Abstract
Current neurocognitive models of motor control postulate that accurate action monitoring is crucial for a normal experience of agency—the ability to attribute the authorship of our actions and their consequences to ourselves. Recent studies demonstrated that action monitoring is impaired in Gilles de la Tourette syndrome, a movement disorder characterized by motor and vocal tics. It follows that Tourette syndrome patients may suffer from a perturbed sense of agency, the hypothesis tested in this study. To this end, we recruited 25 Tourette syndrome patients and 25 matched healthy controls in a case-control behavioural and functional magnetic resonance imaging study. As an implicit index of the sense of agency, we measured the intentional binding phenomenon, i.e., the perceived temporal compression between voluntary movements and their external consequences. We found evidence of an impaired sense of agency in Tourette syndrome patients who, as a group, did not show a significant intentional binding. The more reduced was the individual intentional binding, the more severe were the motor symptoms. Specific differences between the two groups were also observed in terms of brain activation patterns. In the healthy controls group, the magnitude of the intentional binding was associated with the activity of a premotor–parietal–cerebellar network. This relationship was not present in the Tourette syndrome group, suggesting an altered activation of the agency brain network for self-generated acts. We conclude that the less accurate action monitoring described in Tourette syndrome also involves the assessment of the consequences of actions in the outside world. We discuss that this may lead to difficulties in distinguishing external consequences produced by their own actions from the ones caused by others in Tourette syndrome patients.
Keywords: Tourette’s syndrome, motor control, cognitive control, social cognition, tic disorder
Zapparoli et al. report behavioural and functional magnetic resonance imaging evidence of an altered sense of agency in Tourette syndrome, correlated with the severity of tics. This altered appreciation of a causal relationship between self-generated actions and their physical effects is discussed in the context of premotor theories of motor awareness.
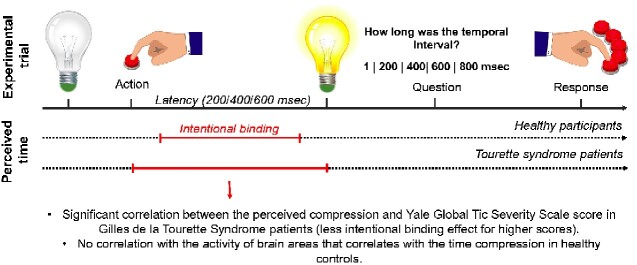
Graphical Abstract
Graphical Abstract.
Introduction
Although much of the functioning of our motor system occurs without awareness (see, for example, Lau et al., 2004; Zapparoli et al., 2018), we are aware that we are actors of our behaviour, and we feel responsible for the external consequences of our motor acts. The feeling of voluntarily controlling our actions and, through them, the consequences in the outside world is called ‘sense of agency’ (Haggard, 2017).
Importantly, an altered sense of agency can be observed in neurological and psychiatric disorders (Frith et al., 2000; Sato and Yasuda, 2005; Moore and Fletcher, 2012) like, for example, in the alien hand syndrome (Pacherie et al., 2006), Parkinson’s disease (Moore et al., 2010; Saito et al., 2017) or functional movement disorders (Kranick et al., 2013). Disordered experiences of agency are also characteristic of schizophrenic patients, who show a tendency to over-attribute the consequences of their movements to themselves (Daprati et al., 1997; Voss et al., 2010). All these conditions are characterized by disturbed motor control of various kinds, raising the possibility that a perturbed sense of agency might be seen also in other movement disorders.
Here, we tested this possibility for Gilles de la Tourette syndrome (GTS), a hyperkinetic movement disorder characterized by motor and vocal tics. A premonitory urge usually precedes tics, typically described as a feeling of ‘urge to move’, or a mounting internal tension, which can be temporarily relieved by tic expression (Cavanna et al., 2017).
Why should the GTS represent a neurological instance of an altered sense of agency?
The sense of agency has recently been associated with the planning premotor phase of action generation (Kühn et al., 2013; Zapparoli et al., 2020; Seghezzi and Zapparoli, 2020). It has been suggested that the arising of the sense of agency may be contingent on the generation of a forward model, a mechanism whereby we estimate the next motor state and the sensory consequences of any planned movement (see Kim et al., 2019 for a discussion of these concepts in the context of GTS). Besides the fact that motoric manifestations characterize the syndrome, recent evidence has shown that such manifestations are associated with alteration of the motor/premotor network. GTS patients show brain hyperactivations in the premotor cortices of the medial wall (supplementary motor area/anterior cingulate cortex) for various aspects of voluntary motor execution and tic suppression [meta-analytical reviews by Zapparoli et al. (2015) and Polyanska et al. (2017)].
The nature of the motor manifestations of GTS and the experience of GTS patients are also telling in these respects. Contrary to tremor or choreic movements that characterize Parkinson’s and Huntington’s disease, tics can be kept under control for a certain time through voluntary suppression (Zapparoli et al., 2019), and their intensity and frequency can be modulated by attention (Herrmann et al., 2019). For this reason, Cavanna et al. (2018) suggested that tics might be considered as “unvoluntary,” halfway between voluntary and involuntary actions (Cavanna, 2018) and proposed that this quasi-voluntary nature of tics may determinean abnormal conscious experience of voluntary action (Cavanna and Nani, 2013). In particular, one possibility is that tics may be hard to distinguish from volitional movements as they may rely on the same brain motor circuits, with a consequent high-level of noise in the sensorimotor system (Ganos et al., 2015).
In line with these concepts, this previous evidence and the ensuing predictions, a recent study tested the hypothesis of an altered sense of agency in GTS to find illusory judgements of agency associated with the syndrome (Delorme et al., 2016). They asked participants to make judgements of control and performance after completing a computerized game, where they moved a cursor on a screen to catch some targets. The control over the cursor could be normal, disrupted, or artificially enhanced. GTS patients reported an illusory perceived sense of agency when their performance was artificially enhanced (Delorme et al., 2016). These findings were based on explicit agency judgements made by the participants on their performance and represent important evidence about the explicit sense of agency in GTS. Yet, it is worth mentioning that the measures used by Delorme et al. (2016), as all explicit meta-cognitive evaluations, may have been prone to biases since they were directly mediated by the conscious control of the participants and introspection (Schüür and Haggard, 2011; Obhi, 2012). Not surprisingly, perhaps, in that study, a correlation was found only with the self-assessment of the severity of the disorder [the Yale Global Tic Severity Scale (YGTSS) global scale] rather than with the specific items of the scale based on objective measures of the motoric signs. This leaves the hypothesis of a functional connection between an altered sense of agency and motoric function not demonstrated yet. A motorically grounded sense of agency is probably what counts the most in our daily life, particularly for pressing interactions with the environment. Moreover, as much as our everyday experiences of agency do not necessarily involve explicit judgements, we regularly experience a flow between the actions that we plan and their external effects. Thus, we have an implicit feeling of agency, not based on explicit judgements (Kühn et al., 2013), that maps into the physiology of premotor planning (Zapparoli et al., 2020).
These considerations gave us the motivation to further explore whether alterations of the sense of agency in GTS could be described using a more ecological setting that leads to the collection of implicit physical measures. A suitable measure is the ‘intentional binding effect’, whereby the temporal interval between voluntary actions and their consequences is perceived to be shorter than its real duration in comparison with the same effects when passively generated (Haggard et al., 2002). As GTS is frequently associated with abnormal activations of brain regions typically involved in motor control (see, for example, Zapparoli et al., 2015, 2016, 2017; Polyanska et al., 2017), and because the normal intentional binding maps into the premotor network (Zapparoli et al., 2020), we hypothesized that an experiment based on the intentional binding phenomenon could reveal an abnormal implicit agency experience in GTS. This abnormality would map into the physiology of the premotor circuitry.
