Abstract
The re-proliferation of quiescent cancer cells is considered to be the primary contributor to prostate cancer (Pca) recurrence and progression. In this study, we investigated the inhibitory effect of safranal, a monoterpene aldehyde isolated from Crocus sativus (saffron), on the re-proliferation of quiescent Pca cells in vitro and in vivo. The results showed that safranal efficiently blocked the re-activation of quiescent Pca cells by downregulating the G0/G1 cell cycle regulatory proteins CDK2, CDK4, CDK6, and phospho-Rb at Ser807/811 and elevating the levels of cyclin-dependent kinase inhibitors, p21 and p27. Further investigation on the underlying mechanisms revealed that safranal suppressed the mRNA and protein expression levels of Skp2, possibly through the deregulation of the transcriptional activity of two major transcriptional factors, E2F1 and NF-κB subunits. Moreover, safranal inhibited AKT phosphorylation at Ser473 and deregulated both canonical and non-canonical NF-κB signaling pathways. Safranal suppressed the tumor growth of quiescent Pca cell xenografts in vivo. Furthermore, safranal-treated tumor tissues exhibited a reduction in Skp2, E2F1, NF-κB p65, p-IκBα (Ser32), c-MYC, p-Rb (Ser807), CDK4, CDK6, and CDK2 and an elevation of p27 and p21 protein levels. Therefore, our findings demonstrate that safranal suppresses cell cycle re-entry of quiescent Pca cells in vitro and in vivo plausibly by repressing the transcriptional activity of two major transcriptional activators of Skp2, namely, E2F1 and NF-κB, through the downregulation of AKT phosphorylation and NF-κB signaling pathways, respectively.
Keywords: safranal, prostate cancer, cell cycle re-entry, quiescent cancer cells, cancer recurrence, NF-κB, E2F1, Skp2
Introduction
Tumor heterogeneity stems from the complex process of growth and diversity of cells such as quiescent cancer cells (QCCs), a subpopulation that transiently retreats from the cell cycle and arrests in the G0 phase (Yeh and Ramaswamy, 2015). QCCs have been clinically garnering attention as they can re-enter the cell cycle, resulting in cancer progression, recurrence, metastasis, and treatment resistance (Krall et al., 2018; Luskin et al., 2018; Recasens and Munoz, 2019). Prostate cancer (Pca) recurrence following primary treatment remains a leading clinical challenge, occurring in up to 50% of patients after 10 years of post-radical prostatectomy or radiotherapy (Hull et al., 2002). Hence, prevention of QCC re-awakening is an emerging paradigm for the treatment of Pca recurrence.
Consistent with the proposed treatment strategy for Pca recurrence, current research focuses on the mechanism of inhibiting the shift from a quiescent to a proliferative state. Mounting evidence indicates that inhibiting Rb-E2F1 signaling deregulates G0/G1-related cyclins, which eventually block QCCs from re-entering the cell cycle (Zetterberg et al., 1995; Yao et al., 2015; Xi et al., 2016; So and Cheung, 2018; Pennycook and Barr, 2020). c-MYC is a crucial regulator in cell cycle re-entry through the histone chaperone Facilitates Chromatin Transcription (Bi et al., 2019, 2020) and its E3 ligase FBXW7 (Xi et al., 2016). Additionally, Skp2 inhibition maintains quiescence (Zhang et al., 2019) and suppresses tumor progression in multiple transgenic mouse models (Agarwal et al., 2008; Lin et al., 2010; Wang et al., 2010). Nevertheless, a few effective leading compounds have impeded the transition from a quiescent to a proliferative state (Xi et al., 2016; Bi et al., 2020), emphasizing the necessity to mine for potential therapeutic options, which could block QCCs’ re-entry and hinder cancer recurrence.
Saffron, an age-old spice from the plant Crocus sativus L., is therapeutically valued in traditional Chinese and Ayurvedic medicine systems (Bhandari, 2015). Increasing evidence has established that safranal, a monoterpene aldehyde isolated from C. sativus (Tarantilis et al., 1994), exerts anticancer activities on various human malignancies (Samarghandian and Shabestari, 2013; Geromichalos et al., 2014; Samarghandian et al., 2014; Jabini et al., 2017; Al-Hrout et al., 2018; Cheriyamundath et al., 2018). However, the underlying antitumor mechanism of safranal relating to QCCs and cancer recurrence has not been established.
Therefore, the present study aimed to investigate the underlying mechanism of safranal and suppress the re-proliferation of quiescent Pca cells in vitro and in vivo, providing convincing evidence for the development of safranal as an agent against prostate cancer recurrence.
Materials and Methods
Cell Lines and Synchronization at Quiescence
Human Pca cell lines LNCaP and PC-3 were acquired from the American Type Culture Collection (Manassas, VA, United States) and cultured as previously described (Xi et al., 2016). Once the LNCaP cells attained a confluence of 70–80%, they were synchronized by changing to serum-free medium and cultured for 7 days to obtain a quiescent LNCaP model. In contrast, once the PC-3 cells reached 100% confluency, the medium was changed and retained for 3 days to achieve contact inhibition. Replenishing the serum for LNCaP cells and passaging PC-3 cells at lower confluency allowed the synchronized cells to re-enter the cell cycle. This model was used to simulate the progressive re-proliferation of Pca cells in vivo.
Chemicals and Reagents
Safranal (≥90.0% purity) was purchased from Sigma Aldrich (St. Louis, MO, United States), dissolved in dimethyl sulfoxide (DMSO, Life Technologies, Carlsbad, CA, United States), and stored at -80°C. Propidium iodide (PI, P4170; Sigma-Aldrich), PrimeScript RT Reagent Kit (RR037A; TAKARA Biotechnology, Shiga, Japan), SYBR Green reagent (S-7563; Life Technologies), and SYBR Green Realtime PCR Master Mix (QPK-201; TOBOYO, Life Science, Osaka, Japan) were used. The antibodies for immunoblotting included those against CDK4 (#3830-1), CDK6 (#3524-1), and p21 (#3733-1) (Epitomics, Cambridge, United Kingdom). We acquired antibodies against p27 (sc528), IKKα/β (sc7607), α-tubulin (sc5286), and Skp2 (sc7164) from Santa Cruz Biotechnology (Dallas, CA, United States), while anti-β-actin antibody (#66009-1-Ig) was purchased from Proteintech (Wuhan, Hu Bei Province, China). The antibodies against p-Rb (Ser807/811, #9308), E2F1 (#3742), phospho-AKT (Ser473, #4060), AKT (#9272), phospho-IKKα/β (Ser176/180, #2697), phospho-IκBα (Ser32, #2859), IκBα (#4812), NF-κB p65 (#8242), and NF-κB2 (p100/52, #3017) were purchased from Cell Signaling Technology (Danvers, MA, United States), while those against lamin A/C (ab108595), CDK2 (ab32147), c-MYC (ab32072), and GAPDH (ab128915) were supplied by Abcam (Cambridge, United Kingdom).
SYBR Green Assay
Quiescent LNCaP (1 × 104 cells/well) and PC-3 (7 × 103 cells/well) were induced to re-enter the cell cycle by seeding in 96-well plates containing a complete medium with the indicated concentrations of safranal. Additionally, the same number of cells was maintained at -80°C as baseline. After 72 h, the medium was discarded, 100 μl of lysis buffer [comprised of 20% radioimmunoprecipitation assay (RIPA) lysis buffer and 0.01% SYBR Green reagent] was added, and the mixture was incubated for 30 min in the dark. The frozen baseline cells were thawed and subjected to the above-mentioned lysis and incubation treatment as well as quantification of fluorescent SYBR Green-stained DNA, and calculation of growth inhibition (GI) concentrations at 50% (GI50) and 90% (GI90), with 95% confidence interval (CI), in LNCaP and PC-3 cells (Table 1) was performed as previously described (Xi et al., 2016).
TABLE 1.
