Abstract
Objectives:
Chemotherapy is used as an indispensable therapy for advanced gastric cancer. Different chemotherapy regimens have been used for this purpose. Toxicity due to the Chemotherapy drugs is one limiting factor. In this study we aim to compare the efficacy and toxicity of two regimens FOLFOX (leucoverin, 5-fluorouracil and oxaliplatin) and modified DCF (mDCF) (docetaxel, cisplatin, and 5-fluorouracil) in patients with advanced gastric adenocarcinoma.
Methods:
In this analytical cross-sectional study, 47 patients treated with FOLFOX regimen and 57 patients treated with mDCF regimen were recruited, Patients in both groups were compared for demographic findings, response rate, mortality rate, overall survival (OS) and progression free survival (PFS).
Results:
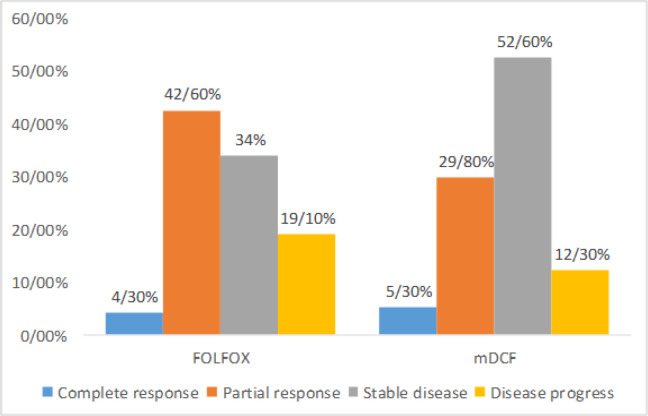
In FOLFOX and mDCF group, complete response (CR) occurred in 4.3% and 5.3%, partial response (PR) in 42.6% and 29.8%, stable disease in 34% and 52.6% and disease progression in 19.1% and 12.3%, respectively (p=0.25). Overall response rate was 48.9% and 56.1%, respectively. There was no significant difference between two regimens in OS and PFS (p=0.22). mDCF compared to FOLFOX had significantly higher hematologic, gastrointestinal complications, as well as creatinine rise, stomatitis and hair loss, but peripheral neuropathy was significantly lower.
Conclusion:
The results of current study showed that in patients with advanced gastric adenocarcinoma, FOLFOX regimen compared to mDCF regimen have similar ORR, OS and PFS. Toxicity rate are also lower in FOLFOX group, thus it seems a better regimen for chemotherapy.
Key Words: Advanced gastric adenocarcinoma- FOLFOX- modified DCF- survival rate- toxicity
Introduction
Gastric cancer is the fourth most common cancer and the second most common cause of cancer-related death in the world (Ferlay et al., 2015; Haghighi et al., 2016). Approximately two-thirds of these cancers occur in the developing countries in Eastern Europe, South America, and Asia (Chen et al., 2014). In Iran, gastric cancers are the most common cause of cancer deaths and their incidence are higher than the global average (Zamani et al., 2013).
Surgery is the only definitive cure for gastric cancer, but a significant proportion of these patients are at the advanced stage at the time of diagnosis, or more than half develop recurrence after surgery. Therefore palliative chemotherapy is now a well-known and effective method compared to other supportive treatments in patients with advanced gastric cancer (Huang et al., 1998; Zabaleta, 2012).
The overall survival (OS) of advanced gastric cancer is very low; the median OS was 7.5 to 12 months after chemotherapy compared with 3 to 5 months in patients receiving supportive therapy. Although overall treatment outcomes have not been satisfactory, chemotherapy has been associated with higher survival and better quality of life compared to supportive treatment (Casaretto et al., 2006; Glimelius et al., 1997; Wagner et al., 2006; Wagner et al., 2010). In addition, meta-analysis studies have shown that combination therapy is also highly effective (Iwase et al., 2011; Koizumi et al., 2012; Kim et al., 2011) and the triple-drug regimen is much better than the single-drug or double-drug regimen (Koizumi et al., 2008; Van Cutsem et al., 2006).
