Abstract
Background
Anaplastic thyroid cancer (ATC) is aggressive with a poor prognosis, partly because of the immunosuppressive microenvironment created by tumor-associated macrophages (TAMs).
Purpose
To understand the relationship between TAM infiltration, tumor vascularization, and corresponding drug delivery by using ferumoxytol-enhanced MRI and macrin in an ATC mouse model.
Materials and Methods
ATC tumors were generated in 6–8-week-old female B6129SF1/J mice through intrathyroid injection to model orthotopic tumors, or intravenously to model hematogenous metastasis, and prospectively enrolled randomly into treatment cohorts (n = 94 total; August 1, 2018, to January 15, 2020). Mice were treated with vehicle or combined serine/threonine-protein kinase B-Raf (BRAF) kinase inhibitor (BRAFi) and anti-PDL1 antibody (aPDL1). A subset was cotreated with therapies, including an approximately 70-nm model drug delivery nanoparticle (DDNP) to target TAM, and an antibody-neutralizing colony stimulating factor 1 receptor (CSF1R). Imaging was performed at the macroscopic level with ferumoxytol-MRI and microscopically with macrin. Genetically engineered BrafV600E/WT p53-null allografts were used and complemented by a GFP-transgenic derivative and human xenografts. Tumor-bearing organs were processed by using tissue clearing and imaged with confocal microscopy and MRI. Two-tailed Wilcoxon tests were used for comparison (≥five per group).
Results
TAM levels were higher in orthotopic thyroid tumors compared with pulmonary metastatic lesions by 79% ± 23 (standard deviation; P < .001). These findings were concordant with ferumoxytol MRI, which showed 136% ± 88 higher uptake in thyroid lesions (P = .02) compared with lung lesions. BRAFi and aPDL1 combination therapy resulted in higher tumor DDNP delivery by 39% ± 14 in pulmonary lesions (P = .004). Compared with the untreated group, tumors following BRAFi, aPDL1, and CSF1R-blocking antibody combination therapy did not show greater levels of TAM or DDNP (P = .82).
Conclusion
In a mouse model of anaplastic thyroid cancer, ferumoxytol MRI showed 136% ± 88 greater uptake in orthotopic thyroid tumors compared with pulmonary lesions, which reflected high vascularization and greater tumor-associated macrophage (TAM) levels. Serine/threonine-protein kinase B-Raf inhibitor and anti–programmed death ligand 1 antibody elicited higher local TAM levels and 43% ± 20 greater therapeutic nanoparticle delivery but not higher vascularization in pulmonary tumors.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Luker in this issue.
Summary
Ferumoxytol MRI, and microscopy with tumor-associated macrophage (TAM)-targeted nanoparticle macrin, showed serine/threonine-protein kinase B-Raf inhibition and immune checkpoint blockade enhanced local TAM accumulation by 46% ± 21 (standard deviation), which correlated with 43% ± 20 greater uptake of a model drug delivery nanoparticle in a mouse model of anaplastic thyroid cancer.
Key Results
■ MRI with the dextran-coated magnetic nanoparticle ferumoxytol quantified tumor-associated macrophage (TAM) levels in mouse models of anaplastic thyroid cancer.
■ Greater TAM levels (by 1.4) were detected in the orthotopic thyroid location compared with disseminated pulmonary lesions (P = .02). TAM density was heterogeneous and correlated with passive accumulation of a model drug delivery nanoparticle (r = 0.95; P < .001).
■ Core TAM accumulation was reduced 39% by cotreatment with a colony stimulating factor 1 receptor–blocking antibody (P = .01).
Introduction
Anaplastic thyroid cancer (ATC) makes up less than 2% of all thyroid malignancies yet accounts for 20%–50% of thyroid cancer mortality, with a median survival of less than 6 months (1). Poor prognosis is associated with metastasis, often to the lungs (1). Targeted therapies and immunotherapies show promise, especially for lesions harboring activating B-Raf kinase (BRAF) V600E mutation, which is common in ATC (2–5) and for which BRAF inhibitor (BRAFi) therapy is used (6). Nevertheless, durable response is rare (6). Thus, there is a need to understand the heterogeneity of treatment response in ATC to overcome drug resistance.
A feature of ATC is the abundance of tumor-associated macrophages (TAMs) in the tumor microenvironment, which correlates with poor prognosis (7,8). “M2-like” immunosuppressive TAM phenotypes are pervasive and can promote drug resistance, especially in response to BRAFi (9) and immune checkpoint blockade (10). Strategies are being tested to eliminate TAM and/or repolarize TAM toward inflammatory phenotypes (11,12). TAM can also influence drug accumulation in solid tumors (13), for instance, enabling delivery of clinically relevant model drug delivery nanoparticle system (DDNP) (14), partly through promoting the enhanced permeability and retention effect. Clinical trials (eg, NCT03181100) are examining DDNP in ATC, including in combination with immune checkpoint blockade by using atezolizumab, a programmed death ligand 1 (PDL1) targeted antibody (aPDL1), but it remains uncertain how drug delivery may be impacted by TAM in this disease (15).
TAM imaging shows promise for monitoring immunomodulatory drug responses, measuring the enhanced permeability and retention effect, and predicting DDNP delivery (16). In particular, dextran-based nanoparticles target tumor-associated phagocytes, especially TAM, and are being tested for companion imaging (16,17). Ferumoxytol is a clinically relevant, magnetic iron oxide nanoparticle with carboxymethyl dextran coating and reported hydrodynamic diameter of 17–31 nm (16). Phagocyte uptake of ferumoxytol, including by TAM, is known to correlate with enhanced T2 signal changes (18), and ferumoxytol-enhanced MRI (hereafter, ferumoxytol MRI) correlates with TAM infiltration (19) and response to liposomal irinotecan DDNP (20) in patients with metastatic solid cancers. More recently, a 64Cu-labeled polyglucose nanoparticle (macrin) was optimized for TAM PET imaging (17). These tools are promising but questions remain. For example, it is unclear how TAM distribute across ATC tumors, how this impacts drug delivery, and whether clinical imaging could guide treatment.
