Abstract
Circulating tumor cells (CTCs) contain metastatic precursors that can initiate new metastases. Recent work shows that association with neutrophils provides a proliferative advantage to CTCs, rendering them more competent in metastasis formation. The study identifies molecular players in CTC-neutrophil interactions, providing potential targets for disrupting formation of these deadly metastatic seeds.
Circulating tumor cells (CTCs) are shed into the vasculature from solid tumors and contain metastasis-initiating cells that are able to extravasate and colonize secondary organs (Yu et al., 2011). It is believed that only a small subset of CTCs shed from the primary tumor succeed in initiating metastasis, and the properties that confer metastatic potential to CTCs are still largely unknown. While the scarcity of CTCs has long hampered their molecular and functional characterization, recent advances in CTC isolation, culture methods, and single-cell analysis have paved the way to a better understanding of CTC biology. In an earlier report by Aceto et al., CTC clusters were found to be much more competent than single CTCs in establishing metastases, due to their ability to resist apoptosis in distant sites (Aceto et al., 2014). A recent study, published in Nature, reports the presence of clusters composed of CTCs and white blood cells (WBCs) in breast cancer patient blood and investigates the mechanisms by which these heterotypic clusters influence tumor progression (Szczerba et al., 2019) (Figure 1).
Figure 1. Interactions with Neutrophils Enhance CTC Proliferation and Metastatic Colonization.
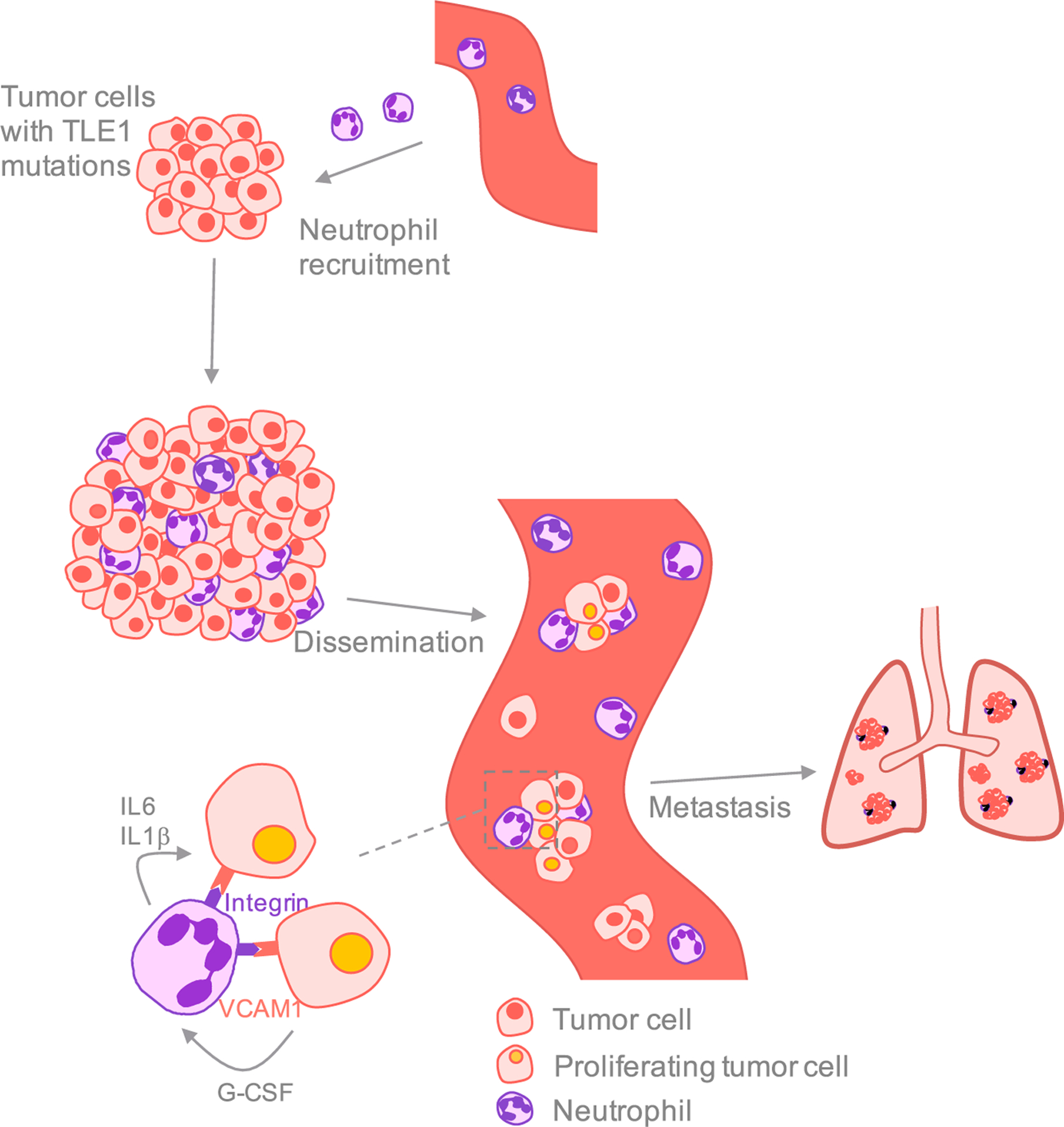
Tumor cells with mutations in specific genes, including TLE1, attract neutrophils to the primary tumor site and increase the number of CTC-neutrophil clusters in the bloodstream. Expression of the cell adhesion molecule VCAM1 in the tumor cells mediates the formation of CTC-neutrophil clusters, presumably by binding to specific integrins present in neutrophils. These neutrophils express IL6 and IL1β, signals that promote CTC cell-cycle progression. Conversely, CTCs express neutrophil-stimulating factors such as G-CSF. CTC-neutrophil clusters are highly efficient in initiating metastasis and their presence correlates with poor outcome.
Using the Parsortix microfluidic device to isolate CTCs, Szczerba and colleagues found that, although the majority of CTCs detected in patients with invasive breast cancer were single, 7.6% and 3.4% of CTCs were part of CTC clusters and CTC-WBC clusters, respectively. CTC-WBC clusters were also found in several mouse models, including tumor transplantation models in both immunocompetent and immunodeficient mice, and in a genetically modified mouse model that develops spontaneous breast tumors. The majority of WBCs found in CTC-WBC clusters were identified as neutrophils, and importantly, patients with CTC-neutrophil clusters had worse progression-free survival than patients with single CTCs and CTC clusters.
