Abstract
Background
Fibroblast growth factor 23 (FGF23), a phosphate-regulating hormone that increases early in the course of chronic kidney disease (CKD), is associated with disease progression in patients with established CKD. Here we aimed to investigate the association between plasma FGF23 and new-onset CKD in the general population.
Methods
We included 5253 individuals without CKD who participated in the Prevention of Renal and Vascular Endstage Disease study, a prospective, population-based cohort. Multi-variable Cox regression was used to study the association of plasma C-terminal FGF23 with new-onset CKD, defined as a combined endpoint of estimated glomerular filtration rate (eGFR) <60 mL/min/ 1.73 m2, urinary 24-h albumin excretion (UAE) >30 mg/24 h or both, or with all-cause mortality.
Results
The median baseline FGF23 was 68 [interquartile range (IQR) 56–85] RU/mL, eGFR was 95 ± 13 mL/min/1.73 m2 and UAE was 7.8 (IQR 5.8–11.5) mg/24 h. After follow-up of 7.5 (IQR 7.2–8.0) years, 586 participants developed CKD and 214 participants died. A higher FGF23 level was associated with new-onset CKD, independent of risk factors for kidney disease and parameters of bone and mineral homoeostasis {fully adjusted hazard ratio (HR) 1.25 [95% confidence interval (CI) 1.10–1.44] per doubling of FGF23; P = 0.001}. In secondary analyses, FGF23 was independently associated with new-onset eGFR <60 mL/min/1.73 m2 [adjusted HR 1.28 (95% CI 1.00–1.62); P = 0.048] or with UAE >30 mg/24 h [adjusted HR 1.24 (95% CI 1.06–1.45); P = 0.01] individually. A higher FGF23 level was also associated with an increased risk of all-cause mortality [fully adjusted HR 1.30 (95% CI 1.03–1.63); P = 0.03].
Conclusions
High FGF23 levels are associated with an increased risk of new-onset CKD and all-cause mortality in this prospective population-based cohort, independent of established CKD risk factors.
Keywords: chronic kidney disease, epidemiology, fibroblast growth factor 23, general population, mortality
ADDITIONAL CONTENT
An author video to accompany this article is available at: https://academic.oup.com/ndt/pages/author_videos.
INTRODUCTION
Chronic kidney disease (CKD) is a highly prevalent disorder, affecting 850 million people worldwide. CKD is accompanied by significant comorbidity, mortality and costs [1]. As such, the importance of early identification of individuals at high risk of developing CKD is well-recognized [2]. However, current risk factors are insufficiently able to identify individuals who are prone to develop CKD in the general population [3].
Fibroblast growth factor 23 (FGF23) is a circulating hormone produced primarily in osteocytes and is a key regulator of mineral homoeostasis. FGF23 promotes urinary phosphate excretion and inhibits the synthesis of active 1,25-dihydroxyvitamin D [4, 5]. Circulating FGF23 levels increase early in the course of CKD, that is, before detectable changes in other parameters of bone and mineral metabolism [6]. Several studies have reported that a higher level of FGF23 is associated with an increased risk of progressive renal function loss in patients with established CKD [7–9]. Moreover, higher FGF23 levels have been linked with adverse cardiovascular outcomes in the general population [10].
The very early increase in the course of CKD suggests that FGF23 could identify individuals in the general population that are at risk of developing new-onset overt kidney disease [6, 11]. However, whether FGF23 is associated with incident CKD, independent of established risk factors, is unclear. Here we investigated whether FGF23 is associated with new-onset CKD or all-cause mortality in participants of the general population-based Prevention of Renal and Vascular Endstage Disease (PREVEND) cohort who were free of CKD at baseline.
MATERIALS AND METHODS
Study population
The current study was performed using data from the PREVEND cohort study, which has been described elsewhere [12]. In summary, between 1997 and 1998, all inhabitants of the city of Groningen, The Netherlands, ages 28–75 years (N = 85 421) were asked to send in a first morning urine sample and a short questionnaire on demographics and cardiovascular disease history. Pregnant women and subjects with type 1 diabetes mellitus were excluded. A total of 40 856 subjects responded (47.8%). After exclusion of subjects with diabetes mellitus Type 1 (defined as the use of insulin) and pregnant women, all remaining subjects with a urinary albumin concentration (UAC) >10 mg/L (N = 7786) in the urine sample and a randomly selected control group with a UAC <10 mg/L (N = 3395) were invited for further investigations in an outpatient clinic. The screening programme was completed by 8592 subjects, making up the full study cohort. The PREVEND study was approved by the Medical Ethics Committee of the University Medical Center Groningen and is performed in accordance with Declaration of Helsinki guidelines. Written informed consent was obtained from all participants.
Between 2001 and 2003, 6894 subjects of the PREVEND study participated in a second round of examinations, which is considered as the baseline for the current study. We excluded subjects with CKD (n = 1082) or unknown CKD status (n = 337) at the time of examination, as well as participants with missing values of plasma FGF23 due to missing blood samples or insufficient plasma volume (n = 222), leaving 5253 participants for analysis.
Data collection
The data collection procedures of the PREVEND study have been described in detail elsewhere [12, 13]. In brief, each examination consisted of two outpatient clinic visits separated by 2 weeks. All participants completed a self-administered questionnaire regarding demographics, cardiovascular and renal disease history, current smoking status and medication use. Information on medication use was supplemented using information from a database of pharmacy dispensing data from all community pharmacies in the city of Groningen since 1999. Blood pressure was measured each visit on the right arm, every minute, for 10 and 8 min, using an automatic Dinamap XL Model 9300 series device (Johnson-Johnson Medical, Tampa, FL, USA). The mean of the last two recordings from each of the two visits was used.
