Abstract
Background
Sarcopenia increases as renal function declines and is associated with higher morbidity and mortality. Myostatin is a negative regulator of muscle growth. Its expression in response to exercise is unclear. In this prespecified substudy of the Renal Exercise (RENEXC) trial, we investigated the effects of 12 months of exercise training on sarcopenia, muscle mass and plasma myostatin and the relationships between physical performance, muscle mass and plasma myostatin.
Methods
A total of 151 non-dialysis-dependent patients (average measured glomerular filtration rate 23 ± 8 mL/min/1.73 m2), irrespective of age or comorbidity, were randomly assigned to either strength or balance in combination with endurance training. Body composition was measured with dual-energy X-ray absorptiometry. Plasma myostatin was analysed using enzyme-linked immunosorbent assay kits.
Results
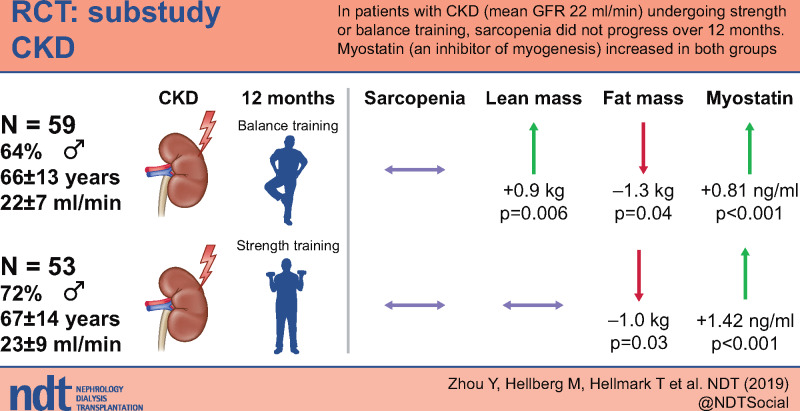
After 12 months, the prevalence of sarcopenia was unchanged, leg and whole-body lean mass increased significantly in the balance group and was unchanged in the strength group. Whole fat mass decreased significantly in both groups. There were no significant between-group differences in sarcopenia or body composition. Plasma myostatin levels increased significantly in both groups, with a significant difference in favour of the strength group. Plasma myostatin was significantly positively related to muscle mass and physical performance at baseline, but these relationships were attenuated after 12 months.
Conclusions
Exercise training seems to be effective in preventing sarcopenia and maintaining muscle mass in non-dialysis-dependent patients with chronic kidney disease (CKD). However, the role of plasma myostatin on muscle mass and physical performance in patients with CKD warrants further study.
Keywords: body composition, chronic kidney disease, exercise training, myostatin, sarcopenia
Graphical Abstract
INTRODUCTION
Sarcopenia is defined as a loss of muscle strength and muscle mass as well as a decline in functional quality [1]. Sarcopenia develops with aging, with loss of renal function and is related to a greater risk of morbidity and mortality [1–3]. Loss of physical performance [3] and muscle mass [4] has been shown to start during chronic kidney disease (CKD) Stage 3 and is associated with a decline in glomerular filtration rate (GFR) [4, 5]. To counteract this decline, Kidney Disease: Improving Global Outcomes guidelines recommend exercise training for patients with CKD [6]. Some randomized controlled trials (RCTs) have reported that exercise not only increases muscle strength, but also can increase muscle mass in patients with CKD [7–9]. However, these studies were performed in relatively small groups of patients (10–20 patients in each group) and with short periods of exercise training (12 weeks) [7–9].
Myostatin is a myokine and a negative regulator of muscle growth [10]. It is predominantly expressed in skeletal muscle [10] and has been found to be overexpressed in uraemic sarcopenia [5, 11]. Consequently, inhibition of myostatin expression has been suggested as a strategy to treat muscle wasting in CKD [11, 12]. However, the literature presents conflicting evidence on the relationship between myostatin and muscle mass both in patients on dialysis and in people in general [13–15]. Furthermore, myostatin was suggested to decrease after exercise training in some studies [16, 17], but the opposite results were found in other studies [18, 19]. We hypothesized that exercise training could increase muscle mass and decrease plasma myostatin.
RENEXC is a RCT with two active exercise training arms, balance or strength training, both in combination with endurance training. The primary hypothesis of RENEXC was that strength training would be superior to balance training in improving physical performance in non-dialysis-dependent patients with CKD Stages 3–5. However, we found that both exercise training groups improved or maintained all aspects of physical performance after 12 months of training without any between-group differences [20].
In this prespecified substudy of RENEXC, we aimed to investigate the effects of 12 months of exercise training on sarcopenia, muscle mass and plasma myostatin and the relationships between physical performance, muscle mass and plasma myostatin.
MATERIALS AND METHODS
Study design
This is a prespecified substudy of RENEXC. RENEXC is a randomized controlled, parallel-group, interventional, single-centre trial with two treatment arms and a 1:1 allocation ratio in patients with CKD not on renal replacement therapy. There were no changes to methods after trial commencement. It is registered as NCT02041156 at www.ClinicalTrials.gov, approved by the Regional Ethical Review Board in Lund (Ref 2011/369) and adheres to the Declaration of Helsinki. Complete study design and primary data analysis of RENEXC have been presented previously [4, 20, 21]. Some information on study design and methods is repeated here for clarity. The inclusion criteria were incident and prevalent patients at the Department of Nephrology, Skåne University Hospital, Lund, Sweden with an estimated GFR <30 mL/min/1.73 m2, age ≥18 years, all renal diagnoses and any number of comorbidities. The exclusion criteria were orthopaedic impediment, severe neurological dysfunction, inability to understand the patient information, renal replacement therapy and estimated start of dialysis within 12 months of study start.
