., Zastrow S., Frohner M., Wirth M. Advances in renal cell carcinoma treatment. Ther. Adv. Urol. 2010;2:11–17. doi: 10.1177/1756287210364959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Liu J., Han X., Zhang X., Lin T., Wang Y., Bai J., Han J. FBXO22 suppresses metastasis in human renal cell carcinoma via inhibiting MMP-9-mediated migration and invasion and VEGF-mediated angiogenesis. Int. J. Biol. Sci. 2019;15:647–656. doi: 10.7150/ijbs.31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. H., Chen M. C., Day C. H., Lin Y. M., Li S. Y., Tu C. C., Padma V. V., Shih H. N., Kuo W. W., Huang C. Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J. Gastroenterol. 2017;23:1171–1179. doi: 10.3748/wjg.v23.i7.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S., Chatterjee K., Ray A. K., DasMahapatra P., Swarnakar S. Regulation of matrix metalloproteinase-2 activity by COX-2-PGE2-pAKT axis promotes angiogenesis in endometriosis. PLoS ONE. 2016;11:e0163540. doi: 10.1371/journal.pone.0163540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Galis Z. S. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler. Thromb. Vasc. Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- Kallakury B. V., Karikehalli S., Haholu A., Sheehan C. E., Azumi N., Ross J. S. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin. Cancer Res. 2001;7:3113–3119. [PubMed] [Google Scholar]
- Kolli-Bouhafs K., Boukhari A., Abusnina A., Velot E., Gies J. P., Lugnier C., Ronde P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Invest. New Drugs. 2012;30:2121–2131. doi: 10.1007/s10637-011-9777-3. [DOI] [PubMed] [Google Scholar]
- Kundu J., Chun K. S., Aruoma O. I., Kundu J. K. Mechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutat. Res. 2014;768:22–34. doi: 10.1016/j.mrfmmm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Lee J., Banu S. K., Subbarao T., Starzinski-Powitz A., Arosh J. A. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol. Cell. Endocrinol. 2011;332:306–313. doi: 10.1016/j.mce.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Lan Tran H. T., Van Ta Q. Regulation of expression of matrix metalloproteinase-9 by JNK in Raw 264.7 cells: presence of inhibitory factor(s) suppressing MMP-9 induction in serum and conditioned media. Exp. Mol. Med. 2009;41:259–268. doi: 10.3858/emm.2009.41.4.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang Y., Kim W. J., Daaka Y. PGE2 promotes renal carcinoma cell invasion through activated RalA. Oncogene. 2013;32:1408–1415. doi: 10.1038/onc.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C. C., Matrisian L. M. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- Nabeshima K., Inoue T., Shimao Y., Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol. Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Rosenberg D. W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhul Amin A. R., Senga T., Oo M. L., Thant A. A., Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: a role for the dual signalling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Sun G. G., Wei C. D., Jing S. W., Hu W. N. Interactions between filamin A and MMP-9 regulate proliferation and invasion in renal cell carcinoma. Asian Pac. J. Cancer Prev. 2014;15:3789–3795. doi: 10.7314/APJCP.2014.15.8.3789. [DOI] [PubMed] [Google Scholar]
- Sung Y. M., He G., Hwang D. H., Fischer S. M. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25:5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- Tavakkoli A., Mahdian V., Razavi B. M., Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J. Pharmacopuncture. 2017;20:179–193. doi: 10.3831/KPI.2017.20.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Dubois R. N. Eicosanoids and cancer. Nat. Rev. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Nagase H., Watanabe T., Nobusue H., Suzuki T., Asami Y., Shinojima Y., Kawashima H., Takagi K., Mishra R., Igarashi J., Kimura M., Takayama T., Fukuda N., Sugiyama H. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci. 2010;101:759–766. doi: 10.1111/j.1349-7006.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A. H., Gao B. T., Goldsmith Z. K., Irvine A. S., Saleh N., Lee R. P., Lendermon J. B., Bheemreddy R., Zhang Q., Brennan R. C., Johnson D., Steinle J. J., Wilson M. W., Morales-Tirado V. M. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17:434. doi: 10.1186/s12885-017-3418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. M., Min K. J., Chae I. G., Chun K. S., Kwon T. K. Silymarin suppresses the PGE2-induced cell migration through inhibition of EP2 activation; G protein-dependent PKA-CREB and G protein-independent Src-STAT3 signal pathways. Mol. Carcinog. 2015;54:216–228. doi: 10.1002/mc.22092. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou B. P. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata S., Tanaka R., Ito Y., Shimizu S. Gelatinases of murine metastatic tumor cells. Biochem. Biophys. Res. Commun. 1989;158:228–234. doi: 10.1016/S0006-291X(89)80202-5. [DOI] [PubMed] [Google Scholar]
- Yasumitsu H., Miyazaki K., Umenishi F., Koshikawa N., Umeda M. Comparison of extracellular matrix-degrading activities between 64-kDa and 90-kDa gelatinases purified in inhibitor-free forms from human schwannoma cells. J. Biochem. 1992;111:74–80. doi: 10.1093/oxfordjournals.jbchem.a123721. [DOI] [PubMed] [Google Scholar]
- Yen J. H., Khayrullina T., Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimer E. M., Tuem K. B., Karim A., Ur-Rehman N., Anwar F. Nigella sativa L. (black cumin): a promising natural remedy for wide range of illnesses. Evid. Based Complement. Alternat. Med. 2019;2019:1528635. doi: 10.1155/2019/1528635. [DOI] [PMC free article] [PubMed] [Google Scholar]