Growth inhibition (GI) concentrations of safranal with 95% confidence interval (CI) in LNCaP and PC-3 cells 72 h after release from quiescence.
| GI% | LNCaP (mM) | 95% CI | PC-3 (mM) | 95% CI |
| GI50 | 0.133 ± 0.034 | 0.049–0.217 | 0.109 ± 0.002 | 0.102–0.115 |
| GI90 | 0.239 ± 0.025 | 0.177–0.301 | 0.317 ± 0.045 | 0.205–0.428 |
Quiescent LNCaP and PC-3 cells were re-activated and treated with various concentrations of safranal, and the rendered GI50 and GI90 with 95% confidence interval were determined using the SYBR Green assay. The GI values represent the mean ± SD of at least three independent experiments.
Flow Cytometric Analysis
Quiescent LNCaP and PC-3 cells were seeded into a six-well plate and treated with either safranal or DMSO for 24 h (PC-3) and 32 h (LNCaP). The cells were harvested and fixed overnight with ice-cold 70% ethanol in phosphate-buffered saline (PBS) at 4°C. Flow cytometric analysis with PI staining was performed as previously described (Li et al., 2018).
Immunoblotting
Protein lysates were prepared by subjecting the cells to an ice-cold RIPA lysis buffer supplemented with protease/phosphatase inhibitor cocktail (#5872, Cell Signaling Technology, Danvers, United States). We conducted protein quantification, electrophoresis, and immunoblotting according to earlier protocols (Wong et al., 2019).
Clonogenic Assay
Quiescent LNCaP (800 cells/well) or PC-3 (1 × 103 cells/well) cells were seeded into six-well plates and treated with either DMSO or safranal (GI50 or GI90) for 24 and 48 h. Thereafter, the media were changed every 4–5 days. After 2 weeks, the colonies were fixed with 4% paraformaldehyde/PBS and stained with 1% crystal violet (Xi et al., 2016).
Real-Time Reverse Transcription PCR
RNA was isolated with Trizol reagent, followed by reverse transcription with PrimeScript RT Reagent Kit and quantification using mRNA-specific primers in a StepOnePlus Real-Time PCR System (ABI) employing the SYBR Green Realtime PCR Master Mix. The details of the protocols were as previously described (Xi et al., 2016), and all results were normalized to TATA box-binding protein (TBP). The sequences of primers used were as follows: c-MYC forward, 5′-GCTGCCAAGAGGGTCA-3′ and reverse, 5′-CGCACAAGAGTTCCGTAG-3′; Skp2 forward, 5′-GAAACGGCTGAAGAGCAAAG-3′ and reverse, 5′-GGAG GCACAGACAGGAAAAG-3′; p21 forward, 5′- CTGGAGACT CTCTGCAGGGTCGAAA-3′ and reverse, 5′-GATTAGGGCTT CCTCTTGGAGAA-3′; p27 forward, 5′-GGCCTCAGAAGACG TCAAAC-3′ and reverse, 5′- ACAGGATGTCCATTCCATGA-3′; E2F1 forward, 5′-AGTTCATCAGCCTTTCCC-3′ and reverse, 5′-AGGTCCCCAAAGTCACAG-33′; NF-κB p65 forward, 5′-CC CACGAGCTTGTAGGAAAGG-3′ and reverse, 5′-GGATTCCC AGGTTCTGGAAAC-3′; NF-κB2 forward, 5′- TGAGAAGGAC ACCCGAAGC-3′ and reverse, 5′-GAGCAGCATTTAGCAGC AAG-3′; and TBP forward, 5′- GAACCACGGCACTGATTTTC-3′ and reverse, 5′-CCCCACCATGTTCTGAATCT-3′.
Preparation of Nuclear and Cytoplasmic Extracts
We used a nuclear and cytoplasmic protein extraction kit (Beyotime Biotechnology, Shanghai, China) as per the manufacturer’s instructions. The isolated proteins were subjected to western blot analysis.
Luciferase Reporter Assay
We sourced the pGM-E2F-Luc reporter plasmid from Genomeditech Biotechnology (Shanghai, China). pML-NFκB-Fluc2-Neo enhanced reporter plasmid (MA0504), renilla luciferase reporter plasmid pML-SV40-hRluc (MA0503), and dual luciferase reporter assay kit were purchased from Meilun Biology Technology Co. Ltd. (Dalian, China). The schematic diagram of pGM-E2F-Luc, pML-NFκB-Fluc2-Neo enhanced, and pML-SV40-hRluc reporter constructs is provided in Supplementary Figure 1. Following a 6-day serum withdrawal for LNCaP and a 2-day contact inhibition for PC-3 cells, the cells were transiently transfected with pGM-E2F-Luc, pML-NFκB-Fluc2-Neo enhanced, and renilla luciferase reporter plasmid pML-SV40-hRluc by using the EZ transfection agent (Life-iLab, Shanghai, China) for 15 h. Next, the transfected cells were concurrently induced to re-enter the cell cycle and treated with GI90 of safranal for a further 24 h. Thereafter, the cell lysates were collected for determination of firefly and renilla luciferase activities. Renilla luciferase served to normalize the values of the experimental reporter gene and acted as an internal control for transfection efficiency.
Implantation of Tumor Xenografts in Nude Mice
The in vivo experiments were approved by the Shanghai University of Traditional Chinese Medicine and animal care was in accordance with the institutional guidelines. Five-week-old male BALB/c nude mice were sourced from the Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China) and housed in a pathogen-free environment. All mice were subcutaneously injected with 3 × 106 quiescent PC-3 cells and then randomly distributed into two groups of six mice for the oral administration of the vehicle control and safranal (100 mg/kg, ig), respectively. Safranal was diluted with normal corn oil and used to pre-treat the mice a day prior to the implantation and for 46 days thereafter. The tumor size and body weight were recorded on alternate days. The mice were then sacrificed to retrieve the tumors, weighed, and photographed.
Immunohistochemistry
The tumor tissues were fixed in 10% neutral-buffered paraformaldehyde, followed by immersion in liquid paraffin, and sectioned (5-μm thickness). Then, the samples were stained with hematoxylin and eosin and with antibodies against Ki-67 (Abcam, ab16667), NF-κB p65 (Santa Cruz, sc514451), p-IκBα (Santa Cruz, sc8404), p21 (Proteintech, #10355-1-AP), CDK4 (Epitomics, #3830-1), CDK6 (Proteintech, #14052-1-AP), CDK2 (Abcam ab32147), p-Rb (Ser807, Abcam, ab184796), E2F1 (St John’s Laboratory, STJ92807), Skp2 (Santa Cruz, sc7164), c-MYC (Abcam, ab32072), and p27 (sc528, Santa Cruz). Finally, the sections were mounted with DPX Mountant (Sigma, 317616) for histological analysis. Staining scores were noted by the intensity and percentage of positively stained cells. The percentage of positive tumor cells was divided into four grades: 0 (<5% positive), 1 (<25% positive), 2 (25–50% positive), 3 for (51–75% positive), and 4 (>75% positive). The intensity of immunostaining was scored as follows: 0 (no staining), 1 (weak staining), 2 (intermediate staining), or 3 (strong staining). Ten random fields were selected and viewed at ×400 in each section to obtain an average score (Li et al., 2020).