The purpose of our study is to understand the relationships between TAM infiltration, tumor vascularization, and drug delivery in murine models of orthotopic and metastatic ATC by combining ferumoxytol MRI with confocal microscopy of optically cleared tissue.
Materials and Methods
All animal research was performed in accordance with guidelines from the Institutional Subcommittee on Research Animal Care and under approval from the Institutional Animal Care and Use Committee at Massachusetts General Hospital (Boston, Mass).
Tumor Cell Culture and Animal Models
The TBP-3743 (TPO-CreER, Braf tm1Mmcm/+, Trp53tm1Brn/tm1Brn) BrafV600E/WTp53−/− murine cell line and the BrafV600E human ATC cell line 8505c were obtained and cultured as previously described (4) with routine mycoplasma and mouse antigen pathogen testing. Six to 8-week-old female B6129SF1/J mice were inoculated with TBP-3743 or stable transductants of green fluorescent protein (GFP)-derivative mClover. We implanted 1 × 105 cells in the left thyroid lobe as previously described (5) or injected 2.5 × 105 cells intravenously to model hematogenous lung metastasis (49 lung TBP, five lung TBP-GFP, 25 thyroid TBP, five thyroid TBP-GFP). We inoculated 8505c cells in 6–8-week-old female severe combined immunodeficient mice (10 mice). Body condition score 2 or less was a euthanasia criterion (20). Experimental design, sample sizes, and explanation for excluding four of 94 mice (August 1, 2018, to January 15, 2020) because of variable disease progression are detailed in Figure E1 (online).
Nanoformulation and Reagents
Fluorophore-conjugated nanoparticles (17) and antibodies (10) were prepared as previously described. Poly (lactic-co-glycolic acid)8.3kDa-b-polyethylene glycol5.5kDa (PLGA-PEG) micelles modeled clinically tested DDNP, with hydrodynamic size measured by dynamic light scattering (Malvern Zetasizer, Spectris plc, Surrey, England, 63 ± 0.8 nm, polydispersity index: 0.16 ± 0.02; ζ-potential: −17 ± 0.3 mV; n = 3), and coencapsulated PLGA-BODIPY-TMR. DDNP did not encapsulate chemotherapy as we focused on delivery (dual drug/NP labeling has been performed previously [13,14,18]). Macrin-VT680XL, Macrin-PacBlue (10,16), and aPDL1-A647 (degree of labeling 5:1) were synthesized as previously described (10). Vasculature was visualized by using fluorescein or rhodamine labeled Griffonia simplicifolia (Bandeiraea simplicifolia) lectin I (Vector Laboratories, Burlingame, Calif) as described previously (12). Modified tissue clearing solution (clear unobstructed brain imaging cocktails, or CUBIC) was used as previously described for volumetric imaging (12).
Monitoring Immunomodulation in ATC
Mice bearing TBP-3743 tumors were randomized following tumor formation (Fig E1 [online]) to be administered combination BRAFi (35 mg/kg in 150 μL of 1.5% methylcellulose/phosphate-buffered saline [PBS], qdx8 via oral gavage; vemurafenib, LC laboratories, Woburn, Mass) and aPDL1 (200 µg/mouse in 200 μL PBS, q2dx4 by intraperitoneal injection; 10F.9G2, bioXcell, West Lebanon, NH). A subset was administered anti-colony stimulating factor 1 receptor (CSF1R)-blocking antibody (AFS98, bioXcell) equivalently dosed with aPDL1. Control mice were administered a vehicle. Twenty-four hours before humanely killing the animals, macrin (10 nmol of dye in 750 μg macrin in 150 μL PBS) and/or DDNP (270 μg) were injected intravenously; lectin (100 μg in 100 μL) was injected intravenously 1 hour before killing the animals. PBS cardiac perfusion was followed by 4% formaldehyde fixation overnight, and tissues were transferred to 10-mL CUBIC solution with 1 μg/mL 4’,6-diamidino-2-phenylindole (DAPI; Life Technologies, Carlsbad, Calif), rocked for 48–72 hours at 37°C, and mounted in glass chambers in fresh CUBIC solution for imaging. DDNP uptake was compared with doxorubicin (15 mg/kg via intravenous injection in 200 μL PBS; λ exitation/λ emission wavelengths, 470 nm/595 nm; LC laboratories) or AF647-aPDL1 (200 μg), co-injected with either macrin and/or lectin, 24 hours before killing the animals.
Confocal Microscopy
Confocal imaging (FV1000MPE; OlympusAmerica, Chelmsford, Mass) was used with 405-, 473-, 559-, and 635-nm lasers and filters as described elsewhere (17), and analyzed with Fiji 2019 (21) and Matlab R2017a (MathWorks, Natick, Mass). Distributions and single-cell uptake were quantified from magnification 2× and 20× images, respectively, by using background subtraction and normalization to adjacent tissue (17).
MRI Parameters
Isoflurane-anesthetized mice were scanned by using a small-animal 4.7-T MRI system (PharmaScan, Bruker BioSpin, Billerica, Mass). Baseline T1- and T2-weighted images were obtained by using an ultrashort echo time (repetition time msec/echo time msec, 3.5/0.013; flip angle, 10°; field of view, 35 × 35 mm; 200 × 200 matrix; slice thickness, 0.175 mm) and multispin multiecho sequence (4000/8.68, 17.36, 26.04, 34.72, 43.40, 52.08, 60.76, 69.44, 78.12, 86.80, 95.48, 104.16, 112.84, 121.52, 130.20, and 138.88; flip angle, 10°; field of view, 25 × 25 mm; 128 × 128 matrix; slice thickness, 0.7 mm; four averages; 20 slices; zero dummy scans; total acquisition time, 30 minutes), respectively. A second set of images with the same sequences were acquired 24 hours after intravenous injection of ferumoxytol (20 mg Fe per kilogram of body weight). A subset of mice was imaged at 4 hours after administration of ferumoxytol. Custom software generated R2 maps as described previously (22).
