Abstract
Retention in care is important in managing HIV among older persons living with HIV (PLWH). We used Theory of Loneliness—loneliness affects emotion-regulatory processes which lead to dysfunctional health behaviors—to test whether social isolation is related to retention in care either directly or indirectly through emotion dysregulation in older PLWH (≥50 years of age; N=144). Retention in care was defined as the proportion of attended scheduled medical visits; visit data were collected prospectively over 12 months from electronic medical records. Self-reported social isolation, emotion dysregulation, and covariates were assessed cross-sectionally at baseline. Most participants were male (60%), African American/Black (86%), and single (59%); 56% were optimally retained in care. Retention was related to monthly income, CD4+ T cell count, and drug use with no direct or indirect effects of social isolation on retention in care. Socioeconomic and behavioral vulnerabilities are closely related to retention in care among older PLWH.
Keywords: older adults, HIV, retention in care, social isolation, emotion dysregulation
Palablas claves: personas mayores, VIH, retención en atención médica, aislamiento social, desregulación emocional
Resumen
Retención en atención médica es importante para el manejo de VIH con personas mayores que viven con VIH (PMVV). Nosotros usamos la Teoría de Soledad- soledad afecta los procesos que regulan emociones y crea comportamientos de salud disfuncionales- para probar si aislamiento social está asociado directamente o indirectamente con la retención en atención médica por desregular emociones en PMVV (≥50 años de edad; N=144). Retención en atención médica fue definido por la proporción de visitas médicas programadas y atendidas; y los datos de visitas atendidas que fueron programadas fueron recopilados prospectivamente por 12 meses de archivos médicos electrónicos. Aislamiento social auto-reportado, desregulación emocional, y covariables fueron evaluados transversalmente de la línea de base. La mayoría de los participantes fueron masculinos (60%), negros/americanos africanos (86%) y solteros (59%); 56% de nuestra muestra fueron retenidos optimamente en atención médica. Retención en atención médica fue asociada con ingresos mensuales, el conteo de linfocitos cd4+, y el consumo de drogas ilegales; no encontramos efectos directos ni indirectos del aislamiento social a la retención en atención médica. Vulnerabilidades socioeconómicas y de comportamiento están vinculados estrechamente a la retención en atención médica para PMVV.
Introduction
Advancements in antiretroviral therapy (ART) have revolutionized HIV management by improving health outcomes and prolonging longevity of persons living with HIV (PLWH). Among older PLWH, retention in care, regular attendance at scheduled HIV clinic visits, remains critical for successful management of HIV. Poor retention in care is associated with numerous health consequences including development of AIDS-defining illnesses, increased odds of mortality, and poorer viral suppression (1–3). Although older PLWH are reported to have greater retention in care than younger PLWH (4), older PLWH face unique psychosocial challenges associated with aging (5–7), such as social isolation, that may hinder adequate retention in care. Understanding these factors and their contributions to retention in care are important in supporting the aging HIV population.
Previous research suggests that older PLWH face barriers to adequate social support (8) and that social support may contribute to retention in care (9, 10). However, studies show that older PLWH have limited and tenuous social connections (11), are socially isolated (5), tend to live alone, have limited and inadequate social networks compared to their younger counterparts (6, 8), and report negative mood stemming from loneliness (7). Socially isolated individuals may have a minimal quantity of social contacts and/or may be deficient in fulfilling and quality relationships, potentially resulting in a diminished sense of social belongingness (12). Social isolation can be assessed based on an individual’s perceived state of loneliness or by an individual’s social network size. Loneliness is a subjective state of social isolation, as it reflects an individual’s perceived discrepancy between actual and desired social relationships (13). On the other hand, social network size is an objective state of social isolation indicated by the number of individuals within a social network or by one’s participation in social activities (14).
Research shows that objectively isolated persons (i.e., small social network size) are not necessarily lonely, and lonely persons are not necessarily isolated in an objective sense, yet some may be both objectively isolated and lonely (15). As such, loneliness and social network size may affect health in different ways (16, 17); to our knowledge, no studies have examined their effects on retention in care among older PLWH. Since the aging HIV population is at risk for social isolation, it is important to assess loneliness and social network size. By assessing two different aspects of isolation concurrently, our findings may provide greater insight into the components of social isolation that drive health management behaviors, such as retention in care, among older PLWH.
The connection between social isolation and retention in care can be tested using the Theory of Loneliness (18) shown in Figure 1. The Theory of Loneliness posits that loneliness makes lonely people feel unsafe in their social environment and that these feelings perpetuate their becoming overly vigilant for social threats, thereby diminishing their capacity to self-regulate emotions and health behaviors (18). In other words, when loneliness becomes a persistent source of distress, it affects emotion-regulatory processes and leads to dysfunctional health behaviors which subsequently affect health outcomes. Emotion dysregulation—defined as difficulties in self-regulation of affective states and in self-control over affect-driven behaviors (19) — is associated with negative affective states, including anxiety and depression, in studies with PLWH (20). Evidence shows that lonely individuals exhibit higher levels of negative affect and lower levels of positive affect than individuals who are not lonely (21, 22), and similar associations, especially with depression, are shown with small social network size (23). Additionally, a recent study showed a moderating effect of emotion dysregulation on the association between depression and antiretroviral therapy (ART) adherence (24), such that the effect of depression on ART non-adherence depended on the level of emotion dysregulation. Based on our current understanding of the relationships among loneliness, social network size, emotion dysregulation, and ART adherence, it is possible that emotion dysregulation is similarly associated with retention in care; however, this remains unexplored. Although the effects of social isolation on emotion dysregulation have not been examined to date, to the extent that loneliness and social network size are associated with negative affective states, it is postulated that they would be associated with emotion dysregulation. In turn, emotion dysregulation may contribute to reduced retention in care.
Figure 1.