Statistical Analysis
All data are presented as mean ± SD values from three independent assays. Statistical analyses were performed with SPSS 21.0 using one-way ANOVA or Student’s t-test. A probability value of P < 0.05 was considered to be statistically significant. Statistical significance was indicated as ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Results
Safranal Inhibits the Re-proliferation of Quiescent Pca Cells
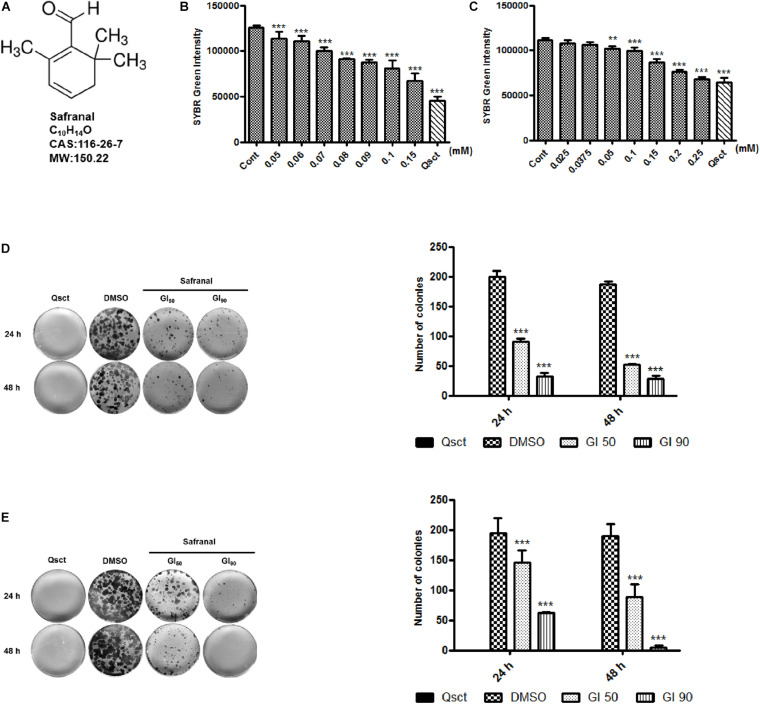
To examine the inhibitory effect of safranal (Figure 1A) on cell cycle re-entry, quiescent LNCaP cells were re-activated by serum replenishment, while quiescent PC-3 cells were reseeded at low confluency, in addition to the indicated concentrations of safranal. SYBR Green, a double-stranded DNA fluorescent dye, was applied to assess the re-synthesis of DNA content with or without safranal treatment. The DNA contents of the LNCaP control group (Figure 1B) and that of the PC-3 control cells (Figure 1C) were notably increased 72 h after re-activation from quiescence. Safranal decreased the DNA re-synthesis of quiescent LNCaP and PC-3 cells in a dose-dependent manner compared with the control group at 72 h, indicative of the inhibition of QCC re-proliferation. The concentrations of safranal-mediated growth inhibition (GI) at 50% (GI50) and 90% (GI90) in LNCaP and PC-3 cells were established based on the SYBR Green assay results (Table 1). Additionally, we monitored the cytotoxicity of safranal on non-malignant prostate stromal cell line WPMY-1, human normal liver cell line HL-7702, and proliferative LNCaP and PC-3 cells (IC50 values listed in the Supplementary Table 1). Safranal was less cytotoxic to the two normal human cell lines and exhibited better inhibitory effect on quiescent Pca re-proliferation compared to the proliferative LNCaP and PC-3 cells. Then, we determined the prolonged effectiveness of safranal on inhibiting cell cycle re-entry using the colony formation assay. Quiescent LNCaP (Figure 1D) and PC-3 cells (Figure 1E) were released from quiescence and treated with GI50 or GI90 of safranal for 24 and 48 h and then maintained in a fresh medium without safranal for an additional 2 weeks. Safranal exerted a long-term effect on Pca re-proliferation and significantly decreased the number and size of colonies in a dose- and time-dependent manner. Overall, these data suggest that safranal exerts an inhibitory effect on quiescent Pca re-proliferation.
FIGURE 1.
Safranal inhibits DNA synthesis and re-proliferation following induction of cell cycle re-entry. Chemical structure of safranal (A). Quiescent LNCaP (B) and PC-3 (C) cells were induced to re-enter the cell cycle, treated with the indicated concentrations of safranal for 72 h, and subjected to the SYBR Green assay to assess their DNA content. Quiescent LNCaP (D) and PC-3 cells (E) were stimulated to re-enter the cell cycle in the presence or absence of safranal (GI50 or GI90) for 24 and 48 h, respectively. The cells were subsequently cultured in fresh full medium for 2 weeks, followed by ethanol fixation, crystal violet staining, and imaging. Representative images and quantification data of LNCaP and PC-3 cell colonies are presented. All data are shown as mean ± SD of triplicate experiments. **P < 0.01, ***P < 0.001 vs. vehicle control. Cont, control cells (non-quiescent cells); Qsct, quiescent cells (7-day serum withdrawal for LNCaP or 3-day contact inhibition for PC-3).
Safranal Blocks Quiescent Pca Cells From Re-entering Cell Cycle by Downregulating G0/G1-Related Proteins
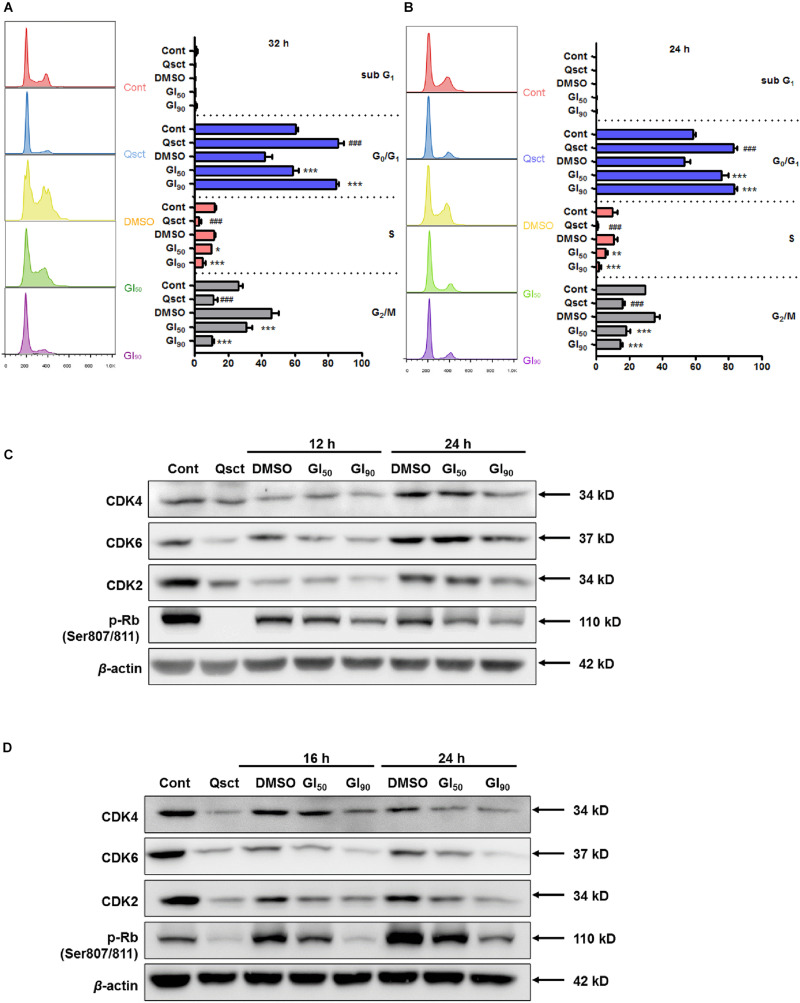
We investigated the role of safranal on cell cycle progression by using PI staining flow cytometric analysis, and we monitored the cell cycle distribution after release from quiescence in the presence or absence of safranal for 32 and 24 h in LNCaP and PC-3 cells, respectively. Experimental quiescence was achieved by a 7-day serum withdrawal for LNCaP cells and 3-day contact inhibition for PC-3 cells, which resulted in quiescence with 85.9% of LNCaP (Figure 2A) and 83.0% of PC-3 cells (Figure 2B) in the G0/G1 phase. Following the re-entry of Pca cells, safranal at GI90 maintained cells in the G0/G1 phase as 84.5% of LNCaP and 83.0% of PC-3 cells approached the level of quiescent cells. In contrast, the control LNCaP and PC-3 cells readily re-entered the cell cycle after leaving quiescence. Our findings imply that safranal significantly retards the cell cycle progression of quiescent Pca cells compared with the DMSO control in a dose- and time-dependent manner (Figures 2A,B).
FIGURE 2.