Measurement of serum creatinine was performed by an isotope dilution mass spectrometry traceable enzymatic method on a Roche modular analyser (Roche Diagnostics, Mannheim, Germany). We measured serum cystatin C concentrations using the Gentian Cystatin C Immunoassay (Gentian, Moss, Norway) on a Roche Diagnostics modular auto-analyser. The cystatin C assay was calibrated using the standard supplied by the manufacturer, which is traceable to the International Federation of Clinical Chemistry Working Group for Standardization of Serum Cystatin C. Glomerular filtration rate (GFR) was estimated using the 2012 combined creatinine cystatin C-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [13].
Participants collected 24-h urine samples on two consecutive days in the week before the visit. UAC was measured by nephelometry (Dade Behring Diagnostic, Marburg, Germany). Urinary 24-h albumin excretion (UAE) was calculated as UAC multiplied by the 24-h urine volume. The two 24-h UAE values of each subject per examination were averaged.
Fasting blood samples were obtained and aliquots of these samples were stored immediately at −80°C for future analysis. C-terminal FGF23 (cFGF23) was measured in ethylenediaminetetraacetic acid plasma using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Quidel, San Diego, CA, USA). This assay uses two antibodies directed against different epitopes within the C-terminal part of FGF23, thus measuring both the intact hormone and C-terminal fragments. This ELISA has intra-assay and interassay coefficients of variation of <5 and <16% in blinded replicated samples, respectively [14].
Study endpoints
The primary outcome of new-onset CKD was defined as the first occurrence of either an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, a UAE >30 mg/24 h or both. Secondary analyses were performed to investigate new-onset eGFR <60 mL/min/1.73 m2 or UAE >30 mg/24 h as separate endpoints and to investigate the association between FGF23 and all-cause mortality in this cohort. Mortality data were obtained from the municipal register. Follow-up of the study cohort continued until 1 January 2011.
Statistical analysis
Normally distributed data are presented as mean and SD and skewed variables as median and interquartile range (IQR). Categorical variables are shown as total number and percentage. FGF23 was log2-transformed for all analyses. Covariates with skewed distributions were ln-transformed when appropriate. Differences in baseline variables across sex-stratified tertiles of FGF23 were assessed using analysis of variance, Kruskal–Wallis and chi-squared tests. Multivariable linear regression was used to identify independent correlates of FGF23. The multivariable model included potential confounders influencing FGF23 levels or the clinical outcomes (incident CKD or mortality): age, sex, body mass index (BMI), mean arterial pressure (MAP), the use of antihypertensive drugs, eGFR, smoking status, history of diabetes, serum calcium, phosphate, parathyroid hormone (PTH), 25-hydroxyvitamin D [25(OH)D], high-sensitivity C-reactive protein (hsCRP), total cholesterol, triglycerides and urine 24-h urea excretion and albuminuria.
Cox proportional hazards models were used to estimate hazard ratios (HRs) for the outcomes of interest described above. Cox analysis was performed using log2FGF23 as a continuous variable and using sex-stratified tertiles of FGF23 as a categorical variable. Crude proportional hazards models were subsequently adjusted for age and sex (Model 2) and further adjusted for mean arterial blood pressure, the use of antihypertensive drugs, BMI, ethnicity, current smoking status, baseline eGFR (CKD-EPI) and baseline albuminuria (Model 3), plasma phosphate, calcium, total cholesterol, diabetes status, PTH, 25(OH)D, hsCRP and urine urea excretion (Model 4) and iron parameters (Model 5). To visualize significant associations between log2FGF23 and outcomes, restricted cubic splines were generated with knots placed at the 10th, 50th and 90th percentiles.
Sensitivity analyses were performed to check the robustness of our results. First, we repeated the primary analysis using log2FGF23 in the subcohort of participants with a baseline eGFR <65 (instead of <60) mL/min/1.73 m2 or a baseline UAE <25 (instead of <30) mg/24 h (n = 5012). Second, we repeated this analysis in the subcohort of participants not using antihypertensive drugs (n = 4354).
Subgroup analyses and multiplicative interaction terms were used to explore the consistency of the association between FGF23 and new-onset CKD for age, sex, BMI, MAP, cardiovascular history, eGFR, UAE, plasma phosphate, calcium and PTH, 25-h urine urea excretion and haemoglobin. For this purpose, biochemical parameters were dichotomized based on cohort medians. Multiple imputations were performed to account for missing data on covariates in our Cox regression models. Numbers of missing cases per variable are presented in Table 1.
Table 1.