Immunofluorescence Staining
Tissue was fixed with 4% paraformaldehyde in PBS at 4°C overnight, transferred to 30% sucrose in PBS after rinsing with PBS for 1 hour, embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, Calif), sectioned at 10 μm, and stained with antimouse F4/80 antibody (BM8; Thermo Fisher, Waltham, Mass) and AF488 Goat anti-Rat IgG (A-11006; Thermo Fisher), and DAPI counterstained. Images were captured by using a BX63 microscope (OlympusAmerica), coverslips were removed, Prussian blue staining was performed by following manufacturer instructions (ab150674; Abcam, Cambridge, Mass), and slides were scanned with NanoZoomer RS2.0 (Hamamatsu, Hamamatsu, Japan). Flow cytometry of fresh tumor-bearing lungs was performed as previously described (Fig E2 [online]) (17).
Statistical Analysis
GraphPad Prism 8.0 (GraphPad Software, La Jolla, Calif) and Matlab R2017a (Mathworks) were used to compute statistics between treatment cohorts (T.S.C.N., a nuclear radiologist with 15 years of experience and training, and M.A.M., with 12 years of experience and training in bioengineering). P values of .05 or less indicated statistical significance. Data are reported as means ± standard deviation, except for mean percentage differences, for which means ± standard error were reported. We also reported two-tailed rank sum, signed rank, and/or Spearman r values. Bonferroni correction was used where appropriate, otherwise hypothesis testing was implied, if not made explicit, because of space limits.
Results
Ferumoxytol MRI of Thyroid and Lung ATC Lesions
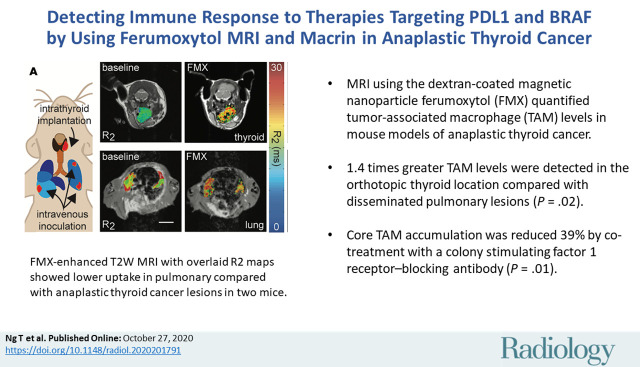
We examined the degree to which an immunocompetent mouse model of ATC accumulated ferumoxytol. At 24 hours after injection, 136% ± 88 greater change in R2 was observed for thyroid (4.9 sec−1 ± 0.8, 35% ± 8) compared with lung tumors (2.1 sec−1 ± 1.8 [P = .02], 13% ± 11; Fig 1, A, B). Differences in changes in R2 values with ferumoxytol injection were not merely correlated to tumor volume (Fig 1, C; r = −0.42; P = .23), which were not consistently different between thyroid (145 mm3 ± 107) and lung (71 mm3 ± 89) tumors (P = .22). Ferumoxytol MRI also showed inconsistent differences in change in R2 in thyroid compared with lung ATC lesions at 4 hours after injection (4.9 sec−1 ± 2.1 vs 2.9 sec−1 ± 1.7, respectively; P = .23; Fig 1, D, E) albeit with limited sample size (3–4 mice per group). Thus, ferumoxytol MRI suggested that thyroid lesions contain a higher density and quantity of TAM reflected by imaging at 24 hours, and heterogeneous vascularization and/or permeability reflected by imaging at 4 hours.
Figure 1:
Ferumoxytol (FMX)-enhanced MRI shows lower uptake in pulmonary compared with thyroid lesions from anaplastic thyroid cancer (ATC). A, Representative T2-weighted images, with overlaid R2 maps of an ATC tumor and multiple lung lesions in separate B6129SF1/J mice before and 24 hours after intravenous injection of ferumoxytol by using the TBP-3743 model (scale bar, 5 mm). Tumor voxels that did not appropriately fit to the monoexponential curve, as previously defined (22), were excluded from analysis (black voxels). Corresponding baseline coronal images are shown at right. B, Ferumoxytol-induced change in R2 (ΔR2) was quantified over the segmented tumor volumes. C, Tumor size and change in R2 did not correlate (data are means across tumors for individual mice; log-linear regression and 95% CI shown in gray). R2 voxel histogram distributions pooled across, D, thyroid and, E, lung tumors, before and after administration with ferumoxytol. For all, data points and error bars are means ± standard deviation.
Immunologic Characterization of Ferumoxytol and Macrin Distribution
To confirm TAM uptake of ferumoxytol in our model, we compared the staining patterns of F4/80 (used to stain for TAM) and Prussian blue (to stain iron of ferumoxytol) and observed instances of colocalization (Fig 2, A). Macrin is similar to ferumoxytol in hydrodynamic diameter (∼20 nm) and carboxymethyl dextran component (17). Prior studies labeled macrin with either 64Cu or the fluorophore VT680XL, for PET or confocal microscopy, and found optical macrin correlated positively with macrin-enhanced PET by greater than 90% selective uptake in TAM in mouse lung tumors (17). Here, we confirmed TAM uptake of macrin in pulmonary ATC lesions by histologic analysis, finding colocalization between macrin and F4/80 (Fig 2, B). Flow cytometry (Figs 2, C, E2 [online]) of tumor-bearing lungs indicated macrin accumulated 22 ± 2 fold higher in macrophages than other cell types (Fig 2, C; P < .001).
Figure 2:
Immunologic assessment of ferumoxytol and macrin cellular uptake. Lungs bearing anaplastic thyroid cancer (ATC) tumors, by using the TBP-3743 model, were excised 24 hours after administration with ferumoxytol and macrin. Within tumors, arrows mark colocalization in cellular staining of, A, iron oxide (by using Prussian blue) and F4/80, and, B, macrin and F4/80. C, Uptake of macrin on a per-cell basis was quantified by flow cytometry, after immunologic definition of cell populations from tumor-bearing lung tissue (Fig E2 [online] shows gating scheme). DAPI = 4’,6-diamidino-2-phenylindole, frac. max = fraction maximum, MΦ = macrophages.