Safranal prevents quiescent Pca cell re-entry by downregulating G0/G1-related proteins. Quiescent LNCaP (A) and PC-3 (B) cells were triggered to re-enter the cell cycle in the presence of dimethyl sulfoxide (DMSO) or safranal (GI50 or GI90). The cells were harvested after 32 and 24 h, respectively, followed by PI staining, and subjected to flow cytometry. Immunoblotting detected the protein levels of CDK2, CDK4, CDK6, and p-Rb (Ser807/811) in treated LNCaP (C) and treated PC-3 cells (D) during cell cycle re-entry at the indicated intervals. β-actin was used as a loading control. Data are shown as mean ± SD of triplicate experiments. ###P < 0.001 vs. non-quiescent control or *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO vehicle control.
Activation of G0/G1 phase-related cyclins and CDK complexes and phosphorylation of retinoblastoma protein (Rb) cause E2F release and promote cell cycle progression (So and Cheung, 2018). According to the preliminary study, G0/G1 phase-related proteins were altered prior to cell cycle distribution, as monitored by flow cytometry. The protein expression levels of CDK2, CDK4, CDK6, and phospho-Rb (Ser807/811) declined in quiescent Pca cells (Figures 2C,D), and almost all of them began to recover at 12 and 16 h after quiescent LNCaP and PC-3 cell re-entry into the cell cycle, respectively. Additionally, safranal significantly suppressed the recovery of these proteins upon resumption of the cell cycle in these cell lines. Overall, these results suggest that safranal blocks quiescent Pca cells from re-entering the cell cycle by downregulating the G0/G1-related proteins.
Safranal Downregulates c-MYC/Skp2/p27 During Cell Cycle Re-entry
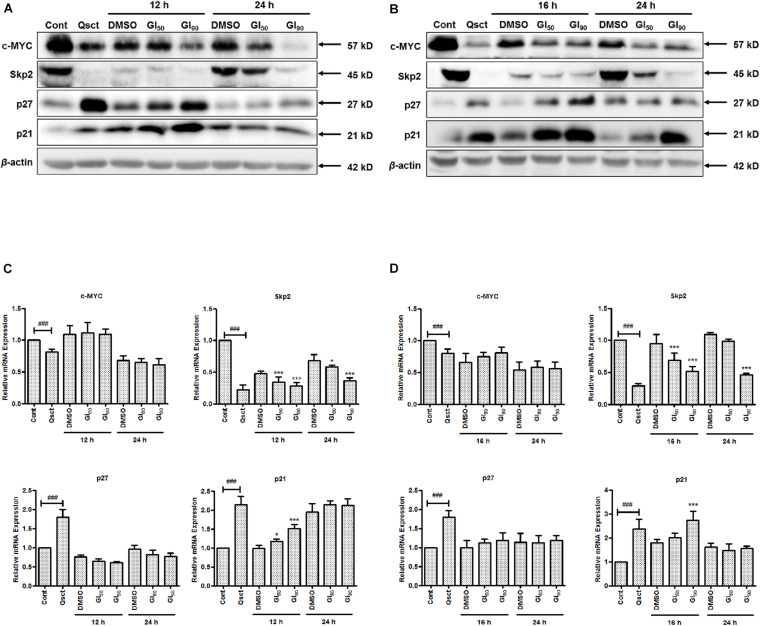
To determine the underlying mechanism of inhibition on quiescent Pca cell cycle re-entry, we examined the protein and mRNA expression levels of c-MYC, Skp2, and CDK inhibitors, namely, p27 and p21, in the presence or absence of safranal. Compared with non-quiescent cells, the protein and mRNA expression levels of c-MYC and Skp2 were significantly decreased in quiescent Pca cells and gradually recovered after release from quiescence. Safranal considerably decreased the protein expression levels of c-MYC and Skp2 in LNCaP (Figure 3A) and PC-3 (Figure 3B) cells. Moreover, the protein levels of p21 and p27, as downstream targets of c-MYC, were dramatically accumulated in the quiescent state and gradually decreased after the cells were released from quiescence. However, safranal significantly promoted the accumulation of p21 in LNCaP and p27 in PC-3 cells after 12 and 16 h, respectively, but not at a later time point (Figures 3A,B). At the mRNA level, safranal notably increased p21 levels after 12 and 16 h in LNCaP and PC-3 cells, respectively, without the concomitant regulation of c-MYC and p27 (Figures 3C,D). Skp2, a member of F-box proteins, was involved in coordinating the G1/S transition and cancer progression. Therefore, we evaluated the mRNA and protein changes of Skp2 and observed that safranal significantly downregulated Skp2 at the transcriptional and protein levels (Figures 3A–D), suggesting that it deregulates Skp2 transcription. These data show that safranal downregulates post-transcriptional c-MYC and the transcription of Skp2, which collectively impairs the degradation of the CDK inhibitors p27 and p21. Hence, further investigation into the underlying mechanism of safranal-induced deregulation of Skp2 was conducted.
FIGURE 3.
Safranal downregulates the post-transcription of c-MYC and the transcription of Skp2 during cell cycle re-entry in Pca cells. Quiescent LNCaP (A,C) and PC-3 (B,D) cells were stimulated to re-enter the cell cycle in the presence or absence of safranal (GI50 or GI90) at the indicated intervals. The protein and mRNA levels of c-MYC, Skp2, p21, and p27 were then analyzed using immunoblotting (A,B) and RT-PCR (C,D) at each interval. β-actin served as a loading control. Cont, control cells in a proliferative state. Results are expressed as mean ± SD of triplicate assays. ###P < 0.001 vs. non-quiescent control or *P < 0.05, ***P < 0.001 vs. dimethyl sulfoxide vehicle control.
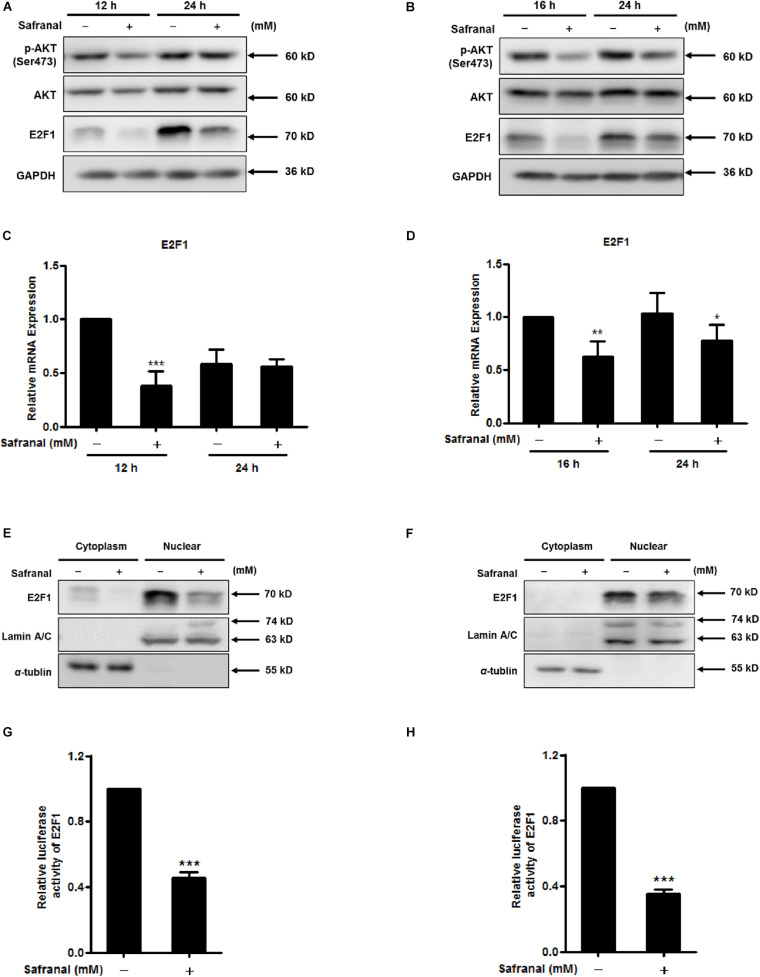
Safranal Reduces AKT Phosphorylation and Suppresses E2F1 Transcriptional Activity
PI3K/AKT signaling positively regulates Skp2 transcription by coupling with E2F1, a critical Skp2 transcription activator (Reichert et al., 2007; Obinata et al., 2017). We evaluated phospho-AKT and E2F1 protein levels during re-entry of quiescent LNCaP (Figure 4A) and PC-3 cells (Figure 4B). Phosphorylation at Ser473 activated AKT (Freudlsperger et al., 2015), safranal inhibited E2F1 and p-AKT (Ser473), and the expression of total AKT remained unchanged. Safranal significantly lowered the mRNA levels of E2F1 at 12 h after the LNCaP cells were released from quiescence, although this was less apparent after 24 h (Figure 4C). In PC-3 cells, E2F1 mRNA levels decreased after 16 and 24 h of cell cycle re-entry (Figure 4D).
