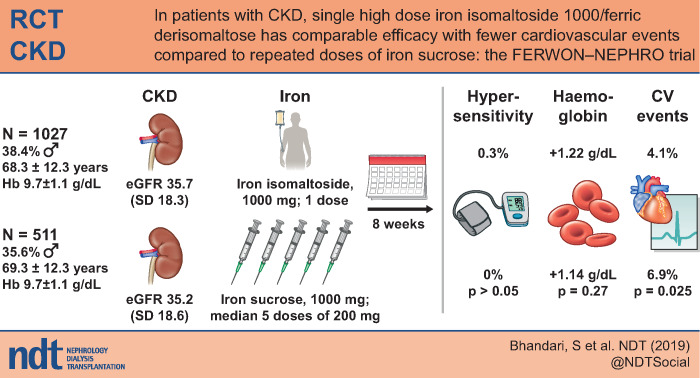
Abstract
Background
The optimal intravenous (IV) iron would allow safe correction of iron deficiency at a single infusion over a short time. The FERWON-NEPHRO trial evaluated the safety and efficacy of iron isomaltoside 1000/ferric derisomaltose (IIM) in patients with non-dialysis-dependent chronic kidney disease and iron deficiency anaemia.
Methods
In this randomized, open-label and multi-centre trial conducted in the USA, patients were randomized 2:1 to a single dose of 1000 mg IIM or iron sucrose (IS) administered as 200 mg IV injections up to five times within a 2-week period. The co-primary endpoints were serious or severe hypersensitivity reactions and change in haemoglobin (Hb) from baseline to Week 8. Secondary endpoints included incidence of composite cardiovascular adverse events (AEs).
Results
A total of 1538 patients were enrolled (mean estimated glomerular filtration rate 35.5 mL/min/1.73 m2). The co-primary safety objective was met based on no significant difference in the incidence of serious or severe hypersensitivity reactions in the IIM and IS groups [0.3% versus 0%; risk difference: 0.29% (95% confidence interval: –0.19; 0.77; P > 0.05)]. Incidence of composite cardiovascular AEs was significantly lower in the IIM versus IS group (4.1% versus 6.9%; P = 0.025). Compared with IS, IIM led to a more pronounced increase in Hb during the first 4 weeks (P ≤ 0.021), and change in Hb to Week 8 showed non-inferiority, confirming that the co-primary efficacy objective was met.
Conclusions
Compared with multiple doses of IS, a single dose of IIM induced a non-inferior 8-week haematological response, comparably low rates of hypersensitivity reactions, and a significantly lower incidence of composite cardiovascular AEs.
Keywords: ferric derisomaltose, iron deficiency anaemia, iron isomaltoside 1000, iron treatment
Graphical Abstract
INTRODUCTION
Anaemia is an important health problem often encountered in patients with non-dialysis-dependent chronic kidney disease (CKD) [1]. The major causes of anaemia in patients with CKD are iron and erythropoietin deficiencies and decreased responsiveness to erythropoietin [2]. Patients with CKD who are treated with erythropoiesis-stimulating agents (ESAs) are likely to develop iron deficiency due to the increased demand for iron to support production of new red blood cells [2]. Thus, iron therapy, alone or in combination with ESA treatment, is essential for effective management of iron deficiency anaemia in patients with CKD. According to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline, patients with non-dialysis-dependent CKD and iron deficiency anaemia should be treated with intravenous (IV) iron or 1–3 months of oral iron depending on the severity of iron deficiency, availability of venous access, response to prior oral iron, side effects with prior iron therapy, expected adherence and cost [3]. For patients with non-dialysis-dependent CKD who require IV iron, the National Institute for Health and Care Excellence guidelines recommend use of high-dose, low-frequency IV iron formulations [4].
Several trials have shown that IV iron improves symptoms, functional capacity and quality of life compared with placebo in the context of heart failure [5–7]. However, to date, there are limited data comparing the relative effects of different IV iron formulations in patients with non-dialysis-dependent CKD.
The FERWON programme was initiated to address the safety and efficacy of high dose iron isomaltoside 1000/ferric derisomaltose (IIM) compared with iron sucrose (IS). The FERWON programme consists of two trials that included a total of 3050 patients with iron deficiency anaemia of mixed aetiologies (FERWON-IDA) or CKD (FERWON-NEPHRO). The results of the FERWON-NEPHRO trial are presented here. The primary objectives were to evaluate the safety and efficacy of IIM compared with IS in patients with non-dialysis-dependent CKD and iron deficiency anaemia with a focus on incidence of serious or severe hypersensitivity reactions and haematological response. The secondary objectives included incidence of composite cardiovascular adverse events (AEs).
MATERIALS AND METHODS
Trial design
This was a randomized, open-label, comparative and multi-centre trial conducted from November 2016 to October 2018. Each individual patient participated in the trial for ∼10 weeks (including a 14-day screening period) during which they attended at least six trial visits (screening visit, baseline visit, three assessment visits and final visit). Patients randomized to IS attended two additional treatment visits to achieve the necessary therapeutic cumulative dose of IS.
The protocols and amendments were approved by the relevant Institutional Review Boards and conducted in accordance with good clinical practice and the Declaration of Helsinki of 1975, as revised in 2013. The trial was registered at ClinicalTrials.gov (NCT02940860). Written informed consent was obtained from all participants.