Different chemotherapy regimens have been introduced for patients with advanced gastric cancer. Oxaliplatin plus 5-fluorouracil (5-FU) and leucoverin, as FOLFOX regimen, is widely used in the treatment of gastrointestinal cancers with 40-50% response rate and survival after treatment to be 10 to 12 months (Oh et al., 2007). Docetaxel plus cisplatin plus 5-FU as the DCF regimen is the other commonly used regimen which has been associated with acceptable survival and better quality of life (Chen et al., 2013).
Despite the efficacy of DCF in progressive gastric cancer, studies have reported that the incidence of grade 3-4 chemotherapy related toxicities is higher than other combination regimens and is therefore not the standard treatment regimen in these patients (Roth et al., 2007; Van Cutsem et al., 2006). However, FOLFOX had acceptable anti tumor activity and tolerable toxicity profile in different treatment protocols and doses (Hacibekiroglu et al., 2015; Haghighi t al., 2016; Yeh et al., 2012; Zhang et al., 2012).
The aim of our study was to evaluate the efficacy of FOLFOX as the first line of treatment in patients with advanced gastric adenocarcinoma compared with mDCF.
Materials and Methods
In this cross-sectional study, all patients between 18-80 years old with advanced gastric adenocarcinoma (non-resectable tumor) receiving FOLFOX-4 or modified DCF chemotherapy regimen during October 2015 and October 2018 were included. Patients with previous history of chemotherapy or other malignancy, severe underlying or infectious disease, with brain metastasis or other causes of severe neuropathy were excluded. The ethics committee of Ardabil University of Medical Sciences have approved the study protocol (Approve no: ARUMS.REC.1396.209).
In all patients, demographic and baseline data including age at the time of diagnosis, gender, clinical findings, disease stage and metastasis as well as demographic, physical exam, laboratory and imaging finings at the end of each chemotherapy cycles were recorded.
Tumor was evaluated using CT studies every four cycles. Patients response to treatment was measured by RECIST criteria (Response Evaluation Criteria In Solid Tumors) using http://www.radiologytutor.com/index.php/cases/oncol/139-recist. CT scan findings were compared before chemotherapy and after each period to define RECIST.
Complete remission (CR) was defined as there was no sign of disease, Partial response (PR) is considered when at least 30 percent of tumor mass was reduced in imaging studies and there was no new lesion or findings regarding the disease progress. Progressive disease (PD) was noted if there was at least 20% increase in largest diameter of the lesion or occurrence of new lesion or metastasis. Stable disease was considered when the treatment response did not reach any criteria of complete or partial response and also is not progressive. Overall response rate (ORR) was the sum of PR and CR.
Progress free survival (PFS) was the duration from the diagnosis of the disease till the recurrence or disease progress or the patient death. Those patients with no changes during the study period were excluded from this evaluation.
The chemotherapy related complications including hematologic, gastrointestinal, neuropathy were recorded in both treatment groups. We also evaluated other drug-related complications.
Statistical analysis
All data were analyzed using the SPSS software 20 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, U.S.A.). Results were presented as mean and standard deviation (SD) or frequency and percentage. Independent T test or Mann Whitney U, Chi square of Fischer’s exact tests were used to compare the findings between two regimens. P-values of <0.05 were considered significant.
Results
In this study, 104 patients received chemotherapy including 47 with FOLFOX regimen and 57 with mDCF. There was no difference between groups regarding baseline findings (Table 1).
Table 1.