Baseline characteristics according to sex-specific tertiles of C-terminal FGF23
| Tertiles of C-terminal FGF23 (RU/mL) |
|||||
|---|---|---|---|---|---|
| Variables | Full cohort | Tertile I | Tertile II | Tertile III | Ptrend |
| ♂: <61.9 | ♂: 61.9–74.2 | ♂: >74.2 | |||
| ♀: <58.4 | ♀: 58.4–82.9 | ♀: >82.9 | |||
| Participants, n | 5253 | 1744 | 1760 | 1749 | |
| Men, % | 47 | 47 | 47 | 47 | |
| Age (years) | 52 ± 12 | 51 ± 11 | 52 ± 11 | 53 ± 12 | <0.001 |
| BMI (kg/m2) | 26.4 ± 4.2 | 25.9 ± 3.8 | 26.4 ± 4.2 | 27 ± 4.5 | <0.001 |
| Race (whites, % | 96 | 94 | 96 | 96 | 0.013 |
| Current smoker, % | 27.6 | 20.6 | 26.6 | 35.4 | <0.001 |
| Systolic blood pressure (mmHg) | 124 ± 17 | 123 ± 17 | 123 ± 17 | 124 ± 17 | 0.001 |
| Diastolic blood pressure (mmHg) | 72 ± 9 | 72 ± 9 | 72 ± 9 | 73 ± 8 | 0.21 |
| Antihypertensive drugs, % | 17.1 | 12.6 | 16.5 | 22.2 | <0.001 |
| Use of ACEIs/ARBs, % | 6.5 | 4.8 | 7.1 | 7.7 | <0.001 |
| Current diabetes, % | 3.9 | 2.6 | 3.4 | 5.8 | <0.001 |
| eGFRCKD-EPI (mL/min×1.73 m2) | 95 ± 13 | 98 ± 13 | 95 ± 13 | 93 ± 14 | <0.001 |
| Plasma total cholesterol (mmol/L)a | 5.4 ± 1.0 | 5.4 ± 1.0 | 5.4 ± 1.0 | 5.4 ± 1.0 | 0.37 |
| Plasma hsCRP (mg/L), median (IQR)b | 1.2 (0.6–2.8) | 1.1 (0.5–2.4) | 1.2 (0.6–2.7) | 1.5 (0.7–3.4) | <0.001 |
| Plasma sodium (mmol/L)c | 140.7 ± 2.0 | 140.7 ± 2.1 | 140.7 ± 1.9 | 140.6 ± 2.1 | 0.03 |
| Plasma potassium (mmol/L)d | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.3 | 0.12 |
| Plasma phosphate (mmol/L)d | 1.01 ± 0.27 | 0.99 ± 0.22 | 1.02 ± 0.28 | 1.03 ± 0.29 | <0.001 |
| Plasma calciumc | 2.30 ± 0.11 | 2.29 ± 0.11 | 2.31 ± 0.11 | 2.30 ± 0.12 | 0.001 |
| Plasma C-terminal FGF23 (RU/mL), median (IQR) | 68.5 (56.1–85.1) | 51.9 (46.7–56.1) | 68.5 (64–73) | 95.4 (85.1–116.7) | <0.001 |
| Plasma PTH (pmol/L), median (IQR)e | 4.8 (4.0–5.7) | 4.7 (3.9–5.6) | 4.8 (4.0–5.7) | 4.9 (4.1–5.9) | 0.009 |
| Plasma 25(OH)D (ng/mL)f | 59.7 ± 26.0 | 60.7 ± 27.0 | 61.2 ± 25.7 | 56.9 ± 25.2 | <0.001 |
| Haemoglobin (mmol/L)g | 8.5 ± 0.7 | 8.5 ± 0.7 | 8.5 ± 0.7 | 8.5 ± 0.8 | 0.70 |
| Plasma iron (μmol/L)h | 15.9 ± 5.6 | 16.6 ± 5.3 | 16.3 ± 5.5 | 15.0 ± 6.0 | <0.001 |
| Plasma transferrin (g/L)h | 2.6 ± 0.4 | 2.5 ± 0.4 | 2.5 ± 0.4 | 2.7 ± 0.4 | <0.001 |
| Plasma ferritin (μg/L), median (IQR)h | 93 (45–169) | 103 (55–180) | 99 (50–175) | 75 (29–148) | <0.001 |
| Urine urea (g/24 h)f | 21.7 ± 6.7 | 22.4 ± 6.9 | 21.8 ± 6.6 | 20.8 ± 6.5 | <0.001 |
| Urine creatinine (mg/24 h)i | 1391 ± 376 | 1407 ± 381 | 1393 ± 374 | 1373 ± 372 | 0.008 |
| Urine albumin (mg/24 h), median (IQR) | 7.8 (5.8–11.5) | 7.8 (5.9–11.3) | 7.7 (5.8–12.3) | 8.0 (5.8–12.3) | 0.34 |
Data are presented as mean ± SD unless stated otherwise. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockade.
Missing in 23 cases.
Missing in 273 cases.
Missing in 112 cases.
Missing in 110 cases.
Missing in 99 cases.
Missing in 91 cases.
Missing in 13 cases.
Missing in 233 cases.
Missing in 71 cases.
Statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA). For all tests, a two-sided P-value <0.05 was regarded as statistically significant. Figures were generated using Graphpad version 6 (GraphPad Software, San Diego, CA, USA). Restricted cubic spline fitting was performed with R 3.5.2 (R Foundation, Vienna, Austria).
RESULTS
The mean age of the study participants was 51 ± 12 years and 48% were male. Overall, 96% of all participants were of Caucasian descent. The mean eGFR of the cohort was 95 ± 13 mL/min/1.73 m2 and the median urine albumin excretion was 7.8 (IQR 5.8–11.5) mg/24 h. The median FGF23 was 68 (IQR 56–85) RU/mL. Baseline characteristics of the study population by sex-specific tertiles of FGF23 are presented in Table 1. Participants in the highest tertile of FGF23 were likely to be older and were more likely to be current smoker, to use antihypertensive drugs or to have a history of diabetes (Table 1). In a multivariable linear regression analysis, FGF23 was positively associated with transferrin (β = 0.23, P < 0.001), female sex, (β = 0.15, P < 0.001), current smoking (β = 0.15, P < 0.001), BMI (β = 0.10, P < 0.001), plasma phosphate (β = 0.08, P < 0.001), plasma calcium (β = 0.07, P = 0.01), the use of antihypertensive drugs (β = 0.03, P = 0.03) and history of diabetes (β = 0.03, P = 0.036). FGF23 was inversely associated with eGFR (β = −0.26, P < 0.001), haemoglobin (β = −0.21, P < 0.001), plasma iron (β = −0.16, P < 0.001), plasma ferritin (β = −0.12, P < 0.001), age (β = −0.09, P < 0.001), plasma 25(OH)D (β = −0.06, P < 0.001), plasma sodium (β = −0.06, P < 0.001), urine urea excretion (β = −0.05, P = 0.006) and plasma C-reactive protein (CRP) (β = −0.04, P = 0.014).