Participants
The trial was conducted at 143 sites (hospitals, private clinics and clinical research centres) in the USA. Adults with haemoglobin (Hb) ≤11 g/dL, serum ferritin ≤100 ng/mL [or ≤300 ng/mL if transferrin saturation (TSAT) ≤30%] and CKD were allowed to participate in the trial. CKD was defined as either estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 at screening (calculated by the Modification of Diet in Renal Disease formula) or eGFR <90 mL/min/1.73 m2 at screening and kidney damage as indicated by abnormalities in urine composition per medical history and/or intermediate/high risk of cardiovascular disease based on the Framingham model. Patients treated with ESAs needed to receive a stable dose (±20%) for 4 weeks before randomization and during the trial. The complete inclusion and exclusion criteria, including changes implemented during the trial’s execution, are shown in Supplementary data, Table S1.
Interventions
Patients were randomized 2:1 to either IIM (Monofer®/Monoferric®, Pharmacosmos A/S, Holbaek, Denmark) administered at baseline as a single dose of 1000 mg infused over 20 min or IS (Venofer®, American Regent, Shirley, NY, USA) administered as 200 mg IV injections repeated according to standard practice or physician choice up to a maximum of five times within the first 2 weeks starting at baseline. A cumulative dose of 1000 mg was recommended. During the trial, the use of iron supplements besides the investigational drug or blood transfusion was prohibited.
Endpoints
The co-primary endpoints were incidence of adjudicated serious or severe hypersensitivity reactions starting during or after the first dose of randomized treatment (safety) and change in Hb from baseline to Week 8 (efficacy). The secondary safety endpoints included incidence of adjudicated composite cardiovascular AEs starting during or after the first dose of randomized treatment. Table 1 details the trial endpoints.
Table 1.
Trial endpoints
| Co-primary safety endpoint | |
| Incidence of adjudicated serious or severe hypersensitivity reactions starting during or after the first dose of randomized treatment | |
| Co-primary efficacy endpoint | |
| Change in Hb from baseline to Week 8 | |
| Secondary safety endpoints | Secondary efficacy endpoints |
|
|
FACIT score range 0–52, the higher the score the better the quality of life; FACIT fatigue score <30 indicates severe fatigue.
Blood samples were drawn at all visits (screening, baseline, Weeks 1, 2, 4 and 8, and two additional treatment visits if applicable) and analysed for Hb, serum ferritin, TSAT and serum phosphate.
Adjudication of serious or severe hypersensitivity reactions and composite cardiovascular AEs were performed in a blinded fashion by an independent Clinical Endpoint Adjudication Committee. The hypersensitivity terms were defined by a standardized set of Medical Dictionary for Regulatory Activities (MedDRA) terms based on discussions with the US Food and Drug Administration. The terms are listed in Supplementary data, Table S2.
Randomization
Patients were randomized in a 2:1 ratio to receive IIM or IS using stratified block randomization according to eGFR at screening (<46, 46–<60 or 60–<90 mL/min/1.73 m2) and baseline cardiovascular risk (history of myocardial infarction, stroke or congestive heart failure; yes/no). The block size was six. The randomization list was prepared by the data management vendor using the randomization module in Informatics ClinPhone RTSM service. Screening, randomization and enrolment of the patients were performed by the investigator or delegated staff at each trial site.
Data analysis sets
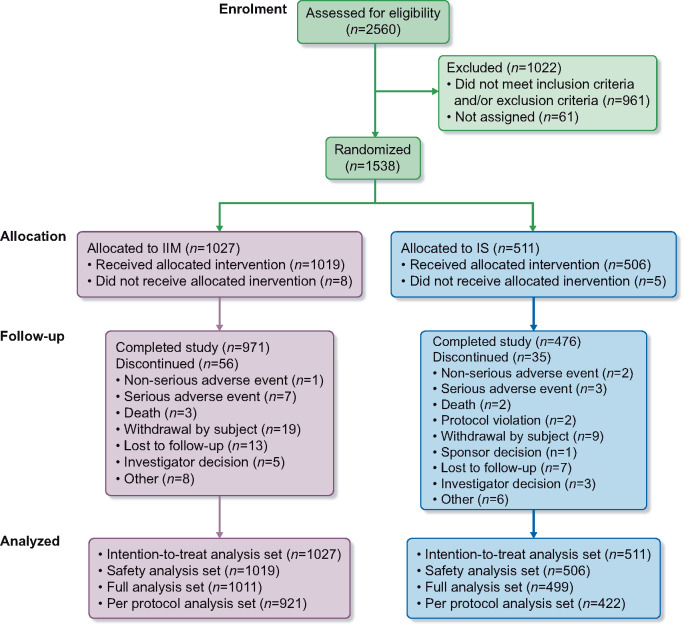
The intention-to-treat analysis set (N = 1538) included all randomized patients and was used to evaluate efficacy (Figure 1). The safety analysis set (N = 1525) included all randomized patients who received at least one dose of the trial drug. This set was used for evaluating safety. The full analysis set (N = 1510) included all randomized patients who received at least one dose of the trial drug, and had at least one post-baseline Hb assessment. This set was used for a sensitivity analysis of the co-primary efficacy endpoint. The per-protocol analysis set (N = 1343) included all patients in the full analysis set who did not have any major protocol deviations. This was used for a sensitivity analysis of the co-primary efficacy endpoint.
FIGURE 1.
Patient disposition.
Power and statistical methods
With 1000 patients treated with IIM and 500 patients treated with IS, there was 100% power to demonstrate non-inferiority of IIM versus IS in the co-primary efficacy endpoint, change in Hb from baseline to Week 8, using a non-inferiority margin of –0.5 g/dL and assuming a common SD of 1.5 g/dL in Hb. Non-inferiority was tested using a mixed model for repeated measurements (MMRM) with a restricted maximum likelihood-based approach. The model included the fixed, categorical effects of treatment, week, treatment-by-week interaction, strata and the continuous covariates of baseline Hb and baseline Hb-by-week interaction. Non-inferiority of IIM versus IS could be claimed if the lower boundary of the 95% confidence interval (CI) was above –0.5 g/dL.
