Abstract
While memories are often thought of as flashbacks to a previous experience, they do not simply conserve veridical representations of the past but must continually integrate new information to ensure survival in dynamic environments. Therefore, ‘drift’ in neural firing patterns, typically construed as disruptive ‘instability’ or an undesirable consequence of noise, may actually be useful for updating memories. In our view, continual modifications in memory representations reconcile classical theories of stable memory traces with neural drift. Here we review how memory representations are updated through dynamic recruitment of neuronal ensembles on the basis of excitability and functional connectivity at the time of learning. Overall, we emphasize the importance of considering memories not as static entities, but instead as flexible network states that reactivate and evolve across time and experience.
Introduction
Memories are neural patterns that guide behavior in familiar situations by preserving relevant information about the past. While this definition is simple in theory, in practice, environments are dynamic and probabilistic, leaving the brain with the difficult task of shaping memory representations to address this challenge. Dynamic environments imply that whatever is learned from a single episode may not hold true for future related experiences and should therefore be updated over time. If not, memory systems will fail to generalize to future retrieval episodes in which conditions may have changed, leading to suboptimal behaviors (Richards and Frankland, 2017). Hence, the ‘goal’ of a memory system is not to remember individual events with the greatest possible precision, but rather to continually adapt its contents in order to build more and more accurate models of the world. To do this, the brain employs ‘memory-updating’, which we define as the process of modifying existing firing patterns to support the integration of new information into previously learned memories. Consistent with this concept, studies on reconsolidation have previously described how memories can become labile during retrieval allowing for memory-updating (Dudai, 2012; Misanin et al., 1968; Nader et al., 2000) and these memory modifications continue indefinitely over an animal’s lifetime (Dudai, 2012; McKenzie and Eichenbaum, 2011; Nadel et al., 2012). While reconsolidation studies have greatly contributed to our understanding of continual learning, these studies typically rely on amnesic pharmacological agents that offer limited insight to how memory modification occurs at the neurophysiological and population level. However, recent studies utilizing approaches to observe and manipulate large-scale neuronal populations have reinvigorated the search for the flexibility of memory traces (Box 1). Indeed, as we will describe in this review, these observations support the concept of continual memory-updating and complement the findings regarding molecular mechanisms in the reconsolidation literature (Tronson and Taylor, 2007). Rather than discrete and fixed neural representations, we propose that memories are stored in flexible activation patterns that are continuously modified over experience, on the order of minutes to lifetimes.
Box 1.
Large-scale neuronal population recordings for studying memory.
Rapid advances over the last several decades have enabled researchers to record the activity of large populations of neurons (usually in rodents, non-human primates, and rarely humans) and relate this activity to mnemonic processes. Multielectrode drives can be chronically or acutely inserted into the brain to measure spiking from many single units through extracellular voltage. Optical techniques such as calcium imaging can monitor fluorescence from calcium indicators expressed in hundreds of neurons simultaneously. Researchers when faced with these large neuronal populations have turned to computational analyses to correlate neural activity with ongoing behavior (for a review see Cunningham and Yu, 2014). As a basis for many of these analyses, the researcher may construct ‘population vectors’ from the neural data. Typically, these vectors will describe the activity rate of each neuron binned with respect to some other variable. For example, a common way to create population vectors from hippocampal place cells is to count spikes for each recorded neuron when the animal is traversing a (binned) spatial location. These population vectors comprise an N × S matrix where N is the number of neurons, S is the number of spatial bins, and each element in the matrix is firing rate. From these data, a researcher could search for activity patterns that may be indicative of memory encoding and retrieval processes. Key findings from this approach have identified hippocampal spiking patterns that correlate with spatiotemporal aspects of experience (Buzsáki and Moser, 2013; Buzsáki and Tingley, 2018; O'Keefe and Dostrovsky, 1971; Pastalkova et al., 2008), reactivate during memory retrieval (Foster, 2017; Joo and Frank, 2018; Lee and Wilson, 2002) and can be causally linked to behavior (Robinson et al., 2020).
Stability versus flexibility in long-term memory
There is consensus that, generally speaking, memories are stored in activity patterns and synaptic weights of neuronal ensembles brain-wide, and that these ensembles are reactivated when recalling the memory (Frankland et al., 2019; Gelbard-Sagiv et al., 2008; Guzowski et al., 1999; Liu et al., 2012; Reijmers et al., 2007; Wilson and McNaughton, 1993). These ensembles can persist over long timespans measured over days to weeks, making them attractive substrates for long-term memory storage (Josselyn et al., 2015; Tonegawa et al., 2015). The foundation for these ideas came from Donald Hebb, whose theories on synaptic plasticity and the stabilization of cell ensembles laid the groundwork for contemporary ideas in memory representations within neuronal networks (Hebb, 1949). For instance, demonstrations of ensemble stability were found in hippocampal place cells (pyramidal neurons that fire according to the animal’s position in space) (O’Keefe and Nadel, 1978) that were found to be stable over many weeks (Thompson and Best, 1990). Stability of neuronal ensembles was also supported by studies using localization of immediate-early gene expression in the hippocampus—exploration of two identical environments 20 min apart induced activity-dependent Arc expression in highly overlapping populations of CA1 neurons (Guzowski et al., 1999). In the amygdala, reactivation of a neuronal ensemble active during learning was correlated with memory recall several days later, indicating a stable neural correlate for fear memory (Reijmers et al., 2007). Based on these and related studies, modern theories suggest that dedicated populations of neurons (‘engram cells’) encode and store memories in the manner of a Hebbian cell ensemble (Hebb, 1949; Josselyn et al., 2015; Tonegawa et al., 2015).
While groundbreaking, the discovery of stable ensembles as substrates for memories is an incomplete account of how memory systems operate over the course of an animal’s lifetime. Above all, these principles do not explain how the brain can integrate new experiences with old memories. In practice, some degree of flexibility must complement persistence in the successful implementation of memory (Richards and Frankland, 2017). The ‘stability-plasticity dilemma’ describes the necessary compromise between these two opposing forces, allowing new learning to occur while preserving existing knowledge (Grossberg, 1982). Indeed, modeling studies have shown that an overly rigid neural network actually encumbers the acquisition of new information. In an inflexible network, existing knowledge can interfere with the encoding of new information (proactive interference) and is also subject to erasure during new learning (catastrophic forgetting) (Hasselmo and Wyble, 1997; McClelland et al., 1995; McCloskey and Cohen, 1989). Therefore, in addition to understanding how memories can persist in neural populations, it is equally important to understand how memory systems can overcome collisions between old and new memories. To that end, dynamic memory ensembles encapsulate how memories can be both persistent and fluid. This idea is in line with the research on reversal learning (Izquierdo et al., 2017), reconsolidation (McKenzie and Eichenbaum, 2011), schema learning (Bartlett, 1932; Gilboa and Marlatte, 2017; Tse et al., 2007), and systems consolidation (Kumaran et al., 2016; McClelland et al., 1995; Nadel et al., 2012), which all describe how previously learned behaviors can be modified to accommodate new learning. In particular, prominent theories on systems consolidation stress the importance of both persistence and flexibility—the hippocampus is often thought of as the flexible learner that trains neocortical networks to store memories long term (McClelland et al., 1995). However, neocortical networks still undergo continual modifications as the animal learns over a lifetime (Kumaran et al., 2016).
Although much is known about the flexibility of behaviors, we know much less about how the memories enabling those behaviors are updated at the neurophysiological level. While the flexibility of memory has been well appreciated in synaptic neurobiology (Holtmaat and Svoboda, 2009; Rumpel and Triesch, 2016; Ziv and Brenner, 2018) and cognitive psychology (Nadel et al., 2012), it has been largely neglected by neurophysiologists with a few recent notable exceptions (Chambers and Rumpel, 2017; Clopath et al., 2017 ; Richards and Frankland, 2017; Rule et al., 2019). We begin by highlighting recent longitudinal observations of slow fluctuations in neural activity and synaptic structure that complement the ‘stable engram’ hypothesis (Josselyn et al., 2015; Tonegawa et al., 2015). Intrinsic fluctuations that alter synaptic connectivity and cellular excitability provide an ever-present reservoir of flexibility in population activity patterns to store a memory. We propose that these slow fluctuations act like a conveyor belt that continuously supplies potential new storage sites of future memories, as suggested by previous studies on 'memory allocation' and 'memory-linking' (Cai et al., 2016; Josselyn and Frankland, 2018; Rashid et al., 2016; Yokose et al., 2017). Neurons encoding these future experiences may overlap with existing engrams, updating past memories. In the following section, we describe how ‘unstable’ ensembles are not in fact paradoxical, but instead are necessary for flexible memory and behavior. Then, we outline the steps of the memory-updating process which entails (1) a partial reactivation of a previously formed engram, (2) recruitment of neuronal populations into existing engrams based on their excitability and functional connectivity, (3) deployment of plasticity processes that modify these networks by integrating the new population, and (4) temporal coordination of neural activity within and across regions for brain-wide memory-updating.
The benefit of dynamism: how drift aids memory flexibility
Representational drift: findings from neurophysiology
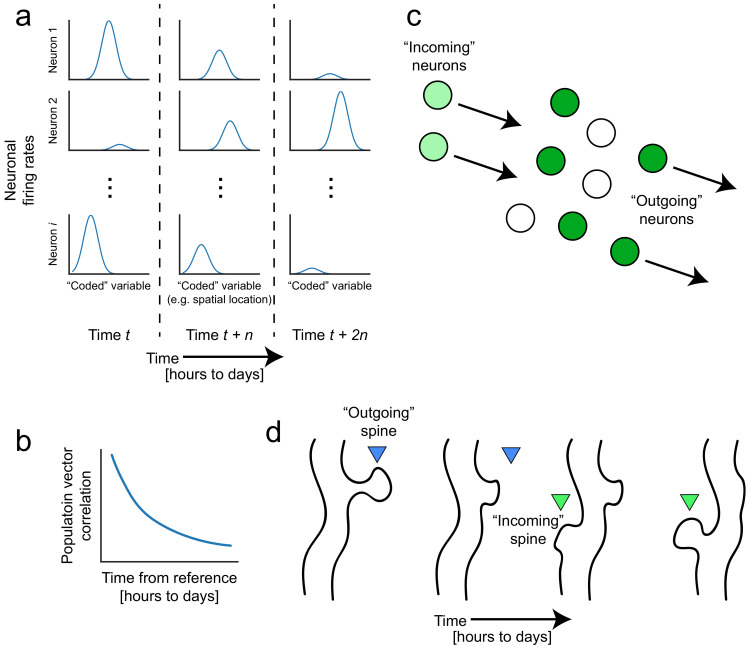
With the advent of functional imaging methods that can enable longitudinal tracking of single neurons over days to weeks, a picture is starting to emerge that memory representations are not as fixed as we might expect. Despite experimenters’ efforts to keep external environments consistent and with no observed changes in the animal’s behavior, the firing patterns of neuronal populations continue to evolve, a phenomenon known as ‘representational drift’ (for reviews see Chambers and Rumpel, 2017; Clopath et al., 2017; Rule et al., 2019). Repeated exposures to the same conditions produce neural representations of these highly familiar experiences that nonetheless fluctuate on the order of hours to weeks, even with stereotypical behavior. For instance, hippocampal ensemble activity deviates over time, as measured by population vector correlations to a reference point, despite no deviations in the behavioral task (Figure 1a and b; Bladon et al., 2019; Hainmueller and Bartos, 2018; Mankin et al., 2012; Manns et al., 2007; Mau et al., 2018; Rubin et al., 2015; Ziv et al., 2013). Similar fluctuations have been reported in barrel cortex (Margolis et al., 2012), motor regions (Liberti et al., 2016; Peters et al., 2017; Rokni et al., 2007), lateral entorhinal cortex (Tsao et al., 2018), and parietal cortex (Driscoll et al., 2017), suggesting that while all these regions differentially contribute to memory, drift may be a ubiquitous feature of neural systems that seems to threaten memory stability. Alternatively, drift could actually reflect the inherent flexibility of the neural code and stem from numerous parallel neurobiological processes including spontaneous synaptic remodeling (Ziv and Brenner, 2018), and dynamic changes in cellular excitability (Figure 1c and d; Chen et al., 2020; Slomowitz et al., 2015). Next, we will consider how these processes could potentially contribute to the way in whichdynamic neural codes can support memory flexibility.
