Abstract
Background
There are limited data on the impact of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on people with multiple sclerosis (MS).
Objective
To better understand SARS-CoV-2 infection in ocrelizumab-treated people with MS.
Methods
Internal Roche/Genentech data sources: Cases of COVID-19 from ongoing Roche/Genentech clinical trials and from post-marketing use of ocrelizumab until July 31, 2020 were identified and assessed using descriptive statistics.
External real-world data (RWD) source: An MS COVID-19 cohort and an ocrelizumab-treated MS COVID-19 cohort were identified and assessed from the OPTUMⓇ de-identified COVID-19 electronic health record (EHR) database.
Results
Roche/Genentech clinical trial data: There were 51 (1.3%) suspected or confirmed cases of COVID-19 identified from 4,000 patients ongoing in 10 Roche/Genentech clinical trials. Of these, 26 (51%) were confirmed COVID-19 and 25 (49%) were suspected COVID-19. Sixteen (31.4%) patients were hospitalized. COVID-19 severity was mild to moderate in most patients (35, 68.6%). Ten (19.6%) patients had severe disease and there were three (5.9%) fatal cases. Most patients (43, 84.3%) recovered or were recovering. There was no association apparent between duration of exposure to ocrelizumab and COVID-19. Among COVID-19 patients with previous serum immunoglobulin status (27/51, 52.9%), all (27/27, 100%) had IgG levels within the normal range.
Roche/Genentech post-marketing safety database data: There were 307 post-marketing cases of COVID-19 in the Roche/Genentech global safety database. Of these, 263 (85.7%) were confirmed and 44 (14.3%) were suspected COVID-19. 100 (32.6%) patients were hospitalized. COVID-19 was asymptomatic, mild or moderate in 143 (46.6%) patients, severe in 52 (16.9%) patients, and critical in 15 (4.9%) patients. There were 17 (5.5%) fatal cases. Information on severity was not reported in 80 (26.1%) cases. Most patients (211, 68.7%) recovered or were recovering at the time of the report.
External RWD data source: As of July 13, 2020, the OPTUMⓇ database included EHRs for almost 1.2 million patients with suspected COVID-19, 130,500 of whom met the criteria for confirmed/clinically diagnosed COVID-19. A total of 357 patients with MS with confirmed COVID-19 were identified. Forty-eight (13.4%) were treated with ocrelizumab, of whom 12 (25.0%) were hospitalized and one died (2.1%). Similar rates of hospitalization, invasive ventilation, and death were observed in the ocrelizumab-treated and non-ocrelizumab-treated MS cohorts.
Across the Roche/Genentech and RWD sources assessed, age, male sex, and the presence of comorbidities such as hypertension were associated with a more severe disease course of COVID-19. There was a higher number of comorbidities present in hospitalized versus non-hospitalized patients.
Conclusions
This assessment provides evidence that COVID-19 in ocrelizumab-treated people with MS is predominantly mild to moderate in severity with most patients not requiring hospitalization; in line with data reported from the general population and MS datasets. Risk factors known to be associated with severe COVID-19 outcomes in the general population also appear to influence COVID-19 severity in ocrelizumab-treated people with MS. Case fatality rates for ocrelizumab-treated people with MS were within published ranges for the general population and other MS cohorts.
Keywords: COVID-19, multiple sclerosis, ocrelizumab, disease-modifying therapy, anti-CD20
Abbreviations: AE, adverse event; BMI, body mass index; CCI, Charlson Comorbidity Index; CCOD, clinical cut-off date; CI, confidence interval; COVID-19, coronavirus disease 2019; CTCAE, Common Terminology Criteria for Adverse Events; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EHR, electronic health record; Ig, immunoglobulin; MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis; RMS, relapsing multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; RT-PCR, real-time polymerase chain reaction; RWD, real-world data; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPMS, secondary progressive multiple sclerosis; WHO, World Health Organization
1. Introduction
Ocrelizumab is a humanized anti-CD20, B cell-depleting, monoclonal antibody approved for the treatment of relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). As of July 31, 2020, an estimated 174,508 people with MS worldwide have been treated with ocrelizumab, including approximately 167,684 in the commercial post-marketing setting, and 6,824 in clinical trials resulting in an exposure of 249,971 patient-years (Roche, data on file, 2020). Although ocrelizumab is associated with an increased risk of certain non-serious infections, including upper respiratory tract infections, no increased risk of serious infections was observed with ocrelizumab treatment in the controlled period of the pivotal clinical trials versus comparators (interferon beta-1a or placebo; Hauser et al., 2017; Montalban et al., 2017). Over a period of approximately seven years in the open-label extensions of these studies, a low and generally stable rate of serious infections, with some year-on-year variation, has been observed (Hauser et al., 2020a; Hauser et al., 2020b).
It is estimated that about one in five individuals worldwide could be at increased risk of severe coronavirus 2019 (COVID-19) (Clark et al., 2020). In the general population, risk factors associated with severe COVID-19 include older age, male sex, and comorbidities such as obesity, diabetes, hypertension, coronary heart disease, chronic pulmonary or kidney disease, and cancer (CDC, 2020a; ISARIC, 2020; Williamson et al., 2020). Additionally, it has been suggested that immunocompromised patients (Fung & Babik, 2020) or patients receiving immunosuppressive treatments (D'Antiga, 2020) may be at increased risk. For patients with MS, it is currently debated whether they are at an increased risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Ghajarzadeh & Bonavita, 2020) or of developing a more severe course of COVID-19 compared with the general population and whether the different disease-modifying therapies (DMTs) play a specific role (Amor et al., 2020; Giovannoni, 2020; Giovannoni et al., 2020). Multiple expert recommendations on the use of DMTs in MS have been published in the context of the COVID-19 pandemic (Brownlee et al., 2020; Thakolwiboon et al., 2020). However, given the limited data available, neurologists are still faced with important challenges in the management of people with MS in the era of COVID-19, and high-quality evidence is needed to better understand the effect of COVID-19 in people with MS (Peeters et al., 2020). Due to this uncertainty around the potential risks that SARS-CoV-2 may pose for people with MS who are receiving immunotherapy, it was recommended that people with MS should not discontinue their DMT during the COVID-19 pandemic without seeking advice from their neurologist (Korsukewitz, 2020).
To better understand COVID-19 in ocrelizumab-treated people with MS, we present the most complete dataset available to date on ocrelizumab-treated people with MS with confirmed or suspected COVID-19 reported in Roche/Genentech clinical trials and in post-marketing pharmacovigilance data, as of July 31, 2020. We also analyzed COVID-19 data from an EHR database in the USA and then discuss our findings in the context of information in the public domain regarding COVID-19 in the general population, people with MS, and ocrelizumab-treated people with MS.
When we wrote our initial report on a pharmacovigilance case series of COVID-19 in people with MS treated with ocrelizumab (April 30, 2020 data cut-off [Hughes et al., 2020]), there were over 3.5 million confirmed COVID-19 cases (243,401 fatal) reported worldwide (World Health Organization 2020a). As we write this update, the figure of confirmed COVID-19 cases now stands at over 38 million (1 million fatal) (World Health Organization 2020b).
2. Methodology
Clinical trial and spontaneous post-marketing case analysis
Suspected or confirmed COVID-19 cases were identified from ongoing Roche/Genentech clinical trials and from spontaneous post-marketing case reports of COVID-related events recorded in the Roche/Genentech global safety database. The validity and seriousness of cases were assessed according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (European Medicines Agency ICH Harmonised Tripartite Guideline E2A, 1995), including a check for duplicate cases.
Clinical trial and post-marketing cases were assessed as confirmed COVID-19 if they had a laboratory confirmation using either a positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) test or a serological test. Post-marketing cases were also conservatively assessed as confirmed if radiological evidence consistent with COVID-19 pneumonia (e.g. ground-glass opacities) was reported or if the cases were described by the reporter as confirmed COVID-19. Cases were considered suspected if only signs or symptoms consistent with COVID-19 (European Centre for Disease Prevention and Control, 2020) were present and SARS-CoV-2 infection was suspected by the reporter.
In the absence of an agreed COVID-19 severity classification (Louapre et al., 2020; National Institutes of Health, 2020; Siddiqi & Mehra, 2020; World Health Organization, 2020c; Wu & McGoogan, 2020), we adopted the following approach:
For clinical trial cases, the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE [v5.0]) grading system was used, which categorizes cases into mild, moderate, severe, life-threatening, or fatal (U.S. Department of Health and Human Services NIH, National Cancer Institute, 2017). Due to reporting heterogeneity, for post-marketing reports, severity categories were assigned based on information provided, using the following classification (Hughes et al., 2020): asymptomatic if it was explicitly stated that no symptoms were present, mild if non-hospitalized symptoms such as low-grade fever or cough were described, moderate if shortness of breath was reported, severe if pneumonia was present, and critical if requiring intensive care and/or mechanical ventilation.