Aims of the study and predictions
In this study, we explored the hypothesis that the sense of agency might be impaired, at the behavioural and/or at the brain activation level, in GTS, a movement disorder characterized by the presence of unwanted movements called tics.
To this end, we studied adult GTS patients and matched healthy controls (HC) by adopting as an implicit measure of agency the intentional binding phenomenon. We took advantage of a paradigm that we recently developed to describe the premotor–parietal correlates of the sense of agency in HC (Zapparoli et al., 2020). Thus, the data of the GTS patients examined here were compared with the behavioural and functional magnetic resonance imaging (fMRI) data of the HC of Zapparoli et al. (2020).
We had specific predictions in mind at the behavioural and at the functional anatomical level, in line with different possible alternative hypotheses/scenarios
A first Scenario A hypothesizes an abnormal intentional binding effect in GTS, both at the behavioural and at the physiological level. The behavioural result could be mirrored by an altered activation of the sense of agency brain network typically recruited by healthy subjects.
Other scenarios are compatible with a normal intentional binding at the behavioural level: this could arise from a truly normal function also at the physiological level (Scenario B). This is essentially equivalent to the demonstration of the null hypothesis.
A third and more nuanced Scenario C would contemplate a normal intentional binding at the behavioural level in GTS. Yet, this may be associated with compensatory brain hyperactivity at the level of premotor/prefrontal regions normally associated with the sense of agency. Given the nature of GTS and the previous demonstration of premotor hyperactivations, one such scenario would be interpretable in the context of common theories of compensation.
Materials and methods
Sample size calculation
In order to determine the sample size of the study, we carried out an a priori power analysis based on the scientific literature. All the details about the sample size calculation are described in the supplementary materials.
Participants
Twenty-five adult HC (mean age: 25.7 ± 3.8 years; mean education level: 15.6 ± 2.5 years; male/female ratio: 12/13) with no history of neurological or psychiatric illness, and 25 GTS patients (GTS, mean age: 26.3 ± 9.4 years; mean education level: 12.2 ± 3.4 years; male/female ratio: 20/5) participated in this study. All the participants were right-handed, as assessed by the Edinburgh handedness inventory (Oldfield, 1971). The study protocol was approved by the local Ethics Committee (IRCCS San Raffaele of Milan; Prot. SOA, 149/INT/2016), and informed written consent was obtained from all subjects according to the Helsinki Declaration (1964). All participants took part in the study after the nature of the procedure had been fully explained.
All subjects completed a neuropsychological and psychopathological assessment and a detailed interview about the severity of their motoric symptoms. The details are described in the supplementary materials and in Table 1.
Table 1.
Demographical, neuropsychological and clinical data
| # | Sex | Age | Edu | MMSE |
FAB |
Raven |
BIS- 11 | Y-BOCS TOT | Beck | Conners | STAI- X-1 | STAI- X-2 | PUTS | YGTSS |
Neuroleptic treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Corr | Raw | Corr | Raw | Corr | Moto | Fon | Soc | ||||||||||||
| 1 | M | 23 | 8 | 29 | 28.19 | 18 | 18 | 31 | 30.5 | 65 | 24 | 13 | 2 | 31 | 40 | 21 | 13 | 11 | 20 | Risperidone (2 mg) |
| 2 | F | 19 | 12 | 24 | 22.59 | 16 | 14.9 | 18 | 16 | 77 | 19 | 22 | 2 | 62 | 56 | 26 | 21 | 21 | 50 | None |
| 3 | M | 22 | 13 | 30 | 30 | 18 | 18 | 35 | 32.5 | 58 | 15 | 12 | 2 | 41 | 50 | 29 | 15 | 5 | 20 | None |
| 4 | M | 20 | 12 | 30 | 30 | 17 | 15.9 | 28 | 26 | 62 | 2 | 10 | 0 | 55 | 53 | 34 | 20 | 17 | 20 | Aripiprazole (22.5 mg); Pimozide (4 mg) |
| 5 | M | 23 | 13 | 30 | 30 | 18 | 18 | 35 | 32.5 | 58 | 13 | 1 | 2 | 31 | 54 | 24 | 17 | 7 | 30 | Aripiprazole (7.5 mg); Pimozide (2 mg) |
| 6 | M | 20 | 13 | 30 | 30 | 18 | 18 | 35 | 32.5 | 68 | 16 | 2 | 2 | 45 | 43 | 25 | 11 | 5 | 40 | Aripiprazole (15 mg) |
| 7 | M | 25 | 13 | 30 | 30 | 17 | 15.6 | 34 | 31.5 | 84 | 13 | 7 | 3 | 27 | 43 | 21 | 20 | 14 | 20 | Pimozide (2 mg) |
| 8 | M | 19 | 13 | 26 | 24.59 | 16 | 14.5 | 35 | 32.5 | 71 | 16 | 8 | 4 | 26 | 44 | 32 | 13 | 5 | 0 | Quetiapine (50 mg) |
| 9 | M | 29 | 10 | 29 | 27.59 | 13 | 12.1 | 30 | 29 | 55 | 19 | 7 | 4 | 34 | 32 | 28 | 6 | 7 | 20 | Pimozide (4 mg) |
| 10 | F | 21 | 13 | 29 | 27.59 | 15 | 13.5 | 22 | 19.5 | 86 | 16 | 5 | 5 | 47 | 36 | 26 | 12 | 11 | 0 | Haloperidol (2 mg); Aripiprazole (15 mg) |
| 11 | M | 29 | 8 | 28 | 27.19 | 16 | 15.5 | 24 | 24 | 63 | 13 | 4 | 1 | 31 | 45 | 19 | 11 | 10 | 30 | Aripiprazole (30 mg); Pimozide (2 mg) |
| 12 | M | 27 | 16 | 30 | 30 | 18 | 18 | 36 | 36 | 57 | 20 | 13 | 0 | 41 | 51 | 27 | 14 | 0 | 20 | Aripiprazole (30 mg) |
| 13 | M | 19 | 12 | 27 | 25.59 | 16 | 14.5 | 30 | 28 | 48 | 17 | 2 | 0 | 53 | 40 | 25 | 12 | 13 | 20 | Aripiprazole (15 mg) |
| 14 | M | 50 | 8 | 29 | 28.97 | 14 | 13.9 | 22 | 23.5 | 67 | 11 | 0 | 2 | 30 | 25 | 27 | 8 | 7 | 40 | Aripiprazole (30 mg) |
| 15 | M | 22 | 13 | 30 | 30 | 18 | 18 | 35 | 32.5 | 61 | 20 | 5 | 4 | 37 | 45 | 36 | 15 | 6 | 20 | Pimozide (4 mg) |
| 16 | M | 28 | 16 | 29 | 27.59 | 18 | 18 | 30 | 26.25 | 70 | 18 | 17 | 1 | 35 | 47 | 34 | 16 | 6 | 40 | Aripiprazole (15 mg) |
| 17 | M | 18 | 8 | 30 | 30 | 16 | 15.3 | 32 | 32 | 76 | 20 | 7 | 4 | 29 | 33 | 21 | 14 | 0 | 20 | Aripiprazole (22.5 mg); Pimozide (4 mg) |
| 18 | M | 22 | 8 | 30 | 30 | 18 | 18 | 32 | 32 | 70 | 17 | 11 | 4 | 33 | 50 | 10 | 0 | 0 | 50 | Aripiprazole (15 mg) |
| 19 | M | 19 | 13 | 30 | 30 | 17 | 15.5 | 36 | 36 | 74 | 10 | 7 | 4 | 34 | 52 | 28 | 17 | 0 | 20 | Pimozide (4 mg) |
| 20 | M | 18 | 8 | 29 | 28.19 | 16 | 15.3 | 34 | 34 | 60 | 0 | 0 | 0 | 24 | 23 | 14 | 3 | 4 | 0 | Aripiprazole (22.5 mg) |
| 21 | F | 34 | 20 | 30 | 30 | 17 | 15.3 | 28 | 24.5 | 86 | 12 | 11 | 4 | 39 | 50 | 29 | 15 | 12 | 30 | None |
| 22 | M | 48 | 13 | 29 | 27.89 | 18 | 18 | 30 | 29.5 | 68 | 18 | 8 | 2 | 41 | 39 | 36 | 17 | 18 | 20 | None |
| 23 | M | 47 | 8 | 27 | 26.62 | 16 | 15.8 | 28 | 26.75 | 67 | 10 | 0 | 1 | 37 | 39 | 22 | 11 | 3 | 0 | Pimozide (4 mg) |
| 24 | F | 24 | 16 | 30 | 30 | 18 | 18 | 34 | 30 | 70 | 19 | 20 | 4 | 59 | 41 | 34 | 16 | 11 | 30 | None |
| 25 | F | 32 | 18 | 30 | 30 | 18 | 18 | 29 | 25.25 | 51 | 21 | 14 | 2 | 50 | 69 | 33 | 11 | 13 | 40 | None |
| Mean | 26.3 | 12.2 | 29.0 | 30.0 | 16.8 | 18.0 | 30.5 | 29.4 | 66.9 | 15.2 | 8.2 | 2.4 | 38.9 | 44.0 | 26.4 | 13.1 | 8.2 | 24.0 | ||
| SD | 9.4 | 3.4 | 1.5 | 0.0 | 1.4 | 0.0 | 4.9 | 5.9 | 10.1 | 5.6 | 6.1 | 1.6 | 10.5 | 10.0 | 6.6 | 5.0 | 5.8 | 14.4 | ||
The majority of patients (n = 19) were on neuroleptics medication. Molecules and dosages are reported in Table 1.