Although ferumoxytol has been reported to influence TAM polarization in some applications, our imaging dose and timeframe did not alter macrophage expression of CD206, a marker of M2-like TAM polarization (6% ± 14 higher; P = .70; Fig E3 [online]). Ferumoxytol coadministration with macrin did not affect accumulation of the latter in macrophages from tumor-bearing lungs (26% ± 13 higher; P = .06; Fig E3 [online]).
Spatial Distribution of Microvasculature and TAM in ATC
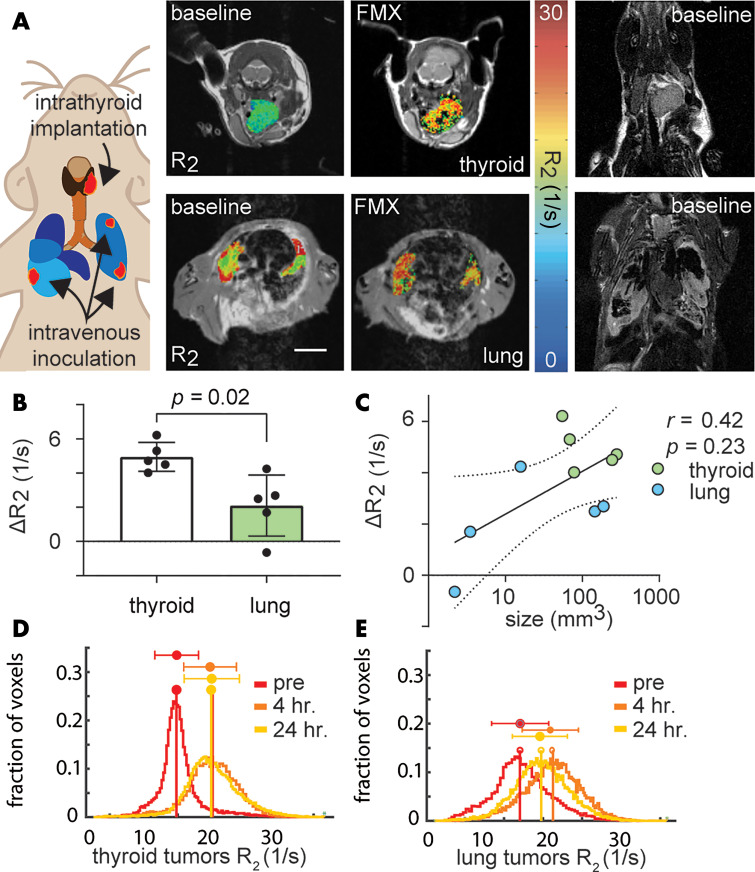
We combined macrin with optical tissue clearing, which enabled volumetric confocal microscopy, to characterize the spatial distribution of microvasculature and TAM within GFP-expressing ATC allografts (Figs 3, E4 [online]). Radial profiles of fluorescence were quantified as a function of distance from the tumor edge, indicating 94% ± 44 elevated macrophage levels in the tumor cores relative to surrounding tissue, averaged across both thyroid and lung lesions (P = .004; Fig 3, B). Lectin similarly showed 73% ± 25 elevated vascularization in thyroid and lung tumors, relative to surrounding tissue (Fig E5 [online]).
Figure 3:
Quantification of tumor-associated macrophages (TAMs) and vasculature in anaplastic thyroid cancer (ATC) lesions by using optical tissue clearing. By using the TBP-3743 model with expression of green fluoresent protein (TBP-GFP) model, tumor cells (GFP positive; GFP+), vasculature (rhodamine-labeled lectin), and TAM (macrin-VT680XL) were imaged in mice bearing thyroid or pulmonary ATC tumors. A, Representative examples of intact thyroids and lungs that were excised, optically cleared, and imaged with confocal microscopy (scale bar, 5 mm). B, Graph shows radial profiles depicting distribution of TAM quantified as a function of distance to the tumor edge from data in A. Thick line and shading denote mean ± standard error of the mean. C, TAM in tumor versus nontumor tissue were quantified from macrin in the TBP (GFP negative; GFP-), and TBP-GFP (GFP+) allograft models, and the 8505c xenograft model. Data are means ± standard deviation. D, Corresponding to C, TAM levels were normalized by their levels in thyroid tumors for each model. Data are means ± standard deviation. Frac. = fraction.
Although both thyroid and lung lesions exhibited elevated macrophage levels relative to surrounding tissue, TAM levels were on average 47% ± 7 higher in the thyroid lesions (P = .02; Fig 3, C, D). To eliminate transgenic GFP as a potential immunogenic confounder (23), we repeated TAM imaging by using tumors of parental cells that lacked GFP. Macrin uptake was lower by 54% ± 4 across thyroid and pulmonary lesions (P < .001; Fig 3, C, Fig E6 [online]) but TAM levels were still 53% ± 15 higher in the former (Fig 3, D; P = .008). We also quantified TAM distribution in an ATC xenograft model. All three tumor models pooled together (GFP-negative and GFP-positive allografts, and xenografts) showed 79% ± 23 greater TAM accumulation in thyroid compared with lung lesions (P < .001; 26 mice; Fig 3, D), although limited sample size in the xenograft is noted (n = 3).
Tumor Penetration of Model Therapeutics
We next examined how vascularization and TAM correlated with delivery of three model classes of drugs, small molecule chemotherapy (doxorubicin), antibody therapy (aPDL1), and a model DDNP of similar size to clinical liposome and polymer nanomedicines. Tumor concentration of doxorubicin nearly matched surrounding tissues (10% ± 4 higher; P = .33; Figs E7, E8 [online]), whereas DDNP and aPDL1 accumulated 36% ± 7 (P = .002) and 80% ± 18 (P = .002) higher in the tumor core compared with the edge, respectively. aPDL1 and DDNP were 2.3- and 3.6-fold more spatially heterogeneous than doxorubicin (Fig E9 [online]; P < .001). DDNP colocalized with macrin-positive TAM in both thyroid and lung tumors (Fig E9 [online]; P < .001). These data show all three model therapeutics could penetrate ATC tumors, and DDNP accumulation occurred in TAM.