Randomization and blinding
Random allocation was generated by SAS ProcPlan (SAS Institute, Cary, NC, USA). The permutation of the blocks and the size of each block were known by only one investigator (P.H.), who had no contact with the patients. Patients were included and allocated sequential treatment according to a list to which only the research physiotherapist had access. The random allocation sequence was generated by P.H., the nephrologists enrolled the patients and the research physiotherapist assigned the intervention. All the recruitment staff except the research physiotherapist were blinded to the randomization.
Intervention
A total of 151 patients, irrespective of age or comorbidity, were randomly assigned to either strength or balance training, both in combination with endurance training. Both groups were prescribed 150 min/week of self-administered exercise training for an intervention period of 12 months and 60 min/week of endurance training was part of the prescription in both groups and was combined with 90 min/week of either strength or balance training.
The primary outcomes were the measures of physical performance at baseline and after 12 months. The prespecific secondary outcomes were body composition and plasma myostatin at baseline and after 12 months as well as the relationships between body composition, physical performance and plasma myostatin. There were no changes to trial outcomes after the trial had commenced.
Physical performance
All physical performance tests have been presented previously in detail [22]. In this study, handgrip strength and isometric quadriceps strength represent strength, functional reach and Berg’s balance test represent balance and the 6-min walk test represents endurance.
Definition of sarcopenia
Sarcopenia was defined as low muscle strength and low muscle mass [1]. Handgrip strength <27 kg for men and <16 kg for women were defined as low muscle strength [1]. An appendicular skeletal muscle index <7 kg/m2 for men and <6 kg/m2 for women was defined as low muscle mass [1].
Dual-energy X-ray absorptiometry
Body composition was measured with dual-energy X-ray absorptiometry and was conducted in a standardized manner at the Department of Diagnostic Radiology, Skåne University Hospital, which is accredited by the Swedish Board for Accreditation and Conformity Assessment (SWEDAC; ISO 15189:2012). The hospital changed from Lunar Prodigy to Lunar iDEXA during the study period, as Lunar iDEX has a superior camera. The analysis software was identical.
Comorbidity
M.H. evaluated comorbidity using the Davies comorbidity score [23].
Laboratory analyses
All laboratory analyses were performed at the Department of Clinical Chemistry, Laboratory Medicine Skåne, which is accredited by SWEDAC (ISO 15189:2012). Measured GFR (mGFR) was assessed with iohexol clearance [24, 25].
Nutritional assessment
Each patient was prescribed continued normal protein intake or a protein-restricted diet consisting of 0.8 or 0.6 g/kg body weight/day by their physician and referred to a renal dietitian for dietary counselling. Subjective global assessment (SGA) [26] and normalized protein catabolic rate (nPCR) were used to describe the patients’ nutritional status. SGA is a subjective evaluation, classifying nutritional status as A (well-nourished), B (moderately, or suspected of being, malnourished) or C (severely malnourished). nPCR was calculated using the equation 6.25 × [(0.028 × urine urea × 24-h urine volume) + (0.031 × weight)]/body weight.
Plasma myostatin
Plasma myostatin was measured using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) at the Nephrology Laboratory, Biomedicine Centre, Lund University. Three samples of known concentration were tested 20 times on one plate to assess the intra-assay precision of the kits. The intra-assay coefficient variants were 5.4, 2.5 and 1.8%, respectively. Three samples of known concentration were tested in 20 separate assays to assess interassay precision. Assays were performed by at least three technicians using two lots of components. The interassay coefficient variants were 6.0, 3.6 and 3.1%, respectively. The plasma was collected fasting and stored at −80°C.