To assess the metastatic potential of CTC-neutrophil clusters, the authors isolated single CTCs, CTC clusters, and CTC-neutrophil clusters spontaneously generated in tumor-bearing mice and individually micromanipulated them to inject an equal number of CTCs from each group into the tail veins of recipient mice. Mice injected with CTCs from CTC-neutrophil clusters succumbed to metastases significantly earlier than mice injected with CTCs that were not associated to WBCs. Accordingly, treating mice with palpable tumors with neutralizing antibodies against the neutrophil surface protein Ly-6G to deplete neutrophils reduced neutrophil infiltration to the primary site, completely abrogated the formation of CTC-neutrophil clusters, and significantly reduced metastasis formation, leading to longer survival. Conversely, mice injected with tumor cells overexpressing G-CSF—a factor involved in neutrophil stimulation—showed augmented neutrophil infiltration to the primary tumor and increased numbers of CTC-neutrophil clusters in the bloodstream, leading to accelerated metastasis development and shorter survival.
To understand the molecular features responsible for the increased lethality of neutrophil-associated CTCs, the researchers took advantage of a recently developed protocol for parallel DNA and RNA sequencing from individual cells (Macaulay et al., 2016). Exome sequencing detected several genes, including TLE1, that were exclusively mutated in patients with CTC-neutrophil clusters. Primary tumors formed by cells engineered to harbor specific TLE1 mutations showed increased levels of neutrophil infiltration and shed significantly more CTC-neutrophil clusters into the bloodstream. Single-cell RNA-seq analysis of CTCs with or without associated neutrophils showed that CTCs from CTC-neutrophil clusters overexpress positive regulators of cell cycle and DNA replication programs, suggesting that interactions with neutrophils provide CTCs with a proliferative advantage. Analysis of the concomitant expression of cytokines and their receptors in CTCs and neutrophils from heterotypic clusters pointed to the IL6 and IL1β signaling pathways as essential components of the CTC-neutrophil crosstalk that leads to increased proliferation of cancer cells. Interestingly, the majority of CTCs present in CTC-neutrophil clusters expressed G-CSF and other cytokines involved in neutrophil stimulation, likely promoting a positive feedback loop that further enhances the proliferative capacity of the tumor cells.