FGF23 and new-onset CKD
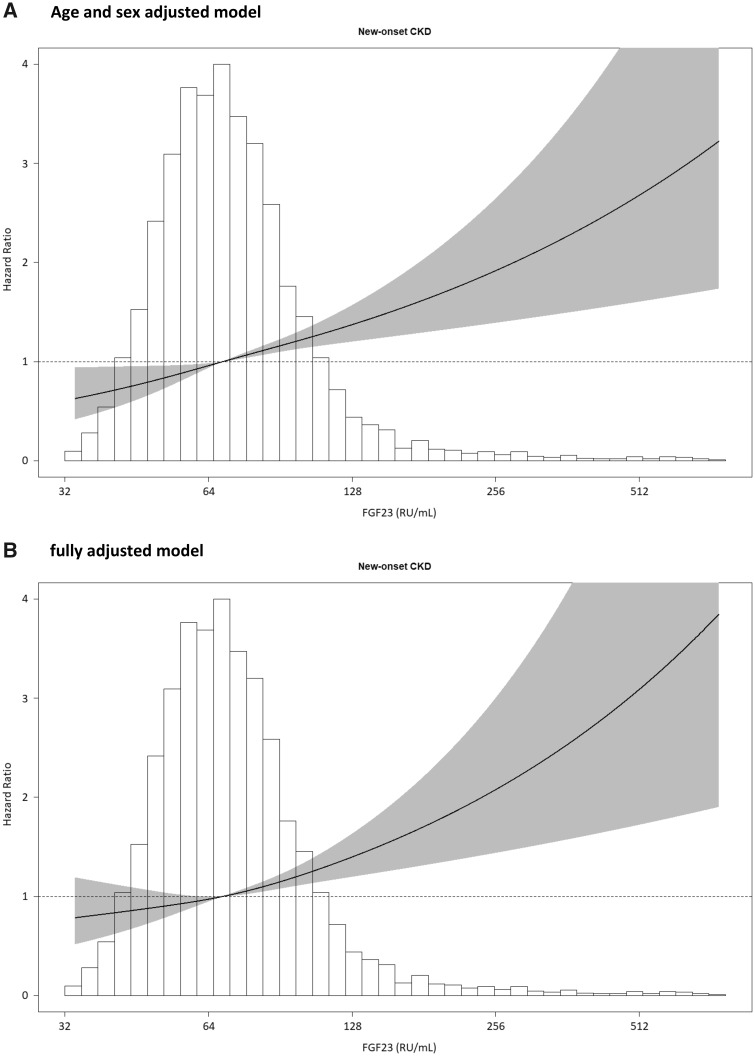
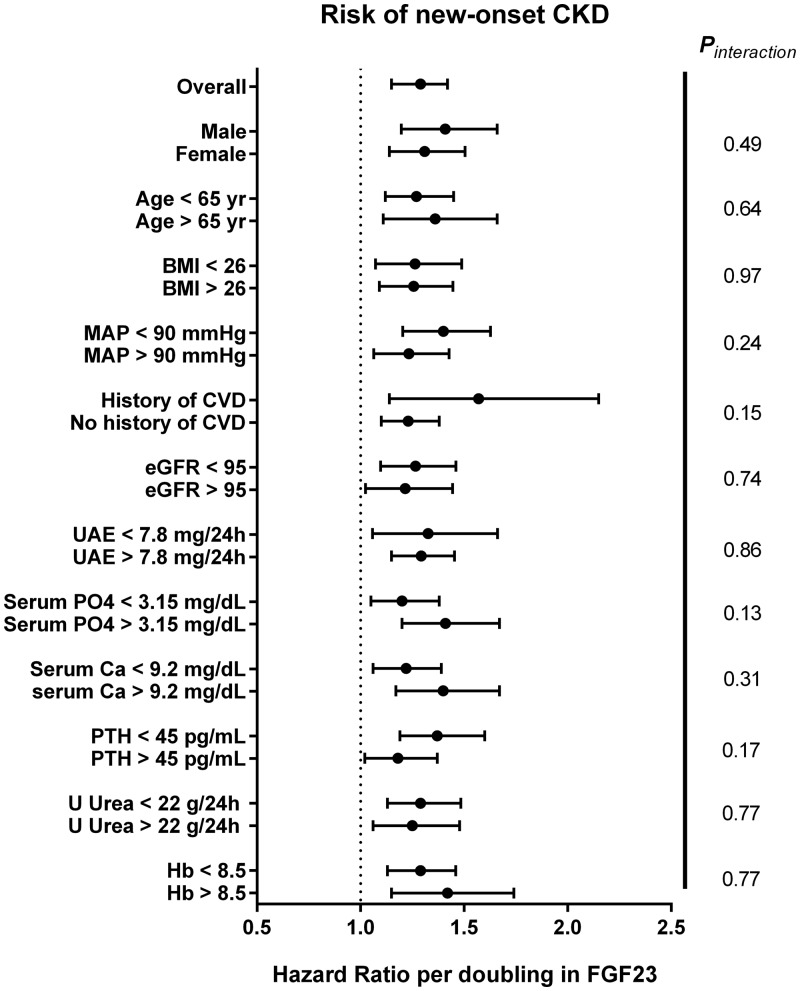
Both eGFR and UAE were measured at baseline and during follow-up examinations. During a median follow-up of 7.5 (IQR 7.2–8.0) years, 586 participants developed CKD: 198 participants developed an eGFR <60 mL/min/1.73 m2, 442 participants developed UAE >30 mg/24 h and 54 of these participants developed both. A higher baseline FGF23 level was significantly associated with a higher risk of developing CKD {HR per doubling of FGF23 1.29 [95% confidence interval (CI) 1.15–1.42]; P < 0.001}, and this association persisted after adjustment for potential confounders (Table 2). In the fully adjusted model, each doubling of FGF23 was associated with a 19% higher risk of new-onset CKD. The association between FGF23 and the risk of new-onset CKD is shown in Figure 1 by restricted cubic splines. In subgroup analyses, no significant effect modification was observed by age, sex, eGFR, serum phosphate, cardiovascular history or other factors (Figure 2). We subsequently focused on the individual determinants of the composite CKD definition, new-onset eGFR <60 mL/min/1.73 m2 or UAE >0 mg/24 h as separate endpoints. These analyses yielded comparable results. When analysed as a continuous variable, higher FGF23 levels were independently associated with either endpoint (Table 3).