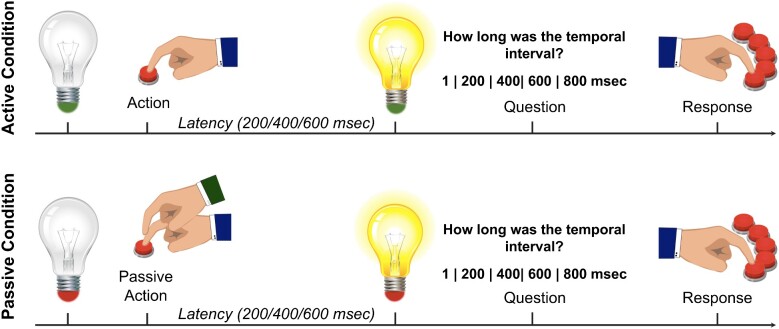
Experimental task
fMRI scans were performed during the execution of a temporal-judgement task (see Fig. 1, see also Zapparoli et al., 2020). There were active and passive conditions. In the active condition, the picture of a turned-off light-bulb with its basis coloured in green was shown. Participants were instructed to turn the light-bulb on by pressing a button with their right index finger on a key-pad placed under the right hand. After the button press, the light-bulb went on with a variable delay of 200, 400, or 600 ms. Participants rated then the perceived temporal interval between their button press and the lightening of the light-bulb. The judgement was reported by means of a visual analogue scale at which they responded using a five-key response key-pad placed under their left hand. Participants had up to 4 s to give their response. They used their fingers, starting from the pinkie to the ring finger, and so on, to select one of five possible response options: 1, 200, 400, 600 and 800 ms. The lowest and the highest response options were included in order to make it possible for the participants to both underestimate and overestimate each presented temporal interval. In the passive condition, the basis of the light-bulb was coloured in red. Subjects were instructed to stay still while an experimenter pressed their right index finger to produce a passive movement that turned the light-bulb on. Participants were then asked to judge the action-outcome delay in the same way as for active trials.
Figure 1.
Experimental paradigm. Graphical representation of the temporal-judgement task performed during fMRI
We administered 60 trials, equally distributed between active and passive trials, with ten trials for each of the three action-outcome delays. The inter-stimulus interval randomly varied between 1500 and 2500 ms.
Before the experiment, participants practiced with the task. They were submitted to a training session composed of ten trials, when they were given feedback on their accuracy trial by trial.
Statistical analyses of the behavioural data
In line with the description of the intentional binding phenomenon (Haggard et al., 2002), the ‘time compression’ (TC), namely the difference between the estimated and the real duration of the action-outcome delay, was taken as an indirect measure of the sense of agency (the greater the compression, the higher the sense of agency).
Linear mixed model
The behavioural data were analysed by using the software SAS (Statistical Analysis System, version 9.4). Four GTS patients were removed from the behavioural analyses due to a technical problem in responses recording.
We included only trials in which participants provided a response (missing trials HC: 6.7%; GTS: 2.8%). The TC measure represented the dependent variable of the model, while the factors ‘Group’ (GTS/HC), ‘Condition’ (active/passive), and ‘Delay’ (200/400/600 ms) were the independent variables. We tested this statistical model by using linear mixed models with random intercept. Significant interactions were explored by means of planned Bonferroni corrected post hoc comparisons.
Effect sizes were calculated by means of Cohen’s d starting from estimated marginal means.
Before applying linear mixed models, we inspected our data distribution by using the Cullen and Frey graph (Cullen and Frey, 1999). This graph is also called the skewness–kurtosis graph, and it provides the best fit for an unknown distribution according to skewness level and kurtosis. The present data had a distribution similar to the normal distribution.
Correlations with clinical data
In order to investigate whether GTS behavioural results might be influenced by their clinical profile, in terms of tics severity and psychopathological comorbidities, TC data were correlated with the different clinical measures indicated in Table 1 by means of non-parametric correlation analyses.
Correlations with neuroleptic medication levels
To assess the possible effects of neuroleptic medication on the behavioural results (given their effects on the motor system even at very low doses), we calculated the correlation between TC data and chlorpromazine equivalent scores (https://cpnp.org/guideline/essentials/antipsychotic-dose-equivalents) by means of non-parametric correlation analyses.
Functional magnetic resonance imaging data acquisition and analysis
All the details about the fMRI data acquisition, data preprocessing and analysis of head motion parameters are described in the supplementary materials.
First level fixed-effect analyses
One HC participant was excluded from the analysis due to technical artefacts. At the first level, we characterized the brain activity recorded between the appearance of the turned-off light-bulb and the lightening of the light-bulb. We included one regressor for each condition (active and passive trials) and each action-outcome delay (200/400/600 ms), for a total of six regressors. Brain activity occurring between the appearance of the evaluation scale and the judgement response was modelled separately for each delay and condition and added to the statistical model, for a total of six non-interest regressors. The parameters obtained from the realignment procedure were added as non-interest regressors as well, to partial out the impact of motion artefacts on the estimates of the beta parameters. Moreover, in order to exclude fMRI scans contaminated by tics, we use the Artifact detection Tools (Withfield-Gabrieli, https://www.nitrc.org/projects/artifact_detect/).
The specific regressors generated by the Artifact detection Tools toolbox were added as non-interest regressors in the first level analyses, in order to exclude the outlier scans that exceeded the movement thresholds. For further details, see the supplementary materials.
For each participant and for each action-outcome delay, we generated a contrast image of the comparison active condition > passive condition (three contrast images per subject overall).
Second level random-effect analysis
Each contrast image was entered in the following second level analyses, conforming to a random-effect approach:
(i) A full factorial analysis (Factor 1: Group (HC/GTS), Factor 2: Delay (200/400/600)), to test the following effects:
• Main effects of the factor condition, to highlight the brain activations of the task independently from the different groups and action-outcome delays.