Outcome for clinical trial cases was captured as recovered/recovering, recovered/recovering with sequelae, not recovered/not resolved or fatal. For post-marketing cases, outcome was classified as recovered, recovering (e.g. “doing well at home”, or “improving”), not recovered at the time of the report, or fatal.
If no information was provided for a given parameter, this was noted as “missing” for clinical trial cases and “not reported” for post-marketing cases.
Real-world data study
The OPTUMⓇ de-identified COVID-19 EHR database contains patients assessed for suspected COVID-19, sourced from patient-level medical and administrative records from hospitals, emergency departments, outpatient centers, and laboratories from across the USA. Confirmed or clinically diagnosed patients are identified from this dataset via the presence of COVID-19 or coronavirus diagnosis codes, or a positive diagnostic test (Rizzo et al., 2020). To identify an MS cohort, ≥3 MS-related encounters from any combination of inpatient, outpatient or DMT use within one year of COVID-19 diagnosis was required and at least one outpatient MS diagnosis code (Culpepper et al., 2019; Earla et al., 2020). Inclusion in the ocrelizumab cohort was defined by an ocrelizumab infusion within six months prior to COVID-19 diagnosis, and all ocrelizumab-treated patients were also considered eligible for the MS cohort.
COVID-19 outcome was categorized using an abbreviated version of the ordinal scale recommended by the WHO (World Health Organization 2020d): not hospitalized; hospitalized (not requiring invasive mechanical ventilation); hospitalized (requiring invasive mechanical ventilation); death. Discharge status of hospitalized patients was also captured. The Charlson Comorbidity Index (CCI) was evaluated to describe the comorbidity burden (Glasheen et al., 2019).
3. Results
Table 1 shows a summary of data from the clinical trial, post-marketing, and RWD study cohorts of ocrelizumab-treated people with MS. Full details for each data source are presented separately in the Appendix (Tables A.1, A.2, A.3).
Table 1.
Demographic data and COVID-19 severity and outcomes in clinical trial, post-marketing, and RWD study cohorts of ocrelizumab-treated people with MS.
| Clinical trials | Post-marketing | RWD study⁎ | |
|---|---|---|---|
| COVID-19 cases, n (%) | |||
| n | 51 | 307 | 48 |
| Confirmed | 26 (51) | 263 (85.7) | 48 (100) |
| Suspected | 25 (49) | 44 (14.3) | - |
| Age, years | |||
| n | 51 | 229 | 48 |
| Mean | 40.7 | 45.0 | 48.5 |
| Range | 19–62 | 16–84 | 19–73 |
| Gender, n (%) | |||
| n | 51 | 307 | 48 |
| Female | 34 (66.7) | 171 (55.7) | 33 (68.8) |
| Male | 17 (33.3) | 94 (30.6) | 15 (31.2) |
| Not reported | - | 42 (13.7) | - |
| Type of MS, n (%) | |||
| n | 51 | 307 | - |
| RMS/RRMS | 38 (74.5) | 128 (41.7) | - |
| PPMS/SPMS | 13 (25.5) | 47 (15.3) | - |
| Not reported | 0 | 132 (43.0) | - |
| Seriousness†, n (%) | |||
| Serious | 16 (31.4) | 131 (42.7) | |
| COVID-19 disease severity, n (%) | |||
| n | 51 | 307 | 48 |
| Asymptomatic/mild/moderate | 35 (68.6) | 143 (46.6) | - |
| Severe | 10 (19.6) | 52 (16.9) | - |
| Critical/Life-threatening | 0 | 15 (4.9) | - |
| Fatal | 3 (5.9) | 17 (5.5) | 1 (2.1) |
| Not reported/missing | 3 (5.9) # | 80 (26.1) | 47 (97.9) |
| Hospitalization | |||
| Hospitalized, n (%) | 16 (31.4) | 100 (32.6) | 12 (25) |
| Mean age of hospitalized patients, years | 42.0 | 50.1 | 55.1 |
| COVID-19 disease outcome, n (%) | |||
| n | 51 | 307 | 48 |
| Recovered/Recovering | 43 (84.3) | 211 (68.7) | - |
| Not recovered | 2 (3.9) | 19 (6.2) | - |
| Died | 3 (5.9) | 17 (5.5) | 1 (2.1) |
| Not reported/missing | 3 (5.9) § | 60 (19.5) | - |
COVID-19, coronavirus 2019; ICH, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis; RMS, relapsing multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; RWD, real-world data; SPMS, secondary progressive multiple sclerosis.
RWD study cases could not be identified at the patient level, so deduplication was not possible. These numbers should not be combined with clinical trials and post-marketing.
The validity and seriousness of cases were assessed according to ICH guidelines (European Medicines Agency ICH Harmonised Tripartite Guideline E2A, 1995); ICH seriousness criteria, in the context of COVID-19, include events that: result in death, are life-threatening, require inpatient hospitalization or prolongation of existing hospitalization, or result in persistent or significant disability/incapacity.
The three missing cases were manually reviewed after the data cut-off: two cases were grade 2 (moderate) and one case was grade 3 (severe).
The three missing cases were manually reviewed after the data cut-off: two cases had recovered/resolved, and one case had not resolved but patient had been discharged from hospital.
COVID-19 was reported in 51 out of 4,000 (1.3%) ocrelizumab-treated patients across 10 clinical trials. Twenty-six (51%) of these cases were confirmed and 25 (49%) were suspected.
In the global safety database, 307 post-marketing cases reporting COVID-19-related events were recorded. Based on an estimated total global exposure to ocrelizumab to July 31, 2020 of 167,684, an incidence rate of 0.18% could be proposed; however, the limitations of post-marketing data affect the ability to define a relevant numerator and a relevant denominator and make such an incidence rate unreliable. Of the 307 cases, 263 (85.7%) were confirmed COVID-19 cases and 44 (14.3%) were suspected.
The OPTUMⓇ database included records for almost 1.2 million patients with suspected COVID-19 in the USA, 130,500 of whom met the criteria for confirmed/clinically diagnosed COVID-19. A total of 357 patients with MS were identified in this confirmed COVID-19 cohort, and of those, 48 (13.4%) patients had been treated with ocrelizumab in the previous six months. With a total exposure to ocrelizumab in the USA to July 31, 2020 of 97,502, an incidence rate of 0.05% could be proposed; however, this figure is not reliable as the OPTUMⓇ dataset does not capture all COVID-19 cases in the USA.
Demographics and MS characteristics
In the clinical trial cohort, the mean age of all COVID-19 patients was 40.7 years; it was 42.0 years in hospitalized patients and 42.8 years in the reference population of 4,000 clinical trial patients (the reference population refers to all patients ongoing in OPERA I, OPERA II, ORATORIO, Phase II, LIBERTO, CONSONANCE, ENSEMBLE, VELOCE at the clinical cut-off date of January 3, 2020; June 19, 2020 for ORATORIO-HAND and July 13, 2020 for OCARINA). In the post-marketing cohort, the mean age was 45.0 years in all cases (age known in 74.6% of cases), while it was 50.1 years in hospitalized patients. In the RWD study, the mean age of COVID-19 cases in the overall ocrelizumab cohort was 48.5 years while it was 55.1 years in the hospitalized ocrelizumab-treated patients.
In the clinical trial cohort, the proportion of male patients with COVID-19 was 33.3% (n=17); for hospitalized patients the proportion was 50.0% (n=8). In the post-marketing cohort, males accounted for 30.6% (n=94) of all cases and 39% (n=39) of hospitalized cases. In the RWD study, the proportion of males among ocrelizumab-treated patients with COVID-19 was 31.2% (n=15).
Relapsing forms of MS were more common than progressive forms (74.5% vs 25.5%, respectively) in the clinical trial cohort and these proportions were similar in hospitalized clinical trial patients (75.0% vs 25.0%, respectively). In the post-marketing cohort, relapsing forms of MS were also more common than progressive forms (41.7% vs 15.3%, respectively). Type of MS was not reported in 132 (43.0%) cases. In hospitalized patients, the proportion of patients with relapsing forms of MS was similar (43.0%) to the post-marketing cohort but the proportion with progressive forms of MS was higher (27.0%). MS type was not reported in 30 (30.0%) cases. Information on MS type was not available in the RWD study.