Statistical analysis
To detect 60% differences at a standard deviation (SD) of 5% and 80% power, we calculated that we needed to include 75 patients in each group to achieve complete data for 50 patients at the end of the intervention. Continuous variables are presented as mean ± SD or median (25th–75th percentiles). Categorical variables are given as frequencies and percentages. Paired t-test was used to compare parametric variables, Wilcoxon signed-rank test was used to compare non-parametric variables and Fisher’s exact test was used to compare binary variables. Linear regression analysis was used to analyse the relationships between variables. In our previous study analysing the primary outcomes of RENEXC, mixed model analyses showed that there were no significant differences between groups for changes in any measures of physical performance after 12 months of exercise training among the 151 randomized patients [20]. In this study, comprising the 112 completers, the results for measures of physical performance were similar to those for the entire group. Therefore we pooled the patients from the two groups to increase the power of the linear regression analyses. A P-value <0.05 was considered statistically significant. Data were analysed using R software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
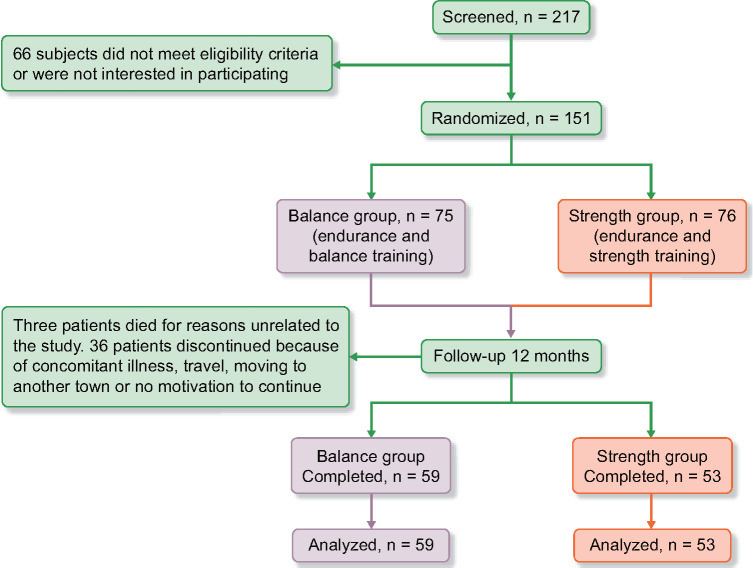
A total of 217 patients were screened, of whom 151 patients were randomized and 112 patients (76 men, 36 women) completed 12 months of exercise training. The recruitment period started in October 2011 and ended in May 2016. The trial ended in May 2017, when the final patient had completed 12 months of exercise training. The 112 completers were included, with a mean age of 67 ± 13 years (range 22–87): 59 patients in the balance group and 53 patients in the strength group. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented in Figure 1. The average mGFR was 23 ± 8 mL/min/1.73 m2. Two patients were in CKD Stage 3a, 8 in Stage 3b, 77 in Stage 4 and 24 in Stage 5. Clinical characteristics are presented in Table 1. The causes of CKD were hypertensive kidney disease [47 (42%)], diabetes nephropathy [21 (19%)], interstitial nephritis [18 (17%)], chronic glomerulonephritis [12 (11%)], polycystic kidney disease [6 (5%)] and other [8 (6%)]. No exercise training–related side effects, unintended effects or harm was reported by the participants during the intervention period in either group.
FIGURE 1.
CONSORT flow over 12 months.
Table 1.
Clinical characteristics of the 112 patients who completed 12 months of exercise training
| Characteristics | Units | n | Balance group | n | Strength group | n | Whole group |
|---|---|---|---|---|---|---|---|
| Age | years | 59 | 66 ± 13 | 53 | 67 ± 14 | 112 | 67 ± 13 |
| Male/female | n (%) | 59 | 38 (64)/21 (36) | 53 | 38 (72)/15 (28) | 112 | 76 (68)/36 (32) |
| Weight | kg | 59 | 79 ± 16 | 53 | 84 ± 19 | 112 | 81 ± 17 |
| Height | m | 59 | 1.71 ± 0.09 | 53 | 1.72 ± 0.1 | 112 | 1.72 ± 0.09 |
| mGFR | mL/min/1.73 m2 | 55 | 22 ± 7 | 49 | 23 ± 9 | 104 | 23 ± 8 |
| P-creatinine | µmol/L | 59 | 242 ± 90 | 53 | 251 ± 92 | 112 | 247 ± 91 |
| P-urea | mmol/L | 59 | 16 ± 5 | 53 | 15 ± 5 | 112 | 15 ± 5 |
| P-PTH | pmol/L | 59 | 11 (9–17) | 53 | 10 (7–16) | 112 | 11 (8–17) |
| P-albumin | g/L | 59 | 37 ± 4 | 53 | 38 ± 3 | 112 | 37 ± 3 |
| B-hemoglobin | g/L | 59 | 129 ± 14 | 53 | 128 ± 14 | 112 | 128 ± 14 |
| P-potassium | mmol/L | 59 | 4.3 ± 0.6 | 53 | 4.1 ± 0.5 | 112 | 4.2 ± 0.5 |
| P-calcium | mmol/L | 59 | 2.3 ± 0.1 | 53 | 2.3 ± 0.1 | 112 | 2.3 ± 0.1 |
| P-Ca×P | mmol2/L2 | 59 | 2.6 ± 0.7 | 53 | 2.5 ± 0.5 | 112 | 2.6 ± 0.6 |
| P-phosphate | mmol/L | 59 | 1.2 ± 0.2 | 53 | 1.1 ± 0.2 | 112 | 1.1 ± 0.2 |
| Base excess | mmol /L | 59 | −1.2 (−2.3 to 0.1) | 53 | −1.4 (−3.2 to 0.0) | 112 | −1.2 (−2.8 to 0.1) |
| P-CRP | mg/L | 59 | 2.9 (1.3 to 5.9) | 53 | 3.5 (1.5 to 6.9) | 112 | 3.1 (1.3 to 6.1) |
| Medication, n (%) | |||||||
| Antihypertensive medication | 55 (93) | 50 (94) | 105 (94) | ||||
| Calcium channel blocker | 33 (56) | 32 (60) | 65 (58) | ||||
| β-blocker | 36 (61) | 37 (70) | 73 (65) | ||||
| RAAS blocker | 37 (63) | 34 (64) | 71 (63) | ||||
| Central antiadrenergic medication | 8 (14) | 5 (9) | 13 (12) | ||||
| Comorbidity, n (%) | |||||||
| Malignancy | 8 (14) | 8 (15) | 16 (14) | ||||
| Ischaemic heart disease | 11 (19) | 11 (20) | 22 (20) | ||||
| Peripheral vascular disease | 10 (17) | 13 (25) | 23 (21) | ||||
| Left ventricular dysfunction | 8 (14) | 3 (6) | 11 (10) | ||||
| Diabetes mellitus | 13 (22) | 17 (32) | 30 (27) | ||||
| Systemic collagen vascular disease | 5 (8) | 5 (9) | 10 (9) | ||||
| Others (e.g. hypertension) | 43 (73) | 40 (75) | 83 (74) | ||||
Data presented as mean ± SD or median (25th–75th percentiles) unless stated otherwise.