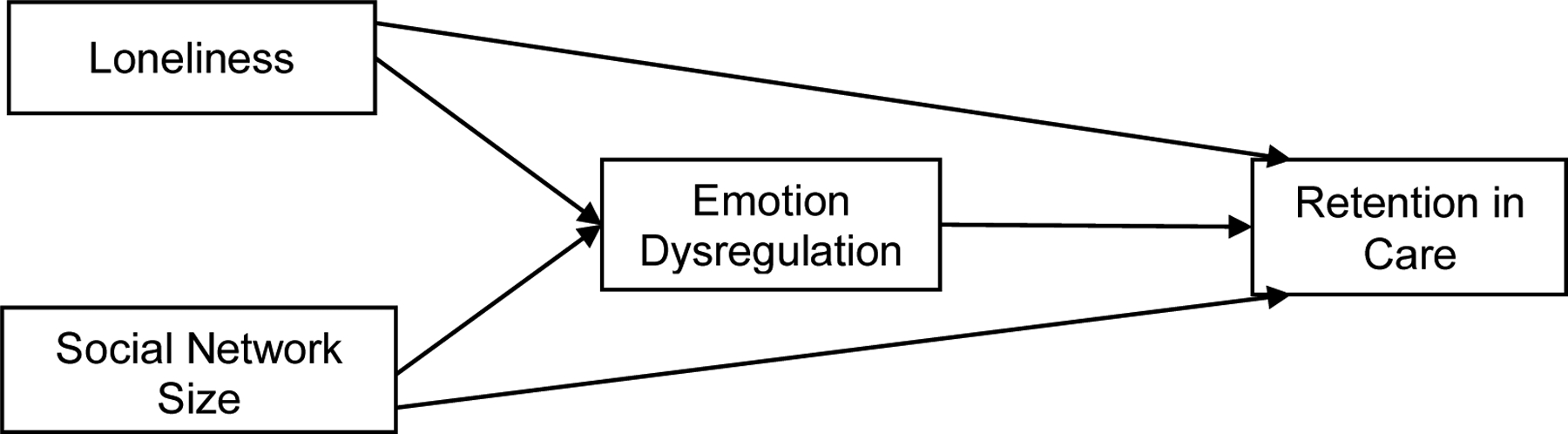
Hypothesized a priori Model of Social Isolation and Retention in Care
In our study, the Theory of Loneliness (18) provided an a priori model and social network size was added along with loneliness to test the associations of both objective and subjective aspects of isolation on retention in care simultaneously. Figure 1 presents a set of hypotheses about the relationships between study variables using the a priori model for Theory of Loneliness. We hypothesized that both indicators of isolation will have direct effects on retention in care and also indirect effects through emotion dysregulation among our sample of older PLWH.
Methods
This study included baseline assessments and a 12-month prospective assessment of retention in care using electronic medical records (EMR). Participants were recruited from a large Ryan White-funded HIV clinic in the metro-Atlanta area that provided access and permission, which facilitated achievement of recruitment goals within the project timeframe. Baseline surveys were collected between August 2016 and April 2017. The recruiting clinic provides comprehensive HIV and primary health care to over 5,800 PLWH in Atlanta, GA. Potential participants were recruited by a graduate student researcher through flyers, word of mouth, and healthcare provider referrals. Eligible individuals were ≥ 50 years of age, diagnosed with HIV, able to speak and understand English, and had at least one HIV care appointment at the recruiting clinic within the last 6 months. We excluded participants if they were unable to pass a post-consent test designed to assess their ability to provide informed consent.
After obtaining informed consent and HIPAA authorization, participants completed baseline study questionnaires through the Research Electronic Data Capture (REDCap). REDCap is a secure, web-based, electronic data capture platform (25) that is compliant with HIPAA policies and procedures. Participants were compensated with $25 in cash upon completion of baseline questionnaires. We obtained data over 12-months post-baseline completion on routine medical visits to assess retention in care, health care utilization (emergency department [ED] visits, hospitalizations) data, and baseline HIV biomarkers (viral load, CD4+ T cell count) from participant EMR. Emory University Institutional Review Board and the clinic’s research oversight committee approved this study.
Measures
Sociodemographic characteristics included age, gender, sexual orientation, race, ethnicity, education level, monthly household income, health care insurance, transportation method, time to the clinic, and past unstable housing status.
Additionally, we assessed covariates/confounders. Details of the measures for covariates/confounders are in Table 1. We selected these variables based on previous literature which demonstrated their significant effects on retention in care. For example, HIV biomarkers, ED visits, hospitalizations, depression, substance use, and attitude towards healthcare provider have been associated with retention in care (1–3, 26).
Table I.
Measures and descriptions of covariates/confounders.