The co-primary safety endpoint, proportion of patients with serious or severe hypersensitivity reactions, was summarized and if the upper boundary of the 95% CI was <3%, the safety objective was met. The risk difference between IIM and IS was assessed by constructing a 95% CI of the risk difference. There was 88% power to demonstrate that the upper limit of the 95% CI for the incidence of serious or severe hypersensitivity reactions was <3%.
The proportion of patients with composite cardiovascular AEs and adverse drug reactions was compared between the treatment groups by Fisher’s exact test. The time to first composite cardiovascular AEs was estimated using the Kaplan–Meier method and significance was tested using a log-rank test.
Incidence of Hb responders to each week (defined as an increase in Hb of at least 1 g/dL from baseline to the week in question) was analysed using a repeated-measures logistic regression model with treatment, visit, strata and treatment-by-visit interaction as fixed effects and baseline value as covariate. The incidence of achievement of a serum ferritin ≥100 ng/mL and a TSAT of 20–50% at any time were analysed using logistic regression with treatment and strata as fixed effects.
An MMRM with treatment, week, treatment-by-week interaction, and strata as factors and baseline value and baseline value-by-week interaction as covariates, was used to compare the average change in Hb, serum ferritin, TSAT and fatigue symptoms.
Incidence of hypophosphataemia (serum phosphate <2.0 mg/dL) and post hoc analyses on the most frequent cardiovascular AEs was performed by a Fisher’s exact test.
All statistical tests were carried out as two-sided on a 5% level of significance.
RESULTS
Patients
A total of 2560 patients were screened, of whom 1538 were randomized 2:1 to IIM (n = 1027) or IS (n = 511). Of the 1538 patients enrolled, 1447 (94%) completed the trial. The details of patient disposition are summarized in Figure 1. There were 576 men (37.5%) and 962 women (62.5%). The majority of patients were white (N = 1106; 71.9%) or black or African American (N = 381; 24.8%). The age ranged from 25 to 97 years (mean: 68.6 years) and mean eGFR was 35.5 mL/min/1.73 m2. Overall demographics, baseline characteristics and laboratory parameters were comparable between the treatment groups (Table 2).
Table 2.
Summary of demographics and baseline anaemia parameters (intention-to-treat analysis set)
| Parameters | IIM | IS |
|---|---|---|
| Age (years) | ||
| N | 1027 | 511 |
| Mean (SD) | 68.3 (12.3) | 69.3 (12.3) |
| Median | 69.0 | 71.0 |
| Gender, n (%) | ||
| Women | 633 (61.6) | 329 (64.4) |
| Men | 394 (38.4) | 182 (35.6) |
| Race, n (%) | ||
| White | 731 (71.2) | 375 (73.4) |
| Black or African American | 264 (25.7) | 117 (22.9) |
| Asian | 16 (1.6) | 11 (2.2) |
| American Indian or Alaska Native | 2 (0.2) | 2 (0.4) |
| Native Hawaiian or other Pacific Islander | 1 (0.1) | 0 (0.0) |
| Other | 13 (1.3) | 6 (1.2) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 476 (46.3) | 248 (48.5) |
| Not Hispanic or Latino | 551 (53.7) | 263 (51.5) |
| Weighta (kg) | ||
| N | 1016 | 506 |
| Mean (SD) | 86.3 (23.4) | 82.6 (20.3) |
| Median | 83.0 | 80.0 |
| Current smoker, n (%) | 118 (11.5) | 53 (10.4) |
| eGFR (mL/min/1.73 m2) | ||
| N | 1027 | 511 |
| Mean (SD) | 35.7 (18.3) | 35.2 (18.6) |
| Median | 33.0 | 30.0 |
| eGFR (mL/min/1.73 m2) and cardiovascular risk,bn (%) | ||
| eGFR <46, no cardiovascular risk | 492 (47.9) | 245 (47.9) |
| eGFR 46–59, no cardiovascular risk | 105 (10.2) | 52 (10.2) |
| eGFR 60–90, no cardiovascular risk | 107 (10.4) | 52 (10.2) |
| eGFR <46, cardiovascular risk | 257 (25.0) | 129 (25.2) |
| eGFR 46–59, cardiovascular risk | 40 (3.9) | 20 (3.9) |
| eGFR 60–90, cardiovascular risk | 26 (2.5) | 13 (2.5) |
| Cardiac disorders, n (%) | 421 (41.0) | 221 (43.2) |
| Vascular disorders, n (%) | 948 (92.3) | 471 (92.2) |
| Diabetes mellitus,cn (%) | 693 (67.5) | 354 (69.3) |
| Treatment with ESAs, % | 5.6 | 5.5 |
| Haemoglobin (g/dL) | ||
| N | 1026 | 511 |
| Mean (SD) | 9.66 (1.14) | 9.71 (1.12) |
| Median | 9.80 | 9.70 |
| Serum ferritin (ng/mL) | ||
| N | 1027 | 511 |
| Mean (SD) | 82.4 (84.0) | 86.2 (80.2) |
| Median | 54.0 | 60.0 |
| Transferrin saturation, % | ||
| N | 1026 | 509 |
| Mean (SD) | 18.51 (29.23) | 17.44 (11.78) |
| Median | 16.00 | 17.00 |
| Serum phosphatea (mg/dL) | ||
| N | 965 | 478 |
| Mean (SD) | 3.97 (0.82) | 4.01 (0.97) |
| Median | 3.90 | 3.85 |
| FACIT fatigue score | ||
| N | 1021 | 509 |
| Mean (SD) | 28.68 (12.39) | 28.22 (12.55) |
| Median | 29.00 | 28.00 |
Based on the safety analysis set.