FIGURE 4.
The inhibitory effects of safranal on AKT phosphorylation and transcriptional activity of E2F1 during quiescent Pca cell re-entry. Quiescent LNCaP (A) and PC-3 (B) cells were initially stimulated to re-enter the cell cycle, and the effects of safranal (GI90) on protein expression levels of p-AKT (Ser473), AKT, and E2F1 at specific intervals were determined by immunoblotting. GAPDH served as a loading control. The mRNA expression levels of E2F1 in LNCaP (C) and PC-3 (D) cells during cell cycle re-entry in the presence or absence of safranal (GI90) were examined by RT-qPCR. E2F1 levels in cell nuclear and cytoplasm extracts of LNCaP (E) and PC-3 cells (F) were analyzed using immunoblotting after treatment with safranal for 12 and 3 h, respectively, following release from the quiescent state. α-Tubulin and lamin A/C served as loading and purity controls for the cytoplasm and nuclear fractions, respectively. Following a 6-day serum withdrawal for LNCaP and 2-day contact inhibition for PC-3 cells, quiescent LNCaP (G) and PC-3 (H) were transfected with pGM-E2F-Luc and renilla luciferase reporter plasmid pML-SV40-hRluc by using the EZ transfection agent for 15 h. The transfected quiescent cells were then induced to re-enter the cell cycle in the presence or absence of safranal (GI90) for 24 h. Cell lysates were collected to assess firefly and renilla luciferase activities using the dual luciferase reporter assay kit. Renilla luciferase served to normalize the values of the experimental reporter gene and acted as an internal control for transfection efficiency. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. dimethyl sulfoxide vehicle control.
To examine the mediatory effect of safranal on nuclear translocation of E2F1 during cell cycle re-entry, nuclear and cytosolic fractions were separated. Safranal decreased the protein expression level of E2F1, which was predominantly distributed in the nucleus of LNCaP and PC-3 cells after release from quiescence (Figures 4E,F). To further investigate the inhibitory effect of safranal on the transcriptional activity of E2F1 during quiescent Pca cell re-entry, dual luciferase reporter assay was performed. Safranal significantly decreased E2F transcriptional activity in LNCaP (Figure 4G) and PC-3 cells (Figure 4H) during QCCs’ re-entry. Overall, these results suggest that safranal reduces AKT phosphorylation and suppresses E2F transcriptional activity.
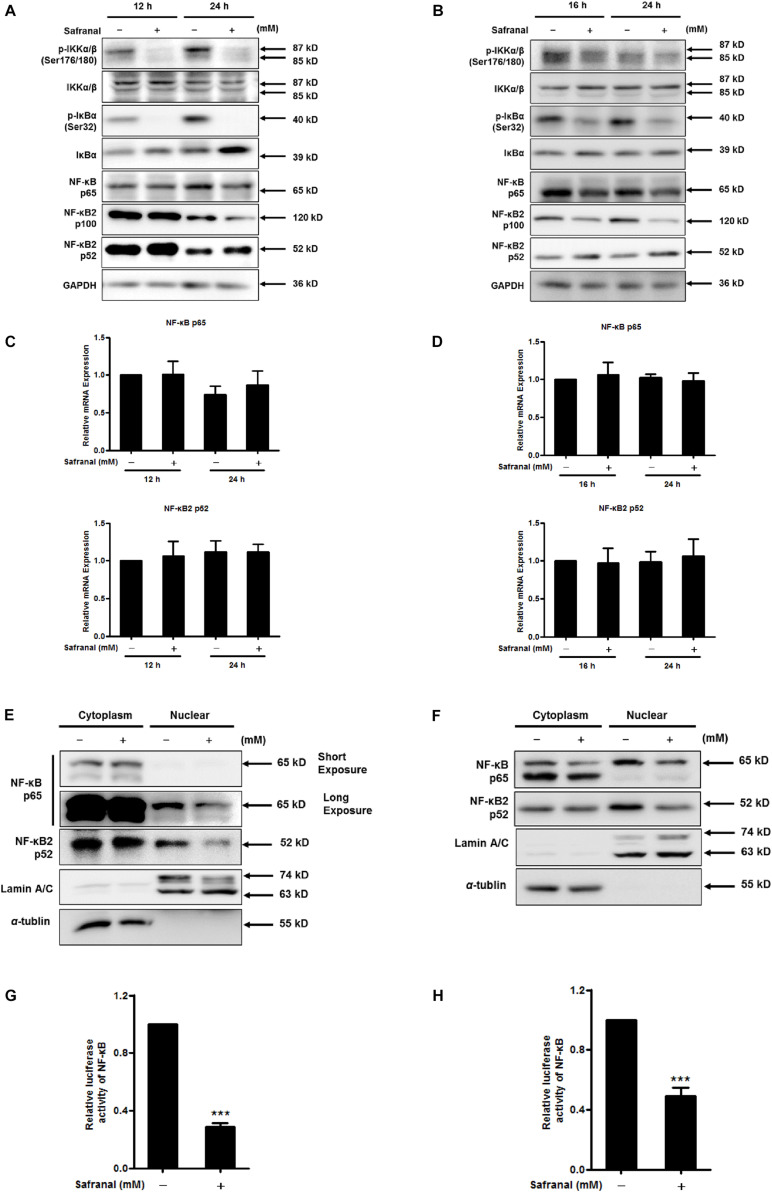
Safranal Deregulates the Canonical and Non-canonical NF-κB Signaling Pathways
NF-κB is another crucial transcriptional activator of Skp2, as blocking IKK/NF-κB turns off Skp2 gene expression (Schneider et al., 2006). IκBs is a switch protein of NF-κB that regulates the canonical NF-κB pathway of NF-κB activation. Once IκBα is phosphorylated by IKK, various NF-κB complexes are translocated to the nucleus, predominantly the p50/RelA dimer. In particular, a non-canonical NF-κB pathway relies on the inducible processing of p100 rather than the degradation of IκBα, thus activating the RelB/p52 NF-κB complex and the downstream target Skp2 (Richmond, 2002). To determine the repressive effect of safranal on NF-κB pathway in the downregulation of Skp2 transcription during the re-entry of quiescent Pca cells into the cell cycle, the transcriptional activity of NF-κB and its corresponding upstream regulators were examined. Safranal notably hindered the phosphorylation of IKKα/β (Ser176/180) without affecting the total protein expression in LNCaP (Figure 5A) and PC-3 cells (Figure 5B). Furthermore, safranal upregulated the protein expression of IκB and substantially inhibited the phosphorylation of IκB (Ser32), and it ultimately downregulated the protein expression of NF-κB p65. Additionally, we examined the expression of p100 NF-κB2 and its processed product p52. Notably, safranal-induced p52 expression was associated with a decrease in the level of the precursor p100, particularly after 24 h in both cell lines. Additionally, we verified that the mRNA level of p65 and p52 remained unchanged post-safranal exposure in LNCaP (Figure 5C) and PC-3 cells (Figure 5D).
FIGURE 5.