Figure 1. Representational drift and intrinsic dynamics supply neural substrates for memory-updating over time.
(a) Example tuning curves of a neuronal population changing its firing patterns over time with respect to an arbitrary external variable (e.g. spatial location) (Mau et al., 2018; Ziv et al., 2013). Each column corresponds to a certain point in time. Some neurons will lose their field while others gain one. This occurs even when the animal is performing stereotyped behavior (Chambers and Rumpel, 2017; Rokni et al., 2007; Rule et al., 2019). (b) Schematic of population similarity over time. As a result of (a), the similarity of population activity to time t decreases over time (Mankin et al., 2012; Mau et al., 2018; Ziv et al., 2013). (c) Schematic of fluidity in ensembles. Intrinsic fluctuations that result in increased excitability in certain neurons (light green circles) bring them up to par with the currently active ensemble (dark green circles) relative to non-active ensembles (white circles). These ‘incoming’ neurons become more likely to encode future memories (Rogerson et al., 2014). At the same time, other ‘outgoing’ neurons lose their association with the network or their synapses are pruned to make room for the incoming cells. (d) Schematic of dynamic synapses. Even in the absence of neuronal activity, synapses are known to be continuously formed and eliminated (Minerbi et al., 2009; Yasumatsu et al., 2008), meaning this is an intrinsic process that occurs regardless of input. Perhaps the underlying source of drift at the population level is intrinsic synaptic volatility (Holtmaat and Svoboda, 2009; Ziv and Brenner, 2018).
Synaptic turnover: contributions to representational drift
Synapses are constantly being remodeled, which could be one underlying factor for drift in ensemble codes (Buzsáki, 2010; Rumpel and Triesch, 2016; Ziv and Brenner, 2018). While neural activity is a well-known mediator of synaptic plasticity (Bi and Poo, 1998; Bliss and Collingridge, 1993), the reverse relationship is also possible—synaptic structure and weights could influence neural activity patterns (Buzsáki, 2010; Fauth et al., 2015; Kalle Kossio et al., 2020; McKenzie et al., 2019; Turrigiano and Nelson, 2004). Along those lines, when action potentials are blocked in cell cultures, synapses continue to turn over across days, demonstrating that spontaneous synaptic remodeling occurs even in the absence of neuronal firing, and that these intrinsic remodeling events could impact the participation of individual neurons within ensembles (Minerbi et al., 2009; Yasumatsu et al., 2008). Hippocampal dendritic spines have been found to completely turn over within weeks, presumably contributing to fluctuations in neuronal firing patterns via rearrangement of synaptic weights (Attardo et al., 2015; Pfeiffer et al., 2018). Supporting this possibility, one experiment described how local activity patterns at CA1 dendrites predicted longitudinal place field stability based on somatic calcium activity. The experimenters found that place cells were more likely to lose their field if the dendritic activity of those cells was more variable on a trial-by-trial basis (Sheffield and Dombeck, 2015). This suggests that the computations occurring in dendritic compartments could be disturbed by synaptic turnover, which in turn could potentially destabilize place fields across the whole population. Recent modeling efforts have shown that synaptic turnover can give rise to drifting ensembles (Kalle Kossio et al., 2020). However, direct evidence for synaptic turnover as a major source for representational drift has not yet been shown and would require technically challenging feats of simultaneously imaging spines and somatic activity over long timescales.
Stable memories from tenacious networks
While seemingly disruptive, there is substantial evidence that constant flux does not preclude memory persistence. Although dendritic spines are routinely formed and eliminated, a significant proportion is estimated to persist over an animal’s lifetime (Yang et al., 2009), suggesting that long-term memories could be stored in the synapses located at those spines. These persistent spines are more common in the neocortex than in the hippocampus (Attardo et al., 2015), consistent with systems consolidation theories suggesting that neocortical networks play a larger role in storing relatively more stable representations. Nonetheless, up to 15–25% of hippocampal CA1 neurons retain the same spatial firing patterns across weeks, which is sufficient for accurate spatial decoding (Shuman et al., 2020; Ziv et al., 2013). Such patterns may persist in part due to the relatively high resilience of larger dendritic spines (Holtmaat and Svoboda, 2009; Holtmaat et al., 2005), which has been observed in both hippocampal (Pfeiffer et al., 2018) and neocortical spines (de Vivo et al., 2017). Additionally, modeling work has suggested that repeated offline reactivation of specific ensembles could maintain potentiated synaptic weights (Fauth and van Rossum, 2019). Thus, long-term memories may be supported by a ‘backbone’ of stable spines and neurons that store gross features while the remainder might continually undergo plasticity to encode more detailed representations (Buzsáki and Mizuseki, 2014; Grosmark and Buzsaki, 2016; Sweeney and Clopath, 2020). A complementary ‘memory indexing’ theory has proposed that hippocampal neurons reinstate neocortical activity patterns for memory retrieval (Goode et al., 2020; Tanaka et al., 2018; Teyler and DiScenna, 1986). In such a regime, neocortical storage sites may house relatively more stable memories that hippocampal computations incrementally modify (Kumaran et al., 2016).
Even with the stability of a ‘backbone’ neural network, a nontrivial portion of neural networks is dynamic. Still, this does not appear to hinder memory or behavior. Performance on a variety of spatial navigation tasks remained stable across days despite continuous reorganization of firing patterns in parietal cortex and hippocampus (Driscoll et al., 2017; Kinsky et al., 2020; Levy et al., 2020). Most surprisingly, motor patterns are unchanged despite drift in neural activity from motor areas (Liberti et al., 2016; Rokni et al., 2007, but see Katlowitz et al., 2018). Perhaps behavioral stability could be attributed to the consistency of the overall population regardless of the activity of individual neurons (Rule et al., 2020). Others have shown that from a population standpoint, the variability of any individual neuron is inconsequential to the fidelity of the overall neural code (Gallego et al., 2020; Gonzalez et al., 2019; Rokni et al., 2007; Rule et al., 2019; Rule et al., 2020). A recent study proposed that downstream readers can hypothetically compensate for drift, suggesting that as long as the interpreter of an upstream neural code is capable of re-weighting its inputs, a stable readout can still be achieved (Kalle Kossio et al., 2020; Rule et al., 2020). Although the neurobiological principles governing this re-weighting have yet to be determined, they may hinge upon neuromodulatory feedback signals such as dopamine or acetylcholine (Duszkiewicz et al., 2019; Hasselmo, 2006). Taken together, these studies indicate that memory retrieval can tolerate some degree of dynamism provided that some core backbone of the collective network remains intact or if downstream readers adjust their outputs in accordance with their fluctuating inputs.
Drift as a mechanism for continuous remodeling
What is the function of drift in memory systems? We posit that drift can slowly and stochastically provide neural substrates that can bind new information (Rumpel and Triesch, 2016), both for forming new memories and for updating old ones. In order to form new memories or update past memories, neural networks are faced with the formidable problem of having to potentiate the appropriate synaptic patterns among the huge number of possibilities that make up the synaptic connectivity space. Structural synaptic turnover through continuous spine degradation and formation could facilitate future learning by maximizing sampling across this synaptic connectivity space (Frank et al., 2018; Holtmaat and Svoboda, 2009; Kappel et al., 2015; Minerbi et al., 2009; Rumpel and Triesch, 2016; Xu et al., 2009), increasing the likelihood of achieving certain spiking patterns, and ultimately potentiating their corresponding synaptic weights. Consistent with this logic, spine turnover is critical for birdsong acquisition, fear conditioning, and spatial navigation in zebra finches and mice (Castello-Waldow et al., 2020; Frank et al., 2018; Roberts et al., 2010). Frank et al., 2018 measured spine turnover in the mouse retrosplenial cortex and found that turnover rates, even before fear conditioning and spatial exploration, positively correlated with individual ability to learn each memory. In other words, high spine turnover rates provided a greater number of new spines available for memory encoding but may have also enabled faster sampling across synaptic space and therefore a quicker arrival to a synaptic connectivity pattern that adequately encoded the new information (Castello-Waldow et al., 2020; Frank et al., 2018; Rumpel and Triesch, 2016; Xu et al., 2009). Changes in synaptic connectivity could also heterogeneously influence the likelihood of spiking (intrinsic excitability) in neuronal subpopulations, which would in turn increase their likelihood of participating in future memory-encoding ensembles (i.e., memory allocation; Box 2; Buzsáki, 2010; Chen et al., 2020; Rogerson et al., 2014; Yiu et al., 2014; Zhou et al., 2009). In this way, the brain could prioritize different rosters of neurons to diversify eligibility for memory encoding or updating (Margolis et al., 2012; Rogerson et al., 2014; Trouche et al., 2016). Such a framework is consistent with systems consolidation where information is constantly redistributed across the neocortical-hippocampal loop (Kumaran et al., 2016; McClelland et al., 1995). In summary, spontaneous synaptic remodeling can supply additional synapses and neurons in which memories can be laid down as they are being experienced, contributing to memory flexibility.
Box 2.
Neuronal excitability and memory allocation.
The memory allocation hypothesis states that neurons with high excitability are more likely to be recruited for memory encoding (Rogerson et al., 2014). Experimental excitation of subpopulations of amygdala neurons biases fear memory storage to those cells, and ablation of that subpopulation abolishes the memory while activation of that subpopulation induces memory retrieval (Han et al., 2007; Rogerson et al., 2016; Yiu et al., 2014; Zhou et al., 2009) In CA1, place cells (as opposed to non-place cells) exhibit electrophysiological properties indicative of high excitability such as lower spiking thresholds (Epsztein et al., 2011) and artificial excitation endows non-place cells with place fields (Bittner et al., 2015; Diamantaki et al., 2018; Lee et al., 2012). Also in CA1, c-fos-positive populations that distinguish between spatial contexts similarly show high mean firing rates (Tanaka et al., 2018). In other brain regions such as prefrontal cortex and nucleus accumbens, highly excitable cells have been found to be critical for reproduction of certain behaviors such as conditioned freezing and reward seeking (Whitaker et al., 2017; Ziminski et al., 2017), demonstrating their role in stable memory encoding and retrieval. In humans, excitability during pre-encoding also appears to predict subsequent memory encoding strength (Urgolites et al., 2020).