Impact of Immunomodulation with BRAFi and aPDL1 on Accumulation of TAM and DDNP
We next tested whether a previously optimized (3,4) immunogenic therapy based on combined BRAFi and aPDL1 enhanced TAM and corresponding DDNP accumulation in ATC lesions. BRAFi and aPDL1 led to 84% ± 25 smaller thyroid tumors (Fig E10 [online]; P = .01) and 59 ± 13 fewer lung lesions (Fig E10 [online]; P = .001), which indicated effective yet incomplete responses. Prior studies showed that tumors eventually grow resistant, mirroring clinical observations (3,4). Combined BRAFi and aPDL1 resulted in higher TAM density in lung tumors by 470% ± 99 (Fig 4, A, B; P = .02).
Figure 4:
Quantification of how combination serine/threonine-protein kinase B-Raf inhibitor (BRAFi) and anti–programmed death ligand 1 (aPDL1) affects tumor-associated macrophage (TAM) accumulation and nanomedicine delivery. A, Representative confocal microscopy of optically cleared tumors reveals macrin accumulation at 24 hours after administration, following an 8-day course of treatment with combination BRAFi and aPDL1 in the murine TBP model of anaplastic thyroid cancer (ATC; scale bar, 5 mm). B, Corresponding to A, the scatter dot plot shows TAM density quantified across single cells from microscopy data by using macrin to identify TAM. Bonferroni correction applied for multiple hypothesis testing (n = 2 hypotheses, corresponding to lung and thyroid, comparing with or without combination BRAFi and aPDL1 treatment). C, Graph shows macrin and drug delivery nanoparticle (DDNP) uptake correlated across treatment groups on a tumor-by-tumor basis (see Fig E11 [online] for individual tumor-by-tumor correlation within individual subjects). D, Scatter dot plots show DDNP uptake quantified by confocal microscopy in TAM (macrin+) and other (macrin-) cells by using the TBP model as a fraction of average uptake in macrin+ cells. See Figure E9 (online) for comparison across drug treatments. For B and D, data are means ± standard deviation.
TAM imaging correlated positively with DDNP uptake on a tumor-by-tumor basis across treatment groups (Fig 4, C; r = 0.95; P < .001) and tumors of individual mice (r = 0.77 ± 0.04; P < .001; Fig E11 [online]). However, treatment did not affect tumor vascularity (Fig E12 [online]; 7% ± 12 lower; P = .69). Imaging (Fig 4, D) suggested enhanced macrin and DDNP uptake was because of greater TAM density rather than greater accumulation on a per-cell basis because the former was higher with treatment (Fig 4, A, B) whereas the latter did not significantly change (6% ± 17; P = .82; Figs 4, D, E13 [online]). This effect was most pronounced toward the tumor core (Fig 5), where enrichment in DDNP accumulation was higher by 39% ± 14 over levels at the tumor edge, in treated compared with untreated lung tumors (Fig 5, C; P = .004). Compared with imaging, flow cytometry of tumor-bearing lungs showed combined BRAFi and aPDL1 did not result in higher bulk macrophage levels averaged across the whole lung (P = .18; Fig 6, A), suggesting localized TAM accumulation. Nonetheless, combined BRAFi and aPDL1 elicited 41% ± 8 lower CD206 levels on CD11bhigh macrophages (consistent with interstitial macrophages as reported previously) (17) but not alveolar macrophages (P = .02; Figs 6, B, E2 [online]).
Figure 5:
Quantification of how combination serine/threonine-protein kinase B-Raf inhibitor (BRAFi) and anti–programmed death ligand 1 (aPDL1) influences the spatial distribution of nanoparticle uptake. A, Macrin and drug delivery nanoparticle (DDNP) were administered following an 8-day course of treatment, and 24 hours later tissue was excised for confocal microscopy of optically cleared TBP tumors in the thyroid and lung. Representative images are shown (scale bar, 5 mm). B, Graphs show macrin and DDNP uptake quantified as a function of distance to the tumor edge. Thick lines and shading are means ± standard error of the mean. C, Scatter dot plots show that, from radial profiles, DDNP accumulation was quantified as a percentage increase at the core compared with the edge of tumors. Data are means ± standard deviation. Bonferroni correction applied for multiple hypothesis testing (n = 2 hypotheses, corresponding to lung and thyroid, comparing with or without combination BRAFi and aPDL1 treatment).