Baseline Findings in FOLFOX and mDCF Groups
| FOLFOX | Modified DCF | P value | |
|---|---|---|---|
| Age (years) | 65.87±12.74 | 63.07±9.27 | 0.19 |
| Gender | |||
| Male | 33 (70.2%) | 46 (80.7%) | 0.21 |
| Female | 14 (29.8%) | 11 (19.35) | |
| Stage | |||
| III | 15 (31.9%) | 20 (35.1%) | 0.73 |
| IV | 32 (68.1%) | 37 (64.9%) | |
| Metastasis | |||
| Liver | 12 (25.5%) | 17 (29.8%) | 0.99 |
| Abdomen and peritoneum | 10 (21.3%) | 7 (12.3%) | |
| Lung | 3 (6.4%) | 2 (3.5%) | |
| More than one Organ | 7 (14.9%) | 11 (19.3%) |
Figure 1 is demonstrating the response rate between groups. mDCF compared to FOLFOX had more cases with stable disease and less progression, but there was no significant difference between groups (p=0.25). ORR was 46.98% in FOLFOX and 35.1% in mDCF, but the difference was not significant (p=0.22).
Figure 1.
Response Rate between FAOLFOX and mDCF Groups
Six patients (12.8%) in FOLFOX group and 10 patients (17.5%) in mDCF group died during the study period (p=0.5). The time between disease diagnosis and death in these patients were 8.33±1.03 months in FOLFOX vs. 6.50±2.32 months in mDCF group (p=0.09).
Overall survival (OS) in FOLFOX and mDCF groups were 12.61±4.05 and 13.50±5.94 months, respectively with no significant difference between groups. FOLFOX compared to mDCF groups had shorter PFS with no significant difference between groups (6.79±2.18 vs. 7.97±3.14, p=0.1).
Chemotherapy related toxicities are demonstrated in Table 2. Hematologic complications including neutropenia, fever and neutropenia and thrombocytopenia were significantly higher in mDCF compared to FOLFOX group. mDCF compared to FOLFOX regimen had significantly more cases with post-chemotherapy nausea and vomiting, creatinine rise, hair loss and facial changes (in all patients) and stomatitis. Neuropathy occurred in both groups with significantly higher incidence in FOLFOX compared to mDCF regimen. Most cases of neuropathy in both groups were grade I and II.
Table 2.
Chemotherapy Related Toxicities between FOLFOX and mDCF Groups
| FOLFOX | Modified DCF | P value | |
|---|---|---|---|
| Hematologic complications | 12 (25.5%) | 41 (71.95) | <0.001 |
| Neutropenia | 9 (19.1%) | 37 (64.9%) | <0.001 |
| Fever and neutropenia | 2 (4.3%) | 19 (33.3%) | <0.001 |
| Thrombocytopenia | 5 (10.6%) | 19 (33.3%) | 0.006 |
| Gastrointestinal complications | 19 (40.4%) | 37 (64.9%) | 0.01 |
| Diarrhea | 5 (10.6%) | 19 (33.3%) | 0.006 |
| Nausea | 12 (25.5%) | 35 (61.4%) | <0.001 |
| Vomiting | 9 (19.1%) | 29 (50.9%) | <0.001 |
| Increase in liver enzymes | 2 (4.3%) | 8 (14%) | 0.09 |
| Creatinine rise | 5 (10.6%) | 15 (26.3%) | 0.04 |
| Stomatitis | 1 (2.1%) | 20 (35.1%) | <0.001 |
| Hair loss | 2 (4.3%) | 57 (100%) | <0.001 |
| Neuropathy | 27 (57.4%) | 9 (15.8%) | <0.001 |
| Neuropathy grade | |||
| I | 14 (29.8%) | 6 (10.5%) | <0.001 |
| II | 10 (21.3%) | 3 (5.3%) | |
| III | 2 (4.3%) | 0 | |
| IV | 1 (2.1%) | 0 |
Discussion
In this retrospective cross-sectional study, we evaluated the outcome of patients with advanced gastric adenocarcinoma following treatment with FOLFOX or mDCF regimens.
The FOLFOX group had a relatively equal CR rate, higher PR, greater disease progression and lower stable disease compared with mDCF. Also, the ORR between the two groups was not statistically significant, despite being higher in the FOLFOX group.