Neurogenesis and plasticity aid memory-updating
Drift may also facilitate the updating of previously learned memories by partially weakening old activity patterns in favor of strengthening new ones. In particular, hippocampal adult neurogenesis has been shown to play an important role in synaptic and circuit remodeling, supporting memory-updating and flexibility (Aimone et al., 2009; Burghardt et al., 2012; Denny et al., 2014; Epp et al., 2016; Frankland et al., 2013; Rangel et al., 2014; Richards and Frankland, 2017; Saxe et al., 2006). The addition of newborn neurons in the dentate gyrus reconfigures existing hippocampal activity by decaying previously potentiated synapses (Alam et al., 2018; Frankland et al., 2013; Kitamura et al., 2009). At first glance, this may appear disruptive for memory, but integration of these newborn neurons into hippocampal circuits can be useful because network-wide synaptic reorganization can erase obsolete patterns while making room for new ones (Alam et al., 2018; Frankland et al., 2013; Richards and Frankland, 2017; Toni et al., 2008). This feature is especially advantageous for memory-updating, which requires circuit reconfiguration in order for previous memories to accommodate potentially conflicting new information. In alignment with this hypothesis, exercise and environmental enrichment (which both increase neurogenesis) enhanced navigational flexibility when a goal was relocated (Epp et al., 2016; Garthe et al., 2016). In these studies, mice with increased neurogenesis were better able to learn by modifying their expectations of where the goal was likely to be, but this ability was abolished when neurogenesis was inhibited (Burghardt et al., 2012; Epp et al., 2016; Garthe et al., 2016). Importantly, neurogenesis was not required for initial memory acquisition, but only when the mice had to reverse their behavior (Burghardt et al., 2012; Epp et al., 2016). This suggests that neurogenesis plays an important role in memory-updating, and not just in acquisition of a new memory (Meshi et al., 2006). Taken together, these findings suggest that after integration of adult-born neurons, the consequent synaptic reorganization (Toni et al., 2008) allows experienced brains to acquire yet more information to integrate with previously learned associations.
Aside from neurogenesis, spontaneous synaptic remodeling is a critical feature for permitting memory-updating through the exploration of synaptic connectivity space using prior knowledge as a starting point. Modeling studies have demonstrated that continual reconfiguration of synaptic weights is necessary for a network to adapt once dynamic conditions are imposed (Ajemian et al., 2013; Duffy et al., 2019; Kappel et al., 2018). One particular study used a neural network to perform a simulated motor task where specific outputs were rewarded (Kappel et al., 2018). The authors then changed the output of a subset of model neurons relative to the motor task, thereby perturbing the neural network’s ability to produce the correct motor actions. However, because the neural network was able to continuously explore alternative solutions through a drift-like synaptic rewiring mechanism, it quickly adapted to the perturbation and regained high accuracy (Kappel et al., 2018). This suggests that slow synaptic turnover may facilitate the ability to draw from a prior knowledge base (by storing connectivity patterns that are slow to decay) while still flexibly exploring related options through stochastic probing of new potential connectivity patterns, built atop existing ones. Such an implementation may underlie flexible behaviors that are based on memories for past outcomes. In other words, as long as a memory is retrievable and the ensemble encoding that memory is sufficiently plastic, it can be updated; but suppressing retrieval (Yokose et al., 2017) or plasticity (Guo et al., 2018) blocks memory-updating. The notion of memory modification after retrieval has also been previously discussed in the context of reconsolidation, when memories are subject to modification after they are retrieved (Dudai, 2012; McKenzie and Eichenbaum, 2011; Misanin et al., 1968; Nader et al., 2000). In the next section, we examine how the brain modifies these memories.
Neural mechanisms of memory-updating
Memory retrieval is associated with a re-visitation of neural state space
There is a large body of evidence showing that memories are not created de novo and instead draw upon prior knowledge stored in biophysical configurations of synapses and population activity (Bliss and Collingridge, 1993; Dragoi and Tonegawa, 2014). As such, memory-updating relies on instantiating an internal representation of past events, comparing expectations to current sensory input, and modifying the representation to improve predictive power (Gershman et al., 2017). For example, in humans, viewing specific images reactivates the firing patterns observed during initial presentation of the stimulus (Gelbard-Sagiv et al., 2008). In rodents, similar neuronal populations are reactivated upon re-exposures to familiar environments (Guzowski et al., 1999; Reijmers et al., 2007; Rubin et al., 2015; Shuman et al., 2020; Thompson and Best, 1990; Ziv et al., 2013), and activation of neuronal populations previously activated during the initial learning will induce memory retrieval (Liu et al., 2012; Ramirez et al., 2013; Robinson et al., 2020).
Reactivation of neural patterns during memory retrieval may constrain the brain to these state spaces, restricting the degrees of freedom for exploring possible solutions for new encoding. These states may act as continuous attractors (Leutgeb et al., 2005; Tsodyks, 1999; Wills et al., 2005) and function as workspaces for memory-updating within that time window (Figure 2). Such a mechanism would imply that learning something associated with a previously learned stimulus would reactivate that previous memory. This view is again consistent with reconsolidation experiments where a reminder cue reinstates a previous memory, creating an opportunity for modification of that memory (Gisquet-Verrier et al., 2015; Hupbach et al., 2007; McKenzie and Eichenbaum, 2011; Nadel et al., 2012). Functional imaging experiments in the human brain found that presentations of reminders triggered reactivation of hippocampal and prefrontal networks, which facilitated learning of new, related items (Schlichting and Preston, 2014; Zeithamova et al., 2012). Rodent studies have also found reactivation of hippocampal ensembles during memory-updating (Dupret et al., 2010; McKenzie et al., 2013; McKenzie et al., 2014). Previous training on memory tasks with one set of stimuli accelerated their ability to form new associations with different stimuli. Upon introduction of these new stimuli, neurons that encoded previous, related stimuli were reactivated, suggesting that this population acted as a scaffold upon which new, related stimuli were embedded (McKenzie et al., 2013; McKenzie et al., 2014). This reactivation facilitates the connection of related experiences across time, allowing for the integration of events through the co-activation of neurons encoding past and present experiences. Such a mechanism is consistent with reports of optogenetic stimulation to co-activate ensembles to update previous memories with experimenter-defined ‘false memories’ (Ohkawa et al., 2015; Ramirez et al., 2013; Vetere et al., 2019).
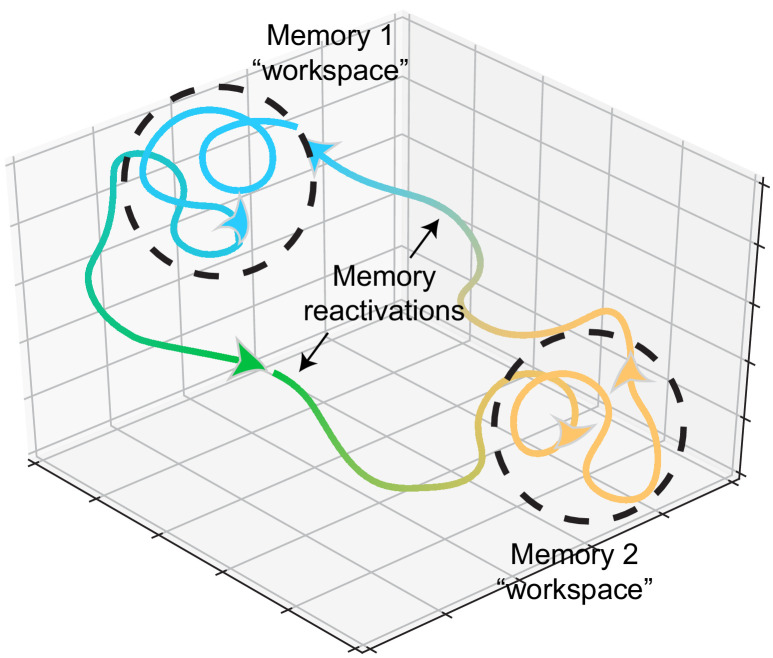
Figure 2. Memory representations occupy regions of state space during experience and learning.
Example trajectories of network states during recollection of two memories, depicted on a neural subspace. The network state is expressed in the firing activity of large neuronal populations. During situations where the environment or context is relatively stationary, the network state exhibits slow drift that constrains learning and plasticity locally (dotted black circles). Upon a major contextual shift, the network state responds with a fast, commensurate shift to a new regime (from Memory 1 to Memory 2, green trajectory) that recalls another memory. The network resides there until another contextual shift kicks it back to Memory 1. In experimental conditions, these contextual shifts are usually experimenter-defined (e.g., placing animals in different enclosures). However, in the wild, they may be shaped by major changes in the animal’s surroundings. In humans, and probably in non-human animals as well, contextual shifts may be internally motivated (i.e., spontaneous recall). Compartmentalization in network state space ensures that learning does not corrupt existing memories while allowing mechanisms for memory modification within local state space regions.
Memory-updating through temporal integration
To determine which experiences get integrated into a past memory, the relevance of input patterns might be weighted by temporal proximity to when the memory was encoded—events that occur close in time can become linked within neural networks (Clewett et al., 2019; Howard et al., 2014; Rogerson et al., 2014; Schlichting and Preston, 2014; Yetton et al., 2019). A functional imaging study in humans found that over two encoding episodes, subjects were more likely to combine their memories of overlapping items (object pairs) if they occurred within 30 min of each other (Zeithamova and Preston, 2017). Within encoding episodes (defined by minutes-long temporal blocks), there is high similarity in hippocampal activity patterns in both humans (Ezzyat and Davachi, 2014; Hsieh et al., 2014) and rats (Bulkin et al., 2020), suggesting that the network resides in the same state space during these episodes. Likewise in mice, two contextual experiences within 5 hr of each other became represented by a common set of hippocampal CA1 neurons, but not when they were separated by 7 days (Cai et al., 2016). Furthermore, not only were those two experiences represented by an overlapping ensemble, but when one of those contexts was subsequently paired with shock, animals transferred the fear from the shocked context to the neutral context. This indicates that the two memories became linked, which was sufficient to update a recent neutral memory with an aversive association (Cai et al., 2016). In another study, mice were fear conditioned with two different tones, separated by 6 hr, and these two tone-shock associations were represented in the amygdala by a common set of neurons (Rashid et al., 2016). When one of the tones was repeatedly presented without shock (a paradigm known to lead to extinction of freezing), it also reduced freezing for the other tone, suggesting that the two memories became linked within those 6 hr. These effects were not seen when longer time intervals separated the two encoding events. Yet another study was able to link a conditioned taste aversion to a fear conditioned response through an overlapping population encoding the two conditioned stimuli (Yokose et al., 2017). These ‘temporal memory-linking’ studies demonstrate that events occurring in close temporal proximity can mutually impact and modify memory representations of surrounding events.
