Abstract
Here we describe novel spherical structures that are induced by cold shock on the lampbrush chromosomes (LBCs) of Xenopus laevis oocytes. We call these structures cold bodies or C-bodies. C-bodies are distributed symmetrically on homologous LBCs, with a pattern similar to that of 5S rDNA. Neither active transcription nor translation is necessary for their formation. Similar protrusions occur on the edges of some nucleoli. Endogenous LBCs as well as those derived from injected sperm form C-bodies under cold shock conditions. The function of C-bodies is unknown.
Keywords: chromatin, cold body, nucleolus, oocyte, sperm, Xenopus
Introduction
Giant chromosomes were first described from shark oocytes in the late 19th century by Rückert [1]. Because they superficially resembled the brushes used for cleaning kerosene lamp chimneys, Rückert named them lampbrush chromosomes (LBCs). Since then, LBCs have been characterized most extensively from amphibian and avian oocytes, but they occur in many other giant oocytes, including those of insects and even some plants [2, 3].
The oocyte nucleus or germinal vesicle (GV) of amphibians is an excellent model for understanding the physiological characteristics of the nucleus [4, 5]. The GV is arrested at the diplotene phase of meiosis I, at which time homologous chromosomes are held together by one or more chiasmata. Each homologue has an axis of condensed regions called chromomeres, which are detectable by the DNA-specific dye 4′,6-diamidino-2-phenylindole (DAPI). In addition, many paired loops extend laterally from the chromomere axis. The lateral loops are engaged in active transcription, as shown by the incorporation of RNA precursors and by immunostaining with specific antibodies (Fig. 1). For example, antibody H14, which detects phosphorylated RNA polymerase II, stains all lateral loops. In addition to the giant chromosomes themselves, each GV contains hundreds of extrachromosomal nucleoli, histone locus bodies, and Cajal bodies.
Figure 1. Part of a lampbrush chromosome bivalent from Xenopus laevis.
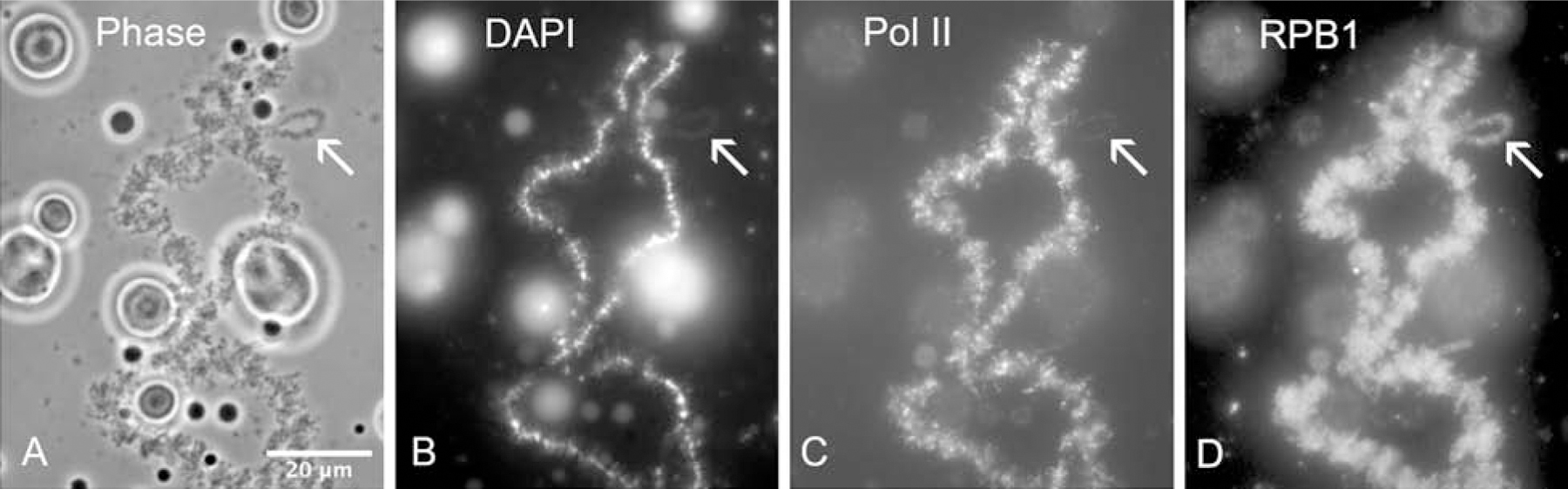
(A) Part of one bivalent from a control GV, showing loops extending laterally from the chromosome axes. An arrow indicates an especially large loop. The loops represent regions of active transcription. Several large extrachromosomal nucleoli are visible in this field. Phase contrast. (B) The same field stained with the DNA-specific dye DAPI. DNA is evident in the chromomere axis of the chromosome, as well as in the core of the nucleoli. (C) Staining with antibody H14 (against phosphorylated pol II) demonstrates active transcription on the lateral loops of the chromosomes. (D) An antibody against RNA polymerase II subunit RPB1 (clone 8WG16) stains the lateral loops.
In earlier experiments we injected sperm heads into the GV of intact oocytes. Over a period of several hours the highly condensed sperm heads swelled, eventually releasing their chromosomes. Even more remarkably, these chromosomes enlarged and came to resemble the endogenous LBCs, complete with active transcription on well-formed lateral loops [6, 7].