| Variable | Measure | Description | Scoring | Cronbach’ s αa |
|---|---|---|---|---|
| Behavioral and provider factors | ||||
| Drug use | 10-item Drug Abuse Screening Test (DAST-10) (27) | Assesses perceptions of problematic drug use during the previous 12 months. |
|
0.80 |
| Alcohol use | Alcohol Use Disorders Identification Test (AUDIT-10) (28) | Assesses perceptions of problematic alcohol use during the previous 12 months. |
|
0.71 |
| Healthcare provider factors | 19-item Attitude Towards the Health Care Provider Scale (ATHCP) (9) | Assesses attitudes toward one’s HIV care team. |
|
0.96 |
| Depressive symptoms | Center for Epidemiologic Studies Depression Scale Revised (CESD-R) (29) | Assesses nine symptoms of depression (dysphoria, anhedonia, appetite, sleep, concentration, worthlessness, fatigue, agitation, and suicidal ideation) in accordance with the DSM-V diagnostic criteria for Major Depressive Disorder. |
|
0.93 |
| Disease status and emergent health care utilization behaviors | ||||
| Variable | Assessment method | Description | Categories/Scoring |
|---|---|---|---|
| Comorbidity burden | Self-reported version of the Charlson Comorbidity Index (CCI) (30). | Participants indicate whether they have a diagnosis for any of 19 conditions. The CCI has strong construct validity and reliability (26). | Comorbidities are assigned a weight based on the adjusted 1 - year mortality risk. AIDS diagnosis was omitted from this study as its weight is outdated in the current ART era. |
| HIV viral load | EMR laboratory data | A single HIV-1 RNA viral load lab value was collected at ± 2 months from the baseline survey completion date. | < 40 copies/mL / ≥40 copies/mL |
| CD4+ T cell count | EMR laboratory data | A single CD4+ T cell count lab value collected at ±2 months from the baseline survey completion date. | <200 cells/mm3 / ≥ 200 cells/mm3 |
| ED visits | EMR admissions data | Total number of ED visits during the 12-month prospective data extraction period. | No ED visits / Any ED visits |
| Acute hospitalizations | EMR admissions data | Total number of hospitalizations during the 12-month prospective data extraction period. | No hospitalizations / any hospitalizations |
Cronbach’s alpha calculated using this study’s sample; DSM-V= Diagnostic and Statistical Manual Five; ART= antiretroviral therapy; ED= emergency department; EMR= electronic medical record
Retention in care was operationalized as visit adherence (32), which is the percentage of attended HIV clinic visits out of the total scheduled HIV clinic visits over a 12-month post-baseline period (range=0–100%). We extracted information on the dates and the status of HIV clinic visits (attended, no-show, or canceled) from participant EMR. HIV clinic visits were defined as ambulatory care visits made with a HIV care provider, including physicians and advance practice providers with prescribing authority. We excluded sub-specialty care visits, annual well-visits, nurse visits, walk-in visits, and phlebotomy visits. The total scheduled HIV clinic visits represented visits with attended and no-show status. Approximately 10% of all retention in care data were randomly re-extracted by the primary author to ensure the data quality.
Emotion dysregulation was measured using the Difficulties in Emotion Regulation Scale (DERS) (33), which has 36 items rated on a 5-point Likert scale (1=almost never to 5=almost always). Higher scores suggest greater difficulty in emotion regulation. DERS has high internal consistency (Cronbach’s α from .80 to .89), test–retest reliability (r = .88), and adequate construct and predictive validity among adolescents, young adults, and community dwelling older adults (34). The DERS has been used in studies with PLWH (20, 35) and the scale had excellent internal consistency for this study’s sample (α=0.93).
Social network size was measured using the Social Network Index (SNI), which assesses whether an individual has regular social contact with 12 types of relationships in person or on the phone at least once every 2 weeks (36). The types of social relationships or activities include spouse, parents, parents-in-law, children, other close family members, close neighbors, friends, colleagues/coworkers, schoolmates, fellow volunteers, members of groups without religious affiliation, and members of religious groups (36). We summed the total number of people with whom the participant had regular contact across the 12 possible social relationships to reflect the overall social network size. A higher score indicates greater social network size. SNI has been used in studies of older adults (36, 37).
Loneliness was measured using Short Form 8a of the Patient-Reported Outcomes Measurement Information System (PROMIS)-Social Isolation (SI) item bank. The PROMIS measures are standardized to allow for comparisons across patient populations and with the general U.S. population (38). The PROMIS-SI scale is validated among persons with chronic illnesses (39) and uses 8-items to assess perceptions of being avoided, excluded, detached, disconnected from, or unknown by others on a 5-point Likert scale (1=never to 5= always). We calculated the total raw score by summing the response values to each question. We then used the score conversion table (39) to convert each total raw score to a T-score metric, which has a mean of 50 and a standard deviation of 10 (38). A higher T-score indicates greater loneliness. The PROMIS-SI had excellent internal consistency for this study (α=0.95).
Data analysis
We computed descriptive statistics, assessed measures of central tendency for all continuous variables, and used bivariate analyses (t-tests and Chi-square tests) to evaluate differences in participant characteristics by visit adherence. Our descriptive statistics revealed that visit adherence followed a non-parametric distribution, such that participants attended either a very high or a very low proportion of their scheduled HIV clinic visits. Using cross-tabulations and Chi-square tests, we aimed to identify a clinically meaningful visit adherence cutoff (70%, 75%, 80%, 85%, or 100%) that was statistically significantly associated with baseline viral suppression and CD4+ T cell count. We chose the cutoff for CD4+ T cell count of 200 cells/mm3, because previous work has shown that retention in care is related to CD4 counts (40) and the possibility of the decline in immune response and poor immune recovery with older age in general (41). For these cross-tabulations, we dichotomized viral load as <40 copies/mL (viral suppression) versus ≥40 copies/mL and CD4+ T cell count as <200 cells/mm3 (non-AIDS defining CD4+ T cell count) versus ≥200 cells/mm3 (AIDS-defining CD4 T cell count). A visit adherence cutoff of 85% generated statistically significant differences (p<.01) in viral suppression and non-AIDS-defining CD4+ T cell count respectively (Appendix A). Therefore, in the following analyses, we dichotomized visit adherence as suboptimal (≤85%) and optimal (>85%) visit adherence.
Next, we used block-wise logistic regression to identify covariates that were statistically significantly associated with visit adherence. In the first block, we entered covariates that were associated (p<.10) with visit adherence from our bivariate analyses. The second block included our main variables of interest (loneliness, social network size, and emotion dysregulation). Variables that were significant predictors (p<.05) of visit adherence in the logistic regression were included in the subsequent path analysis as control variables. We assessed violations of key assumptions for regression and multicollinearity using the variance inflation factor.
Lastly, we used path analysis to test the relationships hypothesized in our a priori model adapted from the Theory of Loneliness. We used robust weighted least squares estimation (WLSMV), which allows for deviations from model assumptions, allows binary outcome variable, and provides overall fit statistics for model testing (42). We assessed the model fit using chi-squared test (χ2), comparative fit index (CFI), Tucker-Lewis index (TLI), and Root Mean Squared Error of Approximation (RMSEA). Non-significant χ2, CFI and TLI of greater than 0.95, and the RMSEA less than 0.08 indicate good model fit (43, 44). We performed descriptive statistics, bivariate analyses, and regression analysis using SPSS version 25.0 and path analysis using MPlus software version 8.2.