Excitability and synaptic dynamics ensure a constant supply of neurons for memory-updating
During memory-updating, how does the network determine which neurons are integrated into an existing memory? Accumulating evidence indicates that excitability and functional connectivity influence the allocation of neurons into memory ensembles. This theory, known as the ‘memory allocation hypothesis’, suggests that the excitability of a neuron predisposes it for encoding a memory (Box 2; Rogerson et al., 2014; Zhou et al., 2009). Moreover, the excitability and connectivity of neuronal populations are constantly changing, which would suggest that different sets of neurons encode or update memories as these neurons increase in prominence from the perspective of the network (Buzsáki, 2010; Chen et al., 2020; Minerbi et al., 2009; Slomowitz et al., 2015). In other words, drift, as a result of constantly fluctuating excitability (Chen et al., 2020; Rogerson et al., 2014; Slomowitz et al., 2015), synapses (Holtmaat and Svoboda, 2009; Rumpel and Triesch, 2016; Xu et al., 2009; Yang et al., 2009; Ziv and Brenner, 2018), and intracellular proteomes (Rogerson et al., 2014), define the degree to which single neurons compete to participate in encoding a memory (Han et al., 2007) based on their excitability and connectivity to an existing memory engram.
During memory-updating, in order to homeostatically maintain ensemble sizes (Stefanelli et al., 2016), other neurons must also decrease their roles in memory encoding, ‘exiting’ the ensemble by reducing their contribution to the memory representation (Figure 1c). Because synaptic potentiation is saturated in engram neurons (Choi et al., 2018), a neuron’s exit from an ensemble might reflect depotentiation, reducing its likelihood of being co-activated with the remainder of the ensemble and potentially allowing it to encode other information. Depotentiation could result from a new competing ensemble suppressing the activity of neurons from a previous ensemble (Han et al., 2007; Rashid et al., 2016). For example, ensembles formed during extinction may be inhibiting ensembles formed during fear learning (Lacagnina et al., 2019). As secondary evidence, optogenetic inhibition of an engram results in recruitment of an alternative engram (Rashid et al., 2016; Schoenenberger et al., 2016; Trouche et al., 2016), implying that the ensemble that might have been ‘next in line’ to compete with the first acts as a fail-safe to encode the present experience, ensuring functional homeostasis. Such competition may also underlie the loss of firing selectivity (e.g., receptive fields) over time in some neurons, decreasing their contribution to reliable memory encoding of the original memory (Liberti et al., 2016; Mankin et al., 2012; Manns et al., 2007; Mau et al., 2018; Rubin et al., 2015; Sheffield and Dombeck, 2015; Trachtenberg et al., 2002; Ziv et al., 2013). The gradual departure of those neurons may allow a transition to another set of neurons that surface with new information to integrate into the existing memory.
During memory-updating, memory allocation on the basis of neuronal excitability promotes the overlap between ensembles encoding memories close in time. High excitability biases neurons encoding one memory to be recruited to encode another event, thereby linking the two memories through a shared neuronal ensemble (‘allocate-to-link’ hypothesis) (Cai et al., 2016; Rashid et al., 2016; Rogerson et al., 2014). But how could associations be made between experiences that are more temporally distant? One possibility is through ensemble reactivation during memory retrieval, which prepares an old ensemble to integrate new ensembles (Yokose et al., 2017). Because memory retrieval involves a reactivation of a neuronal ensemble, excitation of those cells could trigger a physiological state that primes them for integrating the activity patterns of new neurons into their network. In line with this idea, learning increases neuronal excitability (Chandra and Barkai, 2018; Disterhoft et al., 1986). Early studies found that eyeblink conditioning (Disterhoft et al., 1986) and operant conditioning (Saar et al., 1998) increased neuronal excitability in CA1 and piriform cortex by decreasing the magnitude of slow after-hyperpolarizations (due to decreases in the calcium-dependent hyperpolarization current that follows spike bursts). Additionally, recent experiments have shown that during encoding and retrieval episodes, excitability is elevated in specific populations—the engram cells (Cai et al., 2016; Pignatelli et al., 2019). By reactivating these engram cells during memory retrieval, they become re-excited along with newly excited cells at the next encoding event, which could induce synaptic potentiation between these ensembles (Abdou et al., 2018; Choi et al., 2018; Nabavi et al., 2014). In doing so, this would combine memories that are related in content (yet encoded distant in time) in a common set of neurons. Simply put, the brain could recruit neurons encoding new information to fire alongside engram cells into an ensemble that now incorporates elements of new and old firing patterns (Ohkawa et al., 2015). This modified ensemble then represents the updated memory.
Pre-existing temporal motifs influence neuronal recruitment patterns
While excitability could determine the identity of the neurons to be recruited into an ensemble during memory-updating, pre-existing firing patterns (i.e., functional connectivity) at the time of encoding could mediate the temporal structure of the new neural motif destined for eventual potentiation (Dragoi and Tonegawa, 2014; McKenzie et al., 2019). The temporal structure of spiking patterns could then be interpreted by downstream reader regions (Buzsáki and Tingley, 2018). An observed phenomenon that supports the hypothesis of pre-existing temporal patterns is hippocampal ‘preplay’ (Figure 3a). Preplay refers to an ensemble of CA1 neurons that produces reliable sequential activity prior to spatial learning, which is then recapitulated by firing in the same order while exploring physical space (Dragoi and Tonegawa, 2011; Dragoi and Tonegawa, 2014, but see Silva et al., 2015). This finding again implies that memory representations are not created de novo and instead that pre-existing functional connections and premature synaptic patterns can become potentiated by experience (Figure 3b). That is, there is always likely to be a neuronal sequence that occurs more often than others by virtue of the current state of synaptic connectivity (Buzsáki and Mizuseki, 2014), and this sequence becomes most likely to encode the next upcoming memory. In this way, the current state of the network as it drifts is connected to ongoing experience through the storage of information in neuronal ensembles with precise temporal coordination.
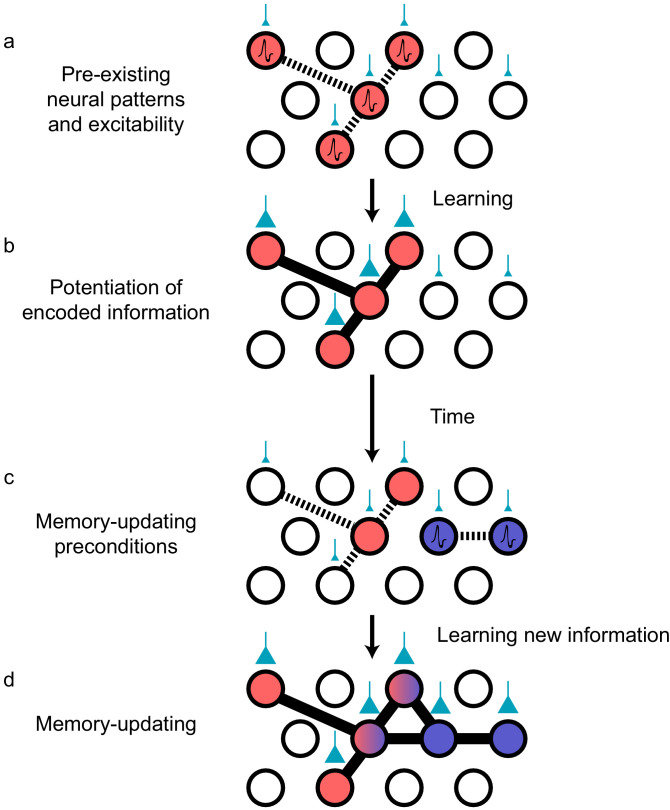
Figure 3. Preconfigured synaptic weights and excitability bias the allocation of memories to certain neuronal populations.
(a) Prior to an encoding event, some neurons will happen to be at the higher end of a distribution of excitability (red circles). These cells may be synaptically connected with each other, or receive shared input from upstream regions (e.g., CA1 receiving input from CA3; cyan boutons with bouton size representing synaptic weight) that result in high functional connectivity (dashed lines). Other cells may also be receiving input but have low excitability (empty circles). (b) During learning, these highly excitable cells increase their functional connectivity (bold lines connecting red circles) through synaptic plasticity. This could be achieved through potentiation of direct synaptic connections in the case of recurrent brain regions (e.g., CA3), or through potentiation of synapses in upstream regions that achieve appropriately timed co-activation of downstream (e.g., CA1) ensembles. (c) Over time, synaptic weights may weaken but some may persist to allow partial reactivation of a prior ensemble during memory recall (red circles). At the same time, just as in (a), a new population of cells may exhibit above-average excitability and functional connectivity (blue circles and dashed lines) at the time of a second learning episode. (d) Learning may potentiate functional connections between the red and blue ensembles to link the two memories.
Neural sequences derived from preplay templates may undergo cyclical refinement, consistent with our views on continuous memory-updating and how pre-existing temporal structure can shape the patterns that emerge. Once a preplay pattern is potentiated, increasing its occurrence rate, it is considered to be ‘replay’ (Dragoi, 2020; Foster, 2017; Liu et al., 2019) and it is likely that replay patterns could then take on the role of preplay templates to integrate upcoming, related events (Dragoi, 2020). Modification of these sequence templates would therefore be an efficient mechanism for flexibly learning new information that is consistent with pre-established networks, without the need for widespread restructuring (McClelland et al., 2020). In support of this idea, pre-existing sequences are relatively preserved after learning but reorganization is still apparent; new neurons are added to a backbone sequence after spatial exploration (Grosmark and Buzsaki, 2016; Figure 3c). One study investigated the properties of neurons that begin to co-fire with an established sequence after spatial exploration of a novel environment (Grosmark and Buzsaki, 2016). This study found that newly recruited neurons contained higher spatial information, suggesting that the neurons that are most informative about spatial regularities in the environment are preferentially added to specific positions along the sequence. Moreover, consistent with the memory allocation theory, the neurons that were recruited tended to have higher firing rates than those that were not (Fernández-Ruiz et al., 2019; Grosmark and Buzsaki, 2016). Finally, the recruitment of neurons is dependent on the degree of temporal coordination between those neurons both prior to and during experience, demonstrating that functional connectivity among the ensemble participants is critical for recruitment (Farooq and Dragoi, 2019; Farooq et al., 2019). Thus, both excitability and functional connectivity bias the composition and temporal coordination of recruited neurons during memory-updating. But to actually induce physical changes in the synaptic strengths of these ensembles, the brain must modify synapses using plasticity proteins in concert with temporally coincident neural activity.