Cardiovascular risk included medical history of myocardial infarction, stroke or congestive heart failure.
Medical history of diabetes mellitus or diabetes-related complications (e.g. diabetic nephropathy).
FACIT, Functional Assessment of Chronic Illness Therapy.
Exposure to iron
A total of 1019 patients received a single administration of IIM at a mean ± SD dose of 993 ± 71 mg, and 506 patients received one to five administrations (mean: 4.6, median: 5 administrations) of IS at a mean cumulative dose of 899 ± 198 mg.
Co-primary safety endpoint: serious or severe hypersensitivity reactions
In the safety analysis set (N = 1525), a total of three serious or severe hypersensitivity reactions occurred in 3/1019 (0.3%; 95% CI 0.06; 0.86) patients in the IIM group. As the upper boundary of the 95% CI was <3%, the co-primary safety endpoint was met. No serious or severe hypersensitivity reactions occurred in the IS group (0%; 95% CI 0.00; 0.73). The risk difference between IIM and IS with respect to adjudicated and confirmed treatment-emergent serious or severe hypersensitivity reactions was estimated as 0.29% (95% CI –0.19; 0.77). The difference between the two treatment groups was not statistically significant as the CI included zero. Narratives of the hypersensitivity reactions are provided in Supplementary data, Table S3.
Composite cardiovascular AEs
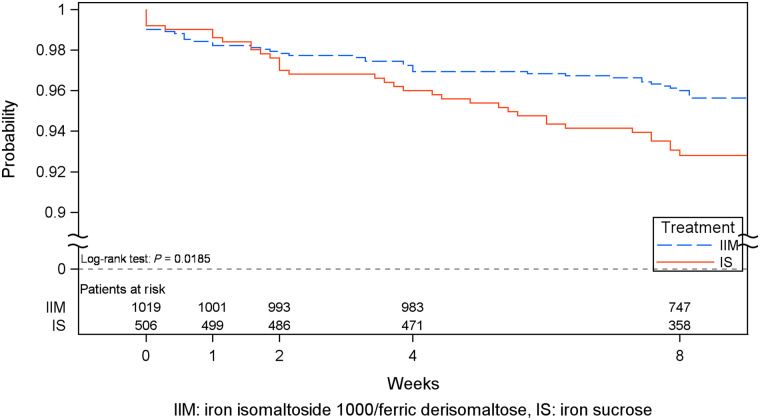
The incidence of adjudicated and confirmed composite cardiovascular AEs was significantly lower in the IIM group compared with the IS group [55 events in 42 (4.1%) patients versus 41 events in 35 (6.9%) patients, P = 0.025). The most frequent cardiovascular AEs were congestive heart failure (0.7% in the IIM group versus 2.2% in the IS group, P = 0.020), hypertension (1.1% versus 2%, P = 0.17) and atrial fibrillation (0.3% versus 1.2%, P = 0.067). The time to first composite cardiovascular AE was significantly longer for IIM versus IS (P = 0.019; Figure 2).
FIGURE 2.
Kaplan–Meier plot of time to first adjudicated and confirmed composite cardiovascular AE (safety analysis set).
Hypophosphataemia
The incidence of hypophosphataemia (serum phosphate <2.0 mg/dL) at any time during the trial was 32/1011 (3.2%) in the IIM and 4/500 (0.8%) in the IS group (P = 0.004). No patient in either group developed severe hypophosphataemia (serum phosphate <1.0 mg/dL).
Adverse drug reactions and other safety data
A total of 83 adverse drug reactions in 48 (4.7%) patients were reported in the IIM group and 43 adverse drug reactions in 27 (5.3%) patients were reported in the IS group (P = 0.62). The most common adverse drug reactions were rash [six events in six (0.6%) patients in the IIM group and one event in one (0.2%) patient in the IS group] and pruritus [five events in five (0.5%) patients in the IIM group and four events in four (0.8%) patients in the IS group]. A total of four serious adverse reactions (drug hypersensitivity, hypersensitivity, acute myocardial infarction and infusion-related reaction) were reported in four (0.4%) patients in the IIM group, while one serious adverse reaction (pyrexia) was reported in one (0.2%) patient in the IS group.
There were three unrelated fatalities in the IIM group and three in the IS group. In the IIM group, three events were reported as septic shock occurring 124 days after treatment, unknown cause of death occurring 3 days after treatment (a patient in the 80s who had a medical history of congestive heart failure, hypertension, hyperlipidaemia, anaemia, CKD, Type 2 diabetes mellitus, secondary hyperparathyroidism, gout, proteinuria and arthritis), and cardiac arrest occurring 55 days after treatment. In the IS group, three events were reported as drug hypersensitivity to angiotensin-converting enzyme inhibitor occurring 30 days after treatment, exacerbation of congestive heart failure occurring 51 days after treatment and cardiac arrest occurring 53 days after treatment. All six deaths were assessed by site investigators as not related to IV iron treatment.
Co-primary efficacy endpoint: change in Hb
The co-primary efficacy analysis (change in Hb concentration from baseline to Week 8) was conducted on the intention-to-treat analysis set (N = 1535), full analysis set (N = 1510) and per-protocol analysis set (N = 1343), and the secondary efficacy analyses were conducted in the intention-to-treat analysis set. The change in Hb concentration from baseline to Week 8 was non-inferior for IIM compared with IS since the lower boundary of the 95% CI for the treatment difference (IIM – IS) was above –0.5 g/dL. In all three analysis sets, superiority of IIM versus IS could not be claimed because the 95% CI included zero (Table 3).