• Conjunction effects of the comparisons active condition > passive condition and passive condition > active condition, to highlight the brain activations shared between the two groups independently from the different action-outcome delays. We tested this conjunction effect to assess whether both groups showed a significant activation of the cerebral network typically involved in voluntary motor control.
• Interaction effect between the condition (active/passive) and the specific time delays (200/400/600 ms).
• Interaction effect between group (GTS/HC), condition (active/passive) and the specific time delays (200/400/600 ms)
(ii) Linear regression analyses of the delay specific contrast images with the delay specific TC measure for each group (GTS/HC). These analyses allowed us to test the hypothesis, for each group, that the activity of some brain regions covaried with the TC measure of the sense of agency in specific time-windows. It is important to note that because the contrast images used in this analysis contained the differential effect between active and passive trials, a differential TC measure between active and passive trials was used as a regressor here. We then compared the correlation coefficients obtained in each group by using the Fisher r-to-z transformation.
All the results reported survive a correction for multiple comparisons: we used the nested-taxonomy strategy recommended by Friston et al. (1996), including regional effects meeting either a clusterwise or voxelwise family-wise error rate (FWER) correction. The voxelwise threshold applied to the statistical maps before the clusterwise correction was P < 0.001 uncorrected, as recommended by Flandin and Friston (2019). For clusters significant at the P < 0.05 FWER-corrected level, we also report the other peaks at P < 0.001.
Data availability
All data, code and materials are available upon request to the corresponding author L.Z.
Results
Behavioural results
Time compression data
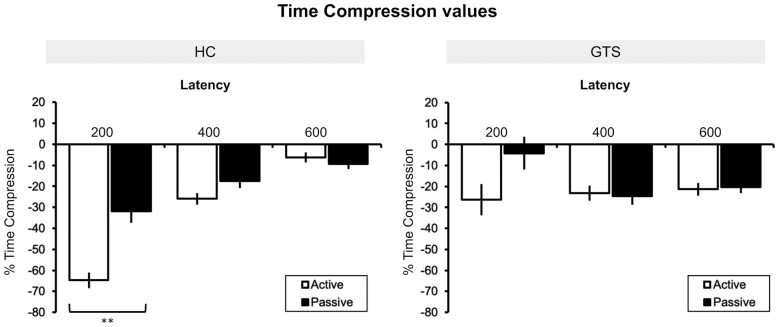
We found a significant effect of the factor ‘Condition’ [F(1, 2569) = 7.49, P = 0.006], a significant effect of the factor ‘Latency’ [F(2, 2569) = 4.97, P < 0.007], a significant ‘Latency × Group’ interaction [F(2, 2569) = 29.68, P < 0.0001] and a significant ‘Condition × Latency × Group’ interaction [F(4, 2569) = 3.76, P = 0.005]. The factor ‘Group’ was not significant [F(1, 2569) = 0.12, P = 0.73] and the ‘Condition × Group’ interaction as well [F(1, 2569) = 0.58, P = 0.45].
To further explore the significant three-way interaction, we run planned Bonferroni corrected post hoc comparisons that showed that the perceived TC (our indirect measure of the sense of agency) was significantly stronger in the active trials compared to the passive ones, only in HC and only when the real temporal interval between the action and the outcome was equal to 200 ms [HC—condition: active, delay: 200 ms, versus condition: passive, delay: 200 ms: t(2569) = −3.58, Bonferroni corrected P = 0.024, Cohen’s d = 0.66; GTS—condition: active, delay: 200 ms, versus condition: passive, delay: 200 ms, group: 2: t(2569) = −2.4, Bonferroni corrected P = 0.1, Cohen’s d = 0.49].
At longer delays, the TC was not significantly different between active and passive conditions, in both groups [HC—condition: active, delay: 400 ms, versus condition: passive, delay: 400 ms: t(2569) = −1.81, Bonferroni corrected P = 0.44, Cohen’s d = 0.33. GTS—condition: active, delay: 400 ms, versus condition: passive, delay: 400 ms: t(2569) = 0.39, Bonferroni corrected P > 0.99, Cohen’s d = 0.08. HC—condition: active, delay: 600 ms, versus condition: passive, delay: 600 ms: t(2569) = 1.01, Bonferroni corrected P > 0.99, Cohen’s d = 0.18. GTS—condition: active, delay: 600 ms, versus condition: passive, delay: 600 ms: t(2569) = −0.34, Bonferroni corrected P > 0.99, Cohen’s d = 0.07]. See Fig. 2.
Figure 2.
Behavioural results: TC values. TC values for the active and passive conditions recorded at 200, 400 and 600 ms of action-outcome delay for HC and GTS patients. Error bars = standard error; asterisks indicate significant results at P < 0.05 Bonferroni corrected. TC is visualized as the percentage of the time delay of the outcome
Correlation between clinical data and behavioural results
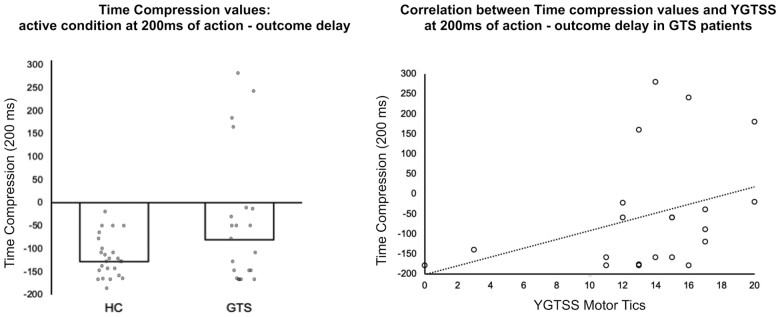
In order to deepen the relationship between TC and clinical data, we performed a correlation analysis between TC values recorded during the active condition at 200 ms (the condition where we found the intentional binding effect in HC) and the severity of the motoric symptoms (measured with the YGTSS scale, subscale motor tics). In particular, we were interested in testing the hypothesis that greater severity of the motoric symptomatology would lead to a reduced sense of agency, and therefore reduced TC values.
We applied a non-parametric correlation analysis since the data were not normally distributed (Shapiro–Wilk’s P-value < 0.05).
We observed a significant positive association between the data: the lower the TC data, the more severe the motoric symptoms (Spearman’s ρ = 0.46; one-tailed P-value = 0.028). See Fig. 3.
Figure 3.
Behavioural results: correlation between behavioural and clinical data. Correlation analysis between the TC values recorded during the active condition at 200 ms (the condition where we found the intentional binding effect in HC) and the severity of the motoric symptoms (measured with the YGTSS scale–subscale motor tics). NB: two sets of data point are overlapping in the scatter-plot graph
Our sample was characterized by a range of comorbid psychiatric disorders, notably Obsessive–Compulsive Disorder and Attention Deficit Hyperactivity Disorder. However, correlation analyses indicate that these confounders are not directly related to TC data (highest Spearman’s ρ = −0.3, lowest P-value = 0.18).
Correlations with neuroleptic medication levels
None of the behavioural data (TC values in the different action-outcome delays) was significantly correlated with the neuroleptic medication levels (highest Spearman’s ρ = 0.168, lowest P-value = 0.466).
Functional magnetic resonance imaging results
Analysis of head motion parameters during the functional magnetic resonance imaging session
There were no significant between-group differences for any of the realignment parameters (see Supplementary Table 1 for the details).
Main effect of the factor condition
Active condition ≥ passive condition (independently from the group and the different action-outcome delay)
The results showed significant activations in a large bilateral brain network, including prefrontal, premotor, motor, somatosensory regions and cerebellum. Further activations were found in the occipital cortices (see Supplementary Table 2a and Fig. 1a).
