Abstract
Alzheimer’s disease and depressive disorder are frequent in old age. Both may be associated with depressed mood and cognitive impairment. Therefore, finding a strategy to clarify the diagnosis underlying subjective complaints of impaired cognition and depressed mood in older persons is of utmost interest. We conducted a cross-sectional retrospective observational clinical cohort study using patient records from 2014 to 2018. From 3758 patients, we included patients aged 60 years and older with a Mini-Mental-Status Examination score of 24 and above. Final analysis included all patients in whom Alzheimer’s disease biomarker analysis was performed (cerebrospinal fluid markers of Alzheimer’s disease or positron emission tomography imaging; n = 179) and patients with depressive disorder in whom Alzheimer’s disease was ruled out by analysis of biomarkers suggestive of Alzheimer’s disease (n = 70). With case–control matching for age, education and gender, performance of patients with Alzheimer’s disease was worse in acquisition, consolidation and recall of verbal information and false-positive answers. None of the results, however, sufficed to differentially diagnose individual patients with Alzheimer’s disease or depressive disorder. With more severe symptoms of depression, patients with biomarker-verified Alzheimer’s disease performed worse in executive testing but were not additionally impaired in verbal episodic memory performance. We conclude that distinguishing between Alzheimer’s disease and depressive disorder is unreliable on clinical grounds and behavioural testing alone. Diagnosing the cause of subjective complaints about deteriorating cognitive function or depressed mood requires additional biomarker assessment, whereas cognitive assessment is needed to define appropriate targets of symptomatic treatment in patients with Alzheimer’s disease and depressive disorder.
Keywords: Alzheimer’s disease, depression, cognition, biomarkers, neuropsychology
Both Alzheimer’s disease and depressive disorder may be associated with depressed mood and cognitive impairment. Distinguishing between the two conditions on clinical and behavioural grounds alone is unreliable. Diagnosing the cause of subjective complaints about deteriorating cognitive function or depressed mood requires additional biomarker assessment.
Graphical Abstract
Graphical Abstract.
Introduction
Two of the most common health conditions among older persons are Alzheimer’s disease (AD) and depressive disorder (DD) (Ritchie and Lovestone, 2002; Alexopoulos, 2005). Both AD and DD may go along with cognitive and affective symptoms. Distinguishing between AD and DD has important therapeutic implications and current and future treatment options warrant to establish an accurate diagnosis as early as possible.
Lesions of medial temporal lobe at the onset of AD result in deficits of episodic memory and spatial orientation (Hodges, 2000). Spread of the disease to the frontal and parietal cortex further impairs executive functions, planning, attention, working memory and visuo-spatial functions (Hodges, 2000). Depressive symptoms are frequent in patients with AD (Fischer, 1996; Kobayashi and Kato, 2011) and may even be present at its onset (Verdaguer et al., 2020).
Depressive disorder is characterized by depressed mood, diminished drive and anhedonia. Despite being frequently reported, characteristics of cognitive impairment in DD are less well understood and no particular pattern is associated with the severity of DD. Accruing evidence over recent years demonstrates that patients with DD may be impaired in several cognitive tasks such as short-term memory, sustained and selective attention, alertness, cognitive flexibility and executive functions (Williams et al., 2000; Landrø et al., 2001; Weiland-Fiedler et al., 2004; Paelecke-Habermann et al., 2005; Lanza et al., 2020). Moreover, different memory processes such as encoding and retrieval are also impaired (Elderkin-Thompson et al., 2007; Taconnat et al., 2010; Mesholam-Gately et al., 2012). It is unclear whether memory deficits are an independent cognitive symptom of DD or secondary to executive dysfunction (Fossati et al., 2002).
The attempt to differentiate between AD and DD on clinical and behavioural grounds is inconclusive (Elderkin-Thompson et al., 2003; Butters et al., 2004; Elderkin-Thompson et al., 2007, 2009; Beblo et al., 2011; Elderkin-Thompson et al., 2011; Mesholam-Gately et al., 2012; Paula et al., 2013). Thus, a conceptual transition to use a biological framework applying biochemical or imaging biomarkers to diagnose AD has been suggested (Dubois et al., 2007; Kester et al., 2009; Blennow et al., 2015; Jack et al., 2018).
A recent consensus article concluded that AD is ruled out if cerebrospinal fluid (CSF)-biomarkers of AD are negative (Molinuevo et al., 2014). Longitudinal data additionally demonstrate that a decrease of Abeta1-42 precedes cognitive impairment (Jack et al., 2013; Villemagne et al., 2013; Young et al., 2014). Thus, normal levels of Abeta1-42 exclude underlying AD as a cause of cognitive impairment. Levels of tau-protein can further support the diagnosis and help to distinguish between patients with age-associated neurodegenerative or even vascular disease (Andreasen et al., 2001; Goossens et al., 2017; Paterson et al., 2018). In cases where CSF-biomarkers are unavailable positron emission tomography (PET) biomarkers can be used to verify or exclude AD (Rice and Bisdas, 2017).