The inhibitory effect of safranal on the activation of the canonical and non-canonical NF-κB pathways during quiescent Pca cell re-entry. Following the induction of quiescent LNCaP (A) and PC-3 (B) cell re-entry into the cell cycle, the effects of safranal (GI90) on the protein expression levels of p-IKKα/β (Ser176/180), IKKα/β, p-IκBα (Ser32), IκBα, NF-κB p65, and p100/p52 at the indicated intervals were determined by immunoblotting. GAPDH served as a loading control. The mRNA expression levels of p65 and p52 in LNCaP (C) and PC-3 (D) cells during cell cycle re-entry in the presence or absence of safranal (GI90) were assessed by RT-qPCR. NF-κB p65 and p52 levels in cell nuclear and cytoplasm extracts of LNCaP (E) and PC-3 cells (F) were analyzed by immunoblotting after treatment with safranal for 24 and 1 h, respectively, following release from the quiescent state. α-Tubulin and lamin A/C were used as loading and purity controls for the cytoplasm and nuclear fractions, respectively. Following a 6-day serum withdrawal for LNCaP (G) and a 2-day contact inhibition for PC-3 cells (H), the cells were transfected with pML-NFκB-Fluc2-Neo enhanced and renilla luciferase reporter plasmid pML-SV40-hRluc by using the EZ transfection agent for 15 h. The transfected quiescent cells were then induced to re-enter the cell cycle in the presence or absence of safranal (GI90) for 24 h. Cell lysates were collected to determine firefly and renilla luciferase activities using the dual luciferase reporter assay kit. Renilla luciferase served to normalize the values of the experimental reporter gene and as an internal control for transfection efficiency. Data are expressed as mean ± SD. ***P < 0.001 vs. dimethyl sulfoxide vehicle control.
Nuclear translocation of NF-κB subunits is the hallmark of NF-κB activity. An evaluation of cell nuclear and cytoplasmic extracts indicated that safranal reduced the protein expression of nuclear NF-κB p65 and p52 during the re-entry of quiescent LNCaP (Figure 5E) and PC-3 cells (Figure 5F). This suggested that safranal deregulated the canonical and non-canonical NF-κB signaling pathways. To further investigate the effect of safranal on NF-κB transcriptional activity during quiescent Pca cell re-entry, dual luciferase reporter assay was performed. Safranal suppressed the NF-κB transcriptional activity after quiescent LNCaP (Figure 5G) and PC-3 cells (Figure 5H) re-entered the cell cycle. Overall, these data support that safranal inhibits the canonical and non-canonical NF-κB signaling pathways during cell cycle re-entry of Pca cells.
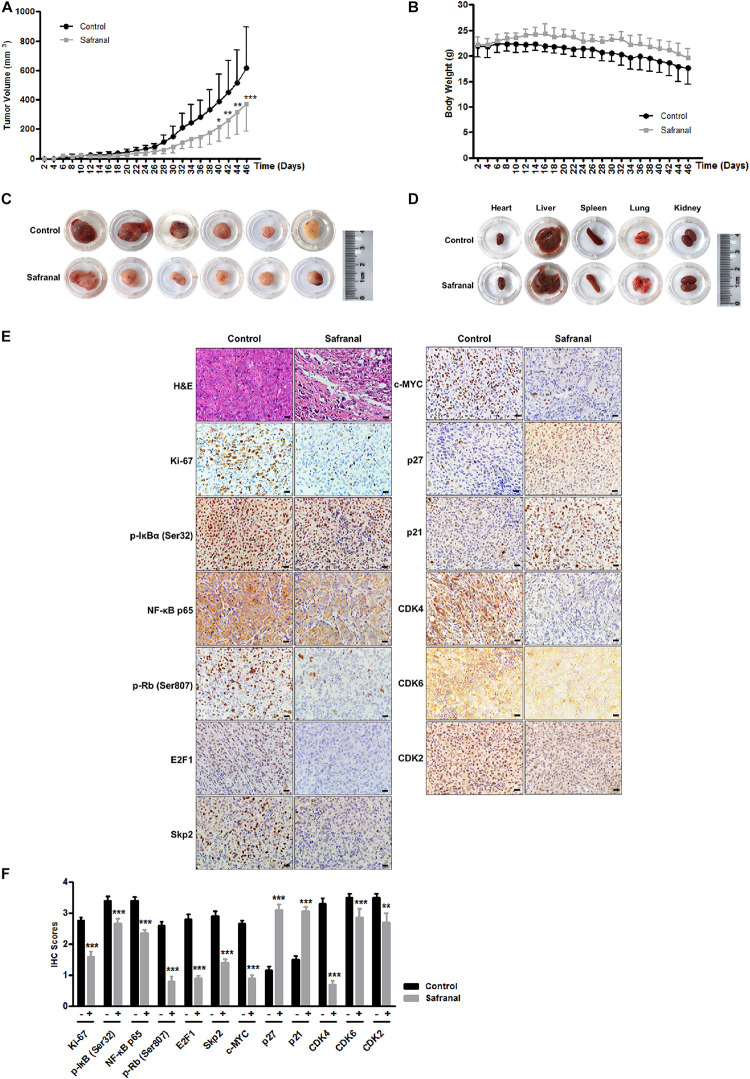
The Potential Effect of Safranal on Suppressing Pca Recurrence in vivo
The re-awakening of QCCs is considered as the main reason of cancer recurrence and progression. Blocking the transition of cancer cells from quiescence to proliferation is critical to prevent cancer recurrence. To further corroborate the potential effects of safranal on suppressing the re-proliferation of quiescent Pca in vivo, we established the xenograft model of quiescent PC-3 cells to mimic the progression of cancer recurrence. All mice were subcutaneously injected with 3 × 106 quiescent PC-3 cells and then randomly distributed into two equal groups of six mice and orally administered with the vehicle control and safranal (100 mg/kg, ig), respectively. Safranal treatment commenced a day prior to tumor inoculation. Daily treatment of safranal significantly suppressed the tumor growth (Figure 6A) despite the unapparent change in the mice body weight (Figure 6B) or pathology of major organs (Figure 6D) compared with the control group. After 46 days of treatment, the tumors in the safranal-treated group (average: 372 mm3) were markedly smaller than those in the control group (average: 618 mm3), with an average reduction of 40% (Figures 6A,C). Moreover, safranal delayed prostate tumor recurrence as the time required for tumor to attain 150 mm3 in the safranal group (38 days) was considerably longer than in the control group (30 days), with an average increase of 26.7%. An examination of resected tumors revealed that the safranal-treated mice had sparse cell density and less Ki-67 positivity (Figures 6E,F). We also confirmed that safranal treatment downregulated the protein expression levels of NF-κB p65, p-IκBα (Ser32), E2F1, Skp2, c-MYC, p-Rb (Ser807), CDK4, CDK6, and CDK2 and elevated p21 and p27 expression in safranal-treated tumor tissues compared with the vehicle control (Figures 6E,F), which is in agreement with the in vitro findings. These data suggest that safranal potentially suppresses Pca recurrence in vivo, reduces Skp2, E2F1, NF-κB p65, p-IκBα (Ser32), c-MYC, p-Rb (Ser807), CDK4, CDK6, and CDK2, and elevates p21 and p27 in safranal-treated tumor tissues.
FIGURE 6.