Deployment of plasticity proteins enables subsequent synaptic potentiation of new ensembles
How do slowly drifting synaptic configurations and spiking patterns actually stabilize to store information? One major factor is the upregulation of experience-dependent plasticity, which can temporarily stabilize memory ensembles. Experience-dependent plasticity can then potentiate activity patterns of memory ensembles to allow for those patterns to be retrieved for the next time they are to be updated. Indeed, there is enhanced expression of plasticity-related proteins such as cAMP responsive element binding protein (CREB) in numerous brain regions following memory retrieval and updating (Hall et al., 2001). Artificial memory retrieval, through activation of engram cells, triggers plasticity in those cells and memory modification at the behavioral level. For instance, after fear-conditioning, chemogenetically activating CREB-expressing amygdala engram neurons induces freezing and also triggers reconsolidation-associated protein signaling cascades that may prime memory-updating (Kim et al., 2014). Blocking plasticity with protein synthesis inhibition prevents memory-updating and impairs learning-associated changes in firing patterns and synaptic turnover (Dragoi and Tonegawa, 2013; Dupret et al., 2010; Kim et al., 2014; Li et al., 2017; Tse et al., 2011). The myriad protein signal pathways triggered by learning also diversifyneuronal electrophysiological properties, such as excitation and inhibition patterns. These signaling cascades may enable complex interplay among a heterogeneous population to support and balance different aspects of memory, such as stability and flexibility (Sweis et al., 2020; Yap and Greenberg, 2018). For example, a recent study found that Fos- and Npas4-expressing granule cell ensembles in the dentate gyrus were respectively responsible for generalization and discrimination of contextual fear memory (Sun et al., 2020). Further study is required to fully understand how constellations of molecular components interact in the cellular and network milieu to support high order cognition.
Temporal coordination of neuronal ensembles promotes neuronal recruitment and memory-updating
In addition to the mobilization of plasticity mechanisms, neurons also need to spike in a temporally coordinated fashion in order to be recruited (Bi and Poo, 1998). Once neurons become coordinated at the millisecond scale, the resultant ensembles can then persist over longer timescales owing to synaptic potentiation (Bi and Poo, 1998; Bliss and Collingridge, 1993). Oscillatory patterns in the brain represent an organizational framework for grouping the spike timing of neuronal subpopulations, which has led to a large body of literature on how rhythms could subserve experience-dependent plasticity, and therefore also memory-updating. In the hippocampus, plasticity is thought to be mediated by temporally coordinated neuronal ensembles within brief time windows such as the sharp-wave ripple (SPW-R) envelope (Buzsáki, 2015) and individual cycles of the 4–12 Hz theta rhythm (Colgin, 2013; Hasselmo et al., 2002; Larson et al., 1986). Both theta oscillations and SPW-Rs are powerful instigators of plasticity and long-term potentiation (LTP). In vitro, theta-paced stimulation is the optimal frequency for inducing LTP in hippocampal slices (Larson et al., 1986). Importantly, the theta phase is critical for determining whether LTP or synaptic depression occurs (Hyman et al., 2003), meaning that not only is the magnitude of theta important, but also the spike timing of neuronal ensembles within theta cycles (Colgin, 2013; Dragoi and Buzsáki, 2006). Recent studies have shown that CA1 neuronal spiking needs to be temporally organized within theta cycles in order to be recruited into SPW-R events after learning (Chenani et al., 2019; Dragoi, 2020; Drieu et al., 2018; Farooq and Dragoi, 2019; Farooq et al., 2019). Thus, theta oscillations corral ensembles for potentiation and memory-updating.
After temporal coordination, SPW-Rs may accelerate the rate of plasticity among the newly recruited neurons during memory-updating to integrate them into the ensemble. Artificial induction of SPW-Rs strengthens Schaffer collateral synapses in vitro (Sadowski et al., 2016), but curiously also causes long-term depression in synapses that were not recently active (Norimoto et al., 2018). This result suggests that recently activated ensembles are preferentially strengthened while connectivity with less relevant neurons is eroded, resulting in enhanced signal-to-noise. Consistent with this view, hippocampal SPW-Rs refine and stabilize place field maps in animals learning novel environments (Dupret et al., 2010; Gridchyn et al., 2020; Roux et al., 2017; van de Ven et al., 2016). This process is facilitated by upregulating SPW-R prevalence during learning. As a rat learns a spatial memory task, SPW-Rs increase in duration, and optogenetically prolonging SPW-Rs increases behavioral performance and recruits neurons that have spatial fields in behaviorally relevant locations (Fernández-Ruiz et al., 2019). This preferential recruitment implies a mechanism for prioritizing integration of neurons that encode pertinent information and consequently serve important functional roles (Kinsky et al., 2020; Michon et al., 2019). SPW-Rs likely bind old and new memories by co-activating the appropriate ensembles. The end product is an updated ensemble whose spiking patterns convey updated information about recently learned stimuli (Dupret et al., 2010; Fernández-Ruiz et al., 2019; Grosmark and Buzsaki, 2016; O'Neill et al., 2008; Roux et al., 2017; van de Ven et al., 2016).
Sharp-wave ripples coordinate cross-regional memory-updating
Hippocampal SPW-Rs could also recruit extrahippocampal neurons, which might help transmit and integrate recent hippocampal computations with knowledge stored in the neocortex to facilitate brain-wide memory-updating (Kumaran et al., 2016; McClelland et al., 1995). The reorganization of neocortical spiking patterns could then readjust local (cortical) synaptic weights in a way that promotes meaningful activation patterns, synthesizing updated memories that may support schemas (Benchenane et al., 2010; Gilboa and Marlatte, 2017; Kumaran et al., 2016; Maingret et al., 2016; Rothschild et al., 2017). This cross-regional dialogue is a signature of systems consolidation, where the hippocampus is thought to train neocortical ensembles by developing and reinforcing population patterns over a lifetime of experiences (Kumaran et al., 2016; McClelland et al., 1995). Indeed, widespread activation of cortical regions is time-locked to hippocampal SPW-Rs (Logothetis et al., 2012), and SPW-R coupling between neocortex and hippocampus becomes upregulated after learning (Khodagholy et al., 2017). After learning, hippocampal SPW-Rs trigger spiking in downstream cortical ensembles, and also couple with cortical oscillatory events such as ripples, spindles, and delta waves (Alexander et al., 2018; Benchenane et al., 2010; Khodagholy et al., 2017; Latchoumane et al., 2017; Maingret et al., 2016; Rothschild et al., 2017; Todorova and Zugaro, 2019). These neocortical oscillatory events might themselves facilitate local plasticity. Although the individual functions of each frequency band remain an active area of research, generally, cross-regional coherence triggers spike pattern reorganization in downstream readers of hippocampal SPW-Rs that could be the neurophysiological readout of memory-updating (Benchenane et al., 2010; Kumaran et al., 2016; Maingret et al., 2016; Rothschild et al., 2017). Recent evidence suggests that SPW-Rs can route specific content, suggesting that different memories can be individually communicated to downstream readers and updated separately (Gridchyn et al., 2020). Hippocampal sequences, and their downstream neo- and subcortical readers, could reflect a modified ensemble structure that balances both parsimony and newly acquired information after undergoing experience-dependent plasticity.
Concluding remarks
In this review, we have discussed forms of memory-updating and their neurophysiological signatures. We have proposed that these processes can be understood through the modification of neuronal ensembles whose membership is determined by both existing circuitry between neurons and intrinsicdynamics within neurons. Our framework is based on the idea that the integration of ensemble activity creates functional affinities (i.e., related memories). The organization of the neurons within these ensembles is heavily influenced by a combination of cellular excitability and functional connectivity (pre-existing temporal activity patterns) and the enactment of plasticity that modifies synaptic weights. While substantial work has shown that ‘stable’ engram neurons underlie memory retrieval (Josselyn et al., 2015; Liu et al., 2012; Tonegawa et al., 2015), they do not necessarily persist indefinitely without modification. Instead, to satisfy the stability–plasticity dilemma, ensemble activities across brain regions must necessarily be fluid in order to update past memories with new information.
While our review discusses how engrams change over time and experience, there is much still unknown about how the brain resolves the end products of memory-updating. How do these new modifications reconcile with established patterns? Previous studies have found that learning induces enduring changes : population activity does not revert to its original state when a familiar rule is reintroduced (Dupret et al., 2010; Grewe et al., 2017; Malagon-Vina et al., 2018; McKenzie et al., 2013). These findings suggest that interleaved learning episodes can have lasting effects on the computational outputs of mnemonic systems, reflecting acquisition of new knowledge (Kumaran et al., 2016). One particularly interesting idea is that this new knowledge might be integrated with the old via the recombination of sub-ensembles, facilitating the formation of new relational topologies (Sweis et al., 2020; Ghandour et al., 2019).
An interesting effect of dynamic ensembles is that ‘tuning’ from neuronal populations relative to the external world deteriorates over time, which explains many observations of apparent neural instability (Chambers and Rumpel, 2017; Driscoll et al., 2017; Mankin et al., 2012; Mau et al., 2018; Rubin et al., 2015; Rule et al., 2019; Ziv et al., 2013). That is, the patterns of neuronal responses related to external stimuli are short-lived. Instead, the internal mappings between neurons persist through time. From the reference frame of the environment, the neural code for some variable (e.g., spatial location) may be drifting, but from the reference frame of the neurons, representations might remain stable due to compensatory adaptation at the network level (Kalle Kossio et al., 2020; Gonzalez et al., 2019; Kinsky et al., 2018; Rule et al., 2019; Rule et al., 2020). Such an outcome may arise from slow, coherent representational shifts such that different brain regions collectively shift in a coordinated manner. Among these regions may be ‘readers’ that interpret the messages of memory ensembles, and if those networks remodel coherently with upstream inputs, this could support the continuity of memories over time (Buzsáki, 2010; Kalle Kossio et al., 2020; Rule et al., 2020) while also providing a means to update those memories. Thus, it is important to consider how inter-cellular activity patterns are modified during learning, and one major future avenue of research should be to determine how memory-updating rearranges internal activity patterns in an organized manner that retains past information while integrating new data. Most importantly, this perspective forces us to re-assess our very notions of stability. Nearly all central nervous system neurons are many synapses away from direct contact with physical stimuli, so if the upstream populations that do supply their input are inherently dynamic, we should rarely expect a neuron to maintain a stable relationship to an external variable even in the context of memory. Instead, we take the view that brain dynamics help generate unique combinatorial patterns onto which both new and familiar experiences are embedded.
While many outstanding questions persist (Box 3), emerging technologies will prove critical for enhancing our understanding of the dynamic brain. In particular, voltage imaging (Adam et al., 2019; Piatkevich et al., 2019) has the advantage of being able to track neurons longitudinally while also providing exquisite temporal resolution and sensitivity to signals that are undetectable with calcium imaging (e.g., subthreshold depolarizations). Combining this with other optical techniques in freely behaving animals (Cai et al., 2016; Ziv et al., 2013) will allow us to advance our understanding of the dynamic brain.
Box 3.
Outstanding questions.
- What is the neurobiological milieu that determines the rate of representational drift? Do neuromodulators, sleep, or neuroendocrine signals exert influence?
- Are drift rates in brain regions related to their local intrinsic synaptic volatility (Attardo et al., 2015)? What are the consequences of these differences and how might this relate to the rate of memory-updating in hippocampal versus neocortical structures (Kumaran et al., 2016)?
- How does ensemble remodeling occur in order to retain stored information while adding new information?
- Downstream of memory representations, what are the circuits and computations that transform these firing patterns into behavioral decisions?