Results
A total of 146 participants were enrolled in the study. Two participants died shortly after the baseline study visit and thus were excluded due to missing data on visit adherence, which resulted in the final sample of 144. The study sample was predominantly African American (AA; 85.6%), male gender (60.3%), and heterosexual (63.0%). Most participants (78.0% and 80.8%) self-reported of completing a high school education/GED or greater and having some type of health care coverage, respectively. Seventeen participants reported unstable housing in the previous 12 months. Details on participant characteristics are provided in Table 2.
Table II.
Participant characteristics.
| Total | ≤85% visit adherence | >85% visit adherence | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | (%) | n | (%) | n | (%) | X2 | p-value |
| All | 146 | (100) | 63 | (43.7) | 81 | (56.3) | ||
| Race/Ethnicity | ||||||||
| African | 125 | (85.6) | 57 | (90.4) | 66 | (81.5) | 2.45 | .29 |
| American/Black | ||||||||
| White/Non-Hispanic | 12 | (8.2) | 3 | (4.8) | 9 | (11.1) | ||
| Other | 9 | (6.2) | 3 | (4.8) | 6 | (7.4) | ||
| Gender | ||||||||
| Born | 88 | (60.3) | 40 | (63.5) | 46 | (56.8) | 0.71 | .70 |
| Male/Identify | ||||||||
| Male | ||||||||
| Born | 55 | (37.7) | 22 | (34.9) | 33 | (40.7) | ||
| Female/Identify | ||||||||
| Female | ||||||||
| Other | 3 | (2.1) | 1 | (1.6) | 2 | (2.5) | ||
| Marital Status | ||||||||
| Never married/Single | 86 | (58.9) | 32 | (50.8) | 53 | (65.4) | 7.79 | .35 |
| Divorced/Separated | 30 | (20.5) | 18 | (28.6) | 12 | (14.8) | ||
| Married/Living with Significant | 14 | (9.6) | 5 | (7.9) | 8 | (9.9) | ||
| Other | ||||||||
| Widow/Widower | 14 | (9.6) | 7 | (11.1) | 7 | (8.6) | ||
| Other | 2 | (1.4) | 1 | (1.6) | 1 | (1.2) | ||
| Sexual Orientation | ||||||||
| Homosexual, Gay, or Lesbian | 30 | (20.5) | 11 | (17.5) | 18 | (22.2) | 2.76 | .60 |
| Heterosexual or Straight | 92 | (63.0) | 43 | (68.3) | 48 | (59.3) | ||
| A Man Who Has Sex with Men | 6 | (4.1) | 3 | (4.8) | 3 | (3.7) | ||
| Bisexual | 16 | (11.0) | 6 | (9.5) | 10 | (12.3) | ||
| Other | 2 | (1.4) | 0 | (0.0) | 2 | (2.5) | ||
| Education | ||||||||
| Some high school or less | 32 | (22.0) | 15 | (23.8) | 15 | (18.5) | 0.83 | .66 |
| High school graduate or GED | 54 | (37.0) | 24 | (38.1) | 30 | (37.0) | ||
| Some college or higher | 60 | (41.0) | 24 | (38.1) | 36 | (44.4) | ||
| Monthly Household Incomea | ||||||||
| ≤$1,000 | 101 | (69.2) | 50 | (79.4) | 50 | (61.7) | 5.20 | .02 |
| >$1,000 | 44 | (30.1) | 13 | (20.6) | 31 | (38.3) | ||
| Health Care Coverage | ||||||||
| No | 29 | (19.9) | 14 | (22.2) | 14 | (17.3) | 0.55 | .46 |
| Yes | 117 | (80.1) | 49 | (77.8) | 67 | (82.7) | ||
| Unstable housing in the past 12 months | ||||||||
| No | 129 | (88.4) | 16 | (25.4) | 1 | (1.2) | 19.87 | <001 |
| Yes | 17 | (11.6) | 47 | (74.6) | 80 | (98.8) | ||
| Transportation to the clinic | ||||||||
| Public | 96 | (65.8) | 46 | (73.0) | 50 | (61.7) | 2.55 | .77 |
| Private vehicle | 39 | (26.7) | 13 | (20.6) | 24 | (29.6) | ||
| Rides from family/friends/others | 3 | (2.0) | 1 | (1.6) | 2 | (2.5) | ||
| Walking | 8 | (5.5) | 3 | (4.8) | 5 | (6.2) | ||
| Transportation time to the clinic | ||||||||
| <30 minutes | 43 | (29.5) | 17 | (27.0) | 25 | (30.9) | 2.27 | .52 |
| 30 minutes - 1 hour | 60 | (41.1) | 24 | (38.1) | 35 | (43.2) | ||
| 1 – 2 hours | 37 | (25.3) | 20 | (31.7) | 17 | (21.0) | ||
| 2 – 3 hours | 6 | (4.1) | 2 | (3.2) | 4 | (4.9) | ||
| Baseline CD4+ T cell countb | ||||||||
| ≥ 200 cells/mm3 | 130 | (90.3) | 52 | (82.5) | 78 | (96.3) | 7.64 | .01 |
| < 200 cells/mm3 | 14 | (9.7) | 11 | (17.5) | 3 | (3.7) | ||
| Baseline HIV-1 RNA viral loada | ||||||||
| < 40 copies/mL | 96 | (66.2) | 35 | (55.6) | 61 | (75.3) | 6.22 | .01 |
| ≥ 40 copies/mL | 49 | (33.8) | 28 | (44.4) | 20 | (24.7) | ||
| Hospitalizationsa | ||||||||
| No | 132 | (91.0) | 54 | (85.7) | 77 | (95.1) | 3.77 | .04 |
| Yes | 13 | (9.0) | 9 | (14.3) | 4 | (4.9) | ||
| ED visitsa | ||||||||
| No | 119 | (82.1) | 52 | (82.5) | 66 | (81.5) | 0.03 | .87 |
| Yes | 26 | (17.9) | 11 | (17.5) | 15 | (18.5) | ||
| Total | ≤85% visit adherence | >85% visit adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | (SD) | Mean | (SD) | Mean | (SD) | t-test | p-value | |
| Age | 50 – 72 | 56.5 | (4.6) | 56.6 | (4.8) | 56.4 | (4.4) | 0.12 | .73 |
| Years since HIV diagnosis | 2 – 34 | 18.1 | (8.4) | 19.1 | (8.0) | 17.3 | (8.7) | 1.72 | .19 |
| DAST-10 | 0 – 9 | 1.2 | (1.9) | 1.7 | (2.3) | 0.8 | (1.3) | 9.00 | .00 |
| AUDIT-10 | 0 – 20 | 2.4 | (3.6) | 3.0 | (4.3) | 1.9 | (2.9) | 3.22 | .08 |
| ATHCP | 35 – 114 | 102.8 | (13.5) | 105.2 | (12.0) | 101.1 | (14.5) | 3.40 | .07 |
| CESD-R | 0 – 47 | 10.99 | (10.33) | 10.92 | (10.12) | 11.04 | (10.55) | 0.00 | .95 |
| CCI | 0 – 8 | 1.3 | (1.5) | 1.2 | (1.4) | 1.3 | (1.7) | 0.18 | .67 |
| DERS | 36 – 136 | 71.2 | (20.1) | 70.6 | (21.0) | 71.9 | (19.7) | 0.14 | .71 |
| SNI | 0 – 48 | 16.1 | 10.6 | 15.3 | (10.7) | 16.5 | (10.4) | 0.45 | .50 |