Analysis of biomarker information thus allows to select patients in whom the diagnosis of AD has to be considered as verified. Similarly, AD is ruled out if biomarker assessment is inconspicuous, i.e. no evidence of tau- or Abeta-pathology. As far as we are aware, biomarkers have not been used to rule out AD pathology in patients with DD. Our goal was to investigate whether the cognitive profile of patients with verified AD is distinct from the cognitive profile of patients with verified DD. We also assessed the impact of depressive symptoms on the cognitive profile of patients with verified AD.
Materials and methods
Our observational clinical cohort study used patient’s records of the gerontopsychiatric services at Ulm University at Bezirkskrankenhaus Günzburg from 2014 to 2018. The study was approved by the ethics committee of Ulm University (289/18) and was conducted according to the guidelines outlined in the declaration of Helsinki (2013).
Study sample
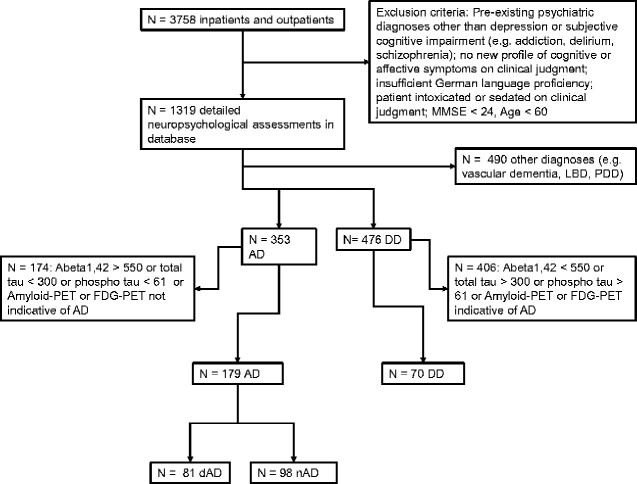
Geriatric Psychiatry services of Ulm University at Bezirkskrankenhaus Günzburg serve as a primary service for a rural catchment area of about 650 000 people. Patients are referred to this unit by surrounding hospitals, general practitioners as well as medical specialists in private practice. Among most frequent complaints presented by the patients are mood changes and memory impairment; however, lacking introspection on behalf of the patients often conflicts with the referral diagnosis or report by proxies. For this reason, a detailed neuropsychological assessment of patients presenting with either mood or cognitive complaints is included in our standard practice (exclusion criteria, Fig. 1). Further diagnostic procedures were initiated in three cases: (i) patients presented with a slowly progressing cognitive decline over many months, (ii) neuropsychological assessment indicated at least a moderate decline in episodic memory and (iii) patients remained worried after initial diagnostic steps and requested further diagnostic measures. The indeterminateness of the decision-making algorithm reflects the hitherto existing vague knowledge on the pattern of cognitive impairment due to DD and AD. We ruled out organic causes of cognitive impairment by performing CSF tap. In cases where CSF tap was contraindicated (e.g. due to anticoagulation) or patients refused the procedure, fluorodeoxyglucose-PET or Am-PET was performed instead. Additional fluorodeoxyglucose-PET or Am-PET was also initiated when clinical judgement and CSF tap results differed.
Figure 1.
Selection process and exclusion criteria. AD: Alzheimer’s disease; DD: depressive disorder; dAD: AD patients with depressive syndrome; nAD: AD patients without depressive syndrome.
Depressed mood was diagnosed phenotypically using criteria outlined in the ICD-10. Similar to a previously reported approach (Kessler et al., 2010), no organic exclusions or diagnostic hierarchy rules were applied in the diagnostic process.
Records of all in- and outpatients aged >60 years from 2014 to 2018 were included in the analysis. Age cut-off was set by following WHO recommendations (‘At the moment, there is no United Nations standard numerical criterion, but the UN agreed cut-off is 60+ years to refer to the older population.’; https://www.who.int/healthinfo/survey/ageingdefnolder/en/).
Differentiation between AD and DD is of particular importance in early stages of disease. A recent meta-analysis found that a cut-off score of 24 in the Mini-Mental State Examination (MMSE) is most appropriate to differentiate between normal and impaired cognition (Creavin et al., 2016). An elaborate definition of patients’ cognitive profiles above this threshold would facilitate an accurate diagnostic process even in the very mild stages of the disease. Other subgroups of these patients have been published previously (Lanza et al., 2020). This study aimed to select only the patients in whom biomarkers were concordant and characteristic for AD (inclusion criterion: Abeta1-42 < 550 pg/ml, total tau > 300 pg/ml and phospho-tau > 61 pg/ml). Same method was applied to rule out underlying AD pathology in patients with DD (inclusion criterion: Abeta1-42 > 550 pg/ml, total tau < 300 pg/ml and phospho-tau < 61 pg/ml). Applying these inclusion and exclusion criteria, 179 patients with diagnosis of AD and 70 patients with diagnosis of DD were analysed.
Demographic variables and neuropsychological data for all patients with verified diagnosis are summarized in Table 1.
Table 1.