Passive condition ≥ active condition (independently from the group and the different action-outcome delay)
The results showed significant bilateral activations in the secondary somatosensory areas and in the middle temporal gyrus (see Supplementary Table 2b and Fig. 1b).
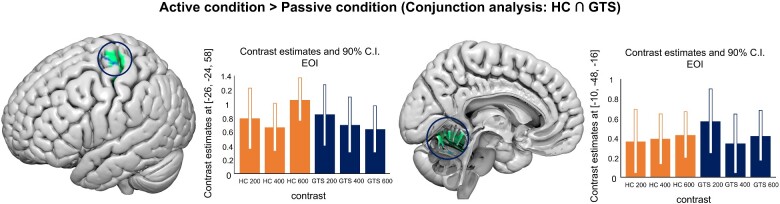
Active condition ≥ passive condition (conjunction analysis: HC GTS)
The results showed similar activations in the left motor/premotor network and in the left cerebellum in both HC and GTS participants (see Table 2 and Fig. 4).
Table 2.
Results of the conjunction analysis between HC and GTS of the comparison active > passive trials (independently from the different action-outcome delay)
| Brain regions | MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere |
Right hemisphere |
|||||||
| x | y | z | Z-score | x | y | z | Z-score | |
| Active condition > passive condition (conjunction analysis) | ||||||||
| Precentral gyrus (6) | −26 | −24 | 58 | 4.1 | ||||
| −28 | −24 | 64 | 4.0 | |||||
| −26 | −22 | 68 | 4.0 | |||||
| Precentral gyrus (4) | −40 | −22 | 56 | 3.3 | ||||
| Cerebellum_4_5 | −12 | −54 | −14 | 3.9 | ||||
| −20 | −52 | −20 | 3.6 | |||||
| Cerebellum_6 | −14 | −58 | −16 | 3.9 | ||||
| −22 | −56 | −18 | 3.8 | |||||
| −26 | −54 | −20 | 3.7 | |||||
Figure 4.
fMRI results: conjunction analysis. Conjunction analysis (HC GTS) for the contrast active condition > passive condition (for all the action-outcome delays)
Passive condition ≥ active condition (conjunction analysis: HC GTS)
No region displayed a significant effect.
Main effect of the factor group
Active condition ≥ passive condition (HC ≥ GTS or GTS ≥ HC)
No region displayed a significant effect.
Passive condition ≥ active condition (HC ≥ GTS or GTS ≥ HC)
No region displayed a significant effect.
Interaction effect between condition (active/passive) and the specific time delays (200/400/600 ms)
No region displayed a significant effect.
Interaction effect between group (GTS/HC), condition (active/passive) and the specific time delays (200/400/600 ms)
No region displayed a significant effect.
Linear regression analyses between functional magnetic resonance imaging blood oxygenation level dependent responses and behavioural data
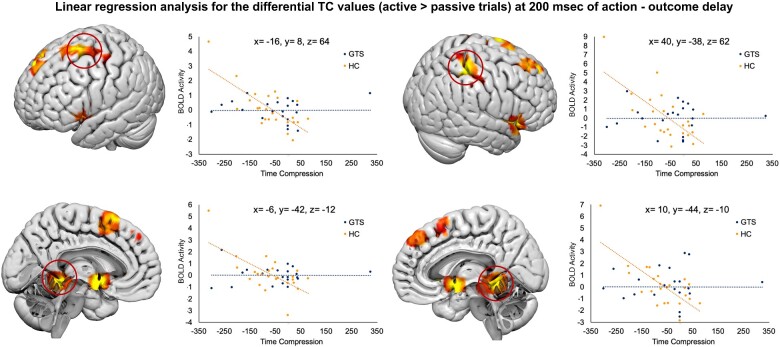
Time compression values (active ≥ passive trials) at 200 ms action-outcome delay
In the HC, we found a significant correlation between the differential TC values of individual participants and the blood oxygen level dependent (BOLD) signal in the left pre-supplementary motor area, in the left precentral gyrus (Brodmann area 6), in the superior parietal lobule (Brodmann area 40) and the postcentral gyrus (Brodmann area 2). We also found significant correlations in the insular cortex, in the cerebellum bilaterally, in the left hippocampus and in the bilateral superior frontal gyrus (Brodmann areas 6 and 8). Further activations were found in the bilateral thalamus and in the left pallidus. Higher negative values of TC (estimated time interval shorter than the real interval) corresponded to higher BOLD activity in these areas for the active trials compared to passive ones in HC. See Table 3 and Fig. 5.
Table 3.
Linear regression analysis between fMRI data collected for trials with action-outcome of 200 ms, with time compression data (at 200 ms action-outcome delay)
| Brain regions | MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere |
Right hemisphere |
|||||||
| x | y | z | Z-score | x | y | z | Z-score | |
| Sup. frontal med. gyrus (9) | 10 | 44 | 44 | 4.0 | ||||
| Inf. frontal orb. gyrus (45) | 58 | 22 | −2 | 3.3 | ||||
| Inf. frontal tri. gyrus (45) | −48 | 24 | 2 | 4.0 | ||||
| −44 | 28 | 4 | 3.7 | |||||
| −54 | 22 | 0 | 3.2 | |||||
| Rolandic opercular gyrus | 60 | 16 | 0 | 3.2 | ||||
| Sup. frontal gyrus (8) | 14 | 26 | 48 | 4.1 | ||||
| 14 | 22 | 50 | 3.8 | |||||
| Sup. frontal gyrus (9) | 18 | 32 | 48 | 4.4 | ||||
| Sup. frontal gyrus (6) | −18 | 4 | 64 | 4.4 | ||||
| −14 | 10 | 54 | 3.5 | |||||
| Precentral gyrus (6) | −28 | −12 | 62 | 3.4 | ||||
| −26 | −8 | 66 | 3.3 | |||||
| Pre-supplementary motor area (6) | −16 | 8 | 64 | 4.5 | ||||
| −4 | 18 | 62 | 3.7 | |||||
| −8 | 10 | 54 | 3.7 | |||||
| −6 | 14 | 54 | 3.4 | |||||
| −4 | 18 | 56 | 3.4 | |||||
| Precentral gyrus (4) | −40 | −20 | 58 | 3.4 | ||||
| −38 | −18 | 62 | 3.2 | |||||
| Postcentral gyrus (2) | 38 | −38 | 62 | 4.4 | ||||
| 34 | −40 | 64 | 4.1 | |||||
| 52 | −30 | 50 | 3.7 | |||||
| 32 | −40 | 68 | 3.6 | |||||
| Sup. parietal lobule (40) | 36 | −42 | 60 | 4.1 | ||||
| Insula | −40 | 12 | 2 | 4.2 | 40 | 22 | −6 | 4.1 |
| −40 | 4 | −8 | 3.5 | 46 | 14 | −4 | 4.5 | |
| −42 | 6 | −4 | 3.4 | |||||
| Hippocampus | −20 | −30 | −4 | 3.3 | ||||
| Parahippocampal gyrus | −18 | −34 | −6 | 3.5 | 22 | −34 | −10 | 4.2 |
| Cerebellum_4_5 | −14 | −44 | −10 | 4.3 | 10 | −46 | −6 | 4.1 |
| 10 | −44 | −10 | 4.0 | |||||
| 12 | −40 | −10 | 3.9 | |||||
| Vermis_3 | −2 | −38 | −10 | 3.3 | ||||
| Pallidum | −20 | −2 | −4 | 4.4 | ||||
| −22 | −8 | 0 | 4.3 | |||||
| −24 | −16 | −2 | 3.5 | |||||
| Thalamus | −18 | −8 | 2 | 4.2 | 16 | −10 | −2 | 4.0 |
| 8 | −4 | −6 | 3.7 | |||||
Figure 5.
fMRI results: linear regression with TC values. Linear regression analysis between the BOLD activity recorded during the task and the differential TC values (active trials–passive trials) when the action-outcome delay was 200 ms and a significant intentional binding was observed in HC (but not in GTS)
Conversely, GTS patients did not show any activation of this sense of agency network: no brain regions were significantly correlated, in terms of functional activity, with the magnitude of the yet limited intentional binding phenomenon, in any action-outcome delays. See Fig. 5.