Figure 6:
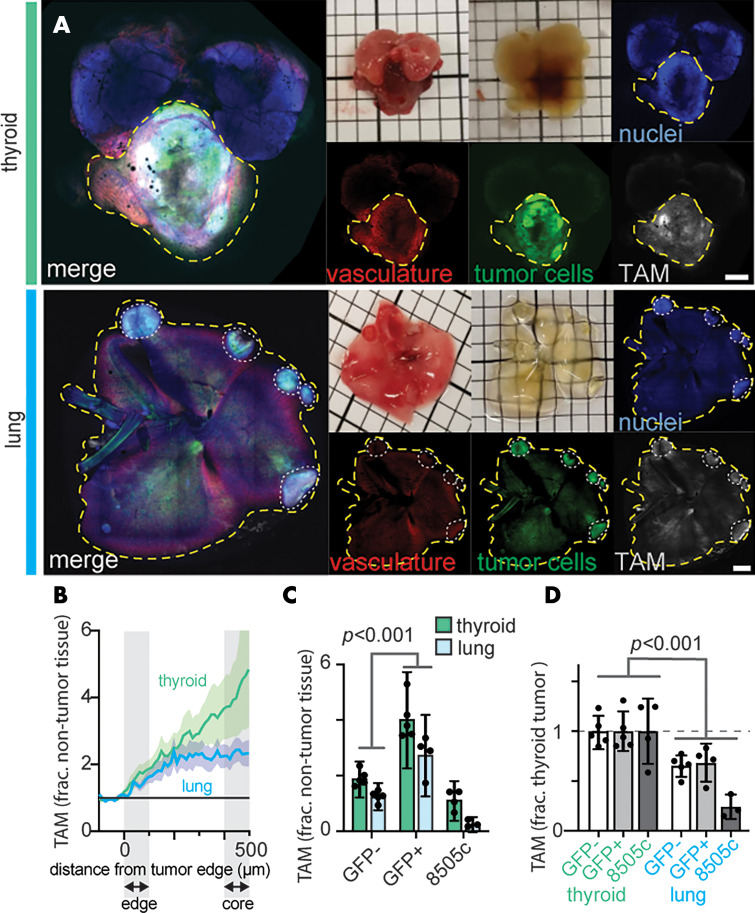
The influence of anti-colony stimulating factor 1 antibody treatment (aCSF1R) on tumor-associated macrophage (TAM) levels following combination threonine-protein kinase B-Raf inhibitor (BRAFi) and anti–programmed death ligand 1 (aPDL1) treatment. By using the TBP model, lungs bearing TBP-3743 tumors were excised 24 hours after treatment with macrin and drug delivery nanoparticle (DDNP), which were co-injected intravenously after an 8-day treatment course of aPDL1, BRAFi, and CSF1R-blocking antibody, as defined in Figure E1 (online). A, Scatter dot plot shows macrophage abundance quantified by flow cytometry of tumor-bearing lungs; the two macrophage populations were normalized to their abundances in the control group (see Fig E2 [online] for gating schemes and absolute abundance). B, Plot shows expression of CD206 quantified in interstitial macrophages by flow cytometry. C, As in Figure 5, confocal microscopy of cleared tumor-bearing lungs quantified TAM in tumor cores relative to edges. D, Scatterplot shows total TAM levels in tumors correlated with total DDNP uptake, shown as average values across tumors in individual mice. A–C, Bonferroni correction applied. Data are means ± standard deviation.
CSF1R-blocking antibody resulted in lower interstitial macrophages in lungs of tumor-bearing mice treated with combined BRAFi and aPDL1 by 84% ± 5 (Fig 6, A; P = .01). It reversed the effects of combined BRAFi with aPDL1 on TAMs within tumor cores and on CD206 expression among remaining interstitial macrophages, so that levels of either did not differ from untreated control mice (Fig 6, B, C; P = .82 and P = .31, respectively). However, CSF1R-blocking antibody did not further reduce tumor sizes compared with reductions with combined BRAFi and aPDL1 alone (Fig E14 [online]; P = .48), and CSF1R-blocking antibody–elicited TAM reduction correlated with lower accumulation of coadministered DDNP (r = 0.95; P < .001; Fig 6, D).
Discussion
Tumor-associated macrophage (TAM) levels vary across patients and tumor regions and reportedly correlate with resistance to immune checkpoint blockade, in some cases more than any other immune cell type examined (24). Most studies have quantified TAM in primary tumors or subcutaneous xenograft models, and it remains unclear how TAM differ across sites of metastasis. Therefore, we applied multiple translational TAM imaging approaches to study the immunogenic response to a combination of an anti–programmed death ligand 1 (aPDL1) immune checkpoint blockade and serine/threonine-protein kinase B-Raf (BRAF) inhibitor (BRAFi)-targeted therapy, both clinically used and tested in the treatment of BRAFV600E anaplastic thyroid cancer (ATC) and other cancers. In data pooled across parental and green fluorescent protein (GFP)-expressing versions of a genetically engineered mouse model of ATC, and a human xenograft model, 44% ± 23 lower TAM density was observed in lung tumors compared with thyroid tumors (P < .001). This was observed by ferumoxytol-enhanced MRI (hereafter, ferumoxytol MRI) in parental allografts (P = .02) and supported by confocal microscopy of macrin in optically cleared organs (P = .02). Combined BRAFi and aPDL1 shifted interstitial but not alveolar macrophage polarization, which was observed by 41% ± 8 lower CD206 expression (P = .02) and higher local TAM in pulmonary ATC tumors by 46% ± 21 (P = .01) in a manner blocked by colony stimulating factor 1 receptor (CSF1R)-blocking antibody (P = .01). Higher TAM correlated with higher accumulation of a model drug delivery nanoparticle (DDNP; r = 0.95; P < .001) similar to those used clinically, including PEGylated liposomal doxorubicin and liposomal irinotecan. Altogether, ferumoxytol MRI and macrin (which can be imaged with PET) may enable measurement of differential TAM accumulation across metastases and in response to therapy, promising to guide clinical prognosis and inform the administration of TAM-targeted drugs, including DDNP (as tested in our model) and TAM-directed therapies targeting CSF1R (12), CD47 (8), toll-like receptors (11), and others (17).
Understanding microenvironmental differences between primary and metastatic lesions, including genetic and proteomic features of tumor cells (23) and distinct immune cell composition (25), is increasingly recognized to be clinically impactful for drug delivery (26) and treatment efficacy (27). Consistent with our imaging results, lower TAM levels have also been found in metastatic lesions compared with primary tumors of a murine breast cancer model (28) and in human melanoma biopsies (29). Similar to our observations in ATC, elevated TAM levels and corresponding drug distribution in the tumor core have been noted with other models, although TAM can be prevalent at the tumor periphery in other cases, and mechanisms governing spatial TAM distribution need to be better understood (12,17,18). In this and past work (10,17), macrin demonstrated pan-macrophage specificity that may combine with other imaging agents to quantify subsets of macrophages or other markers such as PD1 and PDL1 (10). These assays complement the current mainstay of structural imaging of ATC lesions (30) and may be useful for upcoming assessment of immunomodulatory therapies in ATC.