| PROMIS-SI | 34 – 72 | 47.2 | (10.1) | 47.4 | (9.1) | 47.3 | (10.9) | 0.00 | .93 |
n=145;
n=144; SD=standard deviation; ED=Emergency department; DAST-10=10-item Drug Abuse Screening Test; AUDIT-10=10-item Alcohol Use Disorders Identification Test; ATHCP=Attitude Towards the Health Care Provider Scale; CESD-R= Center for Epidemiologic Studies Depression Scale Revised; CCI=Charlson Comorbidity Index; DERS=Difficulties in Emotion Regulation Scale; SNI=Social Network Index; PROMIS-SI= Patient-Reported Outcomes Measurement Information System-Social Isolation
Of 144 participants, 81 had optimal and 63 had suboptimal visit adherence. Over the 12-month period, participants with optimal and suboptimal visit adherence had 3.5 ± 1.6 (range=1–10) and 5.0 ± 2.1 (range=1–12) scheduled HIV clinic visits, respectively. Statistically significant differences between suboptimal and optimal visit adherence were noted in monthly household income (χ2=5.20, p=.02), past unstable housing (χ2=19.87, p<.001), CD4+ T cell count (χ2=7.64, p=.01), viral load (χ2= 6.22, p=.01), hospitalization (χ2= 3.77, p<.05), and drug use (t=9.00, p=.003). Bivariate analysis suggested that unstable housing was related to retention in care, emotion dysregulation, loneliness, and social network size. Depression was related to emotion dysregulation (r=.63, p<.001), loneliness (r=.50, p<.001), and social network size (r=−.17, p=.039). Therefore, due to concerns for multicollinearity, unstable housing and depression were removed from subsequent analyses. There were no statistically significant differences in visit adherence by gender, sexual orientation, race/ethnicity, education levels, transportation method, and time to clinic.
Variables from our bivariate analyses that differed by optimal versus suboptimal visit adherence (p<.10) were entered sequentially into a logistic regression model (Table 3, Model 1). Statistically significant covariates in Model 1 remained significant in Model 2 even after the addition of emotion dysregulation, social network size, and loneliness. Monthly income (B=0.80, p=.07), baseline CD4+ T cell count (B=1.45, p=.05), and drug use (B=−0.28, p=.02) were related to optimal visit adherence and were subsequently controlled for in the path analysis. The relationship between the variables in the full logistic regression model was statistically significant (χ2 [10, N = 144] = 30.55, p <.001) and the model explained 26% of the variance in visit adherence.
Table III.
Logistic regressions on optimal visit adherencea (N=144).
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variable | B | SE | OR | B | SE | OR |
| Monthly income | ||||||
| ≤ $1,000 | REF | REF | ||||
| > $1,000 | 0.81 | 0.43 | 2.25* | 0.80 | 0.44 | 2.23* |
| Viral load | ||||||
| Detectable | REF | REF | ||||
| Undetectable | 0.64 | 0.40 | 1.90 | 0.64 | 0.41 | 1.90 |
| CD4+ T cell count | ||||||
| < 200 cells/mm3 | REF | REF | ||||
| ≥ 200 cells/mm3 | 1.49 | 0.73 | 4.46** | 1.45 | 0.73 | 4.28** |
| Hospitalization | ||||||
| Yes | REF | REF | ||||
| No | 0.81 | 0.67 | 2.26 | 0.78 | 0.67 | 2.17 |
| AUDIT-10 | −0.03 | 0.06 | 0.97 | −0.03 | 0.06 | 0.97 |
| DAST-10 | −0.27 | 0.12 | 0.77** | −0.28 | 0.12 | 0.76** |
| ATHCP | −0.03 | 0.02 | 0.97 | −0.03 | 0.02 | 0.97 |
| DERS | 0.01 | 0.01 | 1.01 | |||
| SNI | 0.01 | 0.02 | 1.01 | |||
| PROMIS-SI | −0.01 | 0.02 | 1.00 | |||
| χ2 | 29.97, df=7, p<.001 | 30.55, df=10, p<.001 | ||||
| Nagelkerke R2 | 25% | 26% | ||||
| Hosmer and Lemeshow test | p=.42 | p=.43 | ||||
| Classification accuracy (% of optimal visit adherence) | 72.2 | 73.6 | ||||
p <.10;
p < .05;
p <.001, two-tailed;
>85% visit adherence; AUDIT-10=10-item Alcohol Use Disorders Identification Test; DAST-10=10-item Drug Abuse Screening Test; ATHCP=Attitude Towards the Health Care Provider Scale; DERS=Difficulties in Emotion Regulation Scale; SNI=Social Network Index; PROMIS-SI= Patient-Reported Outcomes Measurement Information System-Social Isolation
Findings from the path analysis are illustrated in Figure 2 and Table 4 and include standardized path coefficients (β) and standard errors. Loneliness was directly related to emotion dysregulation (β=0.46, p<.001). Higher social network size was related to lower emotion dysregulation (β=−0.16, p=.01). Neither social network size (β=0.04, p=.68) nor loneliness (β=−0.03, p=.82) were directly related to optimal visit adherence. The direct path between emotion dysregulation and optimal visit adherence was non-significant (β=0.11, p=.33). As would be expected from the non-significant paths of social isolation and emotion dysregulation on visit adherence, the indirect effect of emotion dysregulation was also not statistically significant. Monthly income (β=0.54, p=.04), CD4+ T cell count (β=1.05, p=.01), and drug use (β=−0.17, p=.007) were significant covariates for optimal visit adherence. The model reflected very good fit (χ2=8.81, p=.46; CFI=1.00, TLI=1.01, RMSEA<0.001) and explained 28% of variance in emotion dysregulation (p<.001) and 23% of the variance in optimal visit adherence (p=.008).