P, plasma; B, blood; PTH, parathyroid hormone; Ca×P, calcium phosphate product; RAAS, renin–angiotensin–aldosterone system.
Prevalence of sarcopenia and mGFR
The prevalence of sarcopenia was unchanged without a between-group difference. The prevalence of sarcopenia for the whole group was 10 (10%) at baseline and 11 (11%) after 12 months (Table 2). mGFR decreased significantly in both groups without significant between-group difference (Table 2).
Table 2.
Sarcopenia, mGFR and nutritional status of strength group, balance group and whole group
| Balance group |
Strength group |
Whole group |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | Baseline | 12 months | P-value | 95% CI | n | Baseline | 12 months | P-value | 95% CI | n | Baseline | 12 months | P-value | 95% CI |
| mGFR (mL/min/1.73 m2), mean ± SD | 55 | 22.3 ± 6.9 | 21.1 ± 7.8 | 0.003 | 0.7–3.2 | 49 | 23.0 ± 9.1 | 22.0 ± 9.7 | 0.01 | 0.5–3.7 | 104 | 22.6 ± 8.0 | 21.6 ± 8.8 | <0.001 | 1.0–3.0 |
| Sarcopenia, n (%) | 56 | 7 (7) | 8 (8) | 1 | 0.34–4.10 | 47 | 3 (3) | 3 (3) | 1 | 0.13–7.88 | 103 | 10 (11%) | 11 (11) | 1 | 0.41–3.06 |
| Nutritional status | |||||||||||||||
| SGA (A/B/C), n | 59 | 55A/4B/0C | 55A/4B/0C | – | – | 53 | 47A/6B/0C | 46A/6B/1C | – | – | 112 | 102A/10B/0C | 101A/10B/1C | – | – |
| nPCR (g/kg body weight/day), median (25th–75th percentiles) | 45 | 0.9 (0.7–1.1) | 0.9 (0.7–1.5) | 0.4 | −0.83–0.05 | 42 | 0.9 (0.8–1.0) | 0.9 (0.7–1.1) | 0.3 | −0.29–0.06 | 87 | 0.9 (0.7–1.1) | 0.9 (0.7–1.3) | 0.1 | −0.28–0.02 |
| BMI (kg/m2), mean ± SD | 59 | 27.0 ± 4.7 | 26.6 ± 4.1 | 0.1 | −0.05–0.75 | 53 | 28.0 ± 5.2 | 27.7 ± 5.0 | 0.1 | −0.10–0.70 | 112 | 27.4 ± 4.9 | 27.1 ± 4.6 | 0.03 | 0.05–0.60 |
CI, confidence interval. A P-value < 0.05 was considered statistically significant.
Nutritional status
SGA, nPCR and body mass index (BMI) for both groups at baseline and after 12 months are presented in Table 2. After 12 months of exercise training, BMI decreased significantly in the whole group. Neither SGA nor nPCR changed significantly.
Body composition
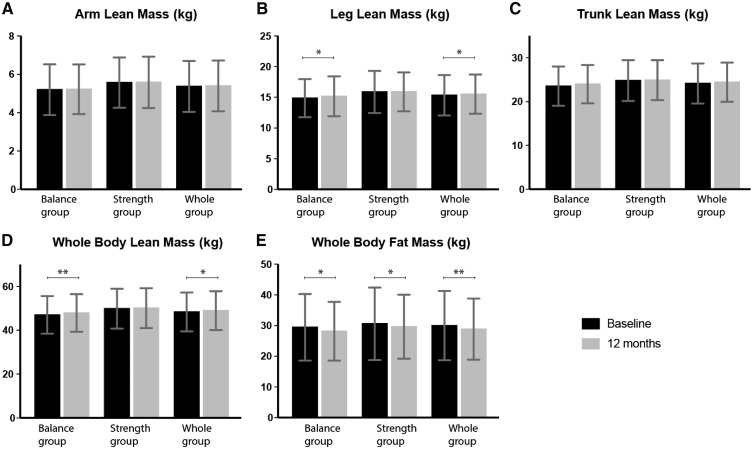
Body composition in both groups at baseline and after 12 months is presented in Figure 2. In the balance group, leg lean mass and whole-body lean mass increased by 0.3 kg (P = 0.02) and 0.9 kg (P = 0.006), respectively, arm lean mass and trunk lean mass were unchanged and whole-body fat mass decreased by 1.3 kg (P = 0.04). In the strength group, arm lean mass, leg lean mass, trunk lean mass and whole-body lean mass were unchanged, while whole-body fat mass decreased by 1 kg (P = 0.03). In the whole group, leg lean mass and whole-body lean mass increased by 0.2 kg (P = 0.01) and 0.6 kg (P = 0.02), respectively, arm lean mass and trunk lean mass were unchanged and whole-body fat mass decreased by 1.2 kg (P = 0.003). There were no significant differences between groups for changes in any measures of body composition.