Demographics and neuropsychological data of all AD and DD
| Neuropsychological assessment | AD (n = 179) | DD (n = 70) | P-value | R 2 |
|---|---|---|---|---|
| Median/IQR | Median/IQR | |||
| Age (years) | 78.0/72.0–83.0 | 73.6/60.0–88.0 | 0.000 | 0.05 |
| GDS | 4.0/2.0–7.75 | 6.0/4.0–11.0 | 0.000 | 0.06 |
| MMSE | 26.0 ± 25.0–28.0 | 27.0 ± 25.0–29.0 | 0.046 | 0.02 |
| CVLT1 | 3.0/2.0–4.0 | 3.0/2.0–5.0 | 0.004 | 0.03 |
| CVLT5 | 5.0/4.0–7.0 | 7.0/5.0–9.75 | 0.000 | 0.10 |
| CVLT total recall | 22.0/18.0–28.0 | 28.0/23.0–35.8 | 0.000 | 0.10 |
| CVLT delayed recall | 1.0/0.0–4.0 | 4.0/3.0–7.0 | 0.000 | 0.17 |
| CVLT delayed cued recall | 4.0/2.0–6.0 | 7.0/5.0–9.0 | 0.000 | 0.15 |
| CVLT recognition | 14.0/12.0–15.0 | 14.0/12.3–15.0 | 0.964 | <0.01 |
| CVLT false positive | 8.0/4.0–11.0 | 2.5/1.0–5.0 | 0.000 | 0.11 |
| Digit span forward | 7.0/5.5–8.0 | 6.5/5.0–8.0 | 0.514 | <0.01 |
| Digit span backward | 4.0/3.0–5.0 | 4.0/3.0–5.8 | 0.481 | <0.01 |
| Block span forward | 6.0/5.0–6.0 | 6.0/5.0–7.0 | 0.019 | 0.02 |
| Block span backward | 5.0/3.0–6.0 | 5.0/4.0–6.0 | 0.456 | <0.01 |
| Clock drawing | 3.0/2.0–4.0 | 2.5/1.0–3.0 | 0.004 | 0.03 |
| TMT-A | 77.0/56.0–112.0 | 58.0/38.5–97.5 | 0.001 | 0.05 |
| TMT-B | 210.0/164.0–268.0 | 162.0/109.0–245.0 | 0.001 | 0.04 |
| Semantic fluency | 13.0/10.5–17.0 | 14.5/12.0–20.0 | 0.020 | 0.02 |
| Phonematic fluency P | 5.0/3.0–8.0 | 6.0/3.0–8.0 | 0.364 | <0.01 |
| Phonematic fluency S | 8.0/4.0–10.0 | 7.0/5.0–10.0 | 0.819 | <0.01 |
Abbreviations: AD, Alzheimer’s disease; CVLT, California Verbal Learning Test; DD, depressive disorder; GDS, Geriatric Depression Scale; MMSE, Mini-Mental Status Examination; TMT-A, Trail-Making Test A; TMT-B, Trail-Making Test B.
Neuropsychological assessments
Clinical Scales
Mini-Mental Status Examination
The MMSE (Folstein et al., 1975) is an assessment of global cognitive function and comprises questions on orientation, attention, short-term memory, language and basic motor skills. The score ranges from 0 to 30. A score below 24 indicates a cognitive impairment.
Geriatric Depression Scale
In this short version of the Geriatric Depression Scale (Burke et al., 1991, 1992), depressive symptoms are assessed using 15 yes/no questions. A score of >5 indicates depression (van Den et al., 2001).
Clock drawing test
The clock drawing test (Shulman et al., 1993) requires participants to draw a face of a clock, indicating time ‘10 min after 11’. It is used as a screening test for cognitive impairment and spatial dysfunction. Scores range from 1 (perfect clock) to 6 (no clock is recognizable).
Specific neuropsychological tests
California Verbal Learning Test
The California Verbal Learning Test (CVLT) (Niemann et al., 2008) is a verbal memory test and assesses immediate and delayed, free and cued recall, as well as recognition. A list of 16 words falling into four different categories (fruit, clothing, drinks and tools) is read five times. After each round, participants are asked to recall as many words as possible. Immediate recall is followed by a free and cued delayed recall after 5 and 20-min intervals. At the end, participants are presented with a yes/no recognition task. Depending on age, sex and education, the score of delayed recall and recognition range from 10 to 15 and 14 to 16, respectively.
Digit and visual span (Wechsler Memory Scale Revised)
The digit span test (Härting et al., 2000) comprises forward and backward tasks. In the digit span, forward participants are instructed to repeat a sequence of digits in the order, in which they were presented. The task is terminated when the longest sequence of eight digits is reached or when participants incorrectly repeat two sequences of the same length. In the digit span backward, the same procedure is applied for repeating the digits in reversed order. Visual span was assessed using spatial sequences tapped on Corsi-block. Same as in digit span, visual span had forward and backward tasks. One point is given for each correct answer with scores ranging from 0 to 12 for digit span and visual span backward and from 0 to 14 for visual span forward. Mean digit span scores for healthy older persons are about 7–9 and 6–8 forward and backward, respectively. Mean visual span scores for healthy older persons are about 5–8 forward and 7–9 backward (Gron et al., 2002; Widmann et al., 2012).