Figure 2. Final Path Diagram on Optimal Retention in Care (N=144).
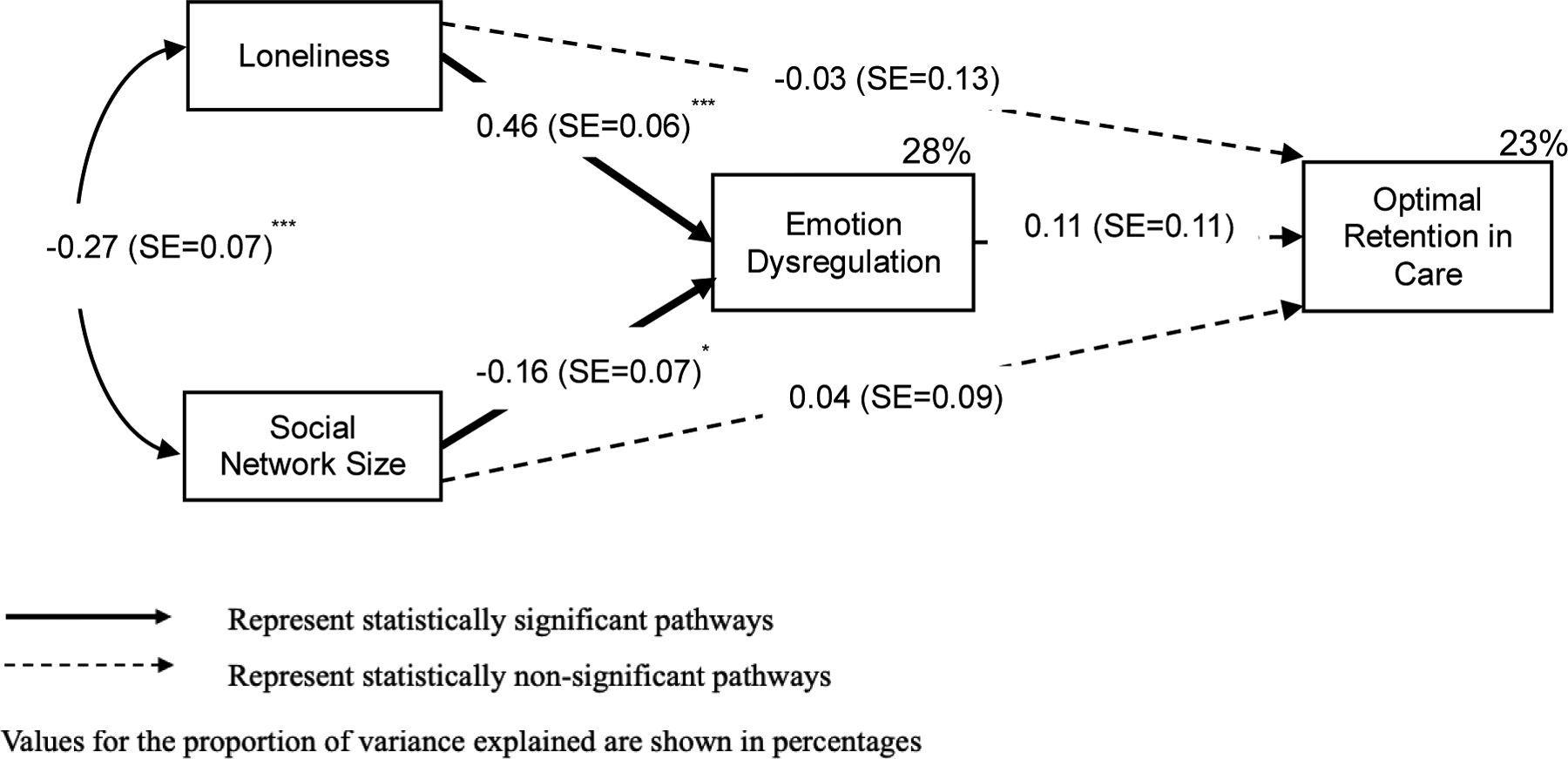
*p<.05, **p <.01***p<.001, two-tailed
Standardized beta coefficients and standard error (SE) are reported. Significant and non-significant paths are illustrated by solid and dotted arrows, respectively. The analysis used robust weighted least-squares estimation method and adjusted for baseline CD4+ T cell count, monthly income, and drug use on optimal retention in care (>85% visit adherence).
Table IV.