In a test of various experimental conditions, we placed oocytes at a temperature of 4°C for 12 or more hours. Although the overall morphology of the transcriptionally active lateral loops did not change much, some parts of the LBCs formed spherical structures visible by light microscopy. We call these spherical structures chromosomal bodies or C-bodies. Cold shock induced similar protrusions on the outside of some nucleoli. Here we describe the morphology of these bodies and perform experiments to analyze their possible function.
Materials and Methods
Oocytes and LBC spreads
Adult frogs Xenopus laevis were purchased from Xenopus 1 (Dexter, MI). Oocytes were removed from adult females by surgery and were held at room temperature (18–24°C) in a Petri dish of OR2 saline [8]. In some cases, oocytes were cold shocked at 4°C. Chromosome spreads were prepared from individual GVs as described previously [4, 6, 7].
Transcription or translation inhibition
To inhibit transcription, oocytes were treated with 20 μg/ml actinomycin D or 10 μg/ml α-amanitin for 23 h [9]. To inhibit translation, oocytes were treated with 10–100 μg/ml cycloheximide for 23 h.
Oocyte injections
Oocyte injections were performed as described previously [9, 10]. To monitor newly synthesized RNAs, each oocyte was injected with 23 nl of 20 mM bromouridine triphosphate (BrUTP). The BrUTP was visualized by immunofluorescence using an antibody against BrUTP. To label specific chromosomal loci and rDNA, the HA-RPB6 plasmid was injected into the oocyte [9]. Sperm heads of Xenopus laevis were injected into the GV as described previously [7].
Immunofluorescence
GV spreads were stained with antibodies as described previously [6, 7, 9]. Primary antibodies used in this study include mouse mAb H14 (IgM) against RNA polymerase (pol) II [11]; mAb 8WG16 (IgG) against RNA polymerase II subunit RPB1; mAb K121 against the trimethylguanosine (TMG) cap of snRNAs [12]; Alexa 488 conjugated anti-BrUTP; mAb No34 against proteins derived from the high-speed pellet of fractionated Xenopus laevis oocyte nuclei [13]; rabbit anti-RPB6. Hemagglutinin (HA)-tagged RPB6 (HA-RPB6) was detected with rat mAb 3F10 (Roche Applied Science, Indianapolis, IN); secondary antibodies were Alexa 488- or Alexa 594-conjugated goat anti-mouse IgG or IgM, goat anti-rat IgG or goat anti-rabbit IgG (Molecular Probes, Eugene, OR). The DNA of the chromosomes was stained with 0.01 μg/ml DAPI (4′,6 diamidino-2-phenylindole) for 4–12 h at room temperature. Slides were mounted in 50% glycerol.
Microscopy
Specimens were examined with a Zeiss 63X 1.25 N.A. planapo objective on the Zeiss Axioplan fluorescence microscope. Images were captured with a Micromax charge-coupled device camera (Princeton Instruments, Trenton, NJ) using the IPLab (3.5.5) image acquisition and analysis program (Scanalytics, Fairfax, VA). Image processing, including pairwise stitching, was performed using Fiji/ImageJ software [14].
Results
Cold shock induces chromosomal bodies in the Xenopus GV
When Xenopus oocytes are placed at 4°C for 12 hours or more, the general morphology of the LBCs persists, including the presence of lateral loops. However, cold temperature causes many spherical or slightly ellipsoidal structures to appear on the LBCs (Fig. 2). They appear either in pairs, attached to corresponding positions on homologous chromosomes, or as single spheres at points where homologous chromosomes are joined (chiasmata). We call these structures chromosomal bodies, or C-bodies for short. The distribution of C-bodies is quite similar to that of the oocyte-type 5S ribosomal DNA, specifically at or near the ends of 16 of the 18 chromosomes [15, 16].
Figure 2. Cold shock induces C-bodies on Xenopus LBCs.
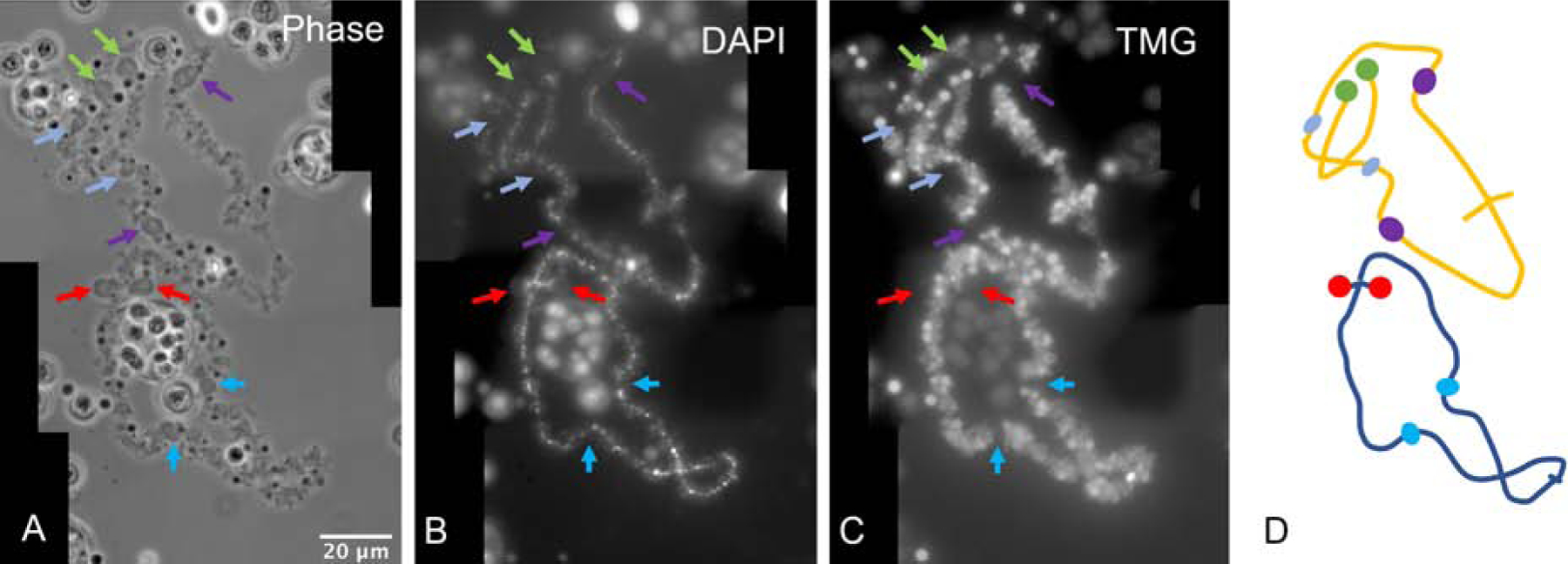
(A) Cold shock induces C-bodies at homologous positions on Xenopus LBCs (arrows). Phase contrast image. (B) The chromomeres of homologous chromosomes are clearly visible after DAPI staining, but the C-bodies are not stained. (C) An antibody against the trimethyl guanosine (TMG) part of snRNAs stains the lateral loops, but not C-bodies. (D) Diagram of A–C. Green and red arrows: terminal C-bodies. Purple and blue arrows: interstitial C-bodies.