The Fisher r-to-z transformation showed that the correlation coefficients (r) calculated for HC participants (indicating the strength of the association between the BOLD activity of the key regions of the sense of agency network and the individually measured TC values) were significantly higher than the same coefficients calculated for the GTS group (pre-supplementary motor area: r HC = −0.73, r GTS = 0.0003, z = −2.89, P-value = 0.004. Parietal site: r HC = −0.68, r GTS = 0.00009, z = −2.58, P-value = 0.01. Cerebellum left: r HC = −0.65, r GTS = 0.0008, z = −2.42, P-value = 0.01. Cerebellum right: r HC = −0.73, r GTS = 0.0003, z = −2.89, P-value = 0.004).
Finally, in order to check whether the abnormal activation of the agency network in the GTS was related to the neuroleptics medication dosage, we correlated the activity of some key regions of this network with the chlorpromazine equivalent scores. The activity of these regions was not significantly associated with the drug dosage (highest Spearman’s ρ = 0.281, lowest P-value = 0.23).
Time compression (active ≥ passive trials) at 400 and 600 ms action-outcome delays
No region displayed a significant correlation with the differential TC values for longer delays between action and the lighting-up of the light-bulb, nor in GTS neither in HC participants.
Discussion
In this paper, we tested the hypothesis that the sense of agency might be impaired, at the behavioural and at the brain activation level, in GTS, a movement disorder characterized by the presence of unwanted movements called tics. Given the ambiguity over voluntariness of tics in GTS, we considered whether the tic disorder might also negatively influence the experience of voluntary action.
We studied GTS patients and matched HC with a recent fMRI temporal-judgement task (Zapparoli et al., 2020). Through this paradigm, we measured the intentional binding phenomenon, an implicit index of the sense of agency (Haggard et al., 2002), which validity is currently assumed on the following grounds: (i) the measure is obtained via the comparison of perceptual judgements, for active versus passive movements, on the physical consequences of movements: subjects report shorter intervals between action and the caused visual effects, and this temporal compression is systematically stronger for active movements (for a review see Moore and Obhi, 2012); (ii) patients with a perturbed sense of agency, like schizophrenic patients with delusion of control, have a perturbed intentional binding (Haggard et al., 2003; Voss et al., 2010); and (iii) the measure correlates with the brain activity of regions involved in intentional motor planning (Kühn et al., 2013; Zapparoli et al., 2020). Importantly, the binding of actions and effects (i.e., TC) occurs only when the subject's motor commands are the cause of the subsequent effect. This situation is different from the scenario of someone being the involuntary cause of and an external event like for involuntary or passive movements: importantly, these produce less binding—less TC—than voluntary actions, or even the reverse effect (Engbert et al., 2007, 2008; Wenke and Haggard, 2009).
Measuring the intentional binding phenomenon might have the potential of shedding further light on the nature of the GTS disorder providing, in return, some face validity to the conjectures that we recently formulated on the nature of the sense of agency (Zapparoli et al., 2020). We found an abnormal intentional binding in our GTS patients and no correlation of the underlying behavioural measure (the differential TC for actives versus passive movements) with the activity of the brain regions that correlate with intentional binding in HC. Most importantly, we also found a correlation between the severity of the motor symptoms in our GTS patients and the reduction of intentional binding. These findings complete the circle of a conceptual validation of the implicit sense of agency as a phenomenon anchored to the functioning of the motor system. Moreover, it provides new hints about the functioning of the motor system for those suffering from GTS.
Abnormal sense of agency in Gilles de la Tourette syndrome
In our study, HC show the expected intentional binding effect, but only at 200 ms of delay between actions and their outcomes. This stringent temporal contiguity probably mimics real-life conditions, where latency of ∼200 ms is the one that can be measured between the time when we press an electricity light-switch and the time that a conventional light-bulb takes to be fully on (Sivak et al., 1994). This binding effect is absent in GTS for any action-outcome delay.
We interpret this result as an indication of a reduced sense of control over voluntary actions in patients with GTS. More precisely, as we accept that the temporal action/perception pattern of the intentional binding phenomenon represents a measurable manifestation of the specific cognitive function that allows one to implicitly distinguish the effects caused by his actions from the ones generated by others’, we interpreted the absence of any intentional binding effect in the patient group as a sign of an absent implicit agency experience in Gilles de la Tourette’s syndrome: GTS patients do not implicitly experience agency in circumstances where they should. In other words, GTS may implicitly treat as non-self-generated a consequence that should be treated as a self-induced one.
Importantly, the action-outcome binding reduction in GTS was related to the severity of the disease in terms of motoric symptoms: the greater the severity of motor tics, as measured by the YGTSS, the more reduced the TC for intentional acts.
We discuss these findings in the domain of general impairment of action monitoring processes in GTS. Kim et al. (2019) have already found evidence of abnormal monitoring of proprioceptive movement-outcomes in GTS patients. In particular, they showed that while the GTS patients were equally accurate and no more variable than a matched control group in executing outward movements, they were significantly less accurate and exhibited greater movement variability than controls when executing return movement. The authors interpreted these findings as consistent with the view that individuals with GTS may experience difficulties in updating the forward model estimates, which are necessary to correct the ongoing motor plans by taking into account the incoming proprioceptive information with our results, we expand this previous evidence, suggesting that less precise action monitoring abilities in GTS may not be limited to proprioceptive movement-outcomes (e.g., the position of the hand in the space after the execution of the movement, Kim et al., 2019) but they may also generalize to the external consequences generated by the actions (e.g., the lightening of a light-bulb after the pressing of a button). We propose that GTS may have an impairment of forward models that permit an accurate monitoring of self-generated actions and their proprioceptive and exteroceptive effects. This would explain why GTS patients do not show an intentional binding effect between actions and their effects in the external environment.
We are aware that further studies might provide evidence on a causal relationship between the altered agency experience in GTS patients and the proposed impairment in forward model estimates. Therefore, other possible explanations, such as the establishment of a causal link between a voluntary action and its effect or the temporal control over the timing of the event, still need to be specifically tested (see, for example, Desantis et al., 2012).
Explicit and implicit agency in Gilles de la Tourette syndrome
Disturbed sense of agency in GTS patients can have manifestations on both sides of the spectrum: explicit over-attribution of the sense of agency to the self, as in Delorme et al. (2016), and reduced intentional binding effect, as in our findings.