Clinical severity, hospitalization, and outcomes
The majority of cases (n=35, 68.6%) in the clinical trial cohort had a mild or moderate presentation, with 10 (19.6%) being classified as severe. Information on severity was initially missing for three (5.9%) patients but, on follow-up, two of these patients had moderate disease severity and one had severe disease. In the post-marketing cohort, among cases for which severity was reported (n=227, 73.9%), COVID-19 was asymptomatic, mild, or moderate in 143 (63.0%) cases, severe in 52 (22.9%) cases, and critical in 15 (6.6%) cases.
Hospitalization was reported for 16 (31.4%) clinical trial cases and 100 (32.6%) post-marketing cases.
Most patients in the clinical trial cohort had recovered or were recovering (n=43, 84.3%), two (3.9%) had not recovered, and in three (5.9%) cases the event had a fatal outcome. Outcome was not initially reported in three (5.9%) cases but, on follow-up, two of the events had resolved and in the third case the event had not resolved but the patient had been discharged from hospital. In the post-marketing cohort, the majority of patients had recovered or were recovering (n=211, 68.7%), 19 (6.2%) patients had not recovered at the time of the report, and 17 (5.5%) patients had died. Outcome was not reported in 60 (19.5%) cases. Case fatality rate in hospitalized patients was 18.8% (3/16) and 11.0% (11/100) in the clinical trials and post-marketing settings, respectively. Details of all fatal cases can be found in the Appendix, Tables A.4 and A.5.
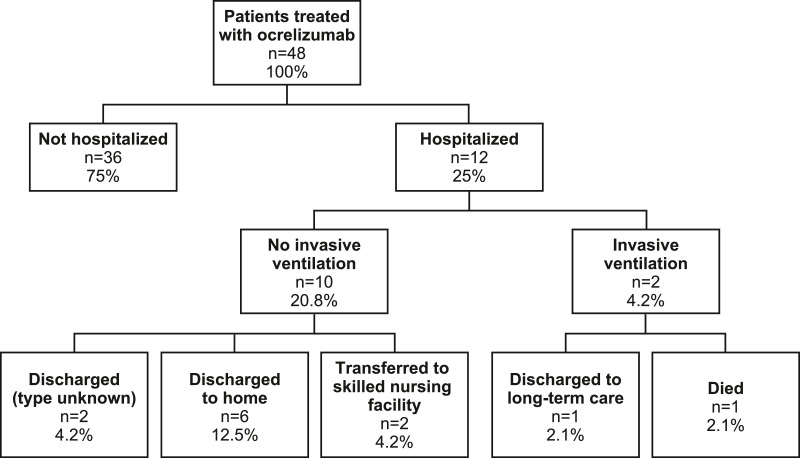
In the RWD study, rates of hospitalization, invasive ventilation, and death were similar between the ocrelizumab-treated and non-ocrelizumab-treated people with MS ( Fig. 1 ). Mortality rate in ocrelizumab-treated people with MS was 2.1% compared with a mortality rate of 3.9% in non-ocrelizumab-treated patients with MS, and 8.3% and 16.0%, respectively, for hospitalized patients. This is an unadjusted comparison and differences in the age and comorbidities of the two cohorts exist ( Table A.3 ). Discharge status for the ocrelizumab-treated cohort is shown in Figure 2 .
Fig. 1.
Rates of hospitalization, invasive ventilation, and death in patients with MS in the RWD study.
Fig. 2.
Discharge disposition at end of COVID-19 hospital stay for patients treated with ocrelizumab in the RWD study.
Comorbidities and risk factors
In clinical trials, the proportion of patients in the COVID-19 cohort with comorbidities (51%) was higher than in the reference population (35.0%). The proportion was even higher in hospitalized COVID-19 patients (56.3%). Underlying respiratory comorbidities (e.g. asthma) were reported in 43.8 % of hospitalized cases, 31.4% of all COVID-19 cases, and in 11.6% of patients in the reference population.
Information on risk factors associated with severe COVID-19 was available for 134/307 post-marketing cases. In 85.8% (n=115) of these cases at least one of the following risk factors was reported: age >50 years (n=82, 61.2%), obesity (n=28, 20.9%), hypertension (n=27, 20.1%), chronic pulmonary disease (n=13, 9.7%), diabetes (n=10, 7.5%), cancer (n=3, 2.2%), coronary heart disease (n=1, 0.7%), and chronic kidney disease (n=1, 0.7%). A higher number of risk factors appeared to be associated with greater disease severity ( Fig. 3 ).
Fig. 3.
COVID severity according to number of risk factors in post-marketing cases.
In the RWD study, MS patients hospitalized for COVID-19 were on average older, proportionally more likely to be male, and had more underlying comorbidities reflected in a higher median CCI and a higher prevalence of many individual comorbidities associated with more severe COVID-19 ( Table A.3 .).
Age
Within the 51 cases with suspected/confirmed COVID-19 in clinical trials, the proportion hospitalized tended to be higher in the age group above 50 years ( Table A.1 ). For those individuals in the post-marketing cohort for whom age was reported (n=229, 74.6%), older age was associated with an increased risk of severe COVID-19. In those aged <50 years (n=142, 46.3%), the majority (n=84, 59.2%) experienced asymptomatic, mild, or moderate disease, whereas 34 (23.9%) experienced severe, critical, or fatal disease. In those aged ≥50 years (n=87, 28.3%), a lesser proportion of patients (n=30, 34.5%) experienced asymptomatic, mild, or moderate disease, and a greater proportion (n=38, 43.7%) experienced severe, critical, or fatal disease than those in the <50-years age group. This pattern of increasing severity remained evident across age groups by decade ( Fig. 4 ).
Fig. 4.
Severity of COVID-19 according to age in patients treated with ocrelizumab in the post-marketing setting.
Expanded Disability Status Scale (EDSS)
In clinical trials, the proportion of patients with EDSS >3.0 was higher for hospitalized patients (43.8%) than for all COVID-19 patients (33.3%). For post-marketing cases, despite the extent of missing EDSS data (n=265, 86.3%), a positive trend for more severe outcomes with higher EDSS was noted ( Table A.6 ). EDSS was not available in the RWD study, but among hospitalized patients the proportion of patients with reported hemiplegia/paraplegia was higher (26.4%) compared with non-hospitalized patients (10.4%) ( Table A.3 ).
Serum immunoglobulin (Ig) levels
Total serum Ig measurements were collected up to January 3, 2020, and were available for 1,883/4,000 (47.2%) patients in the reference clinical trial population; IgG levels were below the lower limit of normal (LLN) (5.65 g/L) in 138/1,883 (7.3%) of these patients, none of whom reported COVID-19. Among COVID-19 patients with previous serum Ig status (27/51, 52.9%) in clinical trials, all (27/27, 100%) had IgG levels within the normal range. With regards to IgA and IgM, 7.4% (2/27) and 48.1% (13/27), respectively, had levels below LLN, compared with 6.4% (120/1,883) and 35.7% (671/1,880) in the reference clinical trial population. Measurements were taken at a median of 29 weeks before COVID-19 infection. At the time of reporting, there were no measurements available during or after COVID-19 infection.
Duration of exposure to ocrelizumab
In clinical trials there was a maximum duration of exposure of eleven years and six months. No association between duration of exposure to ocrelizumab and rates of COVID-19 was apparent. Rates of hospitalization were also not associated with time on treatment with ocrelizumab (Fig. 5 ).
Fig. 5.
Rates of COVID-19 and hospitalization in clinical trials relative to the duration of ocrelizumab exposure by 3-year periods.
Studies: OPERA I, OPERA II, ORATORIO, Phase II, LIBERTO, CONSONANCE, ENSEMBLE, VELOCE, ORATORIO-HAND, OCARINA. CCOD for OPERA I, OPERA II, ORATORIO, Phase II, LIBERTO, CONSONANCE, ENSEMBLE, VELOCE: January 3, 2020; ORATORIO-HAND: June 19, 2020; OCARINA: July 13, 2020.
CCOD, clinical cut-off date; CI, confidence interval; COVID-19, coronavirus 2019.
The maximum duration of exposure in the post-marketing period was three years and four months (ocrelizumab was launched on March 28, 2017). Information on duration of exposure and COVID-19 severity was reported in approximately half of post-marketing cases (n=151, 49.2%); however, the information on ocrelizumab start date and onset of COVID-19 symptoms was frequently imprecise (e.g. “Ocrelizumab therapy started in 2018” or “COVID-19 symptoms began in March or April 2020”), precluding meaningful assessment of the data.