Acknowledgements
We thank Sima Rabinowitz for editorial assistance and Tristan Shuman, Lauren Vetere, Mark Cembrowski, Andrew Alexander, Jon Rueckemann, and Nathaniel Kinsky for detailed feedback on earlier versions of this manuscript. We also thank John Bladon, Marc Howard, Kanaka Rajan, Samuel Levy, David Sullivan, Daniel Sheehan, Daniel Orlin, Zachary Pennington, Yosif Zaki, Lingxuan Chen, Zhe (Phil) Dong, Yu (Susie) Feng, Paul Philipsberg, Lucia Page-Harley, Zoé Christenson Wick, and Nadia Khan for constructive conversations that inspired this manuscript. This work was funded by NIH F32AG067640 to WM; NIH R01 MH052090, ONR MURI N00014-19-1-2571, ONR MURI N00014-16-1-2832, NIH MH120073, NIH R01 MH095297 to MEH; and NIH DP2MH122399, R01 MH120162, One Mind Otsuka Rising Star Award, McKnight Memory and Cognitive Disorders Award, Klingenstein-Simons Fellowship Award in Neuroscience, Mount Sinai Distinguished Scholar Award, Brain Research Foundation Award, and NARSAD Young Investigator Award to DJC.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
William Mau, Email: william.mau@mssm.edu.
Denise J Cai, Email: denise.cai@mssm.edu.
Laura L Colgin, University of Texas at Austin, United States.
Laura L Colgin, University of Texas at Austin, United States.
Funding Information
This paper was supported by the following grants:
NIH F32AG067640 to William Mau.
NIH R01MH052090 to Michael Hasselmo.
Office of Naval Research ONR MURI N00014-19-1-2571 to Michael Hasselmo.
NIH R01MH095297 to Michael E Hasselmo.
NIH DP2MH122399 to Denise Cai.
One Mind Institute One Mind Otsuka Rising Star Award to Denise Cai.
McKnight Foundation McKnight Memory and Cognitive Disorders Award to Denise Cai.
Mount Sinai Health System Mount Sinai Distinguished Scholar Award to Denise Cai.
Brain Research Foundation Brain Research Foundation Award to Denise Cai.
Brain and Behavior Research Foundation NARSAD Young Investigator Award to Denise Cai.
Office of Naval Research MURI N00014-16-1-2832 to Michael Hasselmo.
NIH MH120073 to Michael Hasselmo.
Esther A. and Joseph Klingenstein Fund Klingenstein-Simons Fellowship Award in Neuroscience to Denise Cai.
NIH R01 MH120162 to Denise J Cai.
Additional information
Competing interests
No competing interests declared.
References
- Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu SI, Inokuchi K. Synapse-specific representation of the identity of overlapping memory engrams. Science. 2018;360:1227–1231. doi: 10.1126/science.aat3810. [DOI] [PubMed] [Google Scholar]
- Adam Y, Kim JJ, Lou S, Zhao Y, Xie ME, Brinks D, Wu H, Mostajo-Radji MA, Kheifets S, Parot V, Chettih S, Williams KJ, Gmeiner B, Farhi SL, Madisen L, Buchanan EK, Kinsella I, Zhou D, Paninski L, Harvey CD, Zeng H, Arlotta P, Campbell RE, Cohen AE. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature. 2019;569:413–417. doi: 10.1038/s41586-019-1166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajemian R, D'Ausilio A, Moorman H, Bizzi E. A theory for how sensorimotor skills are learned and retained in noisy and nonstationary neural circuits. PNAS. 2013;110:E5078–E5087. doi: 10.1073/pnas.1320116110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MJ, Kitamura T, Saitoh Y, Ohkawa N, Kondo T, Inokuchi K. Adult neurogenesis conserves hippocampal memory capacity. The Journal of Neuroscience. 2018;38:6854–6863. doi: 10.1523/JNEUROSCI.2976-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AS, Rangel LM, Tingley D, Nitz DA. Neurophysiological signatures of temporal coordination between retrosplenial cortex and the hippocampal formation. Behavioral Neuroscience. 2018;132:453–468. doi: 10.1037/bne0000254. [DOI] [PubMed] [Google Scholar]
- Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 Hippocampus. Nature. 2015;523:592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett FC. Remembering: A Study in Experimental and Social Psychology. New York, USA: Cambridge University Press; 1932. [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. The Journal of Neuroscience. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nature Neuroscience. 2015;18:1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladon JH, Sheehan DJ, De Freitas CS, Howard MW. In a temporally segmented experience hippocampal neurons represent temporally drifting context but not discrete segments. The Journal of Neuroscience. 2019;39:6936–6952. doi: 10.1523/JNEUROSCI.1420-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the Hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bulkin DA, Sinclair DG, Law LM, Smith DM. Hippocampal state transitions at the boundaries between trial epochs. Hippocampus. 2020;30:582–595. doi: 10.1002/hipo.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Mizuseki K. The log-dynamic brain: how skewed distributions affect network operations. Nature Reviews Neuroscience. 2014;15:264–278. doi: 10.1038/nrn3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Tingley D. Space and time: the Hippocampus as a sequence generator. Trends in Cognitive Sciences. 2018;22:853–869. doi: 10.1016/j.tics.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, Flores SE, Kim I, Sano Y, Zhou M, Baumgaertel K, Lavi A, Kamata M, Tuszynski M, Mayford M, Golshani P, Silva AJ. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534:115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello-Waldow TP, Weston G, Ulivi AF, Chenani A, Loewenstein Y, Chen A, Attardo A. Hippocampal neurons with stable excitatory connectivity become part of neuronal representations. PLOS Biology. 2020;18:e3000928. doi: 10.1371/journal.pbio.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Rumpel S. A stable brain from unstable components: Emerging concepts and implications for neural computation. Neuroscience. 2017;357:172–184. doi: 10.1016/j.neuroscience.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Chandra N, Barkai E. A non-synaptic mechanism of complex learning: Modulation of intrinsic neuronal excitability. Neurobiology of Learning and Memory. 2018;154:30–36. doi: 10.1016/j.nlm.2017.11.015. [DOI] [PubMed] [Google Scholar]
- Chen L, Cummings KA, Mau W, Zaki Y, Dong Z, Rabinowitz S, Clem RL, Shuman T, Cai DJ. The role of intrinsic excitability in the evolution of memory: Significance in memory allocation, consolidation, and updating. Neurobiology of Learning and Memory. 2020;173:107266. doi: 10.1016/j.nlm.2020.107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenani A, Sabariego M, Schlesiger MI, Leutgeb JK, Leutgeb S, Leibold C. Hippocampal CA1 replay becomes less prominent but more rigid without inputs from medial entorhinal cortex. Nature Communications. 2019;10:1341. doi: 10.1038/s41467-019-09280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Sim SE, Kim JI, Choi DI, Oh J, Ye S, Lee J, Kim T, Ko HG, Lim CS, Kaang BK. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018;360:430–435. doi: 10.1126/science.aas9204. [DOI] [PubMed] [Google Scholar]
- Clewett D, DuBrow S, Davachi L. Transcending time in the brain: how event memories are constructed from experience. Hippocampus. 2019;29:162–183. doi: 10.1002/hipo.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Bonhoeffer T, Hübener M, Rose T. Variance and invariance of neuronal long-term representations. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372:20160161–20163526. doi: 10.1098/rstb.2016.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Mechanisms and functions of theta rhythms. Annual Review of Neuroscience. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Yu BM. Dimensionality reduction for large-scale neural recordings. Nature Neuroscience. 2014;17:1500–1509. doi: 10.1038/nn.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, Cirelli C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355:507–510. doi: 10.1126/science.aah5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal Memory Traces Are Differentially Modulated by Experience, Time, and Adult Neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantaki M, Coletta S, Nasr K, Zeraati R, Laturnus S, Berens P, Preston-Ferrer P, Burgalossi A. Manipulating hippocampal place cell activity by Single-Cell stimulation in freely moving mice. Cell Reports. 2018;23:32–38. doi: 10.1016/j.celrep.2018.03.031. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, Alkon DL. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. PNAS. 1986;83:2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G. Cell assemblies, sequences and temporal coding in the hippocampus. Current Opinion in Neurobiology. 2020;64:111–118. doi: 10.1016/j.conb.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Buzsáki G. Temporal Encoding of Place Sequences by Hippocampal Cell Assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Development of schemas revealed by prior experience and NMDA receptor knock-out. eLife. 2013;2:e01326. doi: 10.7554/eLife.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Selection of preconfigured cell assemblies for representation of novel spatial experiences. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20120522. doi: 10.1098/rstb.2012.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drieu C, Todorova R, Zugaro M. Nested sequences of hippocampal assemblies during behavior support subsequent sleep replay. Science. 2018;362:675–679. doi: 10.1126/science.aat2952. [DOI] [PubMed] [Google Scholar]
- Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD. Dynamic Reorganization of Neuronal Activity Patterns in Parietal Cortex. Cell. 2017;170:986–999. doi: 10.1016/j.cell.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The restless Engram: consolidations never end. Annual Review of Neuroscience. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Duffy A, Abe E, Perkel DJ, Fairhall AL. Variation in sequence dynamics improves maintenance of stereotyped behavior in an example from bird song. PNAS. 2019;116:9592–9597. doi: 10.1073/pnas.1815910116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nature Neuroscience. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszkiewicz AJ, McNamara CG, Takeuchi T, Genzel L. Novelty and dopaminergic modulation of memory persistence: a tale of two systems. Trends in Neurosciences. 2019;42:102–114. doi: 10.1016/j.tins.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Silva Mera R, Köhler S, Josselyn SA, Frankland PW. Neurogenesis-mediated forgetting minimizes proactive interference. Nature Communications. 2016;7:10838. doi: 10.1038/ncomms10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Brecht M, Lee AK. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron. 2011;70:109–120. doi: 10.1016/j.neuron.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. Similarity breeds proximity: pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81:1179–1189. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U, Sibille J, Liu K, Dragoi G. Strengthened temporal coordination within Pre-existing sequential cell assemblies supports trajectory replay. Neuron. 2019;103:719–733. doi: 10.1016/j.neuron.2019.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U, Dragoi G. Emergence of preconfigured and plastic time-compressed sequences in early postnatal development. Science. 2019;363:168–173. doi: 10.1126/science.aav0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth M, Wörgötter F, Tetzlaff C. Formation and Maintenance of Robust Long-Term Information Storage in the Presence of Synaptic Turnover. PLOS Computational Biology. 2015;11:e1004684. doi: 10.1371/journal.pcbi.1004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth MJ, van Rossum MC. Self-organized reactivation maintains and reinforces memories despite synaptic turnover. eLife. 2019;8:e43717. doi: 10.7554/eLife.43717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, Fermino de Oliveira E, Rocha-Almeida F, Tingley D, Buzsáki G. Long-duration hippocampal sharp wave ripples improve memory. Science. 2019;364:1082–1086. doi: 10.1126/science.aax0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ. Replay comes of age. Annual Review of Neuroscience. 2017;40:581–602. doi: 10.1146/annurev-neuro-072116-031538. [DOI] [PubMed] [Google Scholar]