Table 2.
Associations of FGF23 with new-onset CKD
| Sex-specific tertiles of FGF23 (RU/mL), median (IQR) |
||||||
|---|---|---|---|---|---|---|
| ♂: <61.9 | ♂: 61.9–74.2 | ♂: >74.2 | ||||
| Model | ♀: <58.4 | ♀: 58.4–82.9 | ♀: >82.9 | |||
| New-onset CKD (n = 586) | Ptrend | Log2FGF23 (continuous), median (IQR) | P-value | |||
| 1 | 1.0 (Ref) | 1.11 (0.90–1.38) | 1.73 (1.42–2.12) | <0.001 | 1.29 (1.15–1.42) | <0.001 |
| 2 | 1.0 (Ref) | 1.09 (0.88–1.35) | 1.58 (1.30–1.93) | <0.001 | 1.36 (1.22–1.51) | <0.001 |
| 3 | 1.0 (Ref) | 1.00 (0.89–1.23) | 1.28 (1.15–1.42) | 0.004 | 1.22 (1.08–1.37) | 0.001 |
| 4 | 1.0 (Ref) | 1.01 (0.81–1.27) | 1.27 (1.03–1.57) | 0.01 | 1.19 (1.06–1.34) | 0.004 |
| 5 | 1.0 (Ref) | 1.02 (0.82–1.28) | 1.29 (1.04–1.61) | 0.01 | 1.25 (1.10–1.44) | 0.001 |
Model 1, unadjusted model; Model 2, Model 1 + adjustment for age and sex; Model 3, Model 2 + adjustment for mean arterial blood pressure, the use of antihypertensive drugs, BMI, ethnicity, smoking status, eGFRCKD-EPI and albuminuria; Model 4, Model 3 + adjustment for total cholesterol, history of diabetes, serum phosphate, calcium and plasma PTH, 25(OH)D, hsCRP and 24-h urinary urea excretion; Model 5, Model 4 + adjustment for serum iron, transferrin and ferritin.
FIGURE 1.
Association of FGF23 and the risk of CKD. CKD was defined as eGFR <60 mL/min/1.73 m2, UAE >30 mg/24 h or both. Data were fitted with a Cox proportional hazards regression model based on restricted cubic splines with three knots and adjusted for (A) age and sex, and additionally for (B) mean arterial blood pressure, the use of antihypertensive drugs, BMI, ethnicity, smoking status, eGFRCKD-EPI, 24-h UAE, total cholesterol, history of diabetes, plasma phosphate, calcium, PTH, 25(OH)D, hsCRP and 24-h urine urea excretion.
FIGURE 2.
Forest plot of the association between FGF23 and new-onset CKD in relevant subpopulations. CVD, cardiovascular disease; U Urea, urinary 24-h urea excretion; Hb, haemoglobin. Biochemical parameters were dichotomized based on the cohort mean or median.
Table 3.