Haghighi and colleagues (2016) in their study of elderly patients with advanced gastric cancer treated with FOLFOX-4, observed ORR of 72.6% with stable disease in 13%. In another study of elderly patients, Liu et al., (2008) observed that treatment with FOLFOX-4 was associated with an ORR of 52.5%. In the study of Yeh et al., (2012) the OR was 41.1%, the disease was stable in 26%, and progression occurred in 32.9%. In the study of Baek et al., (2011) FOLFOX regimen showed the lowest CR (2.4%) and PR (23.8%) in 24 patients with advanced gastric cancer compared to other studies, while disease progression was 30.9%. The response rate in different studies varied between 26 and 72% depending on the study location, individual characteristics, and time of diagnosis. However, all studies indicate the acceptable efficacy of this treatment. In the recent new study, Funasaka et al., (2020) reported that FOLFOX6 regimen with response rate of 50% is an effective treatment for gastric cancer. Bhat (2018) also observed that the leucoverin, Oxaliplatin and 5-FU regimen (exactly FOLFOX) has adequate response in advanced gastric cancer patients.
In a meta-analysis, Patients with FolFOx-4 regimen had 12 and 6.7 months OS and PFS respectively, equal to some studies. Chen and colleagues (2013) concluded that the DCF regimen increases PR levels and reduced disease progression. Wang et al. (2016) reported ORR of 48.7% following treatment with DCF in Chinese patients.
Hacibekiroglu et al., (2015) reported rather similar efficacy for FOLFOX-6 and DCF in advanced gastric cancers (OR of 37% and 40.3%, respectively). Kim et al., (2011) also noted that both FOLFOX and DCF regimens have similar improvement rate. Also, in our study both regimens have relatively equal efficacy.
In the present study, FOLFOX-4 regimen had OS of 12 months and PFS of 6.7 months, with OS and PFS equal to some studies (Haghighi et al., 2013; Liu et al., 2008; Yeh et al., 2012). For FOLFOX-4 regimen, Haghighi et al., (2013) reported OS of 11.9 and PFS of 7.3; the mean OS in the study of Liu et al., (2008) was 10 months 11.9 months in Yeh et al., study (2012). However, Baek et al., (2011) reported lower rate of OS and PFS (9.3 and 4.9 months, respectively).
In the current study, Mortality was seen in 12.8% and 17.5% of FOLFOX-4 and modified DCF groups. There was no significant difference between two regimens regarding PFS and OS rate, while the PFS was lower in FOLFOX group.
Similar to our study, Hacibekiroglu et al., (2015) also found that the both FOLFOX and DCF had relatively similar survival rates. However, Kim et al., (2011) reported shorted duration of disease progression for DCF compared to FOLFOX (4 vs. 15 months) with longer OS (48 vs. 37 months); however, there was no significant statistical difference between the two regimens. However, Liu et al., (2018) observed that DOF regimen (Oxaliplatin instead of cisplatin) compared to FOLFOX regimen was more effective in advanced gastric cancer patients.
Chemotherapy-related toxicities are one of the causes that limit the use of different chemotherapy regimens. Therefore, researchers are trying to introduce regimens that have the least or acceptable toxicity with higher efficiency. The DCF regimen has higher rate of grade 3-4 toxicities than other therapies and so has limited its use (Roth et al., 2007; Van Cutsem et al., 2006).
In our study, hematologic and gastrointestinal complications were significantly higher in modified DCF, while peripheral neuropathy was significantly higher in FOLFOX-4. In other studies, the FOLFOX regimen was more acceptable due to its lower chemotherapy-related toxicities. Haghighi et al., (2016) reported only one case of grade 3 neuropathy in FOLFOX group.
Similar to the present study, Hacibekiroglu et al., (2015) reported higher hematologic and gastrointestinal toxicities in DCF compared to FOLFOX regimen. Similarly, Kim et al., (2011) stated that mucositis grade 3-4 and leukopenia were more frequent in patients treated with DCF. Unlike these findings, Liu et al., (2018) observed that both DOF and FOLFOX regimen have acceptable toxicities comparable with each other, but in elder patients, DOF has significantly more neuropathy.