- Frank AC, Huang S, Zhou M, Gdalyahu A, Kastellakis G, Silva TK, Lu E, Wen X, Poirazi P, Trachtenberg JT, Silva AJ. Hotspots of dendritic spine turnover facilitate clustered spine addition and learning and memory. Nature Communications. 2018;9:422. doi: 10.1038/s41467-017-02751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Köhler S, Josselyn SA. Hippocampal neurogenesis and forgetting. Trends in Neurosciences. 2013;36:497–503. doi: 10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Köhler S. The neurobiological foundation of memory retrieval. Nature Neuroscience. 2019;22:1576–1585. doi: 10.1038/s41593-019-0493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego JA, Perich MG, Chowdhury RH, Solla SA, Miller LE. Long-term stability of cortical population dynamics underlying consistent behavior. Nature Neuroscience. 2020;23:260–270. doi: 10.1038/s41593-019-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Roeder I, Kempermann G. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus. 2016;26:261–271. doi: 10.1002/hipo.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human Hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Monfils MH, Norman KA, Niv Y. The computational nature of memory modification. eLife. 2017;6:e23763. doi: 10.7554/eLife.23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandour K, Ohkawa N, Fung CCA, Asai H, Saitoh Y, Takekawa T, Okubo-Suzuki R, Soya S, Nishizono H, Matsuo M, Osanai M, Sato M, Ohkura M, Nakai J, Hayashi Y, Sakurai T, Kitamura T, Fukai T, Inokuchi K. Orchestrated ensemble activities constitute a hippocampal memory engram. Nature Communications. 2019;10:2637. doi: 10.1038/s41467-019-10683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Marlatte H. Neurobiology of Schemas and Schema-Mediated Memory. Trends in Cognitive Sciences. 2017;21:618–631. doi: 10.1016/j.tics.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Lynch JF, Cutolo P, Toledano D, Ulmen A, Jasnow AM, Riccio DC. Integration of new information with active memory accounts for retrograde amnesia: a challenge to the consolidation/Reconsolidation hypothesis? The Journal of Neuroscience. 2015;35:11623–11633. doi: 10.1523/JNEUROSCI.1386-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez WG, Zhang H, Harutyunyan A, Lois C. Persistence of neuronal representations through time and damage in the hippocampus. Science. 2019;365:821–825. doi: 10.1126/science.aav9199. [DOI] [PubMed] [Google Scholar]
- Goode TD, Tanaka KZ, Sahay A, McHugh TJ. An Integrated Index: Engrams, Place Cells, and Hippocampal Memory. Neuron. 2020;107:805–820. doi: 10.1016/j.neuron.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Gründemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, Lüthi A, Schnitzer MJ. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543:670–675. doi: 10.1038/nature21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridchyn I, Schoenenberger P, O'Neill J, Csicsvari J. Assembly-Specific disruption of hippocampal replay leads to selective memory deficit. Neuron. 2020;106:291–300. doi: 10.1016/j.neuron.2020.01.021. [DOI] [PubMed] [Google Scholar]
- Grosmark AD, Buzsaki G. Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science. 2016;351:1440–1443. doi: 10.1126/science.aad1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S. How does a brain build a cognitive code? Psychological Review. 1982;87:1–51. doi: 10.1037/0033-295X.87.1.1. [DOI] [PubMed] [Google Scholar]
- Guo N, Soden ME, Herber C, Kim MT, Besnard A, Lin P, Ma X, Cepko CL, Zweifel LS, Sahay A. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nature Medicine. 2018;24:438–449. doi: 10.1038/nm.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature Neuroscience. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hainmueller T, Bartos M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature. 2018;558:292–296. doi: 10.1038/s41586-018-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. European Journal of Neuroscience. 2001;13:1453–1458. doi: 10.1046/j.0953-816x.2001.01531.x. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Computation. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Current Opinion in Neurobiology. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP. Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function. Behavioural Brain Research. 1997;89:1–34. doi: 10.1016/S0166-4328(97)00048-X. [DOI] [PubMed] [Google Scholar]
- Hebb D. The Organization of Behavior. New York: Wiley & Sons; 1949. [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Reviews Neuroscience. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Howard MW, MacDonald CJ, Tiganj Z, Shankar KH, Du Q, Hasselmo ME, Eichenbaum H. A Unified Mathematical Framework for Coding Time, Space, and Sequences in the Hippocampal Region. Journal of Neuroscience. 2014;34:4692–4707. doi: 10.1523/JNEUROSCI.5808-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 2014;81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learning & Memory. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. The Journal of Neuroscience. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A. The neural basis of reversal learning: An updated perspective. Neuroscience. 2017;345:12–26. doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HR, Frank LM. The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nature Reviews Neuroscience. 2018;19:744–757. doi: 10.1038/s41583-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Köhler S, Frankland PW. Finding the engram. Nature Reviews Neuroscience. 2015;16:521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Frankland PW. Memory allocation: mechanisms and function. Annual Review of Neuroscience. 2018;41:389–413. doi: 10.1146/annurev-neuro-080317-061956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalle Kossio YF, Goedeke S, Klos C, Memmesheimer R-M. Drifting assemblies for persistent memory. bioRxiv. 2020 doi: 10.1101/2020.08.31.276147. [DOI] [PMC free article] [PubMed]
- Kappel D, Habenschuss S, Legenstein R, Maass W. Network plasticity as bayesian inference. PLOS Computational Biology. 2015;11:e1004485. doi: 10.1371/journal.pcbi.1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel D, Legenstein R, Habenschuss S, Hsieh M, Maass W. A dynamic connectome supports the emergence of stable computational function of neural circuits through Reward-Based learning. Eneuro. 2018;5:ENEURO.0301-17.2018. doi: 10.1523/ENEURO.0301-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlowitz KA, Picardo MA, Long MA. Stable sequential activity underlying the maintenance of a precisely executed skilled behavior. Neuron. 2018;98:1133–1140. doi: 10.1016/j.neuron.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN, Buzsáki G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science. 2017;358:369–372. doi: 10.1126/science.aan6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kwon J-T, Kim H-S, Josselyn SA, Han J-H. Memory recall and modifications by activating neurons with elevated CREB. Nature Neuroscience. 2014;17:65–72. doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- Kinsky NR, Sullivan DW, Mau W, Hasselmo ME, Eichenbaum HB. Hippocampal place fields maintain a coherent and flexible map across long timescales. Current Biology. 2018;28:3578–3588. doi: 10.1016/j.cub.2018.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky NR, Mau W, Sullivan DW, Levy SJ, Ruesch EA, Hasselmo ME. Trajectory-modulated hippocampal neurons persist throughout memory-guided navigation. Nature Communications. 2020;11:1–14. doi: 10.1038/s41467-020-16226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Hassabis D, McClelland JL. What learning systems do intelligent agents need? complementary learning systems theory updated. Trends in Cognitive Sciences. 2016;20:512–534. doi: 10.1016/j.tics.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, Lim SC, Santos SL, Denny CA, Drew MR. Distinct hippocampal engrams control extinction and relapse of fear memory. Nature Neuroscience. 2019;22:753–761. doi: 10.1038/s41593-019-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Research. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Latchoumane CV, Ngo HV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple Phase-Locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95:424–435. doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Lee D, Lin BJ, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012;337:849–853. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the Hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/S0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Progressive transformation of hippocampal neuronal representations in "morphed" environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Levy SJ, Kinsky NR, Mau W, Sullivan DW, Hasselmo ME. Hippocampal spatial memory representations in mice are heterogeneously stable. Hippocampus. 2020;388:23272. doi: 10.1002/hipo.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma L, Yang G, Gan W-B. REM sleep selectively prunes and maintains new synapses in development and learning. Nature Neuroscience. 2017;20:427–437. doi: 10.1038/nn.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti WA, Markowitz JE, Perkins LN, Liberti DC, Leman DP, Guitchounts G, Velho T, Kotton DN, Lois C, Gardner TJ. Unstable neurons underlie a stable learned behavior. Nature Neuroscience. 2016;19:1665–1671. doi: 10.1038/nn.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dolan RJ, Kurth-Nelson Z, Behrens TEJ. Human replay spontaneously reorganizes experience. Cell. 2019;178:640–652. doi: 10.1016/j.cell.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. Hippocampal–cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nature Neuroscience. 2016;19:959–964. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- Malagon-Vina H, Ciocchi S, Passecker J, Dorffner G, Klausberger T. Fluid network dynamics in the prefrontal cortex during multiple strategy switching. Nature Communications. 2018;9:1–13. doi: 10.1038/s41467-017-02764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. PNAS. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DJ, Lütcke H, Schulz K, Haiss F, Weber B, Kügler S, Hasan MT, Helmchen F. Reorganization of cortical population activity imaged throughout long-term sensory deprivation. Nature Neuroscience. 2012;15:1539–1546. doi: 10.1038/nn.3240. [DOI] [PubMed] [Google Scholar]
- Mau W, Sullivan DW, Kinsky NR, Hasselmo ME, Howard MW, Eichenbaum H. The Same Hippocampal CA1 Population Simultaneously Codes Temporal Information over Multiple Timescales. Current Biology. 2018;28:1499–1508. doi: 10.1016/j.cub.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, Lampinen AK. Integration of new information in memory: new insights from a complementary learning systems perspective. Philosophical Transactions of the Royal Society B: Biological Sciences. 2020;375:20190637. doi: 10.1098/rstb.2019.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M, Cohen NJ. Catastrophic interference in connectionist networks: the sequential learning problem. Psychology of Learning and Motivation. 1989;24:109–165. doi: 10.1016/S0079-7421(08)60536-8. [DOI] [Google Scholar]
- McKenzie S, Robinson NT, Herrera L, Churchill JC, Eichenbaum H. Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. Journal of Neuroscience. 2013;33:10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Huszár R, English D, Kim K, Yoon E, Buzsáki G. Preexisting hippocampal network dynamics constrain optogenetically induced place fields. bioRxiv. 2019 doi: 10.1101/803577. [DOI] [PMC free article] [PubMed]
- McKenzie S, Eichenbaum H. Consolidation and reconsolidation: two lives of memories? Neuron. 2011;71:224–233. doi: 10.1016/j.neuron.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nature Neuroscience. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Michon F, Sun JJ, Kim CY, Ciliberti D, Kloosterman F. Post-learning hippocampal replay selectively reinforces spatial memory for highly rewarded locations. Current Biology. 2019;29:1436–1444. doi: 10.1016/j.cub.2019.03.048. [DOI] [PubMed] [Google Scholar]
- Minerbi A, Kahana R, Goldfeld L, Kaufman M, Marom S, Ziv NE. Long-term relationships between synaptic tenacity, synaptic remodeling, and network activity. PLOS Biology. 2009;7:e1000136. doi: 10.1371/journal.pbio.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Hupbach A, Gomez R, Newman-Smith K. Memory formation, consolidation and transformation. Neuroscience & Biobehavioral Reviews. 2012;36:1640–1645. doi: 10.1016/j.neubiorev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Norimoto H, Makino K, Gao M, Shikano Y, Okamoto K, Ishikawa T, Sasaki T, Hioki H, Fujisawa S, Ikegaya Y. Hippocampal ripples down-regulate synapses. Science. 2018;359:1524–1527. doi: 10.1126/science.aao0702. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The Hippocampus as a spatial map. preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nature Neuroscience. 2008;11:209–215. doi: 10.1038/nn2037. [DOI] [PubMed] [Google Scholar]