We used two antibodies to further examine the distribution of C-bodies on the chromosomes: antibody No34 that marks specific chromosomal loci [9, 13], and an antibody against RPB6, a subunit common to the three RNA polymerases, which labels both specific loci and C-bodies (Fig. 3). A close inspection reveals that the No34 and RPB6 signals overlap. The No34 signal is at the base of the C-body and coincides with the chromomere, whereas the RPB6 signal is throughout the C-body.
Figure 3. C-bodies are associated with specific chromosomal loci.
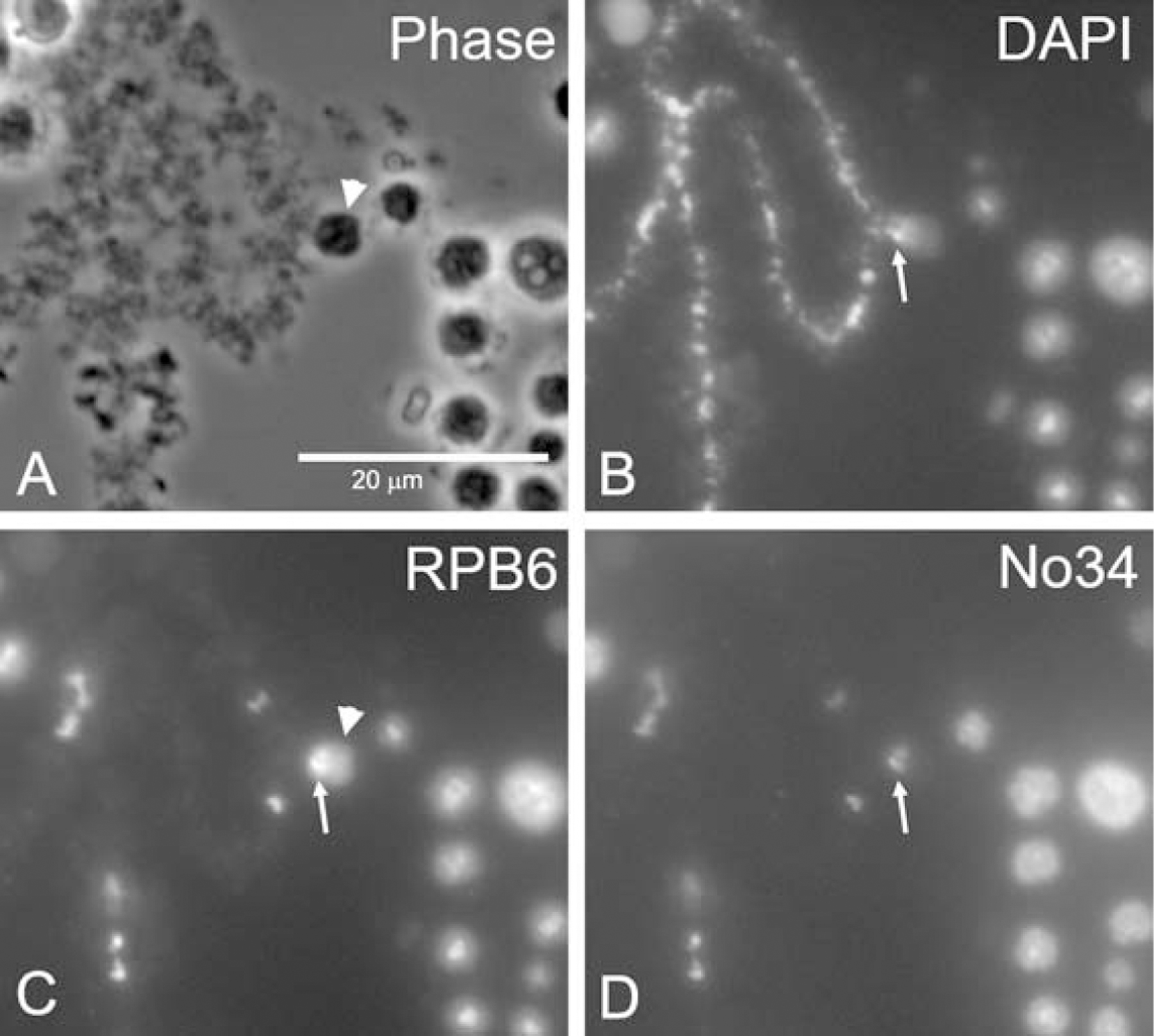
(A) Phase contrast image of a C-body (arrowhead) on a LBC. (B) DAPI labels the chromosome axes. (C) RPB6 labels C-bodies (arrowhead) with enriched signal on the chromosome (arrow). (D) Antibody No34 labels the same loci as RPB6.