4. Discussion
Evidence regarding COVID-19 in people with MS from clinical trials is scarce, so data from all possible sources should be considered. This report derived its findings from numerous data sources, including ongoing ocrelizumab clinical trials, post-marketing safety reports and OPTUMⓇ, a RWD set. The results show that where severity was reported, the majority of SARS-CoV-2 infections resulted in mild-to-moderate disease, which did not require hospitalization, and in most cases, infections had either resolved or were resolving. No association between duration of exposure to ocrelizumab and rates of COVID-19 was observed. All COVID-19 cases with available Ig status had an IgG level within the normal range.
As expected from known risk factors for a more severe course of COVID-19 in the general population and in cohorts of people with MS (older age, disability status, male, and comorbidities) (CDC, 2020a; ISARIC, 2020; Parrotta et al., 2020; Williamson et al., 2020), ocrelizumab-treated people with MS with known risk factors appeared to experience a more severe disease course. Hospitalization was required in 25–33% of cases in the clinical trials, post-marketing safety reports and in the OPTUMⓇ RWD study, which is relatively consistent with the rates reported for patients receiving ocrelizumab in other MS cohorts (Loonstra et al., 2020; Louapre et al., 2020). However, it should be noted that approximately one-quarter of the hospitalized cases in clinical trials had only moderate COVID-19. This suggests a potential referral bias likely driven by the general concern at the start of the pandemic and reflected in several guidelines, that patients on immunosuppressive/depleting drugs were at potential risk for severe disease and ocrelizumab infusions should be delayed (Brownlee et al., 2020; Giovannoni et al., 2020; Hamdy et al., 2020).
Overall, the case fatality rates in ocrelizumab-treated people with MS were similar to other data sources for people with MS, ranging from 1.6% to 4.9% (COViMS Registry, 2020; Loonstra et al., 2020; Louapre et al., 2020; Sormani et al., 2020). Where ocrelizumab-specific case fatality rates were presented, this ranged from 0.0% to 5.2% (COViMS Registry, 2020; Loonstra et al., 2020; Louapre et al., 2020; Sormani et al., 2020). In the post-marketing cohort, fatal cases occurred mainly in patients with underlying risk factors and one of the three fatal cases from clinical trials occurred in a 60-year-old male with multiple risk factors. The other two fatal clinical trial cases occurred in patients in their 20s. Whilst it is much less common for COVID-19 to be associated with a fatal outcome in patients of this age, both cases were reported in Mexico, and although it is not possible to exclude a potential contribution of ocrelizumab, it should be noted that Mexico has been very badly affected by the pandemic and has experienced a higher case fatality rate (Cortés-Tellés et al., 2020; Johns Hopkins University, 2020) and a higher proportion of deaths in younger patients than has been seen in Europe or the US (Ioannidis et al., 2020).
As population mortality estimates are subject to considerable variability, it is useful to consider published mortality rates in patients requiring hospitalization, which range from 9.7% to 27.6% (Chawla et al., 2020; ISARIC, 2020; Louapre et al., 2020; Richardson et al., 2020; Salje et al., 2020). The largest body of evidence to date for hospitalized patients is from the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC), which presented data for 88,463 patients (including 76,421 with recorded outcomes), of whom 52,000 patients recovered and 24,421 died (27.6% of all patients, 32.0% of patients with recorded outcomes).
Global efforts for more harmonized data collection in MS are underway (Peeters et al., 2020), but the current differences in study design, methodology, and classification of severity between reported COVID-19 case series (categories used and assignment criteria) make direct comparisons challenging. Especially for COVID-19 mortality outside of clinical trials, accurate estimates are difficult to determine and the variability of reported case fatality rates is well documented and likely related to factors including frequency of testing, demography, underlying comorbidities, and differences in definitions for confirmed disease and COVID-19-related deaths (Johns Hopkins University, 2020; World Health Organization 2020e). These COVID-specific limitations apply to this assessment, and post-marketing data and RWD are also associated with well-recognized non-COVID-specific limitations. Adverse event reporting is voluntary and post-marketing pharmacovigilance reports often provide incomplete information. There is commonly a reporting bias for more significant outcomes, which may result in an overrepresentation of more severe cases. Reporting rates can be influenced by various external factors and this may be particularly relevant in a pandemic situation. Conversely, the current strain on healthcare systems may reduce reporting or reduce the quality of reports leading to higher levels of missing data. Thus, the determination of COVID-19 prevalence, severity, and potential risks related to MS (and to the use of specific DMTs) is challenging given the impossibility to estimate the at-risk patient population as a denominator. The RWD study in this analysis employed a convenience sampling methodology of suspected COVID-19 patients, thus precluding evaluation of patients without COVID-19. Furthermore, as inclusion in the study required encounters at certain health settings, selection bias into the dataset is likely to exist whereby patients with symptoms of COVID-19 and certain comorbidities or drug treatments may be more likely to attend a health setting.
The immunology of a SARS-CoV-2 infection, the host-pathogen interaction and the course of COVID-19 are not yet fully understood (Vabret et al., 2020). The innate immune response serves as a first line of defense and is fundamental in the immunity to viruses in which T cells play an essential role (Chen & Subbarao, 2007). The adaptive humoral and cellular immune response is critical for the clearance of viruses and is a major part of the memory response that prevents reinfection. The role and interplay of the innate and adaptive immune system in response to SARS-CoV-2 is complex (Tay et al., 2020) and it has been postulated that the immunological pathways themselves are likely to contribute to disease severity and death via a state of hyperinflammation (Gustine & Jones, 2020; Mehta et al., 2020). During the COVID-19 pandemic, many neurologists have adopted a cautious approach to treating MS by delaying or stopping DMTs, due to concerns that DMTs may increase the risk of a SARS-CoV-2 infection or the severity of COVID-19. To date, most published data on COVID-19 outcomes in people with MS are based on smaller case series including patients with various DMTs (Zheng et al., 2020). In general, the emerging data seem to suggest that patients treated with DMTs are not at higher risk of contracting SARS-CoV-2 and seem to have similar risks and outcomes to the general population (Zheng et al., 2020). Risks of infection and hospitalization, and COVID-19 outcome have been reported and some studies suggested a potential increased risk in people treated with anti-CD20 therapies relative to other DMTs (Kieseier et al., 2020; Louapre et al., 2020; Simpson-Yap et al., 2020; Sormani et al., 2020, Safavi et al., 2020; Sahraian et al., 2020). Case reports of patients treated with B cell-depleting agents have, in general, shown similar COVID-19 outcomes to the general population (Ghajarzadeh et al., 2020; Iannetta et al., 2020; Meca-Lallana et al., 2020; Montero-Escribano et al., 2020; Novi et al., 2020; Suwanwongse et al., 2020; Woo et al., 2020; Wurm et al., 2020). Failure or attenuation of the development of anti-SARS-CoV-2 antibodies after COVID-19 in ocrelizumab-treated patients has been reported in the recent literature (Conte et al., 2020; Lucchini et al., 2020; Meca-Lallana et al., 2020; Thornton & Harel, 2020), but is also observed in the general population (Wu et al., 2020). This reduced humoral response was not associated with a more severe disease in most of these cases. In this context, it is interesting to note a recent report on two cases of X-linked agammaglobulinemia patients who developed pneumonia as COVID-19 manifestation, but recovered despite complete absence of B cells from peripheral blood (Soresina et al., 2020); however, it should be noted that these patients were treated with intravenous immunoglobulins, which may offer some protection against SARS-CoV-2 (Díez et al., 2020). The long-term effect of a reduced humoral response on potential reinfection and potential implications for future vaccine readiness is unclear (Baker et al., 2020a). A recent study on the effect of ocrelizumab on vaccine responses in people with MS showed that the humoral response to non-live vaccines after ocrelizumab treatment is present, albeit attenuated compared with untreated or interferon beta-treated patients, but vaccination can still be expected to be protective (Bar-Or et al., 2020; Killestein et al., 2020). In addition, the development of a protective immune response against infection involves multiple mechanisms, in which T and B cells are variably involved. Whether or how ocrelizumab interferes with such mechanisms and may affect the ability of upcoming vaccines to induce disease protection, and whether antibody production will be the appropriate or sole immune correlate of protection, remain open questions for future studies.
To better understand how immunosuppression in general and DMTs in people with MS affect susceptibility to SARS-CoV-2 infection and COVID-19 outcome, further research is required, especially regarding the potential effects of immune-modulation on the hyperinflammatory phase of the disease. It will be critical to understand whether neutralizing antibodies and/or SARS-CoV-2-specific T-cell responses are sufficient to prevent clinical disease and transmission and if the protective adaptive immune responses following natural infection (or future vaccination) are long-lasting, offering extended immunity against reinfection (Cox & Brokstad, 2020). Depending on the mode of action of a DMT for MS, this may lead to different considerations for patient management. Current risk categorizations of DMTs are mostly based on theoretical considerations around the mode of action and the biology of COVID-19 (Amor et al., 2020; Baker et al., 2020b; Giovannoni et al., 2020; Zheng et al., 2020). In future, the potential impact of each DMT on SARS-CoV-2 vaccines will need to be considered and may depend on the type of vaccine used (Zheng et al., 2020).