Trail-Making Tests A and B (TMT-A and TMT-B)
The Trail-Making Test (TMT) (Reitan and Wolfston, 1985) assess visual attention and mental flexibility. Participants are required to connect encircled numbers in ascending order from 1 to 25 (TMT-A) and 25 encircled numbers and letters in an alternating ascending/alphabetic order (TMT-B). Participants are asked to work as quickly and accurately as possible. A large discrepancy between the time needed to complete TMT-A and TMT-B is an indicator of deficits in mental flexibility. Mean scores for TMT-A and TMT-B for healthy older persons range from 25 to 50 s and 50 to 110 s, respectively (Gron et al., 2002; Widmann et al., 2012).
Fluency tasks (Regensburg Verbal Fluency Test; RWT)
RWT (Aschenbrenner et al., 2000) assesses semantic and phonetic verbal fluency. The task requires to name as many words as possible belonging to a category ‘animals’ (semantic fluency) and words starting with letter ‘P’ and with letter ‘S’ (phonemic fluency). One minute is given to complete each task. Mean scores for semantic and phonetic fluency are about 18–28 (Riepe et al., 2010) and 6– 8, respectively (Gron et al., 2002; Widmann et al., 2012).
Statistical analyses
All statistical data analyses were performed using SPSS (SPSS 25.0 for Windows, Armonk, NY, 2017). The normality of distribution was assessed with the Kolmogorov–Smirnov Test. Since majority of parameters were not normally distributed, the non-parametric Mann–Whitney–U-test was used to calculate the group differences. Effect sizes were calculated as R2 to indicate the proportion of variance shared by the two variables. Values of R2 = 0.01 indicate a small effect size, R2 = 0.09 indicate a medium effect size and R2 = 0.25 a large effect size (Cohen, 1992).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
Results
Cognitive symptoms in patients with AD and patients with DD
Cognitive performance in patients with AD and DD is summarized in Table 1. Performance in both patients with AD and patients with DD is below normal. Results in healthy controls have been reported in a previous analysis (Lanza et al., 2020).
Cognitive symptoms in patients with AD and patients with DD
Patients with AD and DD were matched for age (±3 years), education (±one school level) and MMSE score (±1 point). On average, patients with AD were significantly worse in the CVLT 5 (P = 0.000), total recall over all learning trials (P = 0.000), delayed recall (P = 0.000), delayed cued recall (P = 0.000) and false-positive answers (P = 0.000). Similarly, clock drawing test (P = 0.044), and the TMT-A (P = 0.021) was worse than in patients with DD. The results are summarized in Table 2.
Table 2.
Matched data for sex, education, age and MMSE in patients with AD versus DD
| Neuropsychological assessment | AD (n = 63) | DD (n = 63) | P-value | R 2 |
|---|---|---|---|---|
| Median/IQR | Median/IQR | |||
| Age (years) | 76.0/70.0–80.0 | 75.0/69.0–80.0 | 0.756 | <0.01 |
| GDS | 5.0/2.0–8.5 | 6.0/4.0–10.0 | 0.045 | 0.03 |
| MMSE | 26.0 ± 25.0–28.0 | 27.0 ± 25.0–29.0 | 0.138 | 0.02 |
| CVLT1 | 3.0/2.0–4.0 | 3.0/2.0–4.0 | 0.075 | 0.03 |
| CVLT5 | 5.0/4.0–7.0 | 7.0/5.0–9.0 | 0.000 | 0.13 |
| CVLT total recall | 21.0/18.0–28.0 | 27.5/22.8–34.3 | 0.000 | 0.11 |
| CVLT delayed recall | 1.0/0.0–3.8 | 4.0/2.8–7.0 | 0.000 | 0.21 |
| CVLT delayed cued recall | 4.0/2.0–6.0 | 6.5/5.0–9.0 | 0.000 | 0.16 |
| CVLT recognition | 14.0/11.3–15.0 | 14.0/12.8–15.0 | 0.520 | <0.01 |
| CVLT false positive | 6.5/3.3–12.8 | 2.5/1.0–5.3 | 0.000 | 0.10 |
| Digit span forward | 7.0/6.0–8.0 | 6.0/5.0–8.0 | 0.371 | 0.01 |
| Digit span backward | 4.0/3.0–5.0 | 4.0/3.0–5.3 | 0.649 | <0.01 |
| Block span forward | 6.0/5.0–7.0 | 6.0/5.0–7.0 | 0.168 | 0.02 |
| Block span backward | 5.0/3.0–6.0 | 5.0/4.0–6.0 | 0.833 | <0.01 |
| Clock drawing | 3.0/2.0–4.0 | 3.0/2.0–3.0 | 0.044 | 0.03 |
| TMT-A | 71.0/54.0–119.0 | 58.5/40.5–101.0 | 0.021 | 0.04 |
| TMT-B | 199.0/154.0–249.0 | 170.5/119.5–248.0 | 0.077 | 0.03 |
| Semantic fluency | 14.0/11.0–17.0 | 15.0/12.0–20.0 | 0.078 | 0.03 |
| Phonematic fluency P | 5.0/3.0–7.0 | 6.0/3.0–8.0 | 0.138 | 0.02 |
| Phonematic fluency S | 7.5/5.0–10.8 | 7.0/5.0–10.0 | 0.768 | <0.01 |
Abbreviations: AD, Alzheimer’s disease; CVLT, California Verbal Learning Test; DD, depressive disorder; GDS, Geriatric Depression Scale; MMSE, Mini-Mental Status Examination; TMT-A, Trail-Making Test A; TMT-B, Trail-Making Test B.