In the amphibian GV, the ribosomal genes are amplified extrachromosomally [15–17]. These independent ribosomal genes, along with their transcribed ribosomal RNAs and many proteins, form the extrachromosomal nucleoli. We found that under cold shock treatment, C-bodies appeared at the edges of some, but not all nucleoli. C-bodies appear to be associated directly with the small amount of extrachromosomal rDNA (Fig. 4). This association is clearly demonstrated when C-bodies are stained with HA-RPB6 (Fig. 4G). C-bodies are not stained by antibodies against the trimethylguanosine (TMG) cap of snRNAs or RNA polymerase (pol) II. These results suggest the absence of active transcription of pol II, although pol I- or pol III-mediated transcription cannot be ruled out in C-bodies.
Figure 4. C-bodies are associated with rDNA.
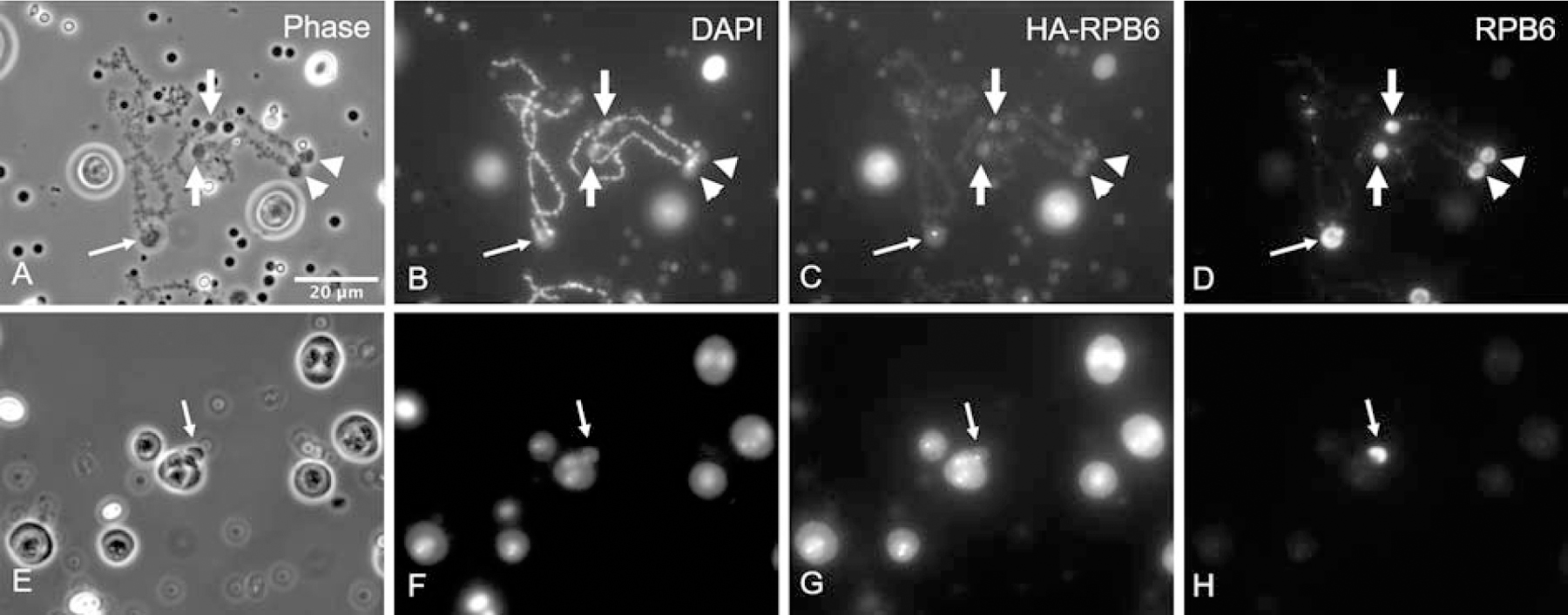
(A–D) C-bodies often appear at the ends of the LBCs, at or near the 5S rDNA loci (arrows). About 16 of the 18 LBCs have one to a few pairs of C-bodies at their ends, consistent with the distribution of 5S ribosomal genes. (E–H) The cold shock treatment can induce nucleolar protrusions with a DAPI-positive structure at their root. HA-RPB6 reveals bright spots on the C-bodies (C) and at the root of the nucleolar protrusions (G). It should be noted that protrusions do not appear on every nucleolus.
Induction of C-bodies does not require active transcription or translation
To test directly whether induction of C-bodies requires active transcription, we treated oocytes with actinomycin D, a transcription inhibitor, and then held them at room temperature or 4°C. After actinomycin treatment, most of the lateral loops disappeared, indicating that transcription was effectively suppressed. No obvious signal was seen with antibody H14 against pol II, in contrast to the signal seen on extended loops without drug treatment. Transcriptional repression not only eliminated the lateral loops, but also shortened the total length of the chromosomes. Despite this, the basic contours of homologous chromosomes are still discernible, including chiasmata (Fig. 5A–D).