5. Conclusions
The present work represents the most complete analysis to date of ocrelizumab-treated people with MS with confirmed or suspected COVID-19 and combines high-quality data from clinical trials and data from a large cohort of patients in the post-marketing setting reported to the Roche/Genentech pharmacovigilance database.
Notwithstanding the limitations of the data sources, the experience of ocrelizumab-treated people with MS with COVID-19 appears in line with the reported data from the general population and MS datasets. During this pandemic, optimal management of MS must remain a key consideration for clinicians, and all treatment decisions should be taken in partnership with patients according to their individual benefit–risk profile.
CRediT authorship contribution statement
Richard Hughes: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Louise Whitley: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Kocho Fitovski: Investigation, Writing - review & editing. Hans-Martin Schneble: Data curation, Writing - review & editing. Erwan Muros: Writing - original draft, Writing - review & editing. Annette Sauter: Formal analysis, Writing - review & editing. Licinio Craveiro: Investigation, Data curation, Writing - review & editing. Paul Dillon: Data curation, Investigation, Methodology, Writing - original draft, Writing - review & editing. Ulrike Bonati: Investigation, Data curation, Writing - review & editing. Nikki Jessop: Data curation, Writing - review & editing. Rosetta Pedotti: Writing - review & editing. Harold Koendgen: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing.
Conflict of interest disclosures
R Hughes was an employee of F. Hoffmann-La Roche Ltd.
L Whitley is a Senior Partner at TranScrip Partners LLP and aconsultant to F. Hoffmann-La Roche Ltd
K Fitovski is an employee of F. Hoffmann-La Roche Ltd.
H-M Schneble is an employee of F. Hoffmann-La Roche Ltd.
E Muros is an employee of F. Hoffmann-La Roche Ltd.
A Sauter is an employee of F. Hoffmann-La Roche Ltd.
L Craveiro is an employee of F. Hoffmann-La Roche Ltd.
P Dillon is an employee of F. Hoffmann-La Roche Ltd.
U Bonati is an employee of F. Hoffmann-La Roche Ltd.
N Jessop is an employee of F. Hoffmann-La Roche Ltd.
R Pedotti is an employee of F. Hoffmann-La Roche Ltd.
H Koendgen is an employee of F. Hoffmann-La Roche Ltd.
Acknowledgments
Acknowledgments
We thank all patients, their families, and the investigators who participated in the trials. Editorial assistance for this manuscript was provided by Martha Hoque of Articulate Science, UK, and funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. The authors had full editorial control of the manuscript and provided their final approval of all content.
Funding
This work was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Appendix
Table A.1.
Patient demographics and adverse event summary in ocrelizumab-treated people with MS in clinical trials with suspected or confirmed COVID-19.
| Reference population (n=4,000) | All COVID-19 cases (n=51) | Confirmed COVID-19 cases (n=26) | Hospitalized COVID-19 cases (n=16) | |
|---|---|---|---|---|
|
Age class*, years, n (%) n 18–20 >20–40 >40–60 >60 |
4,000 34 (0.9) 1,688 (42.2) 2,066 (51.7) 212 (5.3) |
51 2 (3.9) 20 (39.2) 27 (52.9) 2 (3.9) |
26 1 (3.8) 9 (34.6) 16 (61.5) 0 |
16 1 (6.3) 5 (31.3) 10 (62.5) 0 |
|
Age class*, years, n (%) n ≤50 >50 |
4,000 2,895 (72.4) 1,105 (27.6) |
51 40 (78.4) 11 (21.6) |
26 20 (76.9) 6 (23.1) |
16 11 (68.8) 5 (31.3) |
|
Age†, years n Mean (SD) Median Range |
4,000 42.8 (11.1) 43.0 18–68 |
51 40.7 (11.3) 42.0 19–62 |
26 40.8 (11.2) 42.0 19–60 |
16 42.0 (11.6) 42.5 20–60 |
|
Gender, n (%) n Male Female |
4,000 1,600 (40.0) 2,400 (60.0) |
51 17 (33.3) 34 (66.7) |
26 12 (46.2) 14 (53.8) |
16 8 (50.0) 8 (50.0) |
|
Type of MS, n (%) n RMS/RRMS PPMS SPMS |
4,000 2,733 (68.3) 950 (23.8) 317 (7.9) |
51 38 (74.5) 10 (19.6) 3 (5.9) |
26 18 (69.2) 6 (23.1) 2 (7.7) |
16 12 (75.0) 3 (18.8) 1 (6.3) |
|
Geographical location, n (%) n Brazil Canada France Germany Great Britain Italy Mexico Netherlands Poland Russia Spain Sweden Ukraine United States of America |
4,000 63 (1.6) 209 (5.2) 244 (6.1) 146 (3.7) 117 (2.9) 375 (9.4) 139 (3.5) 74 (1.9) 450 (11.3) 107 (2.7) 145 (3.6) 30 (0.8) 207 (5.2) 674 (16.9) |
51 2 (3.9) 1 (2.0) 3 (5.9) 2 (3.9) 3 (5.9) 4 (7.8) 7 (13.7) 1 (2.0) 3 (5.9) 3 (5.9) 6 (11.8) 2 (3.9) 4 (7.8) 10 (19.6) |
26 2 (7.7) 0 1 (3.8) 2 (7.7) 1 (3.8) 2 (7.7) 5 (19.2) 1 (3.8) 1 (3.8) 2 (7.7) 1 (3.8) 1 (3.8) 2 (7.7) 5 (19.2) |
16 0 0 0 1 (6.3) 1 (6.3) 1 (6.3) 3 (18.8) 1 (6.3) 0 3 (18.8) 1 (6.3) 1 (6.3) 2 (12.5) 1 (12.5) |
|
BMI at study entry, n (%) n <30 30–<35 ≥35 Missing |
4,000 3,184 (79.6) 445 (11.1) 281 (7.0) 90 (2.3) |
51 42 (82.4) 2 (3.9) 6 (11.8) 1 (2.0) |
26 21 (80.8) 2 (7.7) 2 (7.7) 1 (3.8) |
16 13 (81.3) 1 (6.3) 1 (6.3) 1 (6.3) |
|
Race, n (%) n American Indian or Alaska Native Asian Black of African American Native Hawaiian or other Pacific Islander White Other Multiple Unknown Not reported Missing |
4,000 31 (0.8) 35 (0.9) 95 (2.4) 7 (0.2) 3,450 (86.3) 55 (1.4) 69 (1.7) 216 (5.4) 22 (0.6) 20 (0.5) |
51 1 (2.0) 0 1 (2.0) 1 (2.0) 40 (78.4) 0 3 (5.9) 5 (9.8) 0 0 |
26 1 (3.8) 0 0 1 (3.8) 20 (76.9) 0 2 (7.7) 2 (7.7) 0 0 |
16 1 (6.3) 0 0 1 (6.3) 12 (75.0) 0 1 (6.3) 1 (6.3) 0 0 |
| Serious AE, n (%) | ||||
| n | N/A | 51 | 26 | 16 |
| Yes | 16 (31.4) | 13 (50.0) | 14 (87.5) | |
| No | 32 (62.7) | 13 (50.0) | 0 | |
| Missing | 3 (5.9) | 0 | 2 (12.5) | |
| COVID-19 severity (highest AE grade), n (%) | N/A | |||
| n | 51 | 26 | 16 | |
| Mild | 14 (27.5) | 7 (26.9) | 0 | |
| Moderate | 21 (41.2) | 9 (34.6) | 3 (18.8) | |
| Severe | 10 (19.6) | 8 (30.8) | 8 (50.0) | |
| Life-threatening | 0 | 0 | 0 | |
| Fatal | 3 (5.9) | 2 (7.7) | 3 (18.8) | |
| Missing | 3 (5.9)# | 0 | 2 (12.5) | |
| AE outcome for COVID-19, n (%) | N/A | |||
| n | 51 | 26 | 16 | |
| Fatal | 3 (5.9) | 2 (7.7) | 3 (18.8) | |
| Not recovered/not resolved | 2 (3.9) | 1 (3.8) | 1 (6.3) | |
| Recovered/resolved | 35 (68.6) | 16 (61.5) | 8 (50.0) | |
| Recovered/resolved with sequelae | 2 (3.9) | 2 (7.7) | 2 (12.5) | |
| Recovering/resolving | 6 (11.8) | 5 (19.2) | 0 | |
| Missing | 3 (5.9)§ | 0 | 2 (12.5) | |
|
Patients with ≥1 comorbidity, n (%) n Respiratory, thoracic and mediastinal disorders Metabolism and nutrition disorders Vascular disorders Cardiac disorders |
1,400 (35.0) 464 (11.6) 732 (18.3) 562 (14.1) 156 (3.9) |
26 (51.0) 16 (31.4) 12 (23.5) 10 (19.6) 3 (5.9) |
15 (57.7) 11 (42.3) 8 (30.8) 5 (19.2) 1 (3.8) |
9 (56.3) 7 (43.8) 4 (25.0) 3 (18.8) 1 (6.3) |
|
EDSS, n (%) n ≤3 >3–5 >5–6.5 ≥7 Missing |
4,000 2,279 (57.0) 853 (21.3) 740 (18.5) 113 (2.8) 15 (0.4) |
51 34 (66.7) 12 (23.5) 5 (9.8) 0 0 |
26 18 (69.2) 7 (26.9) 1 (3.8) 0 0 |
16 9 (56.3) 6 (37.5) 1 (6.3) 0 0 |
| Confirmation, n (%) | N/A | |||
| n | 51 | 26 | 16 | |
| PCR and/or antibody | 25 (49.0) | 25 (96.2) | 11 (68.8) | |
| Imaging criteria | 1 (2.0) | 1 (3.8) | 1 (6.3) | |
| Clinical criteria | 20 (39.2) | 0 | 4 (25.0) | |
| Clinical and epidemiological | 5 (9.8) | 0 | 0 | |
| AE duration (days) | ||||
| n | N/A | 40 | 20 | 13 |
| Mean (SD) | 26.68 (16.70) | 28.85 (17.01) | 31.85 (17.68) | |
| Median | 20.0 | 26.50 | 31.00 | |
| Range | 8.0–80.0 | 9.0–80.0 | 14.0–80.0 | |
| AE onset time since first dose of ocrelizumab (y) | N/A | |||
| n | 48 | 26 | 14 | |
| Mean (SD) | 3.90 (2.86) | 3.58 (2.75) | 4.72 (3.03) | |
| Median | 2.79 | 2.72 | 5.24 | |
| Range | 0.0–10.9 | 0.0–8.4 | 0.7–8.4 | |
Countries shown include at least one patient with COVID-19.