Table 3.
Change in Hb (g/dL) from baseline to Weeks 1, 2, 4 and 8
| Treatment | n | LS mean (95% CI) | Difference | Non-inferiora | Superiority test |
|---|---|---|---|---|---|
| IIM – IS | P-value | ||||
| Estimate (95% CI) | |||||
| Intention-to-treat analysis set | |||||
| IIM (N = 1027) | |||||
| IS (N = 511) | |||||
| Week 1 | |||||
| IIM | 1001 | 0.43 | 0.22 (0.12; 0.31) | – | <0.0001 |
| IS | 494 | 0.21 | |||
| Week 2 | |||||
| IIM | 980 | 0.75 | 0.25 (0.14; 0.36) | – | <0.0001 |
| IS | 474 | 0.50 | |||
| Week 4 | |||||
| IIM | 957 | 1.06 | 0.15 (0.02; 0.28) | – | 0.021 |
| IS | 469 | 0.91 | |||
| Week 8 (co-primary efficacy endpoint) | |||||
| IIM | 967 | 1.22 | 0.08 (–0.06; 0.23) | Yes | 0.27 |
| (1.14; 1.31) | |||||
| IS | 475 | 1.14 | |||
| (1.03; 1.26) | |||||
| Week 8, full analysis set | |||||
| IIM (N = 1011) | 966 | 1.23 | 0.08 (–0.06; 0.23) | Yes | 0.26 |
| (1.15; 1.31) | |||||
| IS (N = 499) | 475 | 1.15 | |||
| (1.03; 1.27) | |||||
| Week 8, per-protocol analysis set | |||||
| IIM (N = 921) | 885 | 1.26 | 0.03 (–0.12; 0.19) | Yes | 0.66 |
| (1.17; 1.35) | |||||
| IS (N = 422) | 406 | 1.23 | |||
| (1.10; 1.36) | |||||
Non-inferiority could be claimed if the lower boundary of the 95% CI is above −0.5 g/dL.
LS mean, least square mean; N, total number of patients; n, number of patient with data at the specific visit.
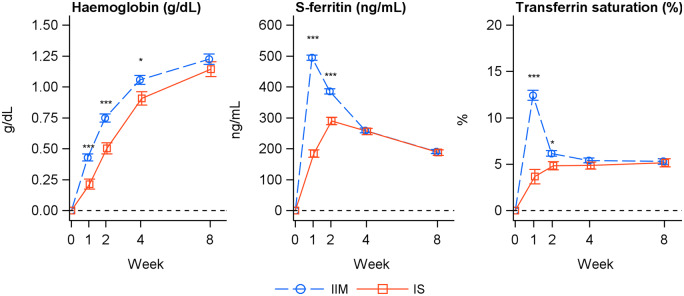
The increase in Hb from baseline to Weeks 1, 2 and 4 was significantly greater for IIM compared with IS (P ≤ 0.021; Figure 3, Table 3). The proportion of responders (patient with an Hb increase of ≥1 g/dL from baseline) was significantly higher in the IIM group compared with the IS group at Weeks 1, 2 and 4 (Table 4), and the time to achieve a Hb increase of ≥1 g/dL was significantly faster for IIM versus IS (P = 0.017).
FIGURE 3.
Change in Hb (g/dL), serum ferritin (ng/mL) and TSAT (%) from baseline to Weeks 1, 2, 4 and 8 (intention-to-treat analysis set). Estimated (LS mean and SE) from a mixed model with repeated measures with treatment, strata and time as factors, treatment × time and baseline value × time interactions and baseline value as covariate. *P < 0.05; ***P < 0.001.
Table 4.
Incidence of responders (intention-to-treat analysis set)
| IIM | IS | |
|---|---|---|
| Patients with Hb increase of ≥1 g/dL from baseline to Weeks 1, 2, 4 or 8 | ||
| Week 1 | ||
| Responders, E/N (%) | 200/1002 (20.0) | 78/494 (15.8) |
| Odds ratioa (95% CI) | 1.37 (1.00; 1.87) | |
| P-value | 0.048 | |
| Week 2 | ||
| Responders, E/N (%) | 339/980 (34.6) | 112/474 (23.6) |
| Odds ratioa (95% CI) | 1.81 (1.39; 2.36) | |
| P-value | <0.0001 | |
| Week 4 | ||
| Responders, E/N (%) | 430/957 (44.9) | 174/469 (37.1) |
| Odds ratioa (95% CI) | 1.41 (1.11; 1.79) | |
| P-value | 0.0048 | |
| Week 8 | ||
| Responders, E/N (%) | 474/967 (49.0) | 226/475 (47.6) |
| Odds ratioa (95% CI) | 1.01 (0.80; 1.27) | |
| P-value | 0.94 | |
| Patients with serum ferritin ≥100 ng/mL and TSAT of 20–50% at any time from Weeks 1 to 8 | ||
| Responders, E/N (%) | 873/1012 (86.3) | 388/500 (77.6) |
| Odds ratioa (95% CI) | 1.82 (1.38; 2.40) | |
| P-value | <0.0001 | |
IIM/IS.
E, number of responders; N, number of patients.