Path analysis on optimal visit adherence.
| Endogenous variable | Exogenous variable | Standardized coefficient | SE | Disturbance | R2 |
|---|---|---|---|---|---|
| Emotion dysregulation | 0.72 | 0.28 | |||
| ON | Social network size | −0.16* | 0.07 | ||
| Loneliness | 0.46*** | 0.06 | |||
| Visit adherence | 0.99 | 0.23 | |||
| ON | Social network size | 0.04 | 0.09 | ||
| Loneliness | −0.03 | 0.13 | |||
| Emotion dysregulation | 0.11 | 0.11 | |||
| Monthly income | 0.54* | 0.26 | |||
| CD4+ T cell count | 1.05** | 0.42 | |||
| Drug usse | −0.17** | 0.06 | |||
| Overall | 0.51 |
Model fit statistics: χ2=8.81 (p=.46), CFI=1.00, TLI=1.01, RMSEA<0.001 (90% CI: 0.00–0.09); SE=standard error; The analysis used robust weighted least-squares estimation method and adjusted for baseline CD4+ T cell count, monthly income, and drug use on optimal (>85%) visit adherence.
p<.05,
p <.01
p<.001, two-tailed
Discussion
Retention in care is important to effectively manage HIV and other comorbidities that are common among older PLWH. This study is among the first to utilize the Theory of Loneliness (18) to explore the relationships among social isolation (loneliness and social network size), emotion dysregulation, and retention in care among older PLWH. We found that higher levels of loneliness and smaller social network sizes were related to greater emotion dysregulation. Our findings on the association between loneliness and emotion dysregulation are consistent with past research that utilized depressive symptoms as an indicator for emotion dysregulation (21). Additionally, findings support the relationship between social network size and emotion dysregulation (23).
Our results indicated that more than half of our sample were optimally retained in care. We found that participants were more likely to be sub-optimally retained in care if they had an income lower than the federal poverty level, had an AIDS-defining CD4+ T cell count, and reported problematic drug use. Previous literature supports the association of these factors with retention in care (26, 45). Socioeconomically challenging environments, such as unstable housing and financial insecurity, may trigger a cascade of stressors that serve as underlying risk factors and barriers to suboptimal retention in care. Past research suggests that individuals under these circumstances may prioritize their basic needs over attendance at routine medical visits (46).
Our main findings showed that emotion dysregulation does not explain a relationship between measures of social isolation and retention in care. This finding is consistent with a cross-sectional retrospective study that assessed the effects of depressive symptoms, one indicator for emotion dysregulation, on visit adherence (9), yet contradicts another study that found a significant relationship between depression and ART adherence (47). It may be plausible that emotion dysregulation is uniquely related to medication adherence, but not to retention in care. Compared to retention in care, medication adherence may require more complex self-management and involve higher levels of motivation and problem-solving skills, which may be more directly influenced by one’s emotional state. The complexity of ART regimens or daily dosing of ART in addition to administration of multiple medications for other health conditions may also complicate one’s adherence to medications. On the other hand, retention in care is a planned behavior that individuals can prepare for and manage before unfavorable contextual circumstances arise.
Despite some evidence of a link between social isolation and health care utilization among seronegative older adults with other chronic conditions (48), our findings suggest that neither social network size nor loneliness is related to retention in care. The lack of association between social isolation and retention in care was unexpected. Socially isolating environments and negative feelings of loneliness may indicate greater unmet needs and higher levels of emotional strain, which may in turn influence retention in care. It is possible that social isolation may be too distal to affect retention in care directly and that other complex mediating and moderating factors, including motivation, self-efficacy, and knowledge of HIV outcomes (49), may have a more direct role on retention in care.
Limitations
The findings from this study should be interpreted within its limitations. First, this study may be limited by its generalizability regarding the patient population and the recruitment clinic. Our sample was recruited from a single health care clinic in a large metropolitan area and did not assess broad system-level factors related to the clinic or the services provided by the clinic. Previous research showed that the various forms of integrated health services provided by the clinic are associated with improved engagement in care (50). Therefore, it is possible that the kinds of care that the recruiting clinic provides, including its rigorous retention initiatives, may have affected our sample’s retention in care. Additionally, our findings may not be generalizable to those who may be more socioeconomically stable or those with minimal access to health care and should not be applied to individuals who were never in HIV care or those lost to follow up. Second, we tested a unidirectional model and it is possible the relationships and associations we found may be bidirectional and/or there may be additional mediators or moderating factors that affect retention. More additional complex models and analyses would be warranted to address this limitation. Third, HIV clinic visits may have been misclassified in our calculation of visit adherence, and we may have inappropriately categorized visits as HIV care visits. Among older adults, HIV care encounters may include assessment and management of other comorbidities. It was not always apparent if an appointment addressed services related to HIV specifically or other medical issues. It is also possible that participants received their HIV care at other clinics, but these visits were not captured in the study. Furthermore, visit adherence may be a biased estimate for retention in care since a greater number of all scheduled HIV clinic visits can inflate percent visit adherence. Additionally, the visit adherence cutoff used in this study was chosen specifically for our sample and may be an inaccurate cut point for a larger sample of PLWH.
Conclusions
Our study is among the first to test an association between social isolation and retention in care among older PLWH and to assess the potential role of emotion dysregulation in this relationship. The findings suggest that neither social network size nor loneliness are directly related to retention in care. Emotion dysregulation was not related to retention in care but was related to both social network size and loneliness. Low income, problematic drug use, low CD4+ T cell count, and past unstable housing status were related to suboptimal retention in care. This suggests that older PLWH who are sub-optimally retained in care may face a myriad of socioeconomic and behavioral vulnerabilities that play a more direct role in retention in care. More studies are needed to elucidate the effects of these factors on retention in care and health outcomes among older PLWH. Additionally, as the aging population of PLWH continues to grow, interventions that address housing or those that involve intensive outreach services may be beneficial to ensure retention in care and continued HIV and comorbidity health management. Lastly, more research is needed to determine if there are specific retention in care thresholds that can be predictive of optimal goals of care related to not only HIV but also other medical conditions for these aging individuals who may have greater burden of disease.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Nursing Research of the National Institutes of Health under the following Award Numbers: F31NR015975, T32NR014205.