Compensatory mechanisms and separable agency processing systems may explain this scenario. Indeed, Delorme et al. showed that GTS patients exhibited exaggerated self-causality attribution when their performance is artificially enhanced. As the authors suggested, an illusion of agency may result from a compensatory mechanism related to increased cognitive control over tics, which in turn could enhance the experience of control. Yet, it is worth mentioning that these findings were based on explicit agency judgements made by the participants on their performance. In other words, these measures, as all explicit meta-cognitive evaluations, were directly mediated by the conscious control of the participants and introspection (Schüür and Haggard, 2011; Obhi, 2012). One can therefore speculate that GTS patients dispose of compensatory mechanisms, which enhance their experience of agency, but those mechanisms are based on overt control, while they do not occur at an implicit level. Previous theories have already spoken in favour of separable implicit and explicit agency processing systems (Synofzik et al., 2008). For example, Synofzik et al. (2008) distinguished between two distinct forms of agency experience: the ‘judgement of agency’ and the ‘feeling of agency’. The ‘judgement of agency’ refers to the conceptual, interpretative, explicit judgments of being the agent of an outcome (‘Did I do that?’). The ‘feeling of agency’ represents the non-conceptual, implicit feeling of control that accompanies their own actions, in the absence of any conscious thought. While the dominant experimental paradigm addressing the ‘judgement of agency’ involves the request of performing explicit judgments, the ‘feeling of agency’ has been investigated by means of implicit paradigms, which are able to capture this feeling without requiring people to overtly think about their agency. With this regard, it has been proposed that intentional binding is linked to lower-level implicit aspects of the sense of agency, mediated by automatic associative learning mechanisms (Moore et al., 2011). In line with this proposal, Delorme found a correlation between his agency measure and the self-assessment of the severity of the disorder (the YGTSS global scale). Conversely, we showed that the action-outcome binding reduction was related to the severity of the disease in terms of motoric symptoms: the greater the severity of motor tics, as measured by the YGTSS, the more reduced the TC for intentional acts. It looks, therefore, even more plausible that the implicit and explicit agency processing systems are actually separable. The dissociation between implicit and explicit processing is constantly seen for psychological functions, like in measures of biases (Moore et al., 2012), and therefore the sense of agency represents no exception. This possibility would account for the discrepancy between explicit measures and intentional binding results: while GTS patients report exaggerated agency judgments when directly asked about their performance, the implicit experience of agency (i.e., the intentional binding effect) is weaker compared to healthy subjects.
Abnormal activation of the agency brain network in Gilles de la Tourette syndrome
The abnormal functioning of the agency network in GTS patients was also evident from the fMRI data that differed substantially from those of the HC participants. For HC, there was a significant correlation between the magnitude of the intentional binding effect and the BOLD activity of a premotor–parietal circuit. These findings are in line with previous neurofunctional studies investigating the neural correlates of the sense of agency (see, for metanalytical reviews, Sperduti et al., 2011; Seghezzi et al., 2019a, b).
Crucially, GTS patients did not show any noticeable activity within the agency network: in no brain region, there was a significant correlation of the BOLD response with the magnitude of the yet limited intentional binding phenomenon, in any of the action-outcome delays.
Previous studies demonstrated that GTS is characterized by abnormal brain activations at the level of the premotor cortices of the medial wall (supplementary motor area/anterior cingulate cortex), in various aspects of tic generation and tic suppression (Zapparoli et al., 2015; Polyanska et al., 2017). In our results, a similar premotor network was specifically related to the agency experience in HC. Therefore, one can hypothesize a direct resource competition within brain regions involved in both the generation of the sense of agency in tic generation/tic suppression.
How many mechanisms behind the sense of agency? Hints from movement disorders
Previous studies already described an abnormal sense of agency in populations affected by movement disorders. For example, Saito et al. (2017) showed a reduced intentional binding effect over voluntary actions in Parkinson’s disease patients (Saito et al., 2017). An abnormal sense of agency has also been reported in patients with functional movement disorders. In these patients, voluntary actions are associated with reduced intentional binding effect compared to healthy volunteers (Kranick et al., 2013). Our results show that an abnormal sense of agency also characterizes the GTS, adding GTS to the list of movement disorders that can show such symptoms.
How is it possible that so many different forms of movement disorders can be associated with a perturbed intentional binding? Prima facie, one would be tempted to think that different mechanisms, at different points in a distributed neural system, may affect the ability to connect own actions and external consequences with the same temporal patterns shown by healthy subjects. Alternatively, one may postulate that augmented noise, of whatever origin, within the neural system involved in action generation, monitoring and implementation can reduce the ability to connect willed actions and their consequences in time. Finally, the altered intentional binding may be the measurable behavioural phenomenon that may be generated by different causing mechanisms. At present, we do not have an answer to these questions. Yet, their very existence leaves the possibility of discovering more on the sense of agency and the underlying neural mechanisms by comparing the different pathological populations affected. Admittedly, this is left to future studies.
Limitations of the study
At the moment of the fMRI examination, our patients, all adult GTS patients with a chronic enduring disorder, were under treatment, similarly with what reported in several previous imaging studies (for a review, see Martino et al., 2018, Table 2; or Kim et al., 2019). The co-occurrence of medication limits the possibility of making firm conclusions, based on imaging findings, about the ‘true nature of GTS’ as it would be possible by the observation of unmedicated patients. However, our findings remain relevant for a substantial proportion of adult GTS patients from the real world, the medicated ones. According to some estimates, these can be more than 20% of the adult GTS population (Burd et al., 2001).
We believe that our selection criteria were not such to prevent the observation of meaningful fMRI differences between GTS patients and age-matched controls. Our patients were sufficiently ill to need medication, but they still had manifestations compatible with the fMRI examination. The physiology of the more severe patients, with massive uncontrollable tics, remains to be explored, possibly with techniques that have fewer practical constraints than fMRI.
Crucially, our estimate of the impact of neuroleptic medication on all variables considered shows no significant effects on our conclusions.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgement
We thank the technical staff of the IRCCS Galeazzi for making this study possible.
Funding
This work was supported by a grant funded by the Italian Ministry of Health (Ricerca Corrente; Project L3025; PI E.P.) and by the Italian Ministry of Education, Research and University (PRIN; Project 201794KEER; PI E.P.).
Competing interests
The authors declare that they have no competing interests.
Data and materials availability
All data, code and materials are available upon request to the corresponding author L.Z.
