Abstract
Real-Time-reverse-transcription-Polymerase-Chain-Reaction from nasopharyngeal swabs and chest computed tomography (CT) depicting typically bilateral ground-glass opacities with a peripheral and/or posterior distribution are mandatory in the diagnosis of COVID-19. COVID-19 pneumonia may present though with atypical features such as pleural and pericardial effusions, lymphadenopathy, cavitations, and CT halo sign. In these two case-reports, COVID-19 presented as pneumothorax, pneumomediastinum and subcutaneous emphysema in critically ill patients. These disorders may require treatment or can be even self-limiting. Clinicians should be aware of their potential effects on the cardiorespiratory status of critically ill COVID-19 patients. Finally, pneumothorax can be promptly diagnosed by means of lung ultrasound. Although operator dependent, lung ultrasound is a useful bedside diagnostic tool that could alleviate the risk of cross-infection related to COVID-19 patient transport.
Abbreviations: SARS-CoV-2 disease, COVID-19; RT-PCR, Real-Time-reverse-transcription-Polymerase-Chain-Reaction; CT, computed tomography; US, ultrasound; ED, emergency department; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; MV, mechanical ventilation; PEEP, positive end-expiratory-pressure; HFNC, high flow nasal cannula; SpO2, saturation of peripheral oxygen; PaO2/FiO2, partial arterial pressure of oxygen to fractional inspired concentration of oxygen; CRS, cytokine release syndrome
Keywords: COVID-19, Pneumothorax, Pneumomediastinum, Subcutaneous emphysema, Chest computed tomography, Lung ultrasound
Introduction
In December 2019 in Wuhan city, China, a novel coronavirus was identified and subsequently named the Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) [1]. Since then SARS-CoV-2 disease (COVID-19) has been linked to over 1.100.000 deaths worldwide [[2], [3], [4]]. COVID-19 symptoms include fever, sore throat, fatigue, anosmia, cough, shortness of breath, abdominal pain, and myalgias. Diagnosis is confirmed using nasopharyngeal swabs and real-time-reverse-transcription-polymerase-chain-reaction (RT-PCR) [[5], [6], [7]]. In terms of imaging, chest computed tomography (CT) findings include ground-glass opacities with a peripheral and/or posterior distribution and mainly involving the lower lobes, and variable infiltrates and consolidations [[8], [9], [10]]. Pleural and pericardial effusions, lymphadenopathy, cavitations, and CT halo sign were less commonly observed [11]. COVID-19 was previously associated with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema [[12], [13], [14], [15], [16]]. We present two critically ill COVID-19 male patients: the first case with pneumothorax/pneumodiastinum and subcutaneous emphysema, while on mechanical ventilation, and the second case with the same findings, while on high-flow nasal cannula (HFNC).
Case reports
Case 1
A fifty-three year-old previous healthy male was admitted to the intensive care unit (ICU) with COVID-19 pneumonia. The patient was diagnosed in the emergency department (ED) where he presented with recent onset fever (38.5 °C), dry cough, altered level of consciousness and chest pain a few days after being in contact with a recovered COVID-19 case. Throat swab RT-PCR assay confirmed the diagnosis [[5], [6], [7]]. His heart rate and respiration rate were 119 and 32 per minute, respectively. Blood pressure was 96/70 mmHg and arterial oxygen saturation (SpO2) was 73%. The patient was intubated in the ED and his ratio of partial arterial pressure of oxygen to fractional inspired concentration of oxygen (PaO2/FiO2) was 170 post-intubation. Baseline laboratory examinations were unremarkable apart from lymphocytopenia (0.71 × 10/L, normal: 1.1–3.2 × 10⁹/L), and increased serum C-reactive protein [(CRP) 75.1 mg/liter, normal: 0−7 mg/L], lactate dehydrogenase (445 units/liter, normal: 100–190 units/L], and ferritin (802 ng/mL, normal: 23−336 ng/mL). Upon ICU admission, he received acute respiratory distress syndrome (ARDS)-net mechanical ventilation (MV) [tidal volume: 6 mL/kg and positive-end-expiratory-pressure (PEEP) of 13 cm H20] and was placed in the prone position, but deteriorating further to a PaO2/FiO2 ratio of 150. On day-2 he became hemodynamically unstable necessitating noradrenaline administration (0.5 mcg/kg/ min). On physical examination he had neck subcutaneous emphysema and his chest X-ray, which was complemented by lung ultrasound (US), revealed right sided pneumothorax, pneumomediastinum and subcutaneous emphysema. A chest drain was placed bedside (Fig. 1 ). Subsequently, his oxygenation markedly improved (PaO2/FiO2: 230). Also, he received antiviral therapy (lopinavir/ritonavir: 400/100 mg twice daily for two weeks), moxifloxacin (400 mg once daily for five days), dexamethasone 6 mg intravenously that was given for two days (discontinued upon the discovery of pneumothorax and pneumomediastinum), prophylactic anticoagulation and supportive ICU care. He continued to improve and was gradually weaned from MV; while the chest drain was removed on day-10. He was discharged from the ICU on day-15. RT-PCR test and microbiology were negative on day-20. All work-up for systemic and other viral diseases was negative. He was discharged to home isolation in good condition on day-27. Informed consent was obtained from the patient for this case report.