FIGURE 2.
Body composition at baseline and after 12 months. Data are presented as mean ± SD. *P < 0.05, **P < 0.01.
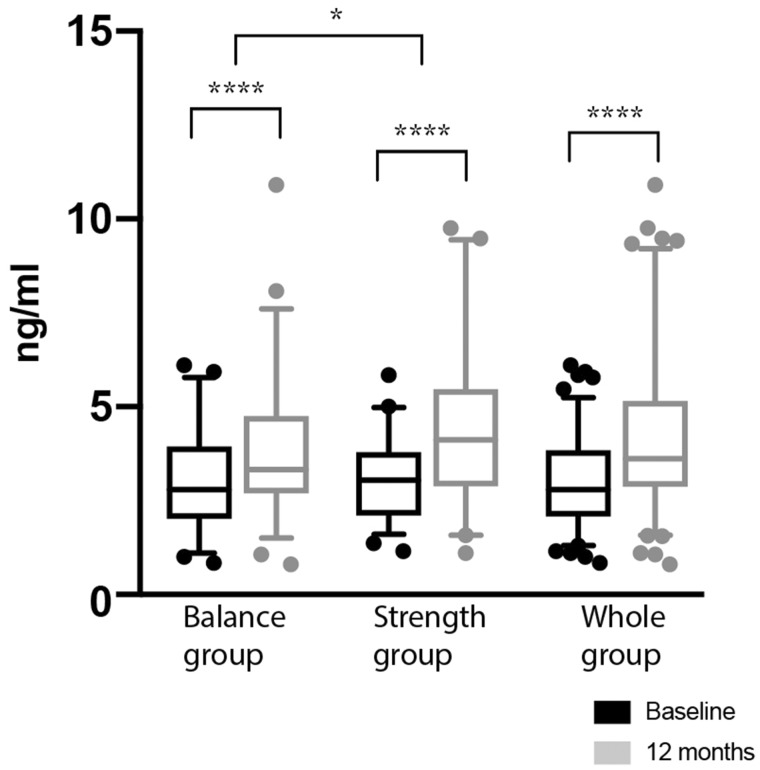
Plasma myostatin
Plasma myostatin levels at baseline and after 12 months are presented in Figure 3. After 12 months of exercise training, plasma myostatin increased by 1.42 ng/mL (P < 0.0001) in the strength group, 0.81 ng/mL (P < 0.0001) in the balance group and 1.1 ng/mL (P < 0.0001) in the whole group. There was a significant difference in favour of the strength group (P = 0.03).
FIGURE 3.
Plasma myostatin at baseline and after 12 months. Data are presented as median ± interquartile range. *P < 0.05, ****P < 0.0001.
Relationships between mGFR, physical performance, body composition and plasma myostatin
Relationships between plasma myostatin and mGFR are presented in Table 3. Plasma myostatin showed no significant relationship with mGFR at baseline or after 12 months. There were no significant relationships between the change in myostatin and baseline mGFR.
Table 3.
Linear regression analyses with mGFR, physical performance, body composition and plasma myostatin
| P-myostatin (ng/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline |
12 months |
Δ |
|||||||
| Eff ± SE | P-value | 95% CI | Eff ± SE | P-value | 95% CI | Eff ± SE | P-value | 95% CI | |
| GFRa (mL/min/1.73 m2) (baseline/12 months) | |||||||||
| mGFR | 0.01 ± 0.01 | 0.6 | −0.02–0.04 | −0.01 ± 0.02 | 0.7 | −0.06–0.04 | 0.005 ± 0.02 | 0.8 | −0.03–0.04 |
| Physical performancea (baseline/12 months) | |||||||||
| HGS mean (kg) | 0.06 ± 0.01 | <0.001 | 0.04–0.07 | 0.05 ± 0.02 | 0.008 | 0.01–0.08 | 0.004 ± 0.01 | 0.8 | −0.02–0.03 |
| IQS mean (kg × m) | 0.2 ± 0.02 | <0.001 | 0.1–0.2 | 0.2 ± 0.04 | <0.001 | 0.1–0.2 | 0.39 ± 0.37 | 0.3 | −0.35–1.12 |
| Functional reach (cm) | 0.06 ± 0.01 | <0.001 | 0.03–0.08 | 0.03 ± 0.03 | 0.3 | −0.02–0.08 | −0.03 ± 0.02 | 0.2 | −0.06–0.01 |
| BBT score (score) | 0.07 ± 0.02 | <0.001 | 0.03–0.1 | 0.05 ± 0.02 | 0.05 | −0.001–0.09 | 0.03 ± 0.02 | 0.3 | −0.02–0.07 |
| 6-MWT (m) | 0.004 ± 0.001 | <0.001 | 0.002–0.006 | 0.003 ± 0.002 | 0.08 | −0.0003–0.01 | 0.00004 ± 0.001 | 1 | −0.002–0.002 |
| Body compositiona (baseline/12 months) | |||||||||
| Arm lean mass (kg) | 0.43 ± 0.08 | <0.001 | 0.28–0.59 | 0.35 ± 0.14 | 0.01 | −0.07–0.63 | 0.04 ± 0.11 | 0.7 | −0.19–0.26 |
| Leg lean mass (kg) | 0.16 ± 0.03 | <0.001 | 0.10–0.22 | 0.08 ± 0.06 | 0.2 | −0.04–0.20 | −0.01 ± 0.05 | 0.9 | −0.10–0.08 |
| Trunk lean mass (kg) | 0.08 ± 0.02 | 0.001 | 0.03–0.13 | 0.05 ± 0.04 | 0.3 | −0.04–0.13 | 0.001 ± 0.03 | 1 | −0.06–0.07 |
The ‘Baseline’ column shows the relationships between p-myostatin at baseline and variables at baseline, the ‘12 months’ column shows the relationships between p-myostatin after 12 months and variables after 12 months and the ‘Δ’ column shows the relationships between the change in p-myostatin (12 months baseline) and variables at baseline.