Cerebrospinal fluid levels of Abeta1-42 (P = 0.000), total tau (P = 0.000), phospho-tau (P = 0.000) and ratio Abeta1-42/Abeta1-40 (P = 0.000) were different in patients with AD and DD as a consequence of the selection process (Supplementary Table 1).
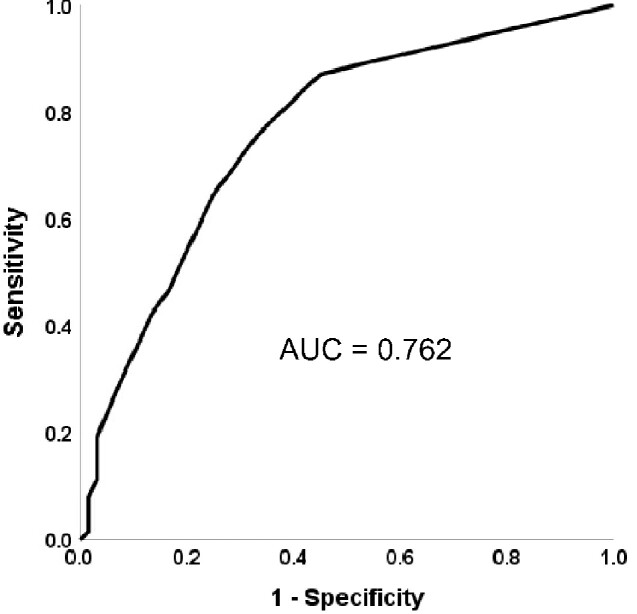
The parameter that strikes out to differentiate the most, the free delayed recall has a medium to strong effect size with R2 = 0.21. Receiver operating characteristic analysis revealed an AUC of 0.762 (Fig. 2).
Figure 2.
Area under the curve of free delayed recall.
Cognitive symptoms in patients with AD with and without concomitant depressive symptoms
To better understand depressive syndrome within AD patients and its impact on CSF values, we analysed the results of all patients with typical CSF constellation for AD (n = 179). We separated this group into two subgroups according to score in the GDS, one group being AD patients with depressive syndrome (dAD, n = 81; GDS > 4), the other group being AD patients without depressive syndrome (nAD, n = 98; GDS < 4).
Calculation of group differences of dAD versus nAD showed significant differences for ratio Abeta1-42/Abeta1-40 (P = 0.030), whereas all other CSF markers were alike (Supplementary Table 2).
Verbal working memory and executive function differed between nAD than dAD. The results are summarized in Table 3.
Table 3.
Neuropsychological assessments in patients with nAD and dAD
| Neuropsychological assessment | nAD (GDS ≤ 4) | dAD (GDS > 4) | P-value | R 2 |
|---|---|---|---|---|
| Median/IQR | Median/IQR | |||
| GDS | 2.0/1.0–3.0 | 8.0/6.0–11.0 | 0.000 | 0.74 |
| MMSE | 26.0 ± 25.0–28.0 | 27.0 ± 25.0–28.0 | 0.451 | <0.01 |
| CVLT1 | 3.0/2.0–4.0 | 3.0/2.0–4.0 | 0.670 | <0.01 |
| CVLT5 | 5.0/4.0–7.0 | 5.0/4.0–7.0 | 0.615 | <0.01 |
| CVLT total recall | 21.0/18.0–28.0 | 22.0/18.0–28.0 | 0.811 | <0.01 |
| CVLT delayed recall | 1.0/0.0–3.0 | 1.0/0.0–5.0 | 0.241 | 0.01 |
| CVLT delayed cued recall | 4.0/2.0–6.0 | 3.5/2.0–6.3 | 0.813 | <0.01 |
| CVLT recognition | 14.0/12.3–15.0 | 14.0/12.0–16.0 | 0.790 | <0.01 |
| CVLT false positive | 8.0/3.0–11.8 | 7.0/4.0–11.0 | 0.853 | <0.01 |
| Digit span forward | 7.0/5.5–8.0 | 7.0/5.0–8.0 | 0.482 | <0.01 |
| Digit span backward | 4.0/3.0–5.0 | 4.0/3.0–5.0 | 0.048 | 0.02 |
| Block span forward | 6.0/5.0–6.0 | 6.0/5.0–6.0 | 0.401 | <0.01 |
| Block span backward | 5.0/3.0–6.0 | 5.0/3.0–6.0 | 0.311 | 0.01 |
| Clock drawing | 3.0/2.0–4.0 | 3.0/2.0–4.0 | 0.050 | 0.02 |
| TMT-A | 70.5/51.3–97.8 | 92.0/65.0–121.5 | 0.004 | 0.05 |
| TMT-B | 199.0/155.3–264.8 | 215.0/177.0–279.0 | 0.050 | 0.03 |
| Semantic fluency | 13.0/11.0–17.8 | 13.0/10.0–16.5 | 0.534 | <0.01 |
| Phonematic fluency P | 5.0/3.0–8.0 | 5.0/3.0–7.0 | 0.819 | <0.01 |
| Phonematic fluency S | 8.0/5.0–11.0 | 7.0/4.0–10.0 | 0.075 | 0.02 |
Abbreviations: CVLT, California Verbal Learning Test; dAD, Alzheimer patients with depressive syndrome; GDS, Geriatric Depression Scale; MMSE, Mini-Mental Status Examination; nAD, Alzheimer patients without depressive syndrome; TMT-A, Trail-Making Test A; TMT-B, Trail-Making Test B.