Associations of FGF23 with eGFR <60 mL/min/1.73 m2 or albuminuria >30 mg/24 h as individual outcomes
| Sex-specific tertiles of FGF23 (RU/mL), median (IQR) |
||||||
|---|---|---|---|---|---|---|
| ♂: <61.9 | ♂: 61.9–74.2 | ♂: >74.2 | ||||
| Model | ♀: <58.4 | ♀: 58.4–82.9 | ♀: >82.9 | |||
| Ptrend | Log2FGF23 (continuous), median (IQR) | P-value | ||||
| eGFR <60 mL/min/1.73 m2 (n = 198) | ||||||
| 1 | 1.0 (Ref) | 1.87 (1.21–2.86) | 3.48 (2.48–5.18) | <0.001 | 1.52 (1.30–1.79) | <0.001 |
| 2 | 1.0 (Ref) | 1.87 (1.22–2.87) | 3.09 (2.08–4.59) | <0.001 | 1.71 (1.45–2.01) | <0.001 |
| 3 | 1.0 (Ref) | 1.38 (1.11–1.72) | 1.73 (1.41–2.13) | 0.006 | 1.25 (1.12–1.38) | 0.03 |
| 4 | 1.0 (Ref) | 1.38 (0.89–2.15) | 1.67 (1.10–2.55) | 0.03 | 1.25 (1.01–1.55) | 0.04 |
| 5 | 1.0 (Ref) | 1.38 (0.89–2.14) | 1.67 (1.08–2.55) | 0.02 | 1.28 (1.00–1.62) | 0.048 |
| Albuminuria >30 mg/24 h (n = 442) | ||||||
| 1 | 1.0 (Ref) | 0.99 (0.78–1.25) | 1.41 (1.12–1.76) | 0.001 | 1.21 (1.06–1.37) | 0.004 |
| 2 | 1.0 (Ref) | 0.95 (0.74–1.20) | 1.27 (1.01–1.58) | 0.03 | 1.29 (1.13–1.47) | <0.001 |
| 3 | 1.0 (Ref) | 0.93 (0.73–1.18) | 1.14 (1.01–1.28) | 0.19 | 1.19 (1.10–1.27) | 0.01 |
| 4 | 1.0 (Ref) | 0.93 (0.73–1.19) | 1.13 (0.90–1.44) | 0.20 | 1.17 (1.02–1.34) | 0.02 |
| 5 | 1.0 (Ref) | 0.94 (0.74–1.20) | 1.16 (0.91–1.49) | 0.17 | 1.24 (1.06–1.45) | 0.01 |
Model 1, unadjusted model; Model 2, Model 1 + adjustment for age and sex; Model 3, Model 2 + adjustment for mean arterial blood pressure, the use of antihypertensive drugs, BMI, ethnicity, smoking status, eGFRCKD-EPI and albuminuria; Model 4, Model 3 + adjustment for total cholesterol, history of diabetes, serum phosphate, calcium and plasma PTH, 25(OH)D, hsCRP and 24-h urinary urea excretion; Model 5, Model 4 + adjustment for serum iron, transferrin and ferritin.
FGF23 and mortality
A total of 214 participants died during a median follow-up of 8.4 (IQR 7.8–8.9) years. Participants who died had a higher median FGF23 level than survivors [74.7 (IQR 65.5–89.9) versus 68.0 (56.0–84.9) RU/mL; P < 0.001]. Cox regression analysis showed a significant association between FGF23 and the risk of premature death [HR 1.31 (95% CI 1.10–1.55) per doubling of FGF23; P = 0.002; Table 4]. This association remained significant after adjustment for age, sex, mean arterial blood pressure, the use of antihypertensive drugs, BMI, ethnicity, smoking status, baseline eGFR and 24-h albuminuria, serum phosphate and calcium, and plasma total cholesterol, diabetes status, PTH, 25(OH)D, hsCRP, urine urea excretion and iron parameters. In the fully adjusted model, each doubling of FGF23 was associated with a 25% higher risk of premature death [HR 1.25 (95% CI 1.03–1.52); P = 0.03; Table 4].
Table 4.
Associations of FGF23 with mortality
| Sex-specific tertiles of FGF23 (RU/mL), median (IQR) |
||||||
|---|---|---|---|---|---|---|
| ♂: <61.9 | ♂: 61.9–74.2 | ♂: >74.2 | ||||
| Model | ♀: <58.4 | ♀: 58.4–82.9 | ♀: >82.9 | |||
| Mortality (n = 214) | Ptrend | Log2FGF23 (continuous), median (IQR) | P-value | |||
| 1 | 1.0 (Ref) | 2.02 (1.36–2.98) | 2.62 (1.80–3.82) | <0.001 | 1.31 (1.10–1.55) | 0.002 |
| 2 | 1.0 (Ref) | 1.91 (1.29–2.83) | 2.08 (1.42–3.03) | 0.001 | 1.30 (1.08–1.56) | 0.006 |
| 3 | 1.0 (Ref) | 1.97 (1.60–2.41) | 2.12 (1.73–2.59) | 0.002 | 1.28 (1.06–1.55) | 0.01 |
| 4 | 1.0 (Ref) | 1.97 (1.32–2.94) | 2.01 (1.35–2.99) | 0.004 | 1.25 (1.03–1.52) | 0.03 |
| 5 | 1.0 (Ref) | 1.97 (1.32–2.95) | 1.99 (1.33–2.98) | 0.008 | 1.30 (1.03–1.63) | 0.03 |
Model 1, unadjusted model; Model 2, Model 1 + adjustment for age and sex; Model 3, Model 2 + adjustment for mean arterial blood pressure, the use of antihypertensive drugs, BMI, ethnicity, smoking status, eGFRCKD-EPI and albuminuria; Model 4, Model 3 + adjustment for total cholesterol, history of diabetes, serum phosphate, calcium and plasma PTH, 25(OH)D, hsCRP and 24-h urinary urea excretion; Model 5, Model 4 + adjustment for serum iron, transferrin and ferritin.
Sensitivity analyses
Our results did not materially change when subjects with an eGFR <65 mL/min/1.73 m2 (instead of <60 mL/min/1.73 m2) or UAE <25 (instead of <30) mg/24 h at baseline were excluded (n = 5012). In these analyses, FGF23 remained associated with new-onset CKD [481 cases; HR 1.32 (95% CI 1.15–1.48)] and mortality [189 deaths; HR 1.25 (95% CI 1.01–1.56)] in the fully adjusted model. Second, we repeated our analyses after exclusion of participants using antihypertensive drugs at baseline (n = 4354) and observed similar associations with new-onset CKD [385 cases; HR 1.23 (95% CI 1.06–1.43)] and mortality [126 deaths; HR 1.28 (95% CI 1.00–1.63)] in the final model.