Univariable.
P-myostatin, plasma myostatin; HGS, handgrip strength; IQS, isometric quadriceps strength; BBT, Berg’s balance test; 6-MWT, 6-minus walking test; Eff, efficiency; CI, confidence interval. A P-value < 0.05 was considered statistically significant.
Relationships between plasma myostatin and physical performance are presented in Table 3. Plasma myostatin showed a significant positive relationship with all measures of physical performance at baseline (P < 0.001). After 12 months, myostatin showed significant positive relationships with handgrip strength (P = 0.008) and isometric quadriceps strength (P < 0.001) only. There were no significant relationships between the change in myostatin and any measures of baseline physical performance.
Relationships between plasma myostatin and lean muscle mass are presented in Table 3. At baseline, plasma myostatin showed a significant positive relationship with arm lean mass (P < 0.001), leg lean mass (P < 0.001) and trunk lean mass (P = 0.001). After 12 months, the only significant positive relationship was with arm lean mass (P = 0.01). There were no significant relationships between the change in myostatin and baseline lean muscle mass.
DISCUSSION
In this prespecified substudy of RENEXC, comprising 112 non-dialysis-dependent patients with CKD Stages 3–5 who completed 12 months of exercise training, the prevalence of sarcopenia was unchanged after 12 months. Leg lean mass increased significantly in the balance group and was maintained in the strength group. Whole-body fat mass decreased significantly in both groups. There were no significant differences between groups for changes in sarcopenia or body composition. Plasma myostatin levels increased significantly in both groups with a significant difference in favour of the strength group. Plasma myostatin was significantly positively related to muscle mass and physical performance at baseline, but these relationships were attenuated after 12 months.
The prevalence of sarcopenia was unchanged in both groups after 12 months. This suggests that exercise training, irrespective of whether it is performed as strength or balance training, seems to prevent the development of sarcopenia in non-dialysis-dependent patients with CKD. This is interesting, as during the natural course of CKD, the prevalence of sarcopenia has been shown to increase with declining GFR [27].
After 12 months, the only significant increase in lean mass was in the legs and whole body in the balance group, which also showed a nonsignificant trend towards a greater increase in trunk lean mass. The balance exercises were predominantly focused on the legs and trunk, like standing on one leg, tandem stance and single-leg deadlift. The strength group’s exercises involved both the arms and the legs, like quadriceps extension, push-ups and biceps curls. Both groups were prescribed endurance training, like walking, jogging and cycling, which also focused on leg muscle. Of note, according to the patients’ training diaries, patients in the balance group performed more minutes of endurance training per week than patients in the strength group [21]. Considering that the balance group spent more time on exercises involving the legs and the trunk, the greater increase in leg muscle mass in the balance group is plausible. In a baseline study from RENEXC, we showed that balance function was related to muscle mass in the legs and trunk [4], which was corroborated by this study. However, most importantly, neither group showed a decrease in muscle mass, suggesting that both strength and balance training in combination with endurance training can attenuate muscle wasting and reduce fat mass in this group of patients. In a previous RCT, compared with usual care, 4 months of caloric restriction only or in combination with aerobic exercise was reported to significantly decrease fat mass in patients with moderate to severe CKD, while 4 months of aerobic exercise only did not [28]. With a longer intervention period of 12 months, we showed that exercise alone could lead to a decrease in fat mass.
Myostatin is a myokine that negatively regulates muscle growth [10]. Interestingly, plasma myostatin increased in both groups after 12 months of exercise training, concomitantly with an increase in whole-body lean mass in the balance group and unchanged muscle mass in the strength group. The increase in plasma myostatin was not related to the small yet significant decline in mGFR. Moreover, the higher levels of plasma myostatin were associated with more lean mass and better physical performance at baseline. Myostatin gene expression has been reported to be directly associated with Interleukin 6 and upregulated by inflammation [29, 30] and is thus considered to be a major cause of protein wasting in patients with CKD and inflammation. However, in noninflamed subjects, irrespective of whether they suffered from CKD or not, myostatin levels have been shown to be within the normal range [31]. Interestingly, in this study, our patients had normal C-reactive protein and albumin levels and thus were not inflamed and consequently had levels of myostatin within the normal range at baseline. There was a significant increase in myostatin after exercise in both groups, which remained within the normal range [32, 33]. This increase in myostatin might well be a normal physiological response to increased muscle mass, since myostatin is mainly expressed in skeletal muscle [10].