Seriousness was based on investigators’ judgement; AE duration was based on resolved AEs only. Patients from blinded studies (ORATORIO-HAND) were considered to be on ocrelizumab.
AE, adverse event; BMI, body mass index; CCOD, clinical cut-off date; COVID-19, coronavirus disease 2019; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; PCR, polymerase chain reaction; PPMS, primary progressive multiple sclerosis; RMS, relapsing multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; SD, standard deviation.
Reference population, age class at CCOD; COVID-19 population, age class at onset of AE.
Reference population, age at CCOD (partial birth dates, where only year is collected, are imputed with first day of the month where day is missing or first month of the year where month is missing. ORATORIO-HAND: day of birth was not collected and is imputed with 15th day of month); COVID-19 population, age at onset of AE.
The three missing cases were manually reviewed after the data cut-off: two cases were grade 2 (moderate) and one case was grade 3 (severe).
The three missing cases were manually reviewed after the data cut-off: two cases had recovered/resolved, and one case had not resolved but patient had been discharged from hospital.
Table A.2.
Demographics, symptom severity, and outcomes for COVID-19 cases in patients treated with ocrelizumab in post-marketing settings.
| All COVID-19 cases (n=307) | Confirmed COVID-19 cases (n=263) | Hospitalized COVID-19 cases*(n=100) | |
|---|---|---|---|
|
Age, years n Mean (range) |
229 45.0 (16–84)† |
200 45.6 (16–84)# |
86 50.1 (18–84)§ |
|
Gender, n (%) n Male Female Not reported |
307 94 (30.6) 171 (55.7) 42 (13.7) |
263 84 (31.9) 143 (54.4) 36 (13.7) |
100 39 (39.0) 54 (54.0) 7 (7.0) |
|
Type of MS, n (%) n Relapsing forms Progressive forms Not reported |
307 128 (41.7) 47 (15.3) 132 (43.0) |
263 106 (40.3) 42 (16.0) 115 (43.7) |
100 43 (43.0) 27 (27.0) 30 (30.0) |
|
Geographical location, n (%) n Australia Austria Belgium Brazil Canada Chile Czech Republic Denmark Dominican Republic Egypt Finland* France Germany Iran Israel Italy Mexico Puerto Rico Saudi Arabia Serbia Spain Switzerland United Kingdom United Arab Emirates United States of America |
307 3 (1) 1 (<1) 11 (3.6) 4 (1.3) 10 (3.3) 4 (1.3) 1 (<1) 1 (<1) 1 (<1) 2 (1) 1 (<1) 10 (3.3) 13 (4.2) 3 (1) 5 (1.6) 18 (5.9) 1 (<1) 1 (<1) 1 (<1) 1 (<1) 37 (12.1) 2 (1) 4 (1.3) 1 (<1) 171 (55.7) |
263 3 (1.1) 1 (<1) 8 (3.0) 2 (1) 6 (2.3) 4 (1.5) 1 (<1) 1 (<1) 1 (<1) 2 (1) 0 10 (3.8) 9 (3.4) 2 (1) 4 (1.5) 17 (6.5) 1 (<1) 1 (<1) 1 (<1) 1 (<1) 32 (12.2) 2 (1) 2 (1) 1 (<1) 151 (57.4) |
100 0 0 4 (4.0) 0 1 (1.0) 1 (1.0) 1 (1.0) 1 (1.0) 0 0 1 (1.0) 7 (7.0) 0 0 0 7 (7.0) 1 (1.0) 1 (1.0) 0 0 16 (16.0) 1 (1.0) 0 1 (1.0) 57 (57.0) |
|
Case seriousness, n (%) n Reported as serious |
307 131 (42.7) |
263 127 (48.3) |
100 100 (100.0) |
|
COVID-19 severity, n (%) n Asymptomatic Mild Moderate Severe Critical Fatal Not reported |
307 10 (3.3) 105 (34.2) 28 (9.1) 52 (16.9) 15 (4.9) 17 (5.5) 80 (26.1) |
263 9 (3.4) 81 (30.8) 20 (7.6) 52 (19.8) 15 (5.7) 17 (6.5) 69 (26.2) |
100 0 3 (3.0) 3 (3.0) 44 (44.0) 15 (15.0) 11 (11.0) 24 (24.0) |
|
Outcome for COVID-19, n (%) n Recovered Recovering Not recovered at time of report Died Not reported |
307 165 (53.7) 46 (15.0) 19 (6.2) 17 (5.5) 60 (19.5) |
263 145 (55.1) 38 (14.4) 17 (6.5) 17 (6.5) 46 (17.5) |
100 53 (53.0) 18 (18.0) 10 (10.0) 11 (11.0) 8 (8.0) |
COVID-19, coronavirus 2019; MS, multiple sclerosis.
All but one of the cases reported as hospitalized were also reported as confirmed, the only hospitalized suspected case had radiological evidence of COVID-19 but reported as “suspected COVID, resolved”.
Calculated on all cases where age was reported (74.6%, n=229).
Calculated on all confirmed cases where age was reported (76.0%, n=200).
Calculated on all hospitalized cases where age was reported (86%, n=86).
Table A.3.