Discussion
Clinically, cognitive and affective symptoms may be present in patients with AD as well as in patients with DD. Neither clinical assessments nor standardized behavioural testing of mood or cognition allows a differential diagnosis. The missing clinical and behavioural gold standards precluded establishing profiles of cognitive impairment due to AD and DD. To our knowledge, this study is the first to use biomarker-verified groups of AD and DD to characterize their respective cognitive profiles.
Our results demonstrate that with similar global cognitive function as assessed with the MMSE, the impairment of verbal episodic memory is much more pronounced in patients with AD than in patients with DD. This applies to established memory measures such as total and delayed recall as well as to false recognitions (Sejunaite et al., 2017, 2018). We hypothesize that due to greater impairment of brain structures crucial for memory storage, patients with AD suffer from greater memory deficits than those with DD (Gron et al., 2002). Although on a group level, the effect sizes of the differences in these measures in AD and DD are moderate, they do not suffice to diagnose individual patients. The receiver operating characteristic curve demonstrates a sensitivity of about 0.6 at a specificity of about 0.8 for distinction between AD and DD using free delayed recall. Thus, even the variable that distinguishes best between AD and DD having a strong effect size for group comparison does not suffice to diagnose single patients.
Executive dysfunction has further influence on memory processes such as encoding, retrieval and learning (Elderkin-Thompson et al., 2007; Taconnat et al., 2010) and may cause additional memory deficits (Fossati et al., 2002). It has been argued that impairment of executive function is one of the hallmarks of cognitive deficits in patients with DD (Elderkin-Thompson et al., 2004; Dotson et al., 2008). However, a recent study found other processes of memory learning and retrieval to be also impaired (Lanza et al., 2020). Overall, group differences in executive function are smaller than memory recall, making these parameters ill-suited to distinguish AD and DD. At least, partly this may be caused by the increased variability of cognitive performance in patients with DD (Gallagher et al., 2015). The overall interpretation is in good harmony with previous reports that a distinct pattern of cognitive impairment in AD and DD cannot be found (desRosiers et al., 1995; Dierckx et al., 2007). This study is the first to support this conclusion in patients with verified AD and verified DD. Thus, the difference between AD and DD regarding cognitive performance is dimensional rather than categorical.
Depressed mood is frequent in patients with AD (Weiner et al., 1997; Wiels et al., 2020). During the course of the disease, nearly half of the patients experience clinically significant depressive syndrome (Starkstein et al., 2005). For this reason, we analysed subgroups of patients with verified AD with and without concomitant depressive symptoms. This study demonstrates that in patients with verified AD concurring depressive symptoms manifest with an additional impairment of executive function, whereas verbal episodic memory is equally impaired in patients with AD with and without concomitant depressive symptoms. Increased impairment of executive function in this subgroup needs to be considered as an additional target that goes beyond the treatment of cognitive symptoms in AD patients, in general.
The format of the medical records used in the study caused some limitations. The number of previous depressive episodes or the age of onset of depressive syndrome could not be reliably determined. This might be relevant, since recent studies have shown, that with each depressive episode, cognitive impairment may persist even after depressive syndrome has vanished (Butters et al., 2000; Beblo et al., 2011). Similarly, exact records on received medication and treatment at the time of neuropsychological assessment was unavailable.
Conclusion
Cognitive impairment and depressive syndrome may be simultaneously present in both AD and DD. Therefore, differential diagnosis solely on clinical and behavioural grounds is unreliable. On the group level, free delayed recall correctly classifies 70% of cases. However, this is insufficient for clinical diagnosis of individual patients. Biomarker analysis is needed to verify the diagnoses of AD and DD. This also applies to the studies appraising depressed mood or impaired cognition as being risk factors for either AD or DD. Standardized neuropsychological and mood assessment in patients with verified AD is important to define targets for a comprehensive therapy of AD. Even clinical trials for both AD and DD need to include this comprehensive approach to not underestimate the treatment effects.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
K.S., C.L., C.S. and I.S. were involved in acquisition of the data, data analysis and drafting and revising the manuscript. K.S., C.L. and M.W.R. were involved in interpretation of the data, drafting and revising the manuscript. All authors approved the final version of the manuscript.
Funding
This study was not funded.
Competing interests
The authors report no competing interest.