Change in serum ferritin and TSAT
The incidence of achievement of serum ferritin ≥100 ng/mL and TSAT of 20–50% at any time from Weeks 1 to 8 was significantly higher in the IIM group compared with the IS group (P < 0.0001; Table 4). The increase in serum ferritin and TSAT from baseline to Weeks 1 and 2 was significantly greater for IIM compared with IS (serum ferritin: P < 0.0001; TSAT: Week 1: P < 0.0001, Week 2: P = 0.013), while the increase from baseline to Weeks 4 and 8 did not differ significantly between treatment groups (Figure 3, Table 5).
Table 5.
Change in serum ferritin (ng/mL) and TSAT (%) from baseline to Weeks 1, 2, 4 and 8 (intention-to-treat analysis set)
| Treatment | n | LS mean | Difference | P-value |
|---|---|---|---|---|
| IIM – IS | ||||
| Estimate (95% CI) | ||||
| Intention-to-treat analysis set | ||||
| IIM (N=1027) | ||||
| IS (N =511) | ||||
| Serum ferritin (ng/mL) | ||||
| Week 1 | ||||
| IIM | 991 | 494.3 | 309.2 (280.7; 337.8) | <0.0001 |
| IS | 485 | 185.0 | ||
| Week 2 | ||||
| IIM | 994 | 385.7 | 95.8 (67.9; 123.7) | <0.0001 |
| IS | 476 | 289.8 | ||
| Week 4 | ||||
| IIM | 968 | 259.4 | 3.5 (–21.2; 28.1) | 0.78 |
| IS | 472 | 256.0 | ||
| Week 8 | ||||
| IIM | 973 | 191.0 | 3.3 (–18.1; 24.7) | 0.76 |
| IS | 480 | 187.7 | ||
| TSAT, % | ||||
| Week 1 | ||||
| IIM | 989 | 12.4 | 8.8 (6.9; 10.7) | <0.0001 |
| IS | 478 | 3.7 | ||
| Week 2 | ||||
| IIM | 989 | 6.2 | 1.3 (0.3; 2.4) | 0.013 |
| IS | 471 | 4.8 | ||
| Week 4 | ||||
| IIM | 964 | 5.4 | 0.5 (–0.4; 1.5) | 0.24 |
| IS | 472 | 4.9 | ||
| Week 8 | ||||
| IIM | 969 | 5.3 | 0.1 (–0.9; 1.2) | 0.81 |
| IS | 477 | 5.2 | ||
LS mean, least-square mean; N, number of patients; n, number of patient with data at the specific visit.
Change in fatigue symptoms
At baseline, more than half of the patients had severe fatigue. The median Functional Assessment of Chronic Illness Therapy fatigue score increased from baseline to Week 8 in both treatment groups (IIM group: from 29 to 40; IS group: from 28 to 39) without significant differences between groups at Weeks 1, 2 or 8 (Supplementary data, Table S4).
DISCUSSION
The ideal IV iron product would allow iron correction and improvement in Hb in a single visit with a short infusion time and minimal side effects, and yet, no IV iron available in the USA currently fulfils these expectations. Due to the structure of IIM it can be administered in high doses, and previous published clinical trials demonstrate the safety and efficacy of IIM in various populations with different comparators [8–20]. In the current FERWON-NEPHRO trial, adjudicated and confirmed serious or severe hypersensitivity reactions occurred with a low incidence and with no significant difference between the IIM and IS groups. These incidences are lower than those published in a previous review of IV irons showing that IS and ferric carboxymaltose were associated with higher incidence of serious or severe hypersensitivity reactions compared with IIM defined by a standardized set of MedDRA terms [21]. One explanation for this difference could be that the hypersensitivity reactions reported in the review were not adjudicated and confirmed by an independent Clinical Endpoint Adjudication Committee as in the present trial. However, by including this blinded and independent adjudication of hypersensitivity reactions, the risk of bias was limited even though it was an open-label design.
Despite being a relatively short-term trial with 8 weeks of follow-up time, the incidence of cardiovascular AEs was significantly lower in the IIM group compared with the IS group (4.1% versus 6.9%; P = 0.025). The patients were stratified on cardiovascular risk and at baseline, 41.0% of the patients in the IIM group and 43.2% in the IS group had a medical history of cardiovascular disease, indicating that the trial was well balanced in this matter. One of the most frequent cardiovascular AE in the IS group was congestive heart failure, which was reported with a significantly lower incidence in the IIM group. This might suggest a short-term benefit of IIM compared with IS with respect to protecting patients from cardiovascular events. The frequency of serious cardiovascular events in the REVOKE trial was 17/67 (25%) in the IS group and 19/69 (28%) in the oral iron group [22], which are higher frequencies than those reported in this trial. However, there were several differences between the trials. The REVOKE trial included patients with more advanced CKD (Stages 3 and 4), the follow-up time was longer and the criteria for the composite cardiovascular events were not the same.
The recent PIVOTAL trial with 2141 haemodialysis patients in a time-to-first event analysis showed that a higher dose IV IS regime led to a significantly lower risk of death or major non-fatal cardiovascular AEs, including myocardial infarction and hospitalization for heart failure, compared with a low dose IV IS regimen. In addition, a higher dose IV iron regime appeared to protect against recurrent events. It was suggested by the authors that the lower ESA doses required with higher dose IV iron and the iron replacement in patients with iron deficiency might have contributed to the improved cardiovascular profile [23].