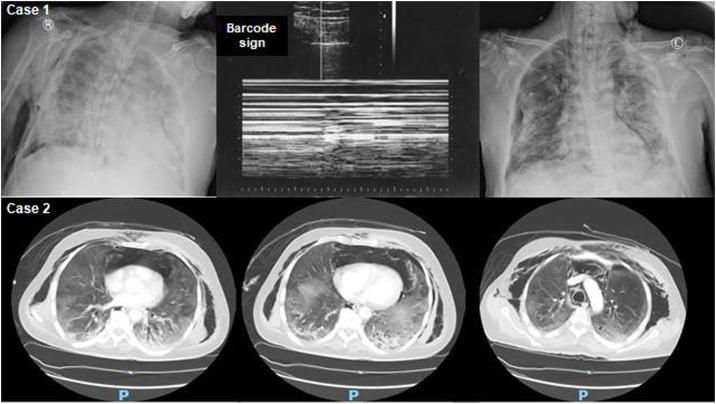
Fig. 1.
Upper panel (Case 1): Chest X-rays showing right-sided pneumothorax, pneumo-mediastinum and subcutaneous emphysema (left) that was confirmed (barcode sign) by the M-mode of the lung ultrasound (middle) and resolution after chest drain insertion (right). Lower panel (Case 2): axial lung window views of chest computed tomography scans depicting bilateral peripheral ground-glass opacities, dependent air-space consolidations, small pneumothoraces, and pneumodiastinum with subcutaneous emphysema.
Case 2
A sixty year-old male diabetic and hypertensive presented to the ED with recent onset anosmia, myalgias, headache, persistent cough, and shortness of breath. Throat swab RT-PCR confirmed COVID-19. His heart rate and respiration rate were 114 and 27 per minute, respectively. Blood pressure was 112/65 mmHg. Baseline laboratory examinations were within normal apart from lymphocytopenia (0.85 × 10⁹/L, normal: 1.1–3.2 × 10⁹/L), and increased C-reactive protein [(CRP) 62.2 mg/L, normal: 0−7 mg/L]. He was admitted to the ICU with an arterial peripheral oxygen saturation (SpO2) of 72; thus, he was started on HFNC (flow: 60 L/min, fraction of inspired oxygen 40%) and proned. His PaO2/FiO2 ratio improved temporarily to 290. However, he subsequently deteriorated (PaO2/FiO2 ratio: 160) and thus was intubated. Post-intubation chest CT scan showed bilateral pneumothoraces, subcutaneous emphysema and pneumomediastinum along with bilateral patchy ground-glass opacities with peripheral distribution (Fig. 1). He received intravenously piperacillin–tazobactam (4.5 g/8 h) for ten days along with empiric antiviral therapy (lopinavir/ritonavir: 400/100 mg twice daily for two weeks), prophylactic anticoagulation and supportive ICU care. Conservative MV strategy with low tidal volumes and PEEP of 8–10 cm H20 was applied and his oxygenation improved gradually. The patient made an uneventful recovery and was extubated on day-14. RT-PCR test and microbiology were negative on day-20. All work-up for systemic and other viral diseases was negative. He was discharged to home isolation in good condition on day-31. Informed consent was obtained by the patient for this case report.
Discussion
Although the majority of COVID-19 patients are asymptomatic or exhibit mild symptoms, a minority can develop life-threatening disease characterized by ARDS, multi-system organ failure, cytokine release syndrome (CRS), and thromboembolic disease [[1], [2], [3], [4],[17], [18], [19], [20], [21], [22]]. Our two cases showed that critically ill COVID-19 patients can also develop pneumothorax, pneumomediastinum, and subcutaneous emphysema.