Safranal suppresses the tumor growth of quiescent PC-3 cells in vivo. Quiescent PC-3 cells were xenografted subcutaneously into the left flank of male nude mice. Then, the mice were orally administrated with either the vehicle control or 100 mg/kg of safranal, commencing a day prior to the xenograft procedure. Tumor volume (A) and mice body weight (B) were measured on alternate days. After a 46-day treatment, the mice were sacrificed, and the tumor (C) and major organs (D) were resected and photographed (scale bar: 20 μm). Paraffin-embedded tumor tissues were stained with hematoxylin and eosin and with antibodies against Ki-67, Skp2, E2F1, NF-κB p65, p-IκBα (Ser32), c-MYC, p-Rb (Ser807), CDK4, CDK6, CDK2, E2F1, Skp2, p21, and p27; representative images (E, ×400) and quantification data (F) are presented. All data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Discussion
It is well-known that re-entry of QCCs into the cell cycle results in cancer recurrence and metastasis (Chen et al., 2012; Li et al., 2015; Tejero et al., 2019). As F-box protein Skp2 is one of the key regulators in cell cycle re-entry (Bashir et al., 2004; Chan et al., 2010), its mRNA and protein expression is at its lowest in the quiescent state and increases as the cell cycle transits to the G1/S phase (Wirbelauer et al., 2000; Bashir et al., 2004), which was also verified in our present study. Safranal downregulates mRNA and protein levels of Skp2 during cell cycle re-entry, which plausibly reduces the risk of Pca recurrence. Further mechanism studies showed that the transcriptional activities of two major transcriptional activators of Skp2, E2F1, and NF-κB were possibly decreased through AKT phosphorylation and the inhibition of the canonical and non-canonical NF-κB signaling pathways. Our in vivo experiments confirm that safranal inhibits the tumorigenicity of quiescent PC-3 cells and represses tumor growth, indicating its potential in suppressing Pca recurrence.
AKT and E2F1 signaling plays a critical role in the proliferation and survival of Pca cells (Suh and Rabson, 2004). The findings of this study indicate that safranal downregulates the expression of p-AKT (ser473), an active site of AKT. Additionally, safranal decreases the mRNA and protein levels of E2F1 in the nucleus and the transcriptional activity of E2F. E2F1 acts as a transcription factor for Skp2 and other genes important for G1/S shift in cell cycle progression (Reichert et al., 2007). When quiescent cells are stimulated to re-enter the cell cycle, there is a surge in the protein levels and activity of E2F1, reaching its peak in late G1 phase to modulate transition to the S phase (Dubrez, 2017). Therefore, inhibition of E2F1 suppresses the re-activation of QCCs and the further advancement of cells to the S phase, exerting multiple inhibitory effects on tumor recurrence and progression. NF-κB, a major nuclear transcription factor involved in the progression of Pca (Sweeney et al., 2004; Shukla et al., 2005) and its inhibition in the bone marrow microenvironment, induces quiescence in breast cancer cells (Ramkissoon et al., 2007). Impairment of NF-κB suppresses the quiescent cancer cells from re-entering the cell cycle, thus preventing its progression to cancer recurrence and metastasis. The NF-κB canonical pathway is triggered by the degradation of IκBα via phosphorylation, releasing sequestered NF-κB complexes (predominantly the p65/p50 dimer) for nuclear translocation (Jeon et al., 2017), followed by the activation of the transcription of Skp2 (Schneider et al., 2006). Additionally, the non-canonical NF-κB pathway largely relies on p100 processing following the nuclear translocation of the p52-RelB dimer. Our data suggest that safranal blocks the canonical and non-canonical NF-κB pathways by deregulating IκB phosphorylation and p100 processing, inhibiting the nuclear translocation of p65 and p52 and thus blocking the transcription of Skp2. Further gain- or loss-of-function studies are required to determine the effect of safranal on PI3K/AKT signaling and NF-κB pathway in regulating cancer recurrence in relation to Skp2 suppression.
Conclusion
In conclusion, we have elucidated that safranal suppresses the re-proliferation of quiescent Pca cells in vitro and exerts a long-term inhibitory effect on clonogenic formation. Mechanism studies showed that safranal inhibits Skp2 transcription, possibly by suppressing the transcriptional activity of E2F1, and NF-κB subunits. Our in vivo study demonstrated that safranal delays the re-growth of quiescent PCa and inhibits tumor progression via the downregulation of Skp2, E2F1, NF-κB p65, p-IκBα (Ser32), c-MYC, p-Rb (Ser807), CDK4, CDK6, and CDK2 expression and elevation of p21 and p27 levels in tumor tissues, concordant with our in vitro findings. Therefore, our study highlights safranal as a potential therapeutic agent for Pca recurrence and provides evidence that pharmacological inactivation of the NF-κB/E2F1–Skp2 axis is a potential therapeutic target against cancer recurrence and progression.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Shanghai University of Traditional Chinese Medicine.
Author Contribions
ZX and HX designed and conceived the study. XJ and YaL performed the experiments and analyzed the data with the assistance of JF. RW and YuL provided technical support in the experiments. YaL and ZX drafted the manuscript. WN and HL revised the manuscript. All the authors approved the final version and agreed for its publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor TL declared a past co-authorship with several of the authors ZX and HX.
Abbreviations
- CDK
cyclin-dependent kinase
- CI
confidence interval
- GI
growth inhibition
- PBS
phosphate-buffered saline
- Pca
prostate cancer
- PI
propidium iodide
- QCCs
quiescent cancer cells
- RT-qPCR
real-time reverse transcription PCR
- Rb
retinoblastoma protein
- Skp2
S-phase kinase-associated protein 2.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (Grant No. 81803571), the China-Morocco Traditional Chinese Medicine Center Construction Project [Grant No. ZY (2018-2020)-GJHZ-1005], and the Key-Area Research and Development Program of Guangdong Province (Grant No. 2020B1 111110003).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2020.598620/full#supplementary-material
References
- Agarwal A., Bumm T. G., Corbin A. S., O’Hare T., Loriaux M., VanDyke J., et al. (2008). Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood 112 1960–1970. 10.1182/blood-2007-09-113860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hrout A., Chaiboonchoe A., Khraiwesh B., Murali C., Baig B., El-Awady R., et al. (2018). Safranal induces DNA double-strand breakage and ER-stress-mediated cell death in hepatocellular carcinoma cells. Sci. Rep. 8:16951. 10.1038/s41598-018-34855-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir T., Dorrello N. V., Amador V., Guardavaccaro D., Pagano M. (2004). Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428 190–193. 10.1038/nature02330 [DOI] [PubMed] [Google Scholar]
- Bhandari P. R. (2015). Crocus sativus L. (saffron) for cancer chemoprevention: a mini review. J. Tradit Complement. Med. 5 81–87. 10.1016/j.jtcme.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi L., Xie C., Jiao L., Jin S., Hnit S. S. T., Mu Y., et al. (2020). CPF impedes cell cycle re-entry of quiescent lung cancer cells through transcriptional suppression of FACT and c-MYC. J. Cell. Mol. Med. 24 2229–2239. 10.1111/jcmm.14897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi L., Xie C., Yao M., Thae Hnit S. S., Vignarajan S., Wang Y., et al. (2019). The histone chaperone complex FACT promotes proliferative switch of G0 cancer cells. Int. J. Cancer 145 164–178. 10.1002/ijc.32065 [DOI] [PubMed] [Google Scholar]
- Chan C. H., Lee S. W., Wang J., Lin H. K. (2010). Regulation of Skp2 expression and activity and its role in cancer progression. ScientificWorldJournal 10 1001–1015. 10.1100/tsw.2010.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li Y., Yu T. S., McKay R. M., Burns D. K., Kernie S. G., et al. (2012). A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488 522–526. 10.1038/nature11287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyamundath S., Choudhary S., Lopus M. (2018). Safranal inhibits hela cell viability by perturbing the reassembly potential of microtubules. Phytother. Res. 32 170–173. 10.1002/ptr.5938 [DOI] [PubMed] [Google Scholar]