- Ohkawa N, Saitoh Y, Suzuki A, Tsujimura S, Murayama E, Kosugi S, Nishizono H, Matsuo M, Takahashi Y, Nagase M, Sugimura YK, Watabe AM, Kato F, Inokuchi K. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Reports. 2015;11:261–269. doi: 10.1016/j.celrep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; 1978. [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat Hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AJ, Lee J, Hedrick NG, O'Neil K, Komiyama T. Reorganization of corticospinal output during motor learning. Nature Neuroscience. 2017;20:1133–1141. doi: 10.1038/nn.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T, Poll S, Bancelin S, Angibaud J, Inavalli VK, Keppler K, Mittag M, Fuhrmann M, Nägerl UV. Chronic 2P-STED imaging reveals high turnover of dendritic spines in the Hippocampus in vivo. eLife. 2018;7:e34700. doi: 10.7554/eLife.34700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkevich KD, Bensussen S, Tseng HA, Shroff SN, Lopez-Huerta VG, Park D, Jung EE, Shemesh OA, Straub C, Gritton HJ, Romano MF, Costa E, Sabatini BL, Fu Z, Boyden ES, Han X. Population imaging of neural activity in awake behaving mice. Nature. 2019;574:413–417. doi: 10.1038/s41586-019-1641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, Ryan TJ, Roy DS, Lovett C, Smith LM, Muralidhar S, Tonegawa S. Engram cell excitability state determines the efficacy of memory retrieval. Neuron. 2019;101:274–284. doi: 10.1016/j.neuron.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the Hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Rangel LM, Alexander AS, Aimone JB, Wiles J, Gage FH, Chiba AA, Quinn LK. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nature Communications. 2014;5:3181. doi: 10.1038/ncomms4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, Yan C, Mercaldo V, Hsiang H-L, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, Deisseroth K, Frankland PW, Josselyn SA. Competition between engrams influences fear memory formation and recall. Science. 2016;353:383–387. doi: 10.1126/science.aaf0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Richards BA, Frankland PW. The persistence and transience of memory. Neuron. 2017;94:1071–1084. doi: 10.1016/j.neuron.2017.04.037. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NTM, Descamps LAL, Russell LE, Buchholz MO, Bicknell BA, Antonov GK, Lau JYN, Nutbrown R, Schmidt-Hieber C, Häusser M. Targeted activation of hippocampal place cells drives Memory-Guided spatial behavior. Cell. 2020;183:1586–1599. doi: 10.1016/j.cell.2020.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, Silva AJ. Synaptic tagging during memory allocation. Nature Reviews Neuroscience. 2014;15:157–169. doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson T, Jayaprakash B, Cai DJ, Sano Y, Lee YS, Zhou Y, Bekal P, Deisseroth K, Silva AJ. Molecular and cellular mechanisms for trapping and activating emotional memories. PLOS ONE. 2016;11:e0161655. doi: 10.1371/journal.pone.0161655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007;54:653–666. doi: 10.1016/j.neuron.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Eban E, Frank LM. A cortical–hippocampal–cortical loop of information processing during memory consolidation. Nature Neuroscience. 2017;20:251–259. doi: 10.1038/nn.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L, Hu B, Eichler R, Stark E, Buzsáki G. Sharp wave ripples during learning stabilize the hippocampal spatial map. Nature Neuroscience. 2017;20:845–853. doi: 10.1038/nn.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A, Geva N, Sheintuch L, Ziv Y. Hippocampal ensemble dynamics timestamp events in long-term memory. eLife. 2015;4:e12247. doi: 10.7554/eLife.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule ME, O’Leary T, Harvey CD. Causes and consequences of representational drift. Current Opinion in Neurobiology. 2019;58:141–147. doi: 10.1016/j.conb.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule ME, Loback AR, Raman DV, Driscoll LN, Harvey CD, O'Leary T. Stable task information from an unstable neural population. eLife. 2020;9:e51121. doi: 10.7554/eLife.51121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, Triesch J. The dynamic connectome. e-Neuroforum. 2016;7:48–53. doi: 10.1007/s13295-016-0026-2. [DOI] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. European Journal of Neuroscience. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Sadowski JH, Jones MW, Mellor JR. Sharp-Wave ripples orchestrate the induction of synaptic plasticity during reactivation of place cell firing patterns in the Hippocampus. Cell Reports. 2016;14:1916–1929. doi: 10.1016/j.celrep.2016.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. PNAS. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. Memory reactivation during rest supports upcoming learning of related content. PNAS. 2014;111:15845–15850. doi: 10.1073/pnas.1404396111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger P, O’Neill J, Csicsvari J. Activity-dependent plasticity of hippocampal place maps. Nature Communications. 2016;7:1–12. doi: 10.1038/ncomms11824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield MEJ, Dombeck DA. Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature. 2015;517:200–204. doi: 10.1038/nature13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman T, Aharoni D, Cai DJ, Lee CR, Chavlis S, Page-Harley L, Vetere LM, Feng Y, Yang CY, Mollinedo-Gajate I, Chen L, Pennington ZT, Taxidis J, Flores SE, Cheng K, Javaherian M, Kaba CC, Rao N, La-Vu M, Pandi I, Shtrahman M, Bakhurin KI, Masmanidis SC, Khakh BS, Poirazi P, Silva AJ, Golshani P. Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nature Neuroscience. 2020;23:229–238. doi: 10.1038/s41593-019-0559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D, Feng T, Foster DJ. Trajectory events across hippocampal place cells require previous experience. Nature Neuroscience. 2015;18:1772–1779. doi: 10.1038/nn.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomowitz E, Styr B, Vertkin I, Milshtein-Parush H, Nelken I, Slutsky M, Slutsky I. Interplay between population firing stability and single neuron dynamics in hippocampal networks. eLife. 2015;4:e04378. doi: 10.7554/eLife.04378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron. 2016;89:1074–1085. doi: 10.1016/j.neuron.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Sun X, Bernstein MJ, Meng M, Rao S, Sørensen AT, Yao L, Zhang X, Anikeeva PO, Lin Y. Functionally distinct neuronal ensembles within the memory engram. Cell. 2020;181:410–423. doi: 10.1016/j.cell.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney Y, Clopath C. Population coupling predicts the plasticity of stimulus responses in cortical circuits. eLife. 2020;9:e56053. doi: 10.7554/eLife.56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweis BM, Mau W, Rabinowitz S, Cai DJ. Dynamic and heterogeneous neural ensembles contribute to a memory engram. Current Opinion in Neurobiology. 2020;67 doi: 10.1016/j.conb.2020.11.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KZ, He H, Tomar A, Niisato K, Huang AJY, McHugh TJ. The hippocampal engram maps experience but not place. Science. 2018;361:392–397. doi: 10.1126/science.aat5397. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behavioral Neuroscience. 1986;100:147–154. doi: 10.1037/0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Research. 1990;509:299–308. doi: 10.1016/0006-8993(90)90555-P. [DOI] [PubMed] [Google Scholar]
- Todorova R, Zugaro M. Isolated cortical computations during delta waves support memory consolidation. Science. 2019;366:377–381. doi: 10.1126/science.aay0616. [DOI] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R. Memory Engram cells have come of age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nature Neuroscience. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nature Reviews Neuroscience. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Trouche S, Perestenko PV, van de Ven GM, Bratley CT, McNamara CG, Campo-Urriza N, Black SL, Reijmers LG, Dupret D. Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nature Neuroscience. 2016;19:564–567. doi: 10.1038/nn.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A, Sugar J, Lu L, Wang C, Knierim JJ, Moser M-B, Moser EI. Integrating time from experience in the lateral entorhinal cortex. Nature. 2018;561:57–62. doi: 10.1038/s41586-018-0459-6. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- Tsodyks M. Attractor neural network models of spatial maps in Hippocampus. Hippocampus. 1999;9:481–489. doi: 10.1002/(SICI)1098-1063(1999)9:4<481::AID-HIPO14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature Reviews Neuroscience. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Urgolites ZJ, Wixted JT, Goldinger SD, Papesh MH, Treiman DM, Squire LR, Steinmetz PN. Spiking activity in the human hippocampus prior to encoding predicts subsequent memory. PNAS. 2020;117:13767–13770. doi: 10.1073/pnas.2001338117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven GM, Trouche S, McNamara CG, Allen K, Dupret D. Hippocampal offline reactivation consolidates recently formed cell assembly patterns during sharp Wave-Ripples. Neuron. 2016;92:968–974. doi: 10.1016/j.neuron.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetere G, Tran LM, Moberg S, Steadman PE, Restivo L, Morrison FG, Ressler KJ, Josselyn SA, Frankland PW. Memory formation in the absence of experience. Nature Neuroscience. 2019;22:933–940. doi: 10.1038/s41593-019-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, Bossert JM, Shaham Y, Bonci A, Hope BT. Bidirectional modulation of intrinsic excitability in rat prelimbic cortex neuronal ensembles and Non-Ensembles after operant learning. The Journal of Neuroscience. 2017;37:8845–8856. doi: 10.1523/JNEUROSCI.3761-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, McNaughton B. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap EL, Greenberg ME. Activity-regulated transcription: bridging the gap between neural activity and behavior. Neuron. 2018;100:330–348. doi: 10.1016/j.neuron.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. Principles of long-term dynamics of dendritic spines. Journal of Neuroscience. 2008;28:13592–13608. doi: 10.1523/JNEUROSCI.0603-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetton BD, Cai DJ, Spoormaker VI, Silva AJ, Mednick SC. Human memories can be linked by temporal proximity. Frontiers in Human Neuroscience. 2019;13:315. doi: 10.3389/fnhum.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu AP, Mercaldo V, Yan C, Richards B, Rashid AJ, Hsiang HL, Pressey J, Mahadevan V, Tran MM, Kushner SA, Woodin MA, Frankland PW, Josselyn SA. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83:722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Yokose J, Okubo-Suzuki R, Nomoto M, Ohkawa N, Nishizono H, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, Sasahara M, Kato F, Inokuchi K. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science. 2017;355:398–403. doi: 10.1126/science.aal2690. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Temporal proximity promotes integration of overlapping events. Journal of Cognitive Neuroscience. 2017;29:1311–1323. doi: 10.1162/jocn_a_01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nature Neuroscience. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziminski JJ, Hessler S, Margetts-Smith G, Sieburg MC, Crombag HS, Koya E. Changes in appetitive associative strength modulates nucleus accumbens, but not orbitofrontal cortex neuronal ensemble excitability. The Journal of Neuroscience. 2017;37:3160–3170. doi: 10.1523/JNEUROSCI.3766-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, Gamal AE, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nature Neuroscience. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Brenner N. Synaptic tenacity or lack thereof: spontaneous remodeling of synapses. Trends in Neurosciences. 2018;41:89–99. doi: 10.1016/j.tins.2017.12.003. [DOI] [PubMed] [Google Scholar]