Stomatitis is another chemotherapy related complication which was significantly higher in mDCF compared to FOLFOX. Similar results were reported by Wang et al., (2014), while Hacibekiroglu et al., (2015) did not find significant difference in stomatitis rate between groups. Salehifar et al., (2019) also find no significant difference between two regimen in stomatitis, visual change and skin reactions.
Another notable finding was hair loss in 100% of mDCF patients, which was seen in only 4.3% of FOLFOX patients. Bhat (2018) also reported less hair loss in similar FOLFOX regimen compared to others. This hair loss and change in the appearance can have a significant impact on the morale and cause depression in these patients.
Mitani et al., (2020) also indicated that FOLOFX6 has acceptable toxicity for chemotherapy-refractory advanced gastric cancer. Given the sum of the side effects of the treatment and the resulting toxicities, a treatment should be chosen that is well-tolerated with lower complications.
In conclusion, the results of current study showed that in patients with advanced gastric adenocarcinoma, FOLFOX regimen compared to mDCF regimen have similar ORR, OS and PFS. Toxicity rate are also lower in FOLFOX group, thus it seems a better regimen for chemotherapy.
Acknowledgments
The present study was extracted from the thesis written by Dr. Mohammad Molaei which was financially supported by Ardabil University of Medical Sciences.
Statement conflict of Interest
Authors declared no conflict of interest.
References
- Baek YH, Choi SR, Jang JS, et al. Modified FOLFOX-4 as first-line and salvage treatment in advanced gastric cancer. Hepatogastroenterology. 2011;58:251–6. [PubMed] [Google Scholar]
- Bhat G. Retrospective study of oxaliplatin, leucovarin and 5 fluoruracil regimen in patients with advanced gastric cancer with poor performance status: A study at a tertiary center of South India. South Asian J Cancer. 2018;7:223–5. doi: 10.4103/sajc.sajc_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaretto L, Sousa PL, Mari JJ. Chemotherapy versus support cancer treatment in advanced gastric cancer: a meta-analysis. Braz J Med Biol Res. 2006;39:431–40. doi: 10.1590/s0100-879x2006000400002. [DOI] [PubMed] [Google Scholar]
- Chen W, Shen J, Pan T, et al. FOLFOX versus EOX as a neoadjuvant chemotherapy regimen for patients with advanced gastric cancer. Exp Ther Med. 2014;7:461–7. doi: 10.3892/etm.2013.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-L, Chen X-Z, Yang C, et al. Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with non-taxane-containing palliative chemotherapy for gastric carcinoma: A Systematic Review and Meta-Analysis. PLoS One. 2013;8:e60320. doi: 10.1371/journal.pone.0060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Funasaka C, Kanemasa Y, Shimoyama T, Cho H, Omuro Y. Clinical efficacy of mFOLFOX6 for advanced gastric cancer. Gan To Kagaku Ryoho. 2020;47:49–53. [PubMed] [Google Scholar]
- Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–8. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- Hacibekiroglu I, Kodaz H, Erdogan B, et al. Comparative analysis of the efficacy and safety of oxaliplatin plus 5-fluorouracil/leucovorin (modified FOLFOX6) with advanced gastric cancer patients having a good or poor performance status. Asian Pac J Cancer Prev. 2015;16:2355–9. doi: 10.7314/apjcp.2015.16.6.2355. [DOI] [PubMed] [Google Scholar]
- Hacibekiroglu I, Kodaz H, Erdogan B, et al. Comparative analysis of the efficacy and safety of modified FOLFOX-6 and DCF regimens as first-line treatment in advanced gastric cancer. Mol Clin Oncol. 2015;3:1160–4. doi: 10.3892/mco.2015.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi S, Kasbkar H, Esmaeilpour K, et al. Oxaliplatin, 5 fluorouracil and leucovorin (FOLFOX4) as first line chemotherapy in elderly patients with advanced gastric cancer. Asian Pac J Cancer Prev. 2016;17:3277–80. [PubMed] [Google Scholar]