Recent studies (3,4) show the potential of targeted and immune-checkpoint therapies in ATC. Almost all combinations tested showed higher TAM infiltration, the most pronounced of which was seen with combination BRAFi and aPDL1 therapy (4) and may be a significant contributor to their mixed outcomes (1). Clinical evidence suggests that aPD1 and aPDL1 can promote TAM accumulation in other cancer types (31). Given that BRAFi and aPDL1 combinations are used together with nanomedicines in clinical trials of ATC (NCT03181100), we sought to take advantage of the observed TAM increases to enhance nanomedicine delivery. Whereas the deleterious effects of TAM infiltration could conceivably be countered with systemic TAM depletion, this approach may face associated systemic toxicities (32). Thus, harnessing TAM infiltration to deliver nanomedicines offers an intriguing synergistic strategy to complement current immunomodulatory treatments.
Our study had limitations. Tumors were generated from the intrathyroid or intravenous cell injection, which can form differently than spontaneous models. However, past comparison of autochthonous and orthotopic lung tumors indicated comparable TAM behavior (33). Xenografts were examined in severe combined immunodeficient hosts, which can be useful for studying TAM (3) but insufficiently model response to aPDL1 and other therapies requiring T-cell activity for efficacy. Therefore, future studies may investigate humanized patient-derived xenograft models with reconstituted immune systems, or ultimately in patients. Heterogeneity across lesions is a challenge both in statistically separating mouse-to-mouse (or patient-to-patient) variability from tumor-to-tumor variability (eg, Fig 4, C, vs Fig E11 [online]) and in guiding clinical decisions. Encouragingly, past ferumoxytol MRI suggested that variance across patients outweighs variance across lesions within patients (34).
In summary, optical and noninvasive molecular imaging revealed that pulmonary tumors harbor lower levels of tumor-associated macrophage (TAM) compared with orthotopic thyroid lesions in an immunocompetent model of anaplastic thyroid cancer (ATC). Combination anti–programmed death ligand 1 immune checkpoint blockade and serine/threonine-protein kinase B-Raf inhibitor caused localized TAM increase, especially in pulmonary lesions. The higher TAM uptake was co-opted for improved nanomedicine delivery and presents a potential synergistic treatment strategy in ATC. Further studies should determine whether these context-dependent and treatment-related differences are observed in patients. Furthermore, mechanisms of TAM accumulation, for instance building on our evidence for the role of colony stimulating factor 1 receptor, and the design of therapies to maximize nanomedicine delivery should be explored. The molecular imaging tools we developed offer clinically translatable companion diagnostic imaging assays to evaluate such strategies.
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments:
We thank Michelle Garlin and Alexandra Dibrindisi and the staff at the Center for Molecular Imaging and Research for their technical assistance.
Study supported by NIH/NCI (grants R00CA207744, U01CA206997, R01CA206890, T32CA079443) and the American Thyroid Association, and the Thyroid Cancer Survivors’ Association; T.S.C.N. supported by a Radiological Society of North America R&E Foundation Research Resident Grant.
Disclosures of Conflicts of Interest: T.S.C.N. disclosed no relevant relationships. V.G. disclosed no relevant relationships. R.L. disclosed no relevant relationships. M.P. disclosed no relevant relationships. Y.I. disclosed no relevant relationships. R.H.K. disclosed no relevant relationships. S.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for board membership from More Health; disclosed money paid to author for consultancy from 2nd MD, Teledoc; money paid to author for expert testimony from Newton Wellesely Hospital; disclosed payment for royalties from UpToDate; and stock options from Novartis. Other relationships: disclosed no relevant relationships. R.W. disclosed no relevant relationships. M.A.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed antibodies via material transfer agreement from Genentech/Roche; disclosed patents pending for Massachusetts General Hospital; disclosed patents issued for Massachusetts Institute of Technology. Other relationships: disclosed no relevant relationships.
Abbreviations:
- aPDL1
- anti-PDL1 antibody
- ATC
- anaplastic thyroid cancer
- BRAF
- serine/threonine-protein kinase B-Raf
- BRAFi
- BRAF inhibitor
- CSF1R
- colony stimulating factor 1 receptor
- DDNP
- drug delivery nanoparticle
- GFP
- green fluorescent protein
- PBS
- phosphate-buffered saline
- PDL1
- programmed death ligand 1
- TAM
- tumor-associated macrophage
References
- 1.Rao SN, Zafereo M, Dadu R, et al. Patterns of Treatment Failure in Anaplastic Thyroid Carcinoma. Thyroid 2017;27(5):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang NS, Shi X, Lei BW, et al. An Update of the Appropriate Treatment Strategies in Anaplastic Thyroid Cancer: A Population-Based Study of 735 Patients. Int J Endocrinol 2019;2019:8428547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brauner E, Gunda V, Vanden Borre P, et al. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 2016;7(13):17194–17211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunda V, Gigliotti B, Ndishabandi D, et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br J Cancer 2018;119(10):1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanden Borre P, Gunda V, McFadden DG, et al. Combined BRAF(V600E)- and SRC-inhibition induces apoptosis, evokes an immune response and reduces tumor growth in an immunocompetent orthotopic mouse model of anaplastic thyroid cancer. Oncotarget 2014;5(12):3996–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer 2008;15(4):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schürch CM, Roelli MA, Forster S, et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 2019;29(7):979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SJ, Li R, Ng TSC, et al. Efficient blockade of locally reciprocated tumor-macrophage signaling using a TAM-avid nanotherapy. Sci Adv 2020;6(21):eaaz8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arlauckas SP, Garris CS, Kohler RH, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 2017;9(389):eaal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodell CB, Arlauckas SP, Cuccarese MF, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng 2018;2(8):578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuccarese MF, Dubach JM, Pfirschke C, et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun 2017;8(1):14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller MA, Zheng YR, Gadde S, et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun 2015;6(1):8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MA, Chandra R, Cuccarese MF, et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci Transl Med 2017;9(392):eaal0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Gunda V, Zhu X, et al. Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer. Proc Natl Acad Sci U S A 2016;113(28):7750–7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MA, Gadde S, Pfirschke C, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med 2015;7(314):314ra183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HY, Li R, Ng TSC, et al. Quantitative Imaging of Tumor-Associated Macrophages and Their Response to Therapy Using 64Cu-Labeled Macrin. ACS Nano 2018;12(12):12015–12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MA, Arlauckas S, Weissleder R. Prediction of Anti-cancer Nanotherapy Efficacy by Imaging. Nanotheranostics 2017;1(3):296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghighi M, Theruvath AJ, Pareek A, et al. Magnetic Resonance Imaging of Tumor-Associated Macrophages: Clinical Translation. Clin Cancer Res 2018;24(17):4110–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullman-Culleré MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 1999;49(3):319–323. [PubMed] [Google Scholar]
- 21.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes SR, Ng TS, Santa-Maria N, Montagne A, Zlokovic BV, Jacobs RE. ROCKETSHIP: a flexible and modular software tool for the planning, processing and analysis of dynamic MRI studies. BMC Med Imaging 2015;15(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin K, He K, Teng F, et al. Heterogeneity in primary tumors and corresponding metastases: could it provide us with any hints to personalize cancer therapy? Per Med 2011;8(2):175–182. [DOI] [PubMed] [Google Scholar]
- 24.Riaz N, Havel JJ, Makarov V, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 2017;171(4):934–949.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motoshima T, Miura Y, Wakigami N, et al. Phenotypical change of tumor-associated macrophages in metastatic lesions of clear cell renal cell carcinoma. Med Mol Morphol 2018;51(1):57–63. [DOI] [PubMed] [Google Scholar]
- 26.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev 2015;91:3–6. [DOI] [PubMed] [Google Scholar]
- 27.Takebayashi K, Mekata E, Sonoda H, et al. Differences in chemosensitivity between primary and metastatic tumors in colorectal cancer. PLoS One 2013;8(8):e73215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makela AV, Foster PJ. Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magn Reson Med 2018;80(3):1138–1147. [DOI] [PubMed] [Google Scholar]
- 29.Bröcker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer 1988;41(4):562–567. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed S, Ghazarian MP, Cabanillas ME, et al. Imaging of Anaplastic Thyroid Carcinoma. AJNR Am J Neuroradiol 2018;39(3):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma. Clin Cancer Res 2017;23(17):5024–5033. [DOI] [PubMed] [Google Scholar]
- 32.Kumar V, Donthireddy L, Marvel D, et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017;32(5):654–668.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016;44(2):343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramanathan RK, Korn RL, Raghunand N, et al. Correlation between Ferumoxytol Uptake in Tumor Lesions by MRI and Response to Nanoliposomal Irinotecan in Patients with Advanced Solid Tumors: A Pilot Study. Clin Cancer Res 2017;23(14):3638–3648. [DOI] [PubMed] [Google Scholar]
- 35.Marwick B, Krishnamoorthy K. cvequality: Tests for the Equality of Coefficients of Variation from Multiple Groups. https://github.com/benmarwick/cvequality. Accessed October 6, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Immunologic assessment of ferumoxytol and macrin cellular uptake. Lungs bearing anaplastic thyroid cancer (ATC) tumors, by using the TBP-3743 model, were excised 24 hours after administration with ferumoxytol and macrin. Within tumors, arrows mark colocalization in cellular staining of, A, iron oxide (by using Prussian blue) and F4/80, and, B, macrin and F4/80. C, Uptake of macrin on a per-cell basis was quantified by flow cytometry, after immunologic definition of cell populations from tumor-bearing lung tissue (Fig E2 [online] shows gating scheme). DAPI = 4’,6-diamidino-2-phenylindole, frac. max = fraction maximum, MΦ = macrophages.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/8adb/7771993/a4ccd4563936/radiol.2020201791.fig2.jpg)
![Quantification of how combination serine/threonine-protein kinase B-Raf inhibitor (BRAFi) and anti–programmed death ligand 1 (aPDL1) affects tumor-associated macrophage (TAM) accumulation and nanomedicine delivery. A, Representative confocal microscopy of optically cleared tumors reveals macrin accumulation at 24 hours after administration, following an 8-day course of treatment with combination BRAFi and aPDL1 in the murine TBP model of anaplastic thyroid cancer (ATC; scale bar, 5 mm). B, Corresponding to A, the scatter dot plot shows TAM density quantified across single cells from microscopy data by using macrin to identify TAM. Bonferroni correction applied for multiple hypothesis testing (n = 2 hypotheses, corresponding to lung and thyroid, comparing with or without combination BRAFi and aPDL1 treatment). C, Graph shows macrin and drug delivery nanoparticle (DDNP) uptake correlated across treatment groups on a tumor-by-tumor basis (see Fig E11 [online] for individual tumor-by-tumor correlation within individual subjects). D, Scatter dot plots show DDNP uptake quantified by confocal microscopy in TAM (macrin+) and other (macrin-) cells by using the TBP model as a fraction of average uptake in macrin+ cells. See Figure E9 (online) for comparison across drug treatments. For B and D, data are means ± standard deviation.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/8adb/7771993/2b68991a52a2/radiol.2020201791.fig4.jpg)

![The influence of anti-colony stimulating factor 1 antibody treatment (aCSF1R) on tumor-associated macrophage (TAM) levels following combination threonine-protein kinase B-Raf inhibitor (BRAFi) and anti–programmed death ligand 1 (aPDL1) treatment. By using the TBP model, lungs bearing TBP-3743 tumors were excised 24 hours after treatment with macrin and drug delivery nanoparticle (DDNP), which were co-injected intravenously after an 8-day treatment course of aPDL1, BRAFi, and CSF1R-blocking antibody, as defined in Figure E1 (online). A, Scatter dot plot shows macrophage abundance quantified by flow cytometry of tumor-bearing lungs; the two macrophage populations were normalized to their abundances in the control group (see Fig E2 [online] for gating schemes and absolute abundance). B, Plot shows expression of CD206 quantified in interstitial macrophages by flow cytometry. C, As in Figure 5, confocal microscopy of cleared tumor-bearing lungs quantified TAM in tumor cores relative to edges. D, Scatterplot shows total TAM levels in tumors correlated with total DDNP uptake, shown as average values across tumors in individual mice. A–C, Bonferroni correction applied. Data are means ± standard deviation.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/8adb/7771993/ff67ac7e1d48/radiol.2020201791.fig6.jpg)