A recent trial showed that treatment with IIM enhances skeletal muscle energetics and improves symptoms in patients with chronic heart failure compared with placebo [24], and several trials have reported that IV iron improves symptoms compared with placebo in the context of heart failure [5–7]. The lower incidence of early-onset cardiovascular AEs observed with IIM in the present trial is difficult to explain in such a short trial. We cautiously speculate that at least two distinct mechanisms could perhaps account for this. First, it may be a positive treatment effect of the faster iron repletion provided by IIM compared with IS leading to the lower incidence of cardiovascular AEs. This mechanism would be consistent with the positive effect of high dose iron treatment demonstrated in the PIVOTAL trial [23] and may relate to improved mitochondrial function of cardiac myocytes from iron therapy as reported previously [25]. Secondly, the lower stability of IS [26] compared with IIM is expected to lead to an increased level of free/labile iron, which generates reactive oxygen species that can cause cell damage [27] and cardiovascular disease via endothelial damage [28]. It is possible that the positive treatment effect of iron is partially countered by the effects of oxidative stress in the IS arm [29] relative to IIM. The current trial only provided 8 weeks of follow-up, thus further research including long-term data are warranted to validate and better understand the observed differences. Nonetheless, the data provide support for the use of IIM when IV iron is indicated for patients with an increased risk of cardiovascular disease. Further understanding of the long-term effects of IIM on cardiovascular outcomes specifically in patients with chronic heart failure will be provided through a large ongoing randomized clinical trial including 1300 patients in the UK (the IRONMAN trial, ClinicalTrials.gov identifier: NCT02642562), which is powered to demonstrate a reduction in cardiovascular death or hospitalization due to worsening of chronic heart failure.
The percentage of patients with adverse drug reactions was comparable for IIM (4.7%) and IS (5.3%), and few serious adverse reactions were reported. The incidence of hypophosphataemia was low in both groups and no patient had a serum phosphate <1.0 mg/dL.
Data from two clinical trials that were specifically designed to evaluate the longer-term safety of IV iron therapy in patients with non-dialysis-dependent CKD had conflicting results. The REVOKE trial, including 136 patients, was terminated early due to little chance of finding differences in measured GFR and increased risk of serious AEs, cardiovascular serious AEs and infection in patients receiving IS compared with those receiving oral iron [22]. In contrast, the FIND-CKD trial, including 626 patients across 193 sites in 20 countries, showed no increase in the risk of cardiovascular events or infections among patients receiving ferric carboxymaltose compared with those receiving oral iron over 56 weeks [30]. Potential explanations for the differences in findings from these trials have been discussed in several commentaries [31–33]. Key differences between the two trials are the difference in oral iron dose (975 mg per day in the REVOKE trial versus 200 mg per day in the FIND-CKD trial), different IV iron compounds and differences in reporting of serious AEs.
As might be expected, a faster and transiently greater Hb response was seen the first 4 weeks with the single dose of 1000 mg IIM compared with up to five doses of 200 mg IS administered over 2 weeks. Likewise, serum ferritin and TSAT showed faster and greater improvements in the IIM group compared with the IS group. The faster haematological response is likely due to the higher doses of IIM given within a shorter time period. The cumulative dose of IIM was also higher (993 mg versus 899 mg), which could contribute to the increased Hb response observed with IIM. However, the current trial design is assessed as clinically relevant, as well as reflecting clinical practice in many countries, and it reflects the intended use of the two IV iron products as IS can only be dosed in lower single doses and hence requires more visits to obtain the same cumulative dose as IIM, which can be dosed in a higher single dose.
Fatigue symptoms improved in both treatment groups during the trial. However, despite the faster and transiently greater response in biochemical efficacy parameters with IIM versus IS, no differences were found between treatment groups in reported fatigue symptoms.
In general, IV irons are considered to provide similar effect in equivalent doses, but they differ in their upper dose limits and safety profiles, such as the effects of ferric carboxymaltose on serum phosphate [34]. Another programme (the PHOSPHARE trials) will provide prospective head-to-head safety data comparing IIM versus ferric carboxymaltose and an improved mechanistic understanding of hypophosphataemia.
In conclusion, IIM and IS infusions were both associated with low rates of serious or severe hypersensitivity reactions, with no difference between groups. Significantly fewer patients treated with IIM had cardiovascular AEs compared with IS-treated patients. The incidence of hypophosphataemia was low and no patient developed severe hypophosphataemia. IIM administered as 1000 mg in a single visit resulted in a faster haematological response and non-inferiority at Week 8 compared with IS, which required multiple visits to obtain full dosing.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the investigators and trial personnel for their contribution to the trial, the statistical support from Biostata, and Jens-Kristian Slott Jensen, Slott Stat and the medical writing support of Eva-Maria Damsgaard Nielsen. Eva-Maria Damsgaard Nielsen is employed at Pharmacosmos A/S.
FUNDING
This work was funded by Pharmacosmos A/S.
AUTHORS’ CONTRIBUTIONS
S.B., P.A.K. and M.W. contributed to the interpretation of data, revision of the manuscript for important intellectual content, approval of the final version for submission and agreement to be accountable for all aspects of the work. M.B. and D.B. contributed to acquisition of data and interpretation of data, revision of the manuscript for important intellectual content, approval of the final version for submission and agreement to be accountable for all aspects of the work. L.L.T. contributed to conception, design, conduct of the trial and interpretation of data, drafting and revision of the manuscript for important intellectual content, approval of the final version for submission and agreement to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
The investigators/institutions received a fee per patient. S.B. has received honorarium, consultancy fees, membership advisory board and travel funding from Pharmacosmos A/S, Vifor Pharma and Astellas. P.A.K. has received personal fees and non-financial support from Pharmacosmos A/S, grants and personal fees from Vifor Pharma and grants from Astellas. M.B. and D.B. have no conflict of interest. L.L.T. is employed by Pharmacosmos A/S. M.W. has received consultancy fees from Pharmacosmos A/S, Diasorin, Amag, Luitpold, Akebia, Amgen, Ardelyx and Sanofi, and grants from Shire.