- Huang JQ, Sridhar S, Chen Y, et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–79. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- Iwase H, Shimada M, Tsuzuki T, et al. A phase II multi-center study of triple therapy with paclitaxel, S-1 and cisplatin in patients with advanced gastric cancer. Oncology. 2011;80:76–83. doi: 10.1159/000328746. [DOI] [PubMed] [Google Scholar]
- Kim JY, Do YR, Park KU, et al. Multicenter phase II trial of S-1, paclitaxel and cisplatin triplet combination chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2011;67:527–32. doi: 10.1007/s00280-010-1353-6. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Goh PG, Kim ES, et al. Comparison of the toxicities and efficacies of the combination chemotherapy regimens in advanced gastric cancer patients who achieved complete response after chemotherapy. Korean J Gastroenterol. 2011;58:311–7. doi: 10.4166/kjg.2011.58.6.311. [DOI] [PubMed] [Google Scholar]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- Koizumi W, Nakayama N, Tanabe S, et al. A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601) Cancer Chemother Pharmacol. 2012;69:407–13. doi: 10.1007/s00280-011-1701-1. [DOI] [PubMed] [Google Scholar]
- Liu ZF, Guo QS, Zhang XQ, et al. Biweekly oxaliplatin in combination with continuous infusional 5-fluorouracil and leucovorin (modified FOLFOX-4 regimen) as first-line chemotherapy for elderly patients with advanced gastric cancer. Am J Clin Oncol. 2008;31:259–63. doi: 10.1097/COC.0b013e31815d43ee. [DOI] [PubMed] [Google Scholar]
- Liu M, Hu G, Wang Y, et al. Comparison of FOLFOX and DOF regimens as first-line treatment in East Asian patients with advanced gastric cancer. Onco Targets Ther. 2018;11:375–81. doi: 10.2147/OTT.S149624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani S, Kadowaki S, Komori A, et al. A phase II study of modified FOLFOX6 for advanced gastric cancer refractory to standard therapies. Adv Ther. 2020;37:2853–64. doi: 10.1007/s12325-020-01358-2. [DOI] [PubMed] [Google Scholar]
- Oh SY, Kwon HC, Seo BG, et al. A phase II study of oxaliplatin with low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) as first line therapy for patients with advanced gastric cancer. Acta Oncol. 2007;46:336–41. doi: 10.1080/02841860600791483. [DOI] [PubMed] [Google Scholar]
- Roth AD, Fazio N, Stupp R, et al. Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin, and fluorouracil as systemic treatment for advanced gastric carcinoma: A randomized phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2007;25:3217–23. doi: 10.1200/JCO.2006.08.0135. [DOI] [PubMed] [Google Scholar]
- Salehifar E, Avan R, Janbabaei G, Mousavi SK, Faramarzi F. Comparison the incidence and severity of side effects profile Of FOLFOX and DCF regimens in gastric cancer patients. Iran J Pharm Res. 2019;18:1032–9. doi: 10.22037/ijpr.2019.1100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. V325 Study Group Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu R, Li J, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19:234–44. doi: 10.1007/s10120-015-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YS, Tsai HL, Ma CJ, et al. A retrospective study of the safety and efficacy of a first-line treatment with modified FOLFOX-4 in unresectable advanced or recurrent gastric cancer patients. Chemotherapy. 2012;58:411–8. doi: 10.1159/000345742. [DOI] [PubMed] [Google Scholar]
- Zabaleta J. Multifactorial etiology of gastric cancer. Methods Mol Biol. 2012;863:411–35. doi: 10.1007/978-1-61779-612-8_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani N, Hajifaraji M, Fazel-tabar Malekshah A, et al. A case-control study of the relationship between gastric cancer and meat consumption in Iran. Arch Iran Med. 2013;16:324–9. [PubMed] [Google Scholar]
- Zhang J, Chen RX, Zhang J, et al. Efficacy and safety of neoadjuvant chemotherapy with modified FOLFOX7 regimen on the treatment of advanced gastric cancer. Chin Med J (Engl) 2012;125:2144–50. [PubMed] [Google Scholar]