Patient demographics and disease history of the MS cohort in the real-world data study, stratified by hospitalization and ocrelizumab use in the six months prior to COVID-19 diagnosis.
|
Stratified by hospitalization |
Stratified by ocrelizumab use |
|||
|---|---|---|---|---|
| Not hospitalized (n=270) | Hospitalized (n=87) | Non-ocrelizumab-treated MS patients (n=309) | Ocrelizumab-treated MS patients (n=48) | |
|
Age, years n Median (IQR) |
270 56.0 (44.0, 66.8) |
87 64.0 (53.5, 71.0) |
309 60.0 (49.0, 69.0) |
48 47.5 (40.5, 60.0) |
|
Gender, n (%) n Male Female |
270 64 (23.7) 206 (76.3) |
87 27 (31.0) 60 (69.0) |
309 76 (24.6) 233 (75.4) |
48 15 (31.2) 33 (68.8) |
|
Weighted CCI n Median (Q1, Q3) |
270 1.0 (0.0, 3.0) |
87 2.0 (1.0, 5.5) |
309 2.0 (0.0, 4.0) |
48 1.0 (0.0, 2.0) |
|
Comorbidities, n (%) n Hypertension Obesity Diabetes |
270 139 (51.5) 67 (24.8) 60 (22.2) |
87 54 (62.1) 26 (29.9) 24 (27.6) |
309 176 (57.0) 83 (26.9) 78 (25.2) |
48 17 (35.4) 10 (20.8) 6 (12.5) |
|
Individual CCI comorbidities, n (%) n AIDS/HIV Any malignancy Cerebrovascular disease Chronic heart failure Chronic pulmonary disease Dementia Diabetes with complications Diabetes without complications Hemiplegia/paraplegia Mild liver disease Moderate/severe liver disease Myocardial infarction Peptic ulcer disease Peripheral vascular disease Renal disease Rheumatic disease |
270 1 (0.4) 58 (21.5) 26 (9.6) 32 (11.9) 68 (25.2) 30 (11.1) 31 (11.5) 56 (20.7) 28 (10.4) 16 (5.9) 2 (0.7) 15 (5.6) 6 (2.2) 37 (13.7) 30 (11.1) 15 (5.6) |
87 0 (0.0) 20 (23.0) 14 (16.1) 22 (25.3) 29 (33.3) 14 (16.1) 17 (19.5) 21 (24.1) 23 (26.4) 6 (6.9) 1 (1.1) 6 (6.9) 3 (3.4) 18 (20.7) 13 (14.9) 6 (6.9) |
309 1 (0.3) 62 (20.1) 37 (12.0) 52 (16.8) 90 (29.1) 42 (13.6) 71 (23.0) 46 (14.9) 50 (16.2) 21 (6.8) 3 (1.0) 20 (6.5) 7 (2.3) 52 (16.8) 42 (13.6) 21 (6.8) |
48 0 (0.0) 16 (33.3) 3 (6.2) 2 (4.2) 7 (14.6) 2 (4.2) 6 (12.5) 2 (4.2) 1 (2.1) 1 (2.1) 0 (0.0) 1 (2.1) 2 (4.2) 3 (6.2) 1 (2.1) 0 (0.0) |
|
Time, years since MS diagnosis n Mean (SD) |
270 5.7 (3.5) |
87 6.4 (4.0) |
309 6.0 (3.7) |
48 5.3 (3.4) |
|
Ocrelizumab in past 6 months, n (%) n Yes |
270 36 (13.3) |
87 12 (13.8) |
- - |
48 48 (100) |
|
Time, years since ocrelizumab initiation n Mean (SD) |
36 1.3 (0.8) |
12 1.2 (0.8) |
- - |
48 1.3 (0.8) |
All ocrelizumab-treated patients were included regardless of whether they met the MS case definition. Five ocrelizumab-treated patients did not meet the MS case definition, and in particular, omitting the requirement for the availability of outpatient data may result in an underreporting of comorbidities for the ocrelizumab cohort.
CCI, Charlson Comorbidity Index; COVID-19, coronavirus 2019; IQR, interquartile range; MS, multiple sclerosis; Q, quartile; SD, standard deviation.
Table A.4.
Patients with fatal outcomes in clinical trial cases of ocrelizumab-treated people with MS.
| Patient number | Age | Sex | MS type | Geographical location | Risk factors | EDSS | Case description |
|---|---|---|---|---|---|---|---|
| 1 | 21 | F | RRMS | Mexico | No | 1.5 | Cause of death atypical pneumonia possibly due to COVID-19 (SARS-CoV-2 test not carried out). BMI at study entry was 28.3 kg/m2. Medical history included hyperpolymenorrhea. |
| 2 | 27 | F | RRMS | Mexico | No | 2.0 | Patient lived with grandparents who both tested positive for SARS-CoV-2 and died of COVID-19. Medical history included polycystic ovaries, depression, and anxiety. Patient was prescribed numerous concomitant medications. |
| 3 | 60 | M | SPMS | Netherlands | >50; Hypertension; chronic pulmonary disease | 6.0 | Mechanical ventilation was not pursued as patient had a ‘do not resuscitate’ order. Medical history included chronic obstructive pulmonary disorder, emphysema, duodenal ulcers, cerebrovascular accident, and depression. |
Some of the fatal cases have already been reported in the literature, so numbers from different sources should not be added together due to the risk of duplicate cases.
BMI, body mass index; COVID-19, coronavirus 2019; EDSS, Expanded Disability Status Scale; F, female; M, male; MS, multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPMS, secondary progressive multiple sclerosis.
Table A.5.
Patients with fatal outcomes in post-marketing cases of ocrelizumab-treated people with MS.
| Patient number | Age | Sex | MS type | Geographical location | Risk factors | EDSS | Case description |
|---|---|---|---|---|---|---|---|
| 1 | 39 | F | RRMS | USA | Diabetes, obesity, cancer | NS | Nursing home resident with severe cognitive impairment, depression, anxiety, and left nephrectomy following kidney cancer. |
| 2 | 43 | M | NS | USA | Hypertension | NS | MS diagnosed in 2003. Several previous DMTs. |
| 3 | 43 | M | PPMS | USA | Hypertension | 9.0 | Nursing home resident. Several previous DMTs. |
| 4 | 44 | F | SPMS | USA | Not reported | 6.0 | Former smoker. No further medical history provided. |
| 5 | 45 | F | NS | Egypt | Not reported | NS | Physician report with no patient or event details. Method of COVID-19 diagnosis not reported. |
| 6 | 50 | F | SPMS | Italy | No | 6.0 | No comorbidities but a 27-year history of MS. Several previous DMTs. |
| 7 | 51 | M | NS | USA | Age >50, hypertension | 6.0 | African American patient with EDSS of 6. Patient had hyperlipidemia and hypothyroidism. |
| 8 | 52 | M | PPMS | USA | Age >50, hypertension, diabetes, obesity | 6.5 | PPMS diagnosed in 1997. Former smoker. |
| 9 | 56 | F | RMS | USA | Age >50, asthma, diabetes, obesity | 6.5 | Patient had renal insufficiency. |
| 10 | 57 | F | SPMS | USA | Age >50, Obesity | 7.0 | Nursing home resident with 34-year history of MS. |
| 11 | 58 | M | NS | Serbia | Age >50 | NS | Patient had dementia. |
| 12 | 60 | M | NS | France | Age >50 | NS | Physician report. No MS or medical history provided. |
| 13 | 62 | M | NS | Spain | Age >50, asthma | NS | Regulatory Health Authority report. No MS history provided. |
| 14 | 63 | F | RRMS | USA | >50; Hypertension; chronic pulmonary disease; CHD | NS | MS history not reported. Several previous DMTs. The patient was a smoker and had a coronary artery stent. |
| 15 | 66 | M | SPMS | USA | Age >50 | NS | Patient had a 33-year history of MS and was a former smoker with a history of prostate and testicular cancer. |
| 16 | 74 | M | NS | Spain | Age >50, hypertension | NS | Regulatory Health Authority report. No MS history provided. |
| 17 | Unk | Unk | NS | France | Not reported | NS | Physician report regarding a patient of unknown demography. No details of the event or the patient were provided. |
Some of the fatal cases have already been reported in the literature, so numbers from different sources should not be added together due to the risk of duplicate cases.
CHD, coronary heart disease; COVID-19, coronavirus 2019; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; F, female; M, male; MS, multiple sclerosis; NS, not specified; PPMS, primary progressive multiple sclerosis; RRMS, relapsing–remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; Unk, unknown.
Table A.6.
EDSS scores for patients treated with ocrelizumab in post-marketing settings.
| EDSS | Severity of COVID-19 | |||||
|---|---|---|---|---|---|---|
| Not reported (n=80) | Asymptomatic, mild, or moderate (n=143) | Severe (n=52) | Critical (n=15) | Fatal (n=17) | ||
| Not reported | 79 (98.8%) | 125 (87.4%) | 40 (76.9%) | 11 (73.3%) | 10 (58.8%) | |
| ≤3 | 0 | 13 (9.1%) | 7 (13.5%) | 3 (20.0%) | 0 | |
| >3–5 | 0 | 0 | 2 (3.8%) | 0 | 0 | |
| >5–6.5 | 1 (1.3%) | 2 (1.4%) | 2 (3.8%) | 1 (6.7%) | 5 (29.4%) | |
| ≥7 | 0 | 3 (2.1%) | 1 (1.9%) | 0 | 2 (11.8%) | |
COVID-19, coronavirus disease 2019; EDSS, Expanded Disability Status Scale.