REFERENCES
- 1. St Peter WL, Guo H, Kabadi S. et al. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol 2018; 19: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehdi U, Toto RD.. Anemia, diabetes, and chronic kidney disease. Diabetes Care 2009; 32: 1320–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 4.National Collaborating Centre for Chronic Conditions, Royal College of Physicians. Guideline on anaemia management in chronic kidney disease. 2015. National Institute for Clinical Excellence. Available at http://www.nice.org.uk/guidance/NG8/evidence (21 January 2020, date last accessed)
- 5. Anker SD, Comin Colet J, Filippatos G. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448 [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, van Veldhuisen DJ, Comin-Colet J. et al. CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jankowska EA, Tkaczyszyn M, Suchocki T. et al. Effects of intravenous iron therapy in iron-deficient patients with systolic heart failure: a meta-analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795 [DOI] [PubMed] [Google Scholar]
- 8. Hildebrandt PR, Bruun NE, Nielsen OW. et al. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. Transfus Altern Transfus Med 2010; 11: 131–137 [Google Scholar]
- 9. Wikstrom B, Bhandari S, Barany P. et al. Iron isomaltoside 1000: a new intravenous iron for treating iron deficiency in chronic kidney disease. J Nephrol 2011; 24: 589–596 [DOI] [PubMed] [Google Scholar]
- 10. Reinisch W, Altorjay I, Zsigmond F. et al. A 1-year trial of repeated high-dose intravenous iron isomaltoside 1000 to maintain stable hemoglobin levels in inflammatory bowel disease. Scand J Gastroenterol 2015; 50: 1226–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reinisch W, Staun M, Tandon RK. et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol 2013; 108: 1877–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johansson PI, Rasmussen AS, Thomsen LL.. Intravenous iron isomaltoside 1000 (Monofer®) reduces postoperative anaemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang 2015; 109: 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhandari S, Kalra PA, Kothari J. et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant 2015; 30: 1577–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalra PA, Bhandari S, Saxena S. et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant 2016; 31: 646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birgegård G, Henry D, Glaspy J. et al. A randomized, noninferiority trial of intravenous iron isomaltoside versus oral iron sulfate in patients with nonmyeloid malignancies and anemia receiving chemotherapy, the PROFOUND trial. Pharmacotherapy 2016; 36: 402–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahlerup JF, Jacobsen BA, van der Woude J. et al. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand J Gastroenterol 2016; 51: 1332–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holm C, Thomsen LL, Norgaard A. et al. Single-dose intravenous iron infusion or oral iron for treatment of fatigue after postpartum haemorrhage: a randomized controlled trial. Vox Sang 2017; 112: 219–228 [DOI] [PubMed] [Google Scholar]
- 18. Holm C, Thomsen LL, Norgaard A. et al. Single-dose intravenous iron infusion versus red blood cell transfusion for the treatment of severe postpartum anaemia: a randomized controlled pilot study. Vox Sang 2017; 112: 122–131 [DOI] [PubMed] [Google Scholar]
- 19. Gybel-Brask M, Seeberg J, Thomsen LL, Johansson PI.. Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron-deficient blood donors: a randomized double-blind placebo-controlled clinical trial. Transfusion 2018; 58: 974–981 [DOI] [PubMed] [Google Scholar]
- 20. Derman R, Roman E, Modiano MR. et al. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol 2017; 92: 286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalra PA, Bhandari S.. Safety of intravenous iron use in chronic kidney disease. Curr Opin Nephrol Hypertens 2016; 25: 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agarwal R, Kusek JW, Pappas MK.. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015; 88: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdougall IC, White C, Anker SD. et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019; 380: 447–458 [DOI] [PubMed] [Google Scholar]
- 24. Charles-Edwards G, Amaral N, Sleigh A. et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency. Circulation 2019; 139: 2386–2398 [DOI] [PubMed] [Google Scholar]
- 25. Hoes MF, Grote Beverborg N, Kijlstra JD. et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jahn MR, Andreasen HB, Futterer S. et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm 2011; 78: 480–491 [DOI] [PubMed] [Google Scholar]
- 27. Haase M, Bellomo R, Haase-Fielitz A.. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol 2010; 55: 2024–2033 [DOI] [PubMed] [Google Scholar]
- 28. Panth N, Paudel KR, Parajuli K.. Reactive oxygen species: a key hallmark of cardiovascular disease. Adv Med 2016; 2016: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nuhu F, Bhandari S.. Oxidative stress and cardiovascular complications in chronic kidney disease, the impact of anaemia. Pharmaceuticals (Basel) 2018; 11: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roger SD, Gaillard CA, Bock AH. et al. FIND-CKD Study Investigators. Safety of intravenous ferric carboxymaltose versus oral iron in patients with nondialysis-dependent CKD: an analysis of the 1-year FIND-CKD trial. Nephrol Dial Transplant 2017; 32: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhandari S, Kalra PA, Coyne DW.. Data confusion. Kidney Int 2015; 88: 1445. [DOI] [PubMed] [Google Scholar]
- 32. Macdougall IC, Roger SD.. New data on the safety of IV iron-but why the discrepancy with FIND-CKD? Kidney Int 2015; 88: 1445–1446 [DOI] [PubMed] [Google Scholar]
- 33. Agarwal R. The author replies. Kidney Int 2015; 88: 1446–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Wyck DB, Mangione A, Morrison J. et al. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion 2009; 49: 2719–2728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.