Figure 5. Induction of C-bodies does not require active transcription or translation.
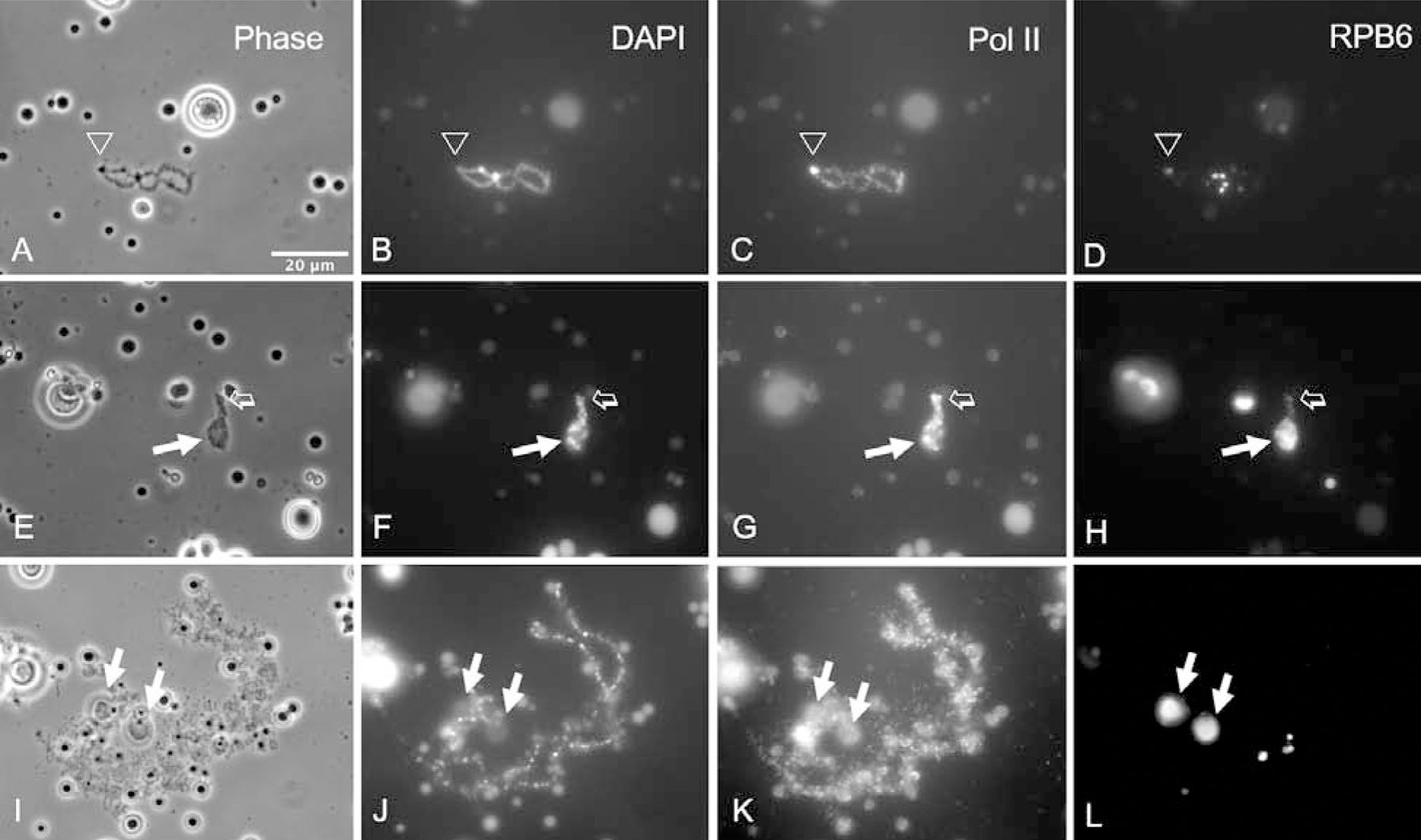
(A–D) When oocytes are treated with actinomycin to inhibit transcription, the chromosomes contract in length and most of the lateral loops disappear. Staining with an antibody against RPB6 is relatively specific for Pol III. One end of the LBC is indicated by an arrowhead. (E–H) When actinomycin-treated oocytes are cold shocked, the LBCs contract even more (open arrow), but C-bodies are induced as usual (solid arrow). (I–L) When oocytes are treated with cycloheximide to inhibit translation, there is relatively little change in morphology of the chromosomes and C-bodies are induced as in controls (arrows).
We found that C-bodies can still form on the very short LBCs from oocytes that have been treated with actinomycin and then cold shocked. Under these conditions a C-body can almost cover the extremely contracted chromosomes (Fig. 5E–H). We also treated LBCs with alpha-amanitin, another transcriptional inhibitor. Although the chromosomes did not contract as much as with actinomycin, C-bodies formed (data not shown). These experiments provide further evidence that C-body formation does not require active Pol II-mediated transcription.
Treatment of oocytes with cycloheximide, a translation inhibitor, does not affect the lateral loops on the LBCs. Furthermore, cold shock still induces C-body formation in cycloheximide-treated oocytes, indicating that C-body formation is not dependent on active translation (Fig. 5I–L). This result is perhaps not unexpected, as lateral loops are involved in transcription, whereas translation takes place in the cytoplasm.