Glossary
- BOLD =
blood oxygen level dependent
- fMRI =
functional magnetic resonance imaging
- GTS =
Gilles de la Tourette syndrome
- HC =
healthy controls
- TC =
time compression
- YGTSS =
Yale Global Tic Severity Scale
References
- Blakemore SJ, Frith C.. Self-awareness and action. Curr Opin Neurobiol 2003; 13: 219–24. [DOI] [PubMed] [Google Scholar]
- Burd L, Kerbeshian PJ, Barth A, Klug MG, Avery PK, Benz B.. Long-term follow-up of an epidemiologically defined cohort of patients with Tourette syndrome. J Child Neurol 2001; 16: 431–7. [DOI] [PubMed] [Google Scholar]
- Cavanna AE. The neuropsychiatry of Gilles de la Tourette syndrome: the état de l’art. Rev Neurol (Paris) 2018; 174: 621–7. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Nani A.. Tourette syndrome and consciousness of action. Tremor Other Hyperkinet Mov (N Y) 2013; 3: tre-03-181-4368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Black KJ, Hallett M, Voon V.. Neurobiology of the premonitory urge in Tourette’s syndrome: pathophysiology and treatment implications. J Neuropsychiatry Clin Neurosci 2017; 29: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen AC, Frey HC.. Probabilistic techniques in exposure assessment. A handbook for dealing with variability and uncertainty in models and inputs. New York/London: Plenum Press; 1999. [Google Scholar]
- Daprati E, Franck N, Georgieff N, Proust J, Pacherie E, Dalery J, et al. Looking for the agent: an investigation into consciousness of action and self-consciousness in schizophrenic patients. Cognition 1997; 65: 71–86. [DOI] [PubMed] [Google Scholar]
- Delorme C, Salvador A, Voon V, Roze E, Vidailhet M, Hartmann A, et al. Illusion of agency in patients with Gilles de la Tourette syndrome. Cortex 2016; 77: 132–40. [DOI] [PubMed] [Google Scholar]
- Desantis A, Hughes G, Waszak F.. Intentional binding is driven by the mere presence of an action and not by motor prediction. PLoS One 2012; 7: e29557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert K, Wohlschläger A, Haggard P.. Who is causing what? The sense of agency is relational and efferent-triggered. Cognition 2008; 107: 693–704. [DOI] [PubMed] [Google Scholar]
- Engbert K, Wohlschläger A, Thomas R, Haggard P.. Agency, subjective time, and other minds. J Exp Psychol Hum Percept Perform 2007; 33: 1261–8. [DOI] [PubMed] [Google Scholar]
- Flandin G, Friston KJ.. Analysis of family-wise error rates in statistical parametric mapping using random field theory. Hum Brain Mapp 2019; 40: 2052–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD.. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage 1996; 4: 223–35. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore SJ, Wolpert DM.. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B 2000; 355: 1771–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganos C, Asmuss L, Bongert J, Brandt V, Münchau A, Haggard P.. Volitional action as perceptual detection: predictors of conscious intention in adolescents with tic disorders. Cortex 2015; 64: 47–54. [DOI] [PubMed] [Google Scholar]
- Haggard P. Sense of agency in the human brain. Nat Rev Neurosci 2017; 18: 196–207. [DOI] [PubMed] [Google Scholar]
- Haggard P, Clark S, Kalogeras J.. Voluntary action and conscious awareness. Nat Neurosci 2002; 5: 382–5. [DOI] [PubMed] [Google Scholar]
- Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N.. Awareness of action in schizophrenia. Neuroreport 2003; 14: 1081–5. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Sprenger A, Baumung L, Alvarez-Fischer D, Münchau A, Brandt V.. Help or hurt? How attention modulates tics under different conditions. Cortex 2019; 120: 471–82. [DOI] [PubMed] [Google Scholar]
- Kim S, Jackson GM, Dyke K, Jackson SR.. Impaired forward model updating in young adults with Tourette syndrome. Brain 2019; 142: 209–19. [DOI] [PubMed] [Google Scholar]
- Kranick SM, Moore JW, Yusuf N, Martinez VT, LaFaver K, Edwards MJ, et al. Action-effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov Disord 2013; 28: 1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Brass M, Haggard P.. Feeling in control: neural correlates of experience of agency. Cortex 2013; 49: 1935–42. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE.. Attention to intention. Science 2004; 303: 1208–10. [DOI] [PubMed] [Google Scholar]
- Martino D, Ganos C, Worbe Y.. Neuroimaging applications in Tourette’s syndrome. Int Rev Neurobiol 2018; 143: 65–108. [DOI] [PubMed] [Google Scholar]
- Moore JW, Fletcher PC.. Sense of agency in health and disease: a review of cue integration approaches. Conscious Cogn 2012; 21: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Obhi SS.. Intentional binding and the sense of agency: a review. Conscious Cogn 2012; 21: 546–61. [DOI] [PubMed] [Google Scholar]
- Moore JW, Dickinson A, Fletcher PC.. Sense of agency, associative learning, and schizotypy. Conscious Cogn 2011; 20: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Middleton D, Haggard P, Fletcher PC.. Exploring implicit and explicit aspects of sense of agency. Conscious Cogn 2012; 21: 1748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Schneider SA, Schwingenschuh P, Moretto G, Bhatia KP, Haggard P.. Dopaminergic medication boosts action-effect binding in Parkinson’s disease. Neuropsychologia 2010; 48: 1125–32. [DOI] [PubMed] [Google Scholar]
- Moretto G, Schwingenschuh P, Katschnig P, Bhatia KP, Haggard P.. Delayed experience of volition in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry 2011; 82: 1324–7. [DOI] [PubMed] [Google Scholar]
- Obhi SS. The troublesome distinction between self-generated and externally triggered action: a commentary on Schüür and Haggard. Conscious Cogn 2012; 21: 587–8. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pacherie E, Green M, Bayne T.. Phenomenology and delusions: who put the ‘alien’ in alien control? Conscious Cogn 2006; 15: 566–77. [DOI] [PubMed] [Google Scholar]
- Polyanska L, Critchley HD, Rae CL.. Centrality of prefrontal and motor preparation cortices to Tourette syndrome revealed by meta-analysis of task-based neuroimaging studies. NeuroImage Clin 2017; 16: 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Takahata K, Yamakado H, Sawamoto N, Saito S, Takahashi R, et al. Altered awareness of action in Parkinson’s disease: evaluations by explicit and implicit measures. Sci Rep 2017; 7: 8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Yasuda A.. Illusion of sense of self-agency: discrepancy between the predicted and actual sensory consequences of actions modulates the sense of self-agency, but not the sense of self-ownership. Cognition 2005; 94: 241–55. [DOI] [PubMed] [Google Scholar]
- Schüür F, Haggard P.. What are self-generated actions? Conscious Cogn 2011; 20: 1697–704. [DOI] [PubMed] [Google Scholar]
- Seghezzi S, , Zapparoli L. Predicting the sensory consequences of self-generated actions: pre-supplementary motor area as supra-modal hub in the sense of agency experience. Brain Sci 2020; 10: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi S, Giannini G, Zapparoli L.. Neurofunctional correlates of body-ownership and sense of agency: a meta-analytical account of self-consciousness. Cortex 2019. a; 121: 169–78. [DOI] [PubMed] [Google Scholar]
- Seghezzi S, Zirone E, Paulesu E, Zapparoli L.. The brain in (willed) action: a meta-analytical comparison of imaging studies on motor intentionality and sense of agency. Front Psychol 2019. b; 10: 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak M, Flannagan MJ, Sato T, Traube EC, Aoki M.. Reaction times to neon, LED, and fast incandescent brake lamps. Ergonomics 1994; 37: 989–94. [DOI] [PubMed] [Google Scholar]
- Sperduti M, Delaveau P, Fossati P, Nadel J.. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct 2011; 216: 151–7. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Vosgerau G, Newen A.. I move, therefore I am: a new theoretical framework to investigate agency and ownership. Conscious Cogn 2008; 17: 411–24. [DOI] [PubMed] [Google Scholar]
- Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P.. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain 2010; 133: 3104–12. [DOI] [PubMed] [Google Scholar]
- Wenke D, Haggard P.. How voluntary actions modulate time perception. Exp Brain Res 2009; 196: 311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapparoli L, Macerollo A, Joyce EM, Martino D, Kilner JM.. Voluntary tic suppression and the normalization of motor cortical beta power in Gilles de la Tourette syndrome: an EEG study. Eur J Neurosci 2019; 50: 3944–57. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Porta M, Gandola M, Invernizzi P, Colajanni V, Servello D, et al. A functional magnetic resonance imaging investigation of motor control in Gilles de la Tourette syndrome during imagined and executed movements. Eur J Neurosci 2016; 43: 494–508. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Porta M, Paulesu E.. The anarchic brain in action: the contribution of task-based fMRI studies to the understanding of Gilles de la Tourette syndrome. Curr Opin Neurol 2015; 28: 604–11. [DOI] [PubMed] [Google Scholar]
- Zapparoli L, Seghezzi S, Scifo P, Zerbi A, Banfi G, Tettamanti M, et al. Dissecting the neurofunctional bases of intentional action. Proc Natl Acad Sci U S A 2018; 115: 7440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapparoli L, Seghezzi S, Zirone E, Guidali G, Tettamanti M, Banfi G, et al. How the effects of actions become our own. Sci Adv 2020; 6: eaay8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapparoli L, Tettamanti M, Porta M, Zerbi A, Servello D, Banfi G, et al. A tug of war: antagonistic effective connectivity patterns over the motor cortex and the severity of motor symptoms in Gilles de la Tourette syndrome. Eur J Neurosci 2017; 46: 2203–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, code and materials are available upon request to the corresponding author L.Z.