Because targeting all neutrophils would have unwanted side effects, the authors explored ways of specifically targeting CTC-neutrophil clusters. They engineered a CRISPR-Cas9-based loss-of-function mini-pool screen in vivo, targeting cell-cell junction pairs simultaneously expressed in CTCs and neutrophils from heterotypic clusters, and demonstrated that disruption of VCAM1 expression in CTCs abrogated their ability to bind to neutrophils.
It is known that increased neutrophil abundance often correlates with poor patient outcome, but whether and how tumor associated neutrophils contribute to tumorigenesis has remained elusive. Recent studies have reported that neutrophils can directly promote tumor progression through various mechanisms, including enhancing angiogenesis, suppressing cytotoxic T cell activity, and producing neutrophil extracellular traps (NETs) that promote metastasis (reviewed by Powell and Huttenlocher, 2016). Using intravital microscopy, Cools-Lartigue et al. showed that neutrophil-produced NETs promote CTC adhesion to capillaries and subsequent extravasation to target organs (Cools-Lartigue et al., 2013). Aceto and colleagues now show that neutrophils present in CTC clusters also support tumor progression by promoting the proliferation of CTCs, both while circulating and upon arriving to distant organs (Szczerba et al., 2019). However, other studies have suggested that neutrophils impair tumor progression by promoting tumor clearance and activating the adaptive immune response. It is believed that context-dependent polarization into antitumor (N1) or protumor (N2) neutrophils could be responsible for this apparent discrepancy (Fridlender et al., 2009; Powell and Huttenlocher, 2016).
The mechanism by which missense mutations in TLE1 lead to the recruitment of neutrophils to the primary tumor and enhance CTC-neutrophil cluster formation remains unclear. It is tempting to speculate that the increased G-CSF levels observed in the CTCs of mice with heterotypic clusters are linked to the activation of NFκB activity that can result from the TLE1 mutation (Ramasamy et al., 2016). Furthermore, increased G-CSF levels could contribute to differentiating neutrophils toward a protumor phenotype (Pylaeva et al., 2016). Tumor cells intravenously injected into naive mice with or without neutrophil depletion failed to show differences in metastatic growth, which seems to indicate that earlier interactions between cancer cells and neutrophils at the primary tumor site are essential for neutrophils to enhance disease progression. This is supported by a study showing that, although depletion of neutrophils during primary tumor growth decreases metastasis, neutrophil depletion after primary tumor removal does not (Coffelt et al., 2015). Another unanswered question is what role other types of leukocytes bound to CTC clusters play in tumor progression. In patient blood, 25% of CTC-WBC clusters involve lymphocytes, and the consequences of such an association remain to be explored.
In summary, the study by Szczerba et al. (2019) provides a mechanism by which tumor cells profit from their interactions with neutrophils during dissemination to improve their metastatic capacity. These findings open the possibility of targeting factors necessary for CTC-neutrophil cluster formation to reduce the metastatic potential of cancer cells.
REFERENCES
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau C-S, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, and de Visser KE (2015). IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, and Ferri L (2013). Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest 123, 3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, and Albelda SM (2009). Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay IC, Teng MJ, Haerty W, Kumar P, Ponting CP, and Voet T (2016). Separation and parallel sequencing of the genomes and transcriptomes of single cells using G&T-seq. Nat. Protoc 11, 2081–2103. [DOI] [PubMed] [Google Scholar]
- Powell DR, and Huttenlocher A (2016). Neutrophils in the tumor microenvironment. Trends Immunol 37, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylaeva E, Lang S, and Jablonska J (2016). The essential role of type I interferons in differentiation and activation of tumor-associated neutrophils. Front. Immunol 7, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Saez B, Mukhopadhyay S, Ding D, Ahmed AM, Chen X, Pucci F, Yamin R, Wang J, Pittet MJ, et al. (2016). Tle1 tumor suppressor negatively regulates inflammation in vivo and modulates NF-κB inflammatory pathway. Proc. Natl. Acad. Sci. U S A 113, 1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. (2019). Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557. [DOI] [PubMed] [Google Scholar]
- Yu M, Stott S, Toner M, Maheswaran S, and Haber DA (2011). Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol 192, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]