C-bodies do not contain newly synthesized RNA
The preceding experiments demonstrate that C-body formation does not require ongoing transcription or translation. We also tested whether C-bodies contain newly synthesized RNA by injecting BrUTP into the GV, and staining with an antibody against BrUTP. At room temperature, BrUTP is incorporated uniformly into the lateral loops. After injecting BrUTP into oocytes and leaving them at 4°C for one day, we found that C-bodies still formed. As expected, a bright BrUTP signal was detected on the lateral loops, but no obvious BrUTP signal was seen in the bodies themselves, indicating that C-body formation does not require newly synthesized RNA (Fig. 6).
Figure 6. C-bodies do not contain newly synthesized RNA.
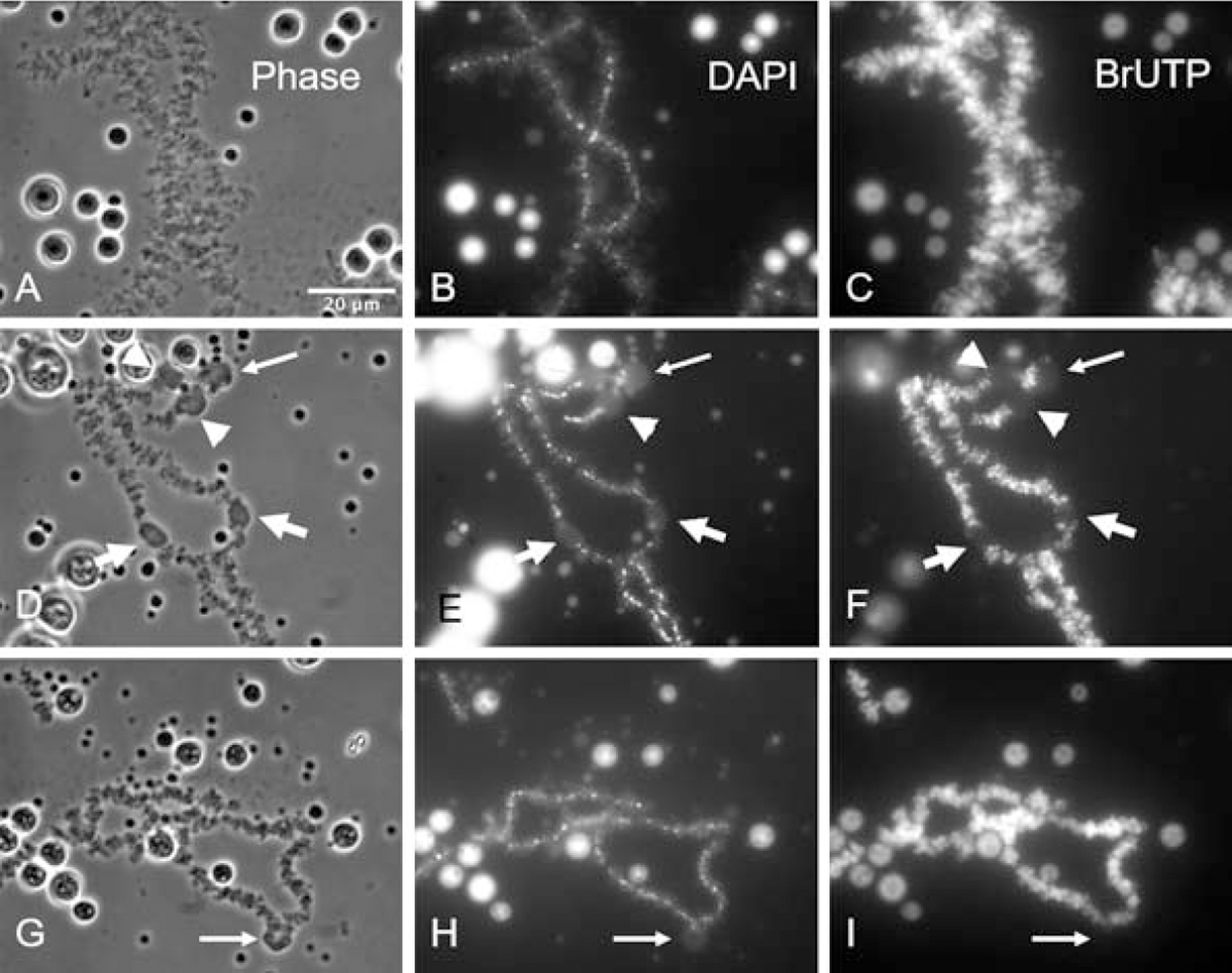
(A–C). BrUTP is incorporated into newly synthesized RNA at room temperature. (D–F) BrUTP is also incorporated into RNA when oocytes are held at 4°C. At the lower temperature the lateral loops of the LBCs are labeled but the induced C-bodies are not. (G–I) The results are similar if BrUTP-injected cells are held at room temperature for 7 hours and then at 4°C for 17 hours. A bright BrUTP signal can be detected on the lateral loops, but there is no signal in the induced C-bodies (thin arrows point to C-bodies at chiasmata, while thick arrowheads or arrows point to paired C-bodies on homologous chromosomes).
C-bodies can be induced in sperm LBCs
In a previous study we injected human sperm heads into the GV of intact oocytes [7]. Over time the sperm heads swelled and each transformed into a cluster of individual LBCs. Induced sperm LBCs are single chromatids, unlike the endogenous LBCs, which are tetrads. Otherwise, they share the characteristics of typical LBCs - extended lateral loops with active transcription.