References
- Amor S, et al. SARS-CoV-2 and multiple sclerosis: not all immune-depleting DMTs are equal or bad. Ann Neurol; 2020;87:794–797. doi: 10.1002/ana.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol; 2020;202(2):149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, et al. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult Scler Relat Disord; 2020;43 doi: 10.1016/j.msard.2020.102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology; 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee W, et al. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology; 2020;94:949–952. doi: 10.1212/WNL.0000000000009507. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2020a. Assessing risk factors for severe COVID-19 illness. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/assessing-risk-factors.html [accessed September 10, 2020].
- Chen J, Subbarao K. The immunobiology of SARS. Annu Rev Immunol; 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- Clark A, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health; 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte WL. Attenuation of antibody response to SARS-CoV-2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult Scler Relat Disord; 2020;44 doi: 10.1016/j.msard.2020.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés-Tellés A, et al. Risk factors for mortality among hospitalized patients with COVID-19. An overview in Mexican population. Tuberc Respir Dis (Seoul) 2020 doi: 10.4046/trd.2020.0095. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COViMS Registry. 2020. The COViMS Database Public Data Update. Available at: www.COViMS.org. [accessed October 15, 2020].
- Cox RJ, Brokstad KA. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol; 2020;20:581–582. doi: 10.1038/s41577-020-00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper WJ, et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology; 2019;92:e1016–e1028. doi: 10.1212/WNL.0000000000007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl; 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- Díez JM, et al. Cross-neutralization activity against SARS-CoV-2 is present in currently available intravenous immunoglobulins. Immunotherapy; 2020;12:1247–1255. doi: 10.2217/imt-2020-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earla JR, et al. Factors associated with prescribing oral disease modifying agents in multiple sclerosis: a real-world analysis of electronic medical records. Mult Scler Relat Disord; 2020;45 doi: 10.1016/j.msard.2020.102334. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control 2020. Available at: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition. [accessed October 8, 2020].
- European Medicines Agency, 1995. ICH Harmonised Tripartite Guideline E2A. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf [accessed October 14, 2020].
- Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin. 2020 doi: 10.1093/cid/ciaa863. Infect Dis; ciaa863. doi: 10.1093/cid/ciaa863. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G. Anti-CD20 immunosuppressive disease-modifying therapies and COVID-19. Mult Scler Relat Disord; 2020;41 doi: 10.1016/j.msard.2020.102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G, et al. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord; 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajarzadeh M, et al. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult Scler Relat Disord; 2020;43 doi: 10.1016/j.msard.2020.102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajarzadeh M, Bonavita S. Are patients with multiple sclerosis (MS) at higher risk of COVID-19 infection? Neurol Sci. 2020;41:2315–2316. doi: 10.1007/s10072-020-04570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasheen WP, et al. Charlson Comorbidity Index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits. 2019;12:188–197. [PMC free article] [PubMed] [Google Scholar]
- Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 2020 doi: 10.1016/j.ajpath.2020.08.009. S0002-9440(20)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy SM, et al. Managing disease-modifying therapies and breakthrough activity in multiple sclerosis patients during the COVID-19 pandemic: toward an optimized approach. Ther Clin Risk Manag. 2020;16:651–662. doi: 10.2147/TCRM.S257714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- Hauser SL, et al. Five years of ocrelizumab in relapsing multiple sclerosis. OPERA studies open-label extension. Neurology; 2020;95:e1854–e1867. doi: 10.1212/WNL.0000000000010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S, et al. Safety of ocrelizumab in multiple sclerosis: updated analysis inpatients with relapsing and primary progressive multiple sclerosis. Eur J Neurol. 2020;27(Suppl 1):331. [Google Scholar]
- Hughes R, et al. COVID-19 in persons with multiple sclerosis treated with ocrelizumab. A pharmacovigilance case series. Mult Scler Relat Disord. 2020;42 doi: 10.1016/j.msard.2020.102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetta M, et al. Mild clinical manifestations of SARS-CoV-2 related pneumonia in two patients with multiple sclerosis under treatment with ocrelizumab. Mult Scler Relat Disord. 2020;45 doi: 10.1016/j.msard.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA, et al. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISARIC (International Severe Acute Respiratory and Emerging Infections Consortium) COVID-19 Report: 4 October 2020. Available at: https://isaric.tghn.org [accessed October 14, 2020].
- Johns Hopkins, Coronavirus Resource Center. Mortality analyses. Available at: https://coronavirus.jhu.edu/data/mortality [accessed November 11, 2020].
- Kieseier, et al. COVID-19 and multiple sclerosis – prevalence and impact of disease-modifying therapies. Presentation LB1252 given at the 8th Joint ACTRIMS-ECTRIMS Meeting. 2020:2020. [Google Scholar]
- Killestein J, et al. Vaccination in B-cell-depleted patients with multiple sclerosis. Neurology; 2020;95:613–614. doi: 10.1212/WNL.0000000000010378. [DOI] [PubMed] [Google Scholar]
- Korsukewitz C, et al. Author correction: Neurological immunotherapy in the era of COVID-19 – looking for consensus in the literature. Nat Rev Neurol. 2020 doi: 10.1038/s41582-020-0392-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra FC, et al. COVID-19 in multiple sclerosis: the Dutch experience. Mult Scler; 2020;26:1256–1260. doi: 10.1177/1352458520942198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol; 2020;77:1–10. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini M, et al. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult Scler Relat Disord; 2020;44 doi: 10.1016/j.msard.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meca-Lallana V, et al. COVID-19 in 7 multiple sclerosis patients in treatment with anti-CD20 therapies. Mult Scler Relat Disord; 2020;44 doi: 10.1016/j.msard.2020.102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet; 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Escribano P, et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord; 2020;42 doi: 10.1016/j.msard.2020.102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban X, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services NIH, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2017. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf [accessed October 14, 2020].
- Novi M, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord. 2020;42 doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta E, et al. COVID-19 outcomes in MS: observational study of early experience from NYU multiple sclerosis comprehensive care center. Neurol Neuroimmunol Neuroinflamm. 2020;7:e835. doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters LM, et al. COVID-19 in people with multiple sclerosis: a global data sharing initiative. Mult Scler. 2020;26:1157–1162. doi: 10.1177/1352458520941485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.7681. [published correction appears. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo S et al. 2020. Descriptive epidemiology of 16,780 hospitalized COVID-19 patients in the United States. doi: 10.1101/2020.07.17.20156265. [accessed October 14, 2020].
- Sahraian MA, et al. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord; 2020;46 doi: 10.1016/j.msard.2020.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi F, et al. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H, et al. Estimating the burden of SARS- CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soresina A, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy and Immunol; 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant; 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S et al. 2020. SS02.04 - First results of the COVID-19 in MS Global Data Sharing Initiative suggest anti-CD20 DMTs are associated with worse COVID-19 outcomes. Available at: https://cslide.ctimeetingtech.com/msdc2020/attendee/confcal/session/calendar/2020-09-26 session SS02-COVID-19. [accessed November 20, 2020].
- Sormani MP et al. 2020. Disease modifying therapies and COVID-19 severity in multiple sclerosis. Available at: 10.2139/ssrn.3631244 [accessed September 11, 2020]. [DOI]
- Suwanwongse K, Shabarek N. Benign course of COVID-19 in a multiple sclerosis patient treated with Ocrelizumab. Mult Scler Relat Disord; 2020;42 doi: 10.1016/j.msard.2020.102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay MZ, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol; 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakolwiboon S, et al. Disease-modifying therapies during the COVID-19 outbreak: a narrative review of international and national recommendations. Int J MS Care; 2020;22:151–157. doi: 10.7224/1537-2073.2020-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JR, Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult Scler Relat Disord; 2020;44 doi: 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N, et al. Immunology of COVID-19: current state of the science. Immunity; 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EJ, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MS, et al. Control of SARS-CoV-2 infection in rituximab-treated neuroimmunological patients. J Neurol. 2020 doi: 10.1007/s00415-020-10046-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2020a. Coronavirus disease (COVID-19) Situation Report –106. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200505covid-19-sitrep-106.pdf?sfvrsn=47090f63_2 [accessed May 14, 2020].
- World Health Organization. 2020b. Available at: covid19.who.int [accessed October 15, 2020].
- World Health Organization. 2020c. Clinical management of COVID-19: interim guidance. 27 May 2020. Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19 [accessed October 14, 2020].
- World Health Organization. 2020d. Available at: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis [accessed October 14, 2020].
- World Health Organization. 2020e. Estimating mortality from COVID-19 Scientific brief 4 August 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 [accessed October 14, 2020].
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA; 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu F, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180:1356–1362. doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm H, et al. Recovery from COVID-19 in a B-cell-depleted multiple sclerosis patient. Mult Scler; 2020;26:1261–1264. doi: 10.1177/1352458520943791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs; 2020;34:879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]