- Dubrez L. (2017). Regulation of E2F1 transcription factor by ubiquitin conjugation. Int. J. Mol. Sci. 18:2188. 10.3390/ijms18102188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudlsperger C., Horn D., Weissfuss S., Weichert W., Weber K. J., Saure D., et al. (2015). Phosphorylation of AKT(Ser473) serves as an independent prognostic marker for radiosensitivity in advanced head and neck squamous cell carcinoma. Int. J. Cancer 136 2775–2785. 10.1002/ijc.29328 [DOI] [PubMed] [Google Scholar]
- Geromichalos G. D., Papadopoulos T., Sahpazidou D., Sinakos Z. (2014). Safranal, a Crocus sativus L constituent suppresses the growth of K-562 cells of chronic myelogenous leukemia. In silico and in vitro study. Food Chem. Toxicol. 74 45–50. 10.1016/j.fct.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Hull G. W., Rabbani F., Abbas F., Wheeler T. M., Kattan M. W., Scardino P. T. (2002). Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J. Urol. 167(2 Pt 1), 528–534. 10.1097/00005392-200202000-00018 [DOI] [PubMed] [Google Scholar]
- Jabini R., Ehtesham-Gharaee M., Dalirsani Z., Mosaffa F., Delavarian Z., Behravan J. (2017). Evaluation of the cytotoxic activity of crocin and safranal, constituents of saffron, in oral squamous cell carcinoma (KB Cell Line). Nutr. Cancer 69 911–919. 10.1080/01635581.2017.1339816 [DOI] [PubMed] [Google Scholar]
- Jeon H. Y., Park C. G., Ham S. W., Choi S. H., Lee S. Y., Kim J. Y., et al. (2017). BRM270, a compound from natural plant extracts, inhibits glioblastoma stem cell properties and glioblastoma recurrence. J. Med. Food 20 838–845. 10.1089/jmf.2017.3929 [DOI] [PubMed] [Google Scholar]
- Krall J. A., Reinhardt F., Mercury O. A., Pattabiraman D. R., Brooks M. W., Dougan M., et al. (2018). The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 10:436. 10.1126/scitranslmed.aan3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Jiang E., Wang X., Shangguan A. J., Zhang L., Yu Z. (2015). Dormant cells: the original cause of tumor recurrence and metastasis. Cell Biochem. Biophys. 72 317–320. 10.1007/s12013-014-0477-4 [DOI] [PubMed] [Google Scholar]
- Li J., Peng W., Yang P., Chen R., Gu Q., Qian W., et al. (2020). MicroRNA-1224-5p inhibits metastasis and epithelial-mesenchymal transition in colorectal cancer by targeting SP1-Mediated NF-kappaB signaling pathways. Front. Oncol. 10:294. 10.3389/fonc.2020.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xi Z., Chen X., Cai S., Liang C., Wang Z., et al. (2018). Natural compound Oblongifolin C confers gemcitabine resistance in pancreatic cancer by downregulating Src/MAPK/ERK pathways. Cell Death Dis. 9 538. 10.1038/s41419-018-0574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. K., Chen Z., Wang G., Nardella C., Lee S. W., Chan C. H., et al. (2010). Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 464 374–379. 10.1038/nature08815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M. R., Murakami M. A., Manalis S. R., Weinstock D. M. (2018). Targeting minimal residual disease: a path to cure? Nat. Rev. Cancer 18 255–263. 10.1038/nrc.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata D., Takayama K., Takahashi S., Inoue S. (2017). Crosstalk of the androgen receptor with transcriptional collaborators: potential therapeutic targets for castration-resistant prostate cancer. Cancers 9 22. 10.3390/cancers9030022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycook B. R., Barr A. R. (2020). Restriction point regulation at the crossroads between quiescence and cell proliferation. FEBS Lett. 594 2046–2060. 10.1002/1873-3468.13867 [DOI] [PubMed] [Google Scholar]
- Ramkissoon S. H., Patel P. S., Taborga M., Rameshwar P. (2007). Nuclear factor-kappaB is central to the expression of truncated neurokinin-1 receptor in breast cancer: implication for breast cancer cell quiescence within bone marrow stroma. Cancer Res. 67 1653–1659. 10.1158/0008-5472.CAN-06-3813 [DOI] [PubMed] [Google Scholar]
- Recasens A., Munoz L. (2019). Targeting cancer cell dormancy. Trends Pharmacol. Sci. 40 128–141. 10.1016/j.tips.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Reichert M., Saur D., Hamacher R., Schmid R. M., Schneider G. (2007). Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 67 4149–4156. 10.1158/0008-5472.CAN-06-4484 [DOI] [PubMed] [Google Scholar]
- Richmond A. (2002). Nf-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2 664–674. 10.1038/nri887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Shabestari M. M. (2013). DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line. Indian J. Urol. 29 177–183. 10.4103/0970-1591.117278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Shoshtari M. E., Sargolzaei J., Hossinimoghadam H., Farahzad J. A. (2014). Anti-tumor activity of safranal against neuroblastoma cells. Pharmacogn. Mag. 10(Suppl. 2), S419–S424. 10.4103/0973-1296.133296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Saur D., Siveke J. T., Fritsch R., Greten F. R., Schmid R. M. (2006). IKKalpha controls p52/RelB at the skp2 gene promoter to regulate G1- to S-phase progression. EMBO J. 25 3801–3812. 10.1038/sj.emboj.7601259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Maclennan G. T., Marengo S. R., Resnick M. I., Gupta S. (2005). Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate 64 224–239. 10.1002/pros.20217 [DOI] [PubMed] [Google Scholar]
- So W. K., Cheung T. H. (2018). Molecular Regulation of Cellular Quiescence: A Perspective from Adult Stem Cells and Its Niches. Methods Mol. Biol. 1686 1–25. 10.1007/978-1-4939-7371-2_1 [DOI] [PubMed] [Google Scholar]
- Suh J., Rabson A. B. (2004). NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J. Cell Biochem. 91 100–117. 10.1002/jcb.10729 [DOI] [PubMed] [Google Scholar]
- Sweeney C., Li L., Shanmugam R., Bhat-Nakshatri P., Jayaprakasan V., Baldridge L. A., et al. (2004). Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin. Cancer Res. 10 5501–5507. 10.1158/1078-0432.CCR-0571-03 [DOI] [PubMed] [Google Scholar]
- Tarantilis P. A., Polissiou M., Manfait M. (1994). Separation of picrocrocin, cis-trans-crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. A 664 55–61. 10.1016/0021-9673(94)80628-4 [DOI] [PubMed] [Google Scholar]
- Tejero R., Huang Y., Katsyv I., Kluge M., Lin J. Y., Tome-Garcia J., et al. (2019). Gene signatures of quiescent glioblastoma cells reveal mesenchymal shift and interactions with niche microenvironment. EBioMedicine 42 252–269. 10.1016/j.ebiom.2019.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bauzon F., Ji P., Xu X., Sun D., Locker J., et al. (2010). Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1± mice. Nat. Genet. 42 83–88. 10.1038/ng.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbelauer C., Sutterluty H., Blondel M., Gstaiger M., Peter M., Reymond F., et al. (2000). The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J. 19 5362–5375. 10.1093/emboj/19.20.5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M., Sun Y., Xi Z., Milazzo G., Poulos R. C., Bartenhagen C., et al. (2019). JMJD6 is a tumorigenic factor and therapeutic target in neuroblastoma. Nat. Commun. 10:3319. 10.1038/s41467-019-11132-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z., Yao M., Li Y., Xie C., Holst J., Liu T., et al. (2016). Guttiferone K impedes cell cycle re-entry of quiescent prostate cancer cells via stabilization of FBXW7 and subsequent c-MYC degradation. Cell Death Dis. 7:e2252. 10.1038/cddis.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Xie C., Kiang M. Y., Teng Y., Harman D., Tiffen J., et al. (2015). Targeting of cytosolic phospholipase A2alpha impedes cell cycle re-entry of quiescent prostate cancer cells. Oncotarget 6 34458–34474. 10.18632/oncotarget.5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh A. C., Ramaswamy S. (2015). Mechanisms of Cancer Cell Dormancy–Another Hallmark of Cancer? Cancer Res. 75 5014–5022. 10.1158/0008-5472.CAN-15-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg A., Larsson O., Wiman K. G. (1995). What is the restriction point? Curr. Opin. Cell Biol. 7 835–842. 10.1016/0955-0674(95)80067-0 [DOI] [PubMed] [Google Scholar]
- Zhang W., Ren Z., Jia L., Li X., Jia X., Han Y. (2019). Fbxw7 and Skp2 regulate stem cell switch between quiescence and mitotic division in lung adenocarcinoma. Biomed. Res. Int. 2019:9648269. 10.1155/2019/9648269 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.