We injected Xenopus sperm heads into the GV and cold shocked the oocytes at different time points thereafter. C-bodies were formed when cold shock was performed early, when the sperm just started to swell in the GV environment (Fig. 7A–C). Many C-bodies surrounded each spermatid, and they stained as usual with the antibody against RPB6. When multiple sperm LBCs are induced and are clustered in the GV, cold-shock treatment can make C-bodies appear on almost all sperm LBCs (Fig. 7D–F). Using different markers, it could be seen that the C-bodies on the sperm LBCs were similar to those formed on the endogenous LBCs. Thus, the ability of GV-induced sperm LBCs to form C-bodies under cold shock conditions appears to be normal.
Figure 7. C-bodies can be induced on sperm LBCs.
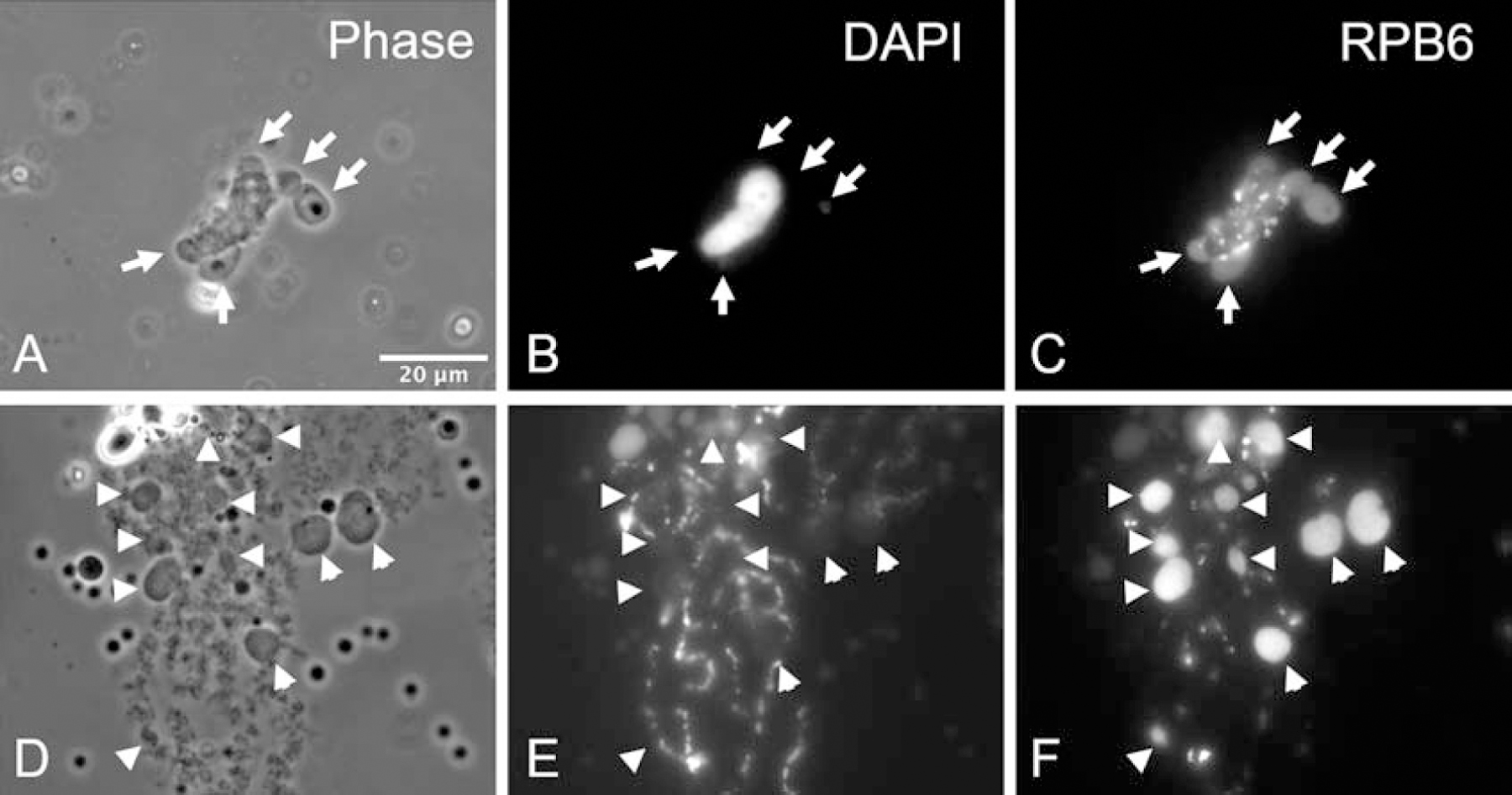
(A–C) A Xenopus sperm head injected into a Xenopus GV swells during the first few hours. After low-temperature treatment, oval or spherical structures appear around the sperm, and these structures are stained by antibody RPB6. (D–F) Sperm injected into a GV eventually give rise to individual LBCs. The C-bodies on these sperm LBCs are comparable in size to those induced on the endogenous LBCs.
Discussion
In this study we found that low temperature induces spherical or protruding structures at specific loci on LBCs in Xenopus laevis oocytes, which we call cold bodies or C-bodies. The formation of C-bodies does not require transcription, translation, or newly synthesized RNA. A previous study showed that similar spherical structures, so called “mini-Cajal bodies,” can be induced in the GV after oocytes recover from heat shock [18]. Since both the low and high temperatures used in these studies are within the normal environmental range, it is likely that C-bodies occur under natural conditions.