Similar results to ours have been reported previously [18, 19] and have been explained by the ‘accelerator–brake’ model, where myostatin and insulin-like growth factor 1 (IGF-1) act as counterregulatory molecules for muscle hypertrophy [34]. In vitro experiments have shown that myostatin expression is increased in coronary myocytes when stretched and that the expression was dependent on IGF-1 [35]. Thus training leads to muscle growth partly due to increased levels of IGF-1 that in turn increase the ‘brake’ function of myostatin. Interestingly, the increase in plasma myostatin was significantly greater in the strength group. In strength training, muscle is stretched more intensively than during balance training, which could have triggered a greater ‘brake’ response, explaining the higher levels of plasma myostatin in the strength group. Consequently one could hypothesize that higher ‘brake’ activity would attenuate an increase in muscle mass in the strength group. In contrast, several studies have shown lower levels of myostatin after training [16, 17, 36–38]. Besides differences in inflammatory status, these conflicting results might be due to differences in training programs, duration and intensity, as well as the degree of uraemia.
The relationships between myostatin, muscle mass and strength have also been studied by other investigators. However, here too the results are far from congruent. Positive relationships between serum myostatin and muscle mass and strength have been shown in healthy men, patients with heart failure and patients on peritoneal dialysis [13, 39, 40]. In contrast, other studies have shown that plasma myostatin was negatively related to muscle mass and strength in elderly women, while in patients on haemodialysis, no significant relationship with muscle mass was found [14, 15, 41]. These contradictory results indicate that the relationships hitherto described between plasma myostatin and muscle mass or physical performance are not robust or clear. The relationships seem to vary depending on the subjects studied. Consequently, one cannot claim that plasma myostatin is a simplistic marker of muscle mass or physical performance. Nor can these findings be entirely explained by the accelerator–brake model and further studies are needed to fully understand the dynamics of plasma myostatin and IGF-1 expression.
Our study has several strengths. Firstly, this exercise training study comprises, to date, the largest group of non-dialysis-dependent patients with CKD Stages 3–5. Second, they are representative of the typical non-dialysis-dependent patient, as the majority are elderly and suffer from a number of comorbidities. Finally, the duration of exercise training was 12 months, which is a rather long period. Our study also has limitations. First, there was no sedentary control group. Second, we did not assay muscle myostatin, which could have provided more accurate myostatin levels than plasma myostatin since myostatin is expressed in skeletal muscle. Finally, since our patients showed no signs of inflammation, and had normal myostatin levels, it would be unlikely that myostatin would have a negative role on muscle mass.
In conclusion, 12 months of either strength or balance training in combination with endurance training seemed to prevent the development of sarcopenia, maintain or increase muscle mass, decrease fat mass and increase plasma myostatin in patients with non-dialysis-dependent CKD Stages 3–5. Higher levels of plasma myostatin, although still within the normal range, were related to more muscle mass and better physical performance, but these relationships were attenuated after 12 months of exercise training. However, the role of plasma myostatin in muscle mass and physical performance in patients with CKD warrants further study.
ACKNOWLEDGEMENTS
We would like to thank renal nurses Carina Holmesson and Marianne Liljenborg, renal dietitians Anita Borgmästars and Tove Diswall, medical secretary Ann-Charlotte Malmberg and biomedical analyst Lena Gunnarsson for their invaluable practical assistance.
FUNDING
This study was supported by grants from the Birgit and Sven-Håkan Ohlsson Trust, Skåne University Hospital’s Research Foundation, the Kidney Trust (Njurstiftelsen), the Swedish Society of Nephrology, the Southern Health Care Region in Sweden and the Anna-Lisa and Sven Eric Lundgren Trust. Y.Z. was supported by a scholarship from the Chinese Scholarship Council.