Our cases both had lymphocytopenia and increased levels of inflammatory mediators, which were previously associated with extensive lung parenchymal injury and CRS [[1], [2], [3], [4],18,19]. Severe COVID-19 pneumonia was associated with bronchial wall thickening, hemorrhagic nodules, pleural effusion, and lymphadenopathy [[11], [12], [13], [14], [15], [16]]. In our institution, chest CT is used in the screening of COVID-19 patients, especially if the RT-PCR is negative [[9], [10], [11]]. Chest CT scan is associated with increased cost, the need to transport unstable patients, and greater risk of viral shedding when compared with portable chest X-ray and/or US [23,24]. Recently, we showed that lung US can reliably depict lung parenchymal abnormalities (i.e., B-lines, pleural line irregularities, and variable consolidations), and identify complications such as pneumothorax in critically ill patients with COVID-19 [25]. Although operator dependent, lung US is a useful bedside diagnostic tool that could minimize the risk of cross-infection related to COVID-19 patient transport. Moreover, ultrasound requires fewer resources and delivers no ionizing radiation.
In our first case, the pneumothorax and pneumomediastinum might have been partially attributed to ventilator-associated volutrauma to an already injured lung or even self-inflicted lung injury. In our second case, these same findings might have been related to the strain of persistent cough. Pneumothorax can be drained at the bedside and spontaneous pneumomediastinum is usually a self-limiting disorder; nevertheless both warrant attention as they can compromise the cardiorespiratory status.
Conclusion
Our cases showed that pneumothorax, pneumodiastinum and subcutaneous emphysema can contribute to the profound hypoxemia in critically ill COVID-19 patients. These rare features of COVID-19 pneumonia were diagnosed by portable chest X-ray, lung US, and CT scan. Lung US is a useful bedside diagnostic tool that could be used in the monitoring of severe COVID-19 pneumonia, mitigating thus the risk of cross-infection related to the transportation of critically ill patients to the radiology department.
Funding
No funding sources.
Competing interests
None declared
Ethical approval
Not required
Contributions
AA, AB, SAA, ZAM, and PGB contributed to the study conception and design, and drafted the initial version of the manuscript. Data collection and analysis were performed by AB, GB, and HB. SAA, ZAM, and DK edited the final version of the manuscript. All authors drafted equally, read, and approved the final version of the manuscript.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He Z.X. Clinical characteristics of coronavirus disease 2019 in China. China medical treatment expert group for Covid-19. N Engl J Med. 2020;382(April (18)):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(March (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(April (16)):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalla A.K., Casto A.M., Huang M.W., Perchetti G.A., Sampoleo R., Shrestha L. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J Clin Microbiol. 2020;58(May (6)) doi: 10.1128/JCM.00557-20. e00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(May (7809)):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(May (18)):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y.H., Dong J.H., An W.M., Lv X.Y., Yin X.P., Zhang J.Z. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARSCoV-2. J Infect. 2020;80(April (4)):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;296(August (2)):E41–E45. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(August (2)):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(July (1)):87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Gao R., Zheng Y., Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020;27(August (5)):taaa062. doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flower L., Carter Jl, Rosales Lopez J., Henry A.M. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep. 2020;13(May (5)) doi: 10.1136/bcr-2020-235861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiro J.E., Sisovic S., Ockert B., Böcker W., Siebenbürger G. Secondary tension pneumothorax in a COVID-19 pneumonia patient: a case report. Infection. 2020;18(June):1–4. doi: 10.1007/s15010-020-01457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ucpinar B.A., Sahin C., Yanc U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: case report. J Infect Public Health. 2020;13(June (6)):887–889. doi: 10.1016/j.jiph.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saudi Ministry of Health . 2020. Coronavirus Diseases 19 (COVID-19) guidelines. (Revised version 1.7)https://covid19.moh.gov.sa) May 25th. [Google Scholar]
- 18.Azkur A.K., Akdis M., Azkur D., Sokolowska M., Van de Veen W., Brüggen M.C. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(July (7)):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faqihi F., Alharthy A., Alodat M., Kutsogiannis D.J., Brindley P.G., Karakitsos D. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: a pilot study. J Crit Care. 2020;(July) doi: 10.1016/j.jcrc.2020.07.001. S0883-9441(20)30602-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(May (19)):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(May (5)):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24:275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fichera G., Stramare R., De Conti G., Motta R., Giraudo C. It’s not over until it’s over: the chameleonic behavior of COVID-19 over a six-day period. Radiol Med. 2020;125(May (5)):514–516. doi: 10.1007/s11547-020-01203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bounsenso D., Pata D., Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med. 2020;8(May (5)):e27. doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alharthy A., Faqihi F., Abuhamdah M., Noor A., Naseem N., Balhamar A. Prospective longitudinal evaluation of point-of-Care LUS in critically ill patients with severe COVID-19 pneumonia. J Ultrasound Med. 2020;(August) doi: 10.1002/jum.15417. 14;10.1002/jum.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]