C-bodies are mostly formed at two specific positions in the nucleus: at or near the ends of the chromosomes and associated with the multiple extrachromosomal nucleoli. It is likely that C-bodies contain a DNA core and associating RNA and protein components. The organization of C-bodies seems similar to that of nucleoli, although the compositions of C-bodies and nucleoli might be very different. The 5S genes In Xenopus are found at the ends of the chromosomes [19] whereas amplified 18S and 28S rDNA genes are found in the extrachromosomal nucleoli [15–17]. What is not clear is whether C-bodies are specifically associated with the DNA at these loci. This question could be addressed for the 5S genes by examining other species in which these genes are not at the ends of the chromosomes.
Previous studies showed that, when the salamander Pleurodeles waltl was moved from 20°C to 8°C for several days, the general structure of LBCs was greatly affected [20, 21]. However, we did not observe dramatic changes of LBC loops except for the formation of C-bodies. There are several possible explanations. Firstly, the cold temperature used in this study was 4°C, whereas previous studies used 8°C. We were unable to observe C-body formation when Xenopus oocytes were placed at 8°C. Secondly, the duration of previous salamander studies was several days, much longer than the duration used in this study. Thirdly, we cannot rule out the possibility of intrinsic difference between Xenopus and Pleurodeles LBCs reactivity to cold shock.
Little is known about the composition of C-bodies and nothing about their ultrastructure. Because of their large size and ease of manipulation, the oocytes and LBCs of Xenopus and other organisms provide an unusually good system in which to address these questions.
Acknowledgments
This work was supported by Grant GM33397 from the National Institute of General Medical Sciences, National Institutes of Health to J.G.G. J.G.G. is American Cancer Society Professor of Developmental Genetics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rückert J Zur Entwickelungsgeschichte des Ovarialeies bei Selachiern. . Anatomische Anzeiger. 1892;7:107–58. [Google Scholar]
- 2.Callan HG. Lampbrush chromosomes. Mol Biol Biochem Biophys. 1986;36:1–252. [PubMed] [Google Scholar]
- 3.Gall JG, Murphy C, Callan HG, Wu ZA. Lampbrush chromosomes. Methods Cell Biol. 1991;36:149–66. [PubMed] [Google Scholar]
- 4.Gall JG, Wu Z. Examining the contents of isolated Xenopus germinal vesicles. Methods. 2010;51(1):45–51. Epub 2010/01/12. doi: 10.1016/j.ymeth.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gall JG, Wu Z, Murphy C, Gao H. Structure in the amphibian germinal vesicle. Exp Cell Res. 2004;296(1):28–34. Epub 2004/05/04. doi: 10.1016/j.yexcr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Gall JG, Murphy C. Assembly of lampbrush chromosomes from sperm chromatin. Mol Biol Cell. 1998;9(4):733–47. Epub 1998/05/16. doi: 10.1091/mbc.9.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JL, Gall JG. Induction of human lampbrush chromosomes. Chromosome Res. 2012;20(8):971–8. Epub 2013/01/09. doi: 10.1007/s10577-012-9331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace RA, Jared DW, Dumont JN, Sega MW. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973;184(3):321–33. Epub 1973/06/01. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- 9.Murphy C, Wang Z, Roeder RG, Gall JG. RNA polymerase III in Cajal bodies and lampbrush chromosomes of the Xenopus oocyte nucleus. Mol Biol Cell. 2002;13(10):3466–76. Epub 2002/10/22. doi: 10.1091/mbc.e02-05-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell. 1999;10(12):4385–402. Epub 1999/12/10. doi: 10.1091/mbc.10.12.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bregman DB, Du L, van der Zee S, Warren SL. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J Cell Biol. 1995;129(2):287–98. Epub 1995/04/01. doi: 10.1083/jcb.129.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krainer AR. Pre-mRNA splicing by complementation with purified human U1, U2, U4/U6 and U5 snRNPs. Nucleic Acids Res. 1988;16(20):9415–29. Epub 1988/10/25. doi: 10.1093/nar/16.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugle B, Scheer U, Franke WW. Ribocharin: a nuclear Mr 40,000 protein specific to precursor particles of the large ribosomal subunit. Cell. 1985;41(2):615–27. Epub 1985/06/01. doi: 10.1016/s0092-8674(85)80034-9. [DOI] [PubMed] [Google Scholar]
- 14.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gall JG. The genes for ribosomal RNA during oogenesis. Genetics. 1969;61(1):Suppl:121–32. [PubMed] [Google Scholar]
- 16.Gall JG. Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc Natl Acad Sci U S A. 1968;60(2):553–60. Epub 1968/06/01. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown DD, Dawid IB. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968;160(3825):272–80. Epub 1968/04/19. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- 18.Handwerger KE, Wu Z, Murphy C, Gall JG. Heat shock induces mini-Cajal bodies in the Xenopus germinal vesicle. J Cell Sci. 2002;115(Pt 10):2011–20. [DOI] [PubMed] [Google Scholar]
- 19.Callan HG, Gall JG, Murphy C. The distribution of oocyte 5S, somatic 5S and 18S + 28S rDNA sequences in the lampbrush chromosomes of Xenopus laevis. Chromosoma. 1988;97:43–54. [Google Scholar]
- 20.Angelier N, Moreau NA, N’Da EA, Lautredou NF. Cold-induced changes in amphibian oocytes. Exp Cell Res. 1989;183(2):508–13. doi: 10.1016/0014-4827(89)90410-2. [DOI] [PubMed] [Google Scholar]
- 21.N’Da E, Bonnanfant-Jais ML, Moreau N, Angelier N. Effects of cold shock treatment on amphibian oocytes: alteration of heterogenous nuclear RNP morphology. Biol Cell. 1990;69(3):139–51. doi: 10.1016/0248-4900(90)90340-9. [DOI] [PubMed] [Google Scholar]