DISCUSSION
In this study we show that plasma FGF23 levels are independently associated with the risk of new-onset CKD in a general population-based cohort. Higher baseline levels of FGF23 were associated with a higher risk of a subsequent eGFR <60 mL/min/1.73 m2 or new-onset albuminuria >30 mg/24 h in PREVEND participants free of CKD at baseline. Analysis of each individual component of the primary endpoint revealed that a higher FGF23 level was also associated with an increased risk of either a reduced eGFR or increased UAE. These associations were independent of established risk factors for CKD, including baseline albuminuria, and other markers of bone and mineral metabolism and iron parameters [13]. FGF23 was also associated with a higher risk of premature mortality in this general population–based cohort.
To our knowledge, this is the first study that has addressed the association between C-terminal FGF23 and incident CKD in a population-based cohort. The relationship between intact FGF23 and incident CKD risk has been investigated in two prior studies. In the Atherosclerosis Risk In Communities (ARIC) cohort, a higher baseline intact FGF23 level was associated with an increased risk of end-stage renal disease, independent of baseline kidney function [15]. Additionally, in a recent study of the Health, Aging and Body Composition (ABC) cohort, which is comprised of older adults, an increased intact FGF23 level was not independently associated with incident CKD [16]. Although participants in the highest quartile of this cohort did have a higher CKD incidence rate than those in the lowest quartile, the elevated risk seemed restricted to individuals with an intact FGF23 level >50 pg/mL and the association with incident CKD lost significance after multivariable adjustment [16]. Participants in the Health ABC cohort were older (age 75 ± 3 years) than those included in the PREVEND cohort (age 52 ± 12 years) and a significantly higher percentage of these older adults developed CKD compared with the PREVEND study (25% versus 11%, respectively). Furthermore, the apparent discrepancy between the two studies may be due to in vitro instability of FGF23 [17]. Intact and C-terminal FGF23 are known to correlate poorly, in particular in the lower ranges that may be expected in the general population [17]. Importantly, compared with the C-terminal FGF23 assay, intact FGF23 is subject to significantly higher intra-individual variation [17]. Another potential explanation for the discrepancy between our findings and the Health ABC cohort study is that the C-terminal assay detects both the intact FGF23 molecule and cleaved C-terminal fragments. Thus it is conceivable that the association between C-terminal FGF23 and incident CKD is driven by an underlying process influencing FGF23 cleavage, particularly inflammation. Although the associations between FGF23 and outcomes in this cohort were not meaningfully influenced by adjustment for baseline hsCRP, this does not fully exclude the possibility of inflammation driving the association.
Our observational data do not allow us to discriminate whether a higher FGF23 level is causally involved in the development of new-onset CKD or whether it is indicative of a more general high-risk profile that is associated with a higher risk of developing CKD. In support of the latter, in univariate analysis, patients in the highest FGF23 tertile were characterized by a high-risk profile; however, the association between FGF23 and incident CKD remained after adjustment for these factors. Previous work from our group showed that intravenous administration of exogenous FGF23 did not aggravate renal fibrosis in an animal model nor did it counteract the renoprotective effect of renin–angiotensin-aldosterone system (RAAS) blockade [18]. On the other hand, FGF23 might play a role in the development of kidney damage. Higher FGF23 levels may be an adaptive response to maintain phosphate homoeostasis during early kidney damage [19]. This hypothesis is supported by data from progressive CKD models in rats [19, 20]. While this adaptive response may appear beneficial, the resulting phosphaturia may induce interstitial inflammation, fibrosis and tubular damage [21] and may thus contribute to the development of overt CKD [19]. Yet this potential explanation is not supported by our observation that adjustment for urea excretion, considered a measure of protein and phosphate intake, did not influence the association between FGF23 and outcomes. In addition, FGF23 has been shown to interact with the RAAS, which in turn plays a major role in the aetiology of renal damage [20, 21], although adjustment for antihypertensive treatment (including RAAS blockers) did not change the relationship between FGF23 and incident CKD in our study. Furthermore, a previous study demonstrated interaction between FGF23 and asymmetric dimethylarginine (ADMA) in patients with CKD, implying that dysregulation of the nitric oxide system and FGF23 might concertedly contribute to CKD progression [22]. Unfortunately, ADMA levels were not available in our cohort. Finally, higher levels of FGF23 may also reflect reduced renal expression of α-Klotho, the obligatory co-factor for renal FGF23 signalling [22, 23]. Animal studies have shown that reduced renal Klotho expression predisposes to kidney injury through pro-fibrotic signalling pathways, including transforming growth factor β1 and Wnt/β-catenin signalling [24–26].
While our study identifies a strong association between C-terminal FGF23 levels and new-onset CKD, the absolute difference in FGF23 between participants who developed CKD during follow-up and those who did not was relatively small. Hence the question remains of how to translate this association to clinical practice. Recently, Isakova et al. [26] showed that the association between FGF23 and mortality in patients with CKD is particularly pronounced in a subgroup of patients with rising FGF23 levels over time. If FGF23 is indeed a marker of subclinical pathology, then a similar relationship between a change in FGF23 over time and kidney-related outcomes may exist, and may be of use to identify individuals with a high risk of kidney disease or kidney disease progression. Unfortunately, serial measurements of FGF23 were not available in this cohort.
Our study has a number of strengths and limitations. Major strengths of this study include the prospective design, the relatively large sample size and the availability of detailed information on potential confounders. However, a number of limitations should also be considered. First, we present findings from an observational study, precluding conclusions on causality. Moreover, even though we extensively adjusted our analyses for potential confounders, the possibility of residual confounding remains. For example, since inflammation may lead to higher FGF23 levels [27] and promote loss of kidney function [28], (subclinical) inflammation may have influenced the association between FGF23 and incident CKD even though adjustment for hsCRP did not influence our results. Lastly, due to the geographical area of recruitment, a large majority of the participants were of Caucasian ethnicity.