Glossary
- AD =
Alzheimer’s disease
- ADL =
activities of daily living
- AUC =
area under the curve
- CSF =
cerebrospinal fluid
- CVLT =
California Verbal Learning Test
- dAD =
Alzheimer patients with depressive syndrome
- DD =
depressive disorder
- ECT =
electroconvulsive therapy
- GDS =
Geriatric Depression Scale
- MCI =
mild cognitive impairment
- MMSE =
Mini-Mental Status Examination
- nAD =
Alzheimer patients without depressive syndrome
- phospho-tau =
phosphorylated tau
Contributor Information
Claudia Lanza, Department of Psychiatry and Psychotherapy II, Ulm University, Ulm, Germany.
Karolina Sejunaite, Department of Psychiatry and Psychotherapy II, Ulm University, Ulm, Germany.
Charlotte Steindel, Department of Psychiatry and Psychotherapy II, Ulm University, Ulm, Germany.
Ingo Scholz, Department of Psychiatry and Psychotherapy II, Ulm University, Ulm, Germany.
Matthias W Riepe, Department of Psychiatry and Psychotherapy II, Ulm University, Ulm, Germany; Division of Geriatrics and Geriatric Psychiatry, Ulm University, Ulm, Germany.
References
- Alexopoulos GS. Depression in the elderly. Lancet 2005; 365: 1961–70. [DOI] [PubMed] [Google Scholar]
- Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 2001; 58: 373–9. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner S, Tucha O, Lange KW.. Regensburger Wortflüssigkeits-Test (RWT). Handanweisung. Göttingen: Hogrefe; 2000. [Google Scholar]
- Beblo T, Sinnamon G, Baune BT.. Specifying the neuropsychology of affective disorders: clinical, demographic and neurobiological factors. Neuropsychol Rev 2011; 21: 337–59. [DOI] [PubMed] [Google Scholar]
- Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H.. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci 2015; 36: 297–309. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Nitcher RL, Roccaforte WH, Wengel SP.. A prospective evaluation of the Geriatric Depression Scale in an outpatient geriatric assessment center. J Am Geriatr Soc 1992; 40: 1227–30. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Roccaforte WH, Wengel SP.. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol 1991; 4: 173–8. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry 2000; 157: 1949–54. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry 2004; 61: 587–95. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull 1992; 112: 155–9. [DOI] [PubMed] [Google Scholar]
- Creavin ST, Wisniewski S, Noel-Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev 2016: CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- desRosiers G, Hodges JR, Berrios G.. The neuropsychological differentiation of patients with very mild Alzheimer’s disease and/or major depression. J Am Geriatr Soc 1995; 43: 1256–63. [DOI] [PubMed] [Google Scholar]
- Dierckx E, Engelborghs S, De Raedt R, De Deyn PP, Ponjaert-Kristoffersen I.. de, Ponjaert-Kristoffersen I. Differentiation between mild cognitive impairment, Alzheimer’s disease and depression by means of cued recall. Psychol Med 2007; 37: 747–55. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, Zonderman AB.. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry 2008; 16: 318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007; 6: 734–46. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Boone KB, Hwang S, Kumar A.. Neurocognitive profiles in elderly patients with frontotemporal degeneration or major depressive disorder. J Int Neuropsychol Soc 2004; 10: 753–71. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Hellemann G, Pham D, Kumar A.. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriat Psychiatry 2009; 24: 459–68. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol 2003; 18: 529–49. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A.. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol 2007; 22: 261–70. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Moody T, Knowlton B, Hellemann G, Kumar A.. Explicit and implicit memory in late-life depression. Am J Geriatr Psychiatry 2011; 19: 249–55. [DOI] [PubMed] [Google Scholar]
- Fischer P. The spectrum of depressive pseudo-dementia. J Neural Transm Suppl 1996; 47: 193–203. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Fossati P, Coyette F, Ergis AM, Allilaire JF.. Influence of age and executive functioning on verbal memory of inpatients with depression. J Affect Disord 2002; 68: 261–71. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Nilsson J, Finkelmeyer A, Goshawk M, Macritchie KA, Lloyd AJ, et al. Neurocognitive intra-individual variability in mood disorders: effects on attentional response time distributions. Psychol Med 2015; 45: 2985–97. [DOI] [PubMed] [Google Scholar]
- Goossens J, Bjerke M, Struyfs H, Niemantsverdriet E, Somers C, van den Bossche T, et al. No added diagnostic value of non-phosphorylated tau fraction (p-taurel) in CSF as a biomarker for differential dementia diagnosis. Alzheimer Res Ther 2017; 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gron G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW.. Subjective memory complaints: objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol 2002; 51: 491–8. [DOI] [PubMed] [Google Scholar]
- Härting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K, Kessler J.. Wechsler Gedächtnistest—Revidierte Fassung: WMS-R; deutsche Adaptation der revidierten Fassung der Wechsler Memory Scale. Bern: Huber; 2000. [Google Scholar]