AUTHORS’ CONTRIBUTIONS
N.C., P.H. and M.H. contributed to the research idea and study design. Y.Z. and M.H. contributed to data acquisition. Y.Z., N.C., P.H., T.H. and M.H. contributed to data analysis and interpretation. Y.Z., P.H. and T.H. contributed to statistical analysis. N.C., P.H. and T.H. supervised and mentored. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Souza VA, Oliveira D, Mansur HN. et al. Sarcopenia in chronic kidney disease. J Bras Nefrol 2015; 37: 98–105 [DOI] [PubMed] [Google Scholar]
- 3. Reese PP, Cappola AR, Shults J. et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol 2013; 38: 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou Y, Hellberg M, Svensson P. et al. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant 2018; 33: 342–348 [DOI] [PubMed] [Google Scholar]
- 5. Wang XH, Mitch WE.. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014; 10: 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 7. Watson EL, Gould DW, Wilkinson TJ. et al. Twelve-week combined resistance and aerobic training confers greater benefits than aerobic training alone in nondialysis CKD. Am J Physiol Renal Physiol 2018; 314: F1188–F1196 [DOI] [PubMed] [Google Scholar]
- 8. Baria F, Kamimura MA, Aoike DT. et al. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol Dial Transplant 2014; 29: 857–864 [DOI] [PubMed] [Google Scholar]
- 9. Castaneda C, Gordon PL, Uhlin KL. et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med 2001; 135: 965–976 [DOI] [PubMed] [Google Scholar]
- 10. McPherron AC, Lawler AM, Lee SJ.. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 1997; 387: 83–90 [DOI] [PubMed] [Google Scholar]
- 11. Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant 2014; 29: 1655–1665 [DOI] [PubMed] [Google Scholar]
- 12. Cheung WW, Rosengren S, Boyle DL. et al. Modulation of melanocortin signaling ameliorates uremic cachexia. Kidney Int 2008; 74: 180–186 [DOI] [PubMed] [Google Scholar]
- 13. Yamada S, Tsuruya K, Yoshida H. et al. Factors associated with the serum myostatin level in patients undergoing peritoneal dialysis: potential effects of skeletal muscle mass and vitamin D receptor activator use. Calcif Tissue Int 2016; 99: 13–22 [DOI] [PubMed] [Google Scholar]
- 14. Koyun D, Nergizoglu G, Kir KM.. Evaluation of the relationship between muscle mass and serum myostatin levels in chronic hemodialysis patients. Saudi J Kidney Dis Transpl 2018; 29: 809–815 [DOI] [PubMed] [Google Scholar]
- 15. Yarasheski KE, Bhasin S, Sinha-Hikim I. et al. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging 2002; 6: 343–348 [PubMed] [Google Scholar]
- 16. Kopple JD, Cohen AH, Wang H. et al. Effect of exercise on mRNA levels for growth factors in skeletal muscle of hemodialysis patients. J Ren Nutr 2006; 16: 312–324 [DOI] [PubMed] [Google Scholar]
- 17. Konopka AR, Douglass MD, Kaminsky LA. et al. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci 2010; 65: 1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han DS, Hsiao MY, Wang TG. et al. Association of serum myokines and aerobic exercise training in patients with spinal cord injury: an observational study. BMC Neurol 2016; 16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc 2004; 36: 574–582 [DOI] [PubMed] [Google Scholar]
- 20. Hellberg M, Höglund P, Svensson P. et al. Randomized controlled trial of exercise in CKD—the RENEXC study. Kidney Int Rep 2019; 4: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hellberg M, Hoglund P, Svensson P. et al. Comparing effects of 4 months of two self-administered exercise training programs on physical performance in patients with chronic kidney disease: RENEXC – a randomized controlled trial. PLoS One 2018; 13: e0207349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hellberg M, Hoglund P, Svensson P. et al. Decline in measured glomerular filtration rate is associated with a decrease in endurance, strength, balance and fine motor skills. Nephrology (Carlton) 2017; 22: 513–519 [DOI] [PubMed] [Google Scholar]
- 23. Davies SJ, Phillips L, Naish PF. et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002; 17: 1085–1092 [DOI] [PubMed] [Google Scholar]
- 24. Du Bois D, Du Bois EF.. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303–311, discussion 312–313 [PubMed] [Google Scholar]
- 25. Krutzen E, Back SE, Nilsson-Ehle I. et al. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med 1984; 104: 955–961 [PubMed] [Google Scholar]
- 26. Detsky AS, McLaughlin JR, Baker JP. et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987; 11: 8–13 [DOI] [PubMed] [Google Scholar]
- 27. Foley RN, Wang C, Ishani A. et al. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 2007; 27: 279–286 [DOI] [PubMed] [Google Scholar]
- 28. Ikizler TA, Robinson-Cohen C, Ellis C. et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol 2018; 29: 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verzola D, Procopio V, Sofia A. et al. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int 2011; 79: 773–782 [DOI] [PubMed] [Google Scholar]
- 30. Zhang L, Pan J, Dong Y. et al. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab 2013; 18: 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Casaburi R, Taylor WE. et al. Skeletal muscle mRNA for IGF-IEa, IGF-II, and IGF-I receptor is decreased in sedentary chronic hemodialysis patients. Kidney Int 2005; 68: 352–361 [DOI] [PubMed] [Google Scholar]
- 32. Paul RG, McMahon CD, Elston MS. et al. GH replacement titrated to serum IGF-1 does not reduce concentrations of myostatin in blood or skeletal muscle. Growth Horm IGF Res 2019; 44: 11–16 [DOI] [PubMed] [Google Scholar]
- 33. Esposito P, La Porta E, Calatroni M. et al. Modulation of myostatin/hepatocyte growth factor balance by different hemodialysis modalities. Biomed Res Int 2017; 2017: 7635459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mak RH, Rotwein P.. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int 2006; 70: 410–412 [DOI] [PubMed] [Google Scholar]
- 35. Shyu KG, Ko WH, Yang WS. et al. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 2005; 68: 405–414 [DOI] [PubMed] [Google Scholar]
- 36. Hittel DS, Axelson M, Sarna N. et al. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 2010; 42: 2023–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watson EL, Viana JL, Wimbury D. et al. The effect of resistance exercise on inflammatory and myogenic markers in patients with chronic kidney disease. Front Physiol 2017; 8: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Konopka AR, Wolff CA, Suer MK. et al. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol Regul Integr Comp Physiol 2018; 315: R461–R468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peng LN, Lee WJ, Liu LK. et al. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J Cachexia Sarcopenia Muscle 2018; 9: 635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furihata T, Kinugawa S, Fukushima A. et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int J Cardiol 2016; 220: 483–487 [DOI] [PubMed] [Google Scholar]
- 41. Fife E, Kostka J, Kroc L. et al. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr 2018; 18: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]