In conclusion, high cFGF23 levels are associated with new-onset CKD in this prospective population-based cohort. The association was independent of traditional risk factors for CKD and parameters of bone and mineral metabolism. Further attention to the role of FGF23 in the development of CKD is warranted.
ACKNOWLEDGEMENTS
The authors thank Marie Pierre Louis and Wendy Dam for their excellent technical assistance.
FUNDING
This study has been supported by the Dutch Kidney Foundation (grants CP1601 and 17OKG18).
CONFLICT OF INTEREST STATEMENT
M.F.E. reports a consultancy agreement with Vifor Fresenius Medical Care Renal Pharma outside the submitted work. M.V. received research support from the Dutch Kidney Foundation (in relation to the submitted work) and has consultancy agreements with Otsuka, AstraZeneca, Vifor Fresenius Medical Care Renal Pharma and Medice and has received research support from Amgen and Fresenius Medical Care (all outside the submitted work). M.H.d.B. has consultancy agreements with Amgen, AstraZeneca, Bayer, Kyowa Kirin, Pharmacosmos, Vifor Fresenius Medical Care Renal Pharma and Sanofi Genzyme and has received grant support from Amgen and Sanofi Genzyme (all outside the submitted work).
REFERENCES
- 1. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Disease: Improving Global Outcomes CKD Work Group. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 2. Gansevoort RT, de Jong PE.. The case for using albuminuria in staging chronic kidney disease. J Am Soc Nephrol 2009; 20: 465–468 [DOI] [PubMed] [Google Scholar]
- 3. Shlipak MG, Day EC.. Biomarkers for incident CKD: a new framework for interpreting the literature. Nat Rev Nephrol 2013; 9: 478–483 [DOI] [PubMed] [Google Scholar]
- 4. Shimada T, Hasegawa H, Yamazaki Y. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2003; 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 5. Shimada T, Yamazaki Y, Takahashi M. et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289: F1088–95 [DOI] [PubMed] [Google Scholar]
- 6. Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol 2010; 21: 1427–1435 [DOI] [PubMed] [Google Scholar]
- 7. Fliser D, Kollerits B, Neyer U. et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 2007; 18: 2600–2608 [DOI] [PubMed] [Google Scholar]
- 8. Titan SM, Zatz R, Graciolli FG. et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol 2011; 6: 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Munoz Mendoza J, Isakova T, Cai X. et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int 2017; 91: 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang M, Gong D, Fan Y.. Elevated fibroblast growth factor-23 and risk of cardiovascular disease or mortality in the general population: a meta-analysis. Int J Cardiol 2016; 222: 342–345 [DOI] [PubMed] [Google Scholar]
- 11. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012; 82: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinto-Sietsma SJ, Janssen WM, Hillege HL. et al. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol 2000; 11: 1882–1888 [DOI] [PubMed] [Google Scholar]
- 13. Eisenga MF, De Jong MA, Van der Meer P. et al. Iron deficiency, elevated erythropoietin, fibroblast growth factor 23, and mortality in the general population of the Netherlands: a cohort study. PLoS Med 2019; 16: e1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rebholz CM, Grams ME, Coresh J. et al. Serum fibroblast growth factor-23 is associated with incident kidney disease. J Am Soc Nephrol 2015; 26: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drew DA, Katz R, Kritchevsky S. et al. Fibroblast growth factor 23: a biomarker of kidney function decline. Am J Nephrol 2018; 47: 242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith ER, Cai MM, McMahon LP, Holt SG.. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 2012; 97: 3357–3365 [DOI] [PubMed] [Google Scholar]
- 18. De Jong MA, Mirkovic K, Mencke R. et al. Fibroblast growth factor 23 modifies the pharmacological effects of angiotensin receptor blockade in experimental renal fibrosis. Nephrol Dial Transplant 2017; 32: 73–80 [DOI] [PubMed] [Google Scholar]
- 19. Kuro-O M. Klotho and endocrine fibroblast growth factors: marker of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant 2019; 34: 15–21 [DOI] [PubMed] [Google Scholar]
- 20. Hasegawa H, Nagano N, Urakawa I. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78: 975–980 [DOI] [PubMed] [Google Scholar]
- 21. Haut LL, Alfrey AC, Guggenheim S. et al. Renal toxicity of phosphate in rats. Kidney Int 1980; 17: 722–731 [DOI] [PubMed] [Google Scholar]
- 22. Tripepi G, Kollerits B, Leonardis D. et al. Competitive interaction between fibroblast growth factor 23 and asymmetric dimethylarginine in patients with CKD. J Am Soc Nephrol 2015; 26: 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urakawa I, Yamazaki Y, Shimada T. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006; 444: 770. [DOI] [PubMed] [Google Scholar]
- 24. Zhou L, Li Y, Zhou D. et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 2013; 24: 771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doi S, Zou Y, Togao O. et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 2011; 286: 8655–8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sugiura H, Yoshida T, Shiohira S. et al. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 2012; 302: F1252–F1264 [DOI] [PubMed] [Google Scholar]
- 27. De Borst MH. Interaction between inflammation, mineral metabolism and the renin-angiotensin system: implications for cardiorenal outcomes in chronic kidney disease. Nephrol Dial Transpl 2019; 34: 547–551 [DOI] [PubMed] [Google Scholar]
- 28. Hiramoto JS, Katz R, Peralta CA. et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2012; 60: 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]