- Hodges JR, Memory in the dementias. In: The oxford handbook of memory. Oxford, New York: Oxford University Press; 2000. p. 441–59. [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum HG, Shahly V, Bromet E, Hwang I, McLaughlin KA, et al. Age differences in the prevalence and co-morbidity of DSM-IV major depressive episodes. Results from the WHO World Mental Health Survey Initiative. Depress Anxiety 2010; 27: 351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MI, van Der V, Blankenstein MA, Pijnenburg YA, van Elk EJ, Scheltens P, et al. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurology 2009; 73: 1353–8. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kato S.. Depression-dementia medius: between depression and the manifestation of dementia symptoms. Psychogeriatrics 2011; 11: 177–82. [DOI] [PubMed] [Google Scholar]
- Landrø NI, Stiles TC, Sletvold H.. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14: 233–40. [PubMed] [Google Scholar]
- Lanza CE, Sejunaite K, Steindel C, Scholz I, Riepe MW.. On the conundrum of cognitive impairment due to depressive disorder in older patients. PLoS One 2020; 15: e0231111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Zillmer EA, Barakat LP, Kumar A, Gur RC, et al. Verbal learning and memory in older adults with minor and major depression. Arch Clin Neuropsychol 2012; 27: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement 2014; 10: 808–17. [DOI] [PubMed] [Google Scholar]
- Niemann H, Sturm W, Thöne-Otto AIT, Willmes K.. CVLT California Verbal Learning Test. German adaptation. Manual. Frankfurt: Pearson Assessment; 2008. [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B.. Attention and executive functions in remitted major depression patients. J Affect Disord 2005; 89: 125–35. [DOI] [PubMed] [Google Scholar]
- Paterson RW, Slattery CF, Poole T, Nicholas JM, Magdalinou NK, Toombs J, et al. Cerebrospinal fluid in the differential diagnosis of Alzheimer’s disease: clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res Ther 2018; 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula JJd, Miranda DM, Nicolato R, Moraes ENd, Bicalho MAC, Malloy-Diniz LF.. Verbal learning on depressive pseudodementia: accentuate impairment of free recall, moderate on learning processes, and spared short-term and recognition memory. Arquivos de neuro-psiquiatria 2013; 71: 596–9. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfston D.. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Reitan Neuropsychology 1985; 4. [Google Scholar]
- Rice L, Bisdas S.. The diagnostic value of FDG and amyloid PET in Alzheimer’s disease—a systematic review. Eur J Radiol 2017; 94: 16–24. [DOI] [PubMed] [Google Scholar]
- Riepe MW, Karl J, Tumani H, von. Arnim CA.. Tau-proteins as gender-specific state markers in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 2010; 30: 93–100. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Lovestone S.. The dementias. Lancet 2002; 360: 1759–66. [DOI] [PubMed] [Google Scholar]
- Sejunaite K, Lanza C, Riepe MW.. Everyday false memories in older persons with depressive disorder. Psychiatry Res 2018; 261: 456–63. [DOI] [PubMed] [Google Scholar]
- Sejunaite K, Lanza C, Riepe MW.. Everyday memory in patients with Alzheimer’s disease: fragmentary and distorted. J Alzheimers Dis 2017; 60: 1489–98. [DOI] [PubMed] [Google Scholar]
- Shulman KI, Pushkar Gold D, Cohen CA, Zucchero CA.. Clock-drawing and dementia in the community. A longitudinal study. Int J Geriat Psychiatry 1993; 8: 487–96. [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG.. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry 2005; 162: 2086–93. [DOI] [PubMed] [Google Scholar]
- Taconnat L, Baudouin A, Fay S, Raz N, Bouazzaoui B, El-Hage W, et al. Episodic memory and organizational strategy in free recall in unipolar depression: the role of cognitive support and executive functions. J Clin Exp Neuropsychol 2010; 32: 719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den HJ, Burchert W, Borner AR, Fricke H, Kuhnel G, Meyer GJ, et al. [1-(11)C]Acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med 2001; 42: 1174–82. [PubMed] [Google Scholar]
- Verdaguer ES, Stafford J, Tuijt R, Orgeta V.. Minor and subthreshold depressive disorders in Alzheimer’s disease: a systematic review and meta-analysis of prevalence studies. J Affect Disorders 2020; 263: 728–34. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 2013; 12: 357–67. [DOI] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, et al. Evidence for continuing neuropsychological impairments in depression. J Affect Disord 2004; 82: 253–8. [DOI] [PubMed] [Google Scholar]
- Weiner MF, Svetlik D, Risser RC.. What depressive symptoms are reported in Alzheimer’s patients? Int J Geriat Psychiatry 1997; 12: 648–52. [PubMed] [Google Scholar]
- Widmann CN, Beinhoff U, Riepe MW.. Everyday memory deficits in very mild Alzheimer’s disease. Neurobiol Aging 2012; 33: 297–303. [DOI] [PubMed] [Google Scholar]
- Wiels W, Baeken C, Engelborghs S.. Depressive symptoms in the elderly-an early symptom of dementia? A systematic review. Front Pharmacol 2020; 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RA, Hagerty BM, Cimprich B, Therrien B, Bay E, Oe H.. Changes in directed attention and short-term memory in depression. J Psychiatr Res 2000; 34: 227–38. [DOI] [PubMed] [Google Scholar]
- Young AL, Oxtoby NP, Daga P, Cash DM, Fox NC, Ourselin S, et al. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain 2014; 137: 2564–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.