We would like to thank Dr. Ashley Anderson, PhD, RN, and Kristine Kulage, MA, MPH, for their constructive feedback and technical assistance with editing the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest. None of the authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Ethical Approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Study protocol was approved by the Institutional Review Board and by the recruiting clinic’s research oversight committee.
Informed Consent. Informed consent was obtained from all individual participants included in the study. Individuals were compensated for their participation in the study.
References
- 1.Giordano TP, Gifford AL, White AC Jr., Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44(11):1493–9. [DOI] [PubMed] [Google Scholar]
- 2.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(2):248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS patient care and STDs. 2009;23(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60(3):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emlet CA. An examination of the social networks and social isolation in older and younger adults living with HIV/AIDS. Health & social work. 2006;31(4):299–308. [DOI] [PubMed] [Google Scholar]
- 6.Shippy RA, Karpiak SE. The aging HIV/AIDS population: fragile social networks. Aging & mental health. 2005;9(3):246–54. [DOI] [PubMed] [Google Scholar]
- 7.Greene M, Hessol NA, Perissinotto C, Zepf R, Hutton Parrott A, Foreman C, et al. Loneliness in Older Adults Living with HIV. AIDS and behavior. 2018;22(5):1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrimshaw EW, Siegel K. Perceived barriers to social support from family and friends among older adults with HIV/AIDS. Journal of health psychology. 2003;8(6):738–52. [DOI] [PubMed] [Google Scholar]
- 9.Bodenlos JS, Grothe KB, Whitehead D, Konkle-Parker DJ, Jones GN, Brantley PJ. Attitudes toward health care providers and appointment attendance in HIV/AIDS patients. The Journal of the Association of Nurses in AIDS Care : JANAC. 2007;18(3):65–73. [DOI] [PubMed] [Google Scholar]
- 10.Waldrop-Valverde D, Guo Y, Ownby RL, Rodriguez A, Jones DL. Risk and protective factors for retention in HIV care. AIDS and behavior. 2014;18(8):1483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gannon BN, Stacciarini JM. Review of the Literature: A Rural-Urban Comparison of Social Networks of Older Adults Living With HIV. The Journal of the Association of Nurses in AIDS Care : JANAC. 2016;27(4):419–29. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson NR Jr. Social isolation in older adults: an evolutionary concept analysis. Journal of advanced nursing. 2009;65(6):1342–52. [DOI] [PubMed] [Google Scholar]
- 13.Peplau A, Perlman D. Perspectives on loneliness In: Peplau APD, editor. Loneliness: a sourcebook of current theory, research, and therapy. New York, NY: Wiley-Interscience; 1982. p. 1–18. [Google Scholar]
- 14.Cornwell EY, Waite LJ. Measuring social isolation among older adults using multiple indicators from the NSHAP study. J Gerontol B Psychol Sci Soc Sci. 2009;64 Suppl 1(Suppl 1):i38–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong-Gierveld J, Van Tilburg TG, Dykstra PA. Loneliness and social isolation In: Perlman D, Vangelisti A, editors. The Cambridge Handbook of Personal Relationships. Cambridge: Cambridge University Press; 2006. p. 485–500. [Google Scholar]
- 16.Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt-Lunstad J, Robles TF, Sbarra DA. Advancing social connection as a public health priority in the United States. The American psychologist. 2017;72(6):517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2010;40(2):218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour research and therapy. 2005;43(10):1281–310. [DOI] [PubMed] [Google Scholar]
- 20.Brandt CP, Zvolensky MJ, Bonn-Miller MOJCT, Research. Distress tolerance, emotion dysregulation, and anxiety and depressive symptoms among HIV+ individuals. 2013;37(3):446–55. [Google Scholar]
- 21.Hawkley L, Preacher K, Cacioppo J. Multilevel modeling of social interactions and mood in lonely and socially connected individuals: The MacArthur social neuroscience studies In: Ong A, van Dulmen M, editors. Oxford Handbook of Methods in Positive Psycholo. Michigan: Oxford University Press; 2007. p. 559–75. [Google Scholar]
- 22.Marroquín B, Czamanski-Cohen J, Weihs KL, Stanton AL. Implicit loneliness, emotion regulation, and depressive symptoms in breast cancer survivors. J Behav Med. 2016;39(5):832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho JH, Olmstead R, Choi H, Carrillo C, Seeman TE, Irwin MR. Associations of objective versus subjective social isolation with sleep disturbance, depression, and fatigue in community-dwelling older adults. Aging & mental health. 2019;23(9):1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt CP, Bakhshaie J, Zvolensky MJ, Grover KW, Gonzalez A. The examination of emotion dysregulation as a moderator of depression and HIV-relevant outcome relations among an HIV+sample. Cognitive behaviour therapy. 2015;44(1):9–20. [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of Adult Retention in HIV Care: A Systematic Review. AIDS and behavior. 2018;22(3):752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner HA. The drug abuse screening test. Addictive behaviors. 1982;7(4):363–71. [DOI] [PubMed] [Google Scholar]
- 28.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcoholism, clinical and experimental research. 2007;31(2):185–99. [DOI] [PubMed] [Google Scholar]
- 29.Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESD-R) In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment: Instruments for adults Lawrence Erlbaum Associates Publishers; 2004. p. 363–77. [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 31.Hall SF. A user’s guide to selecting a comorbidity index for clinical research. Journal of clinical epidemiology. 2006;59(8):849–55. [DOI] [PubMed] [Google Scholar]
- 32.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS patient care and STDs. 2010;24(10):607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gratz KL, Roemer L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology & Behavioral Assessment. 2004;26(1):41–54. [Google Scholar]
- 34.Staples AM, Mohlman J. Psychometric properties of the GAD-Q-IV and DERS in older, community-dwelling GAD patients and controls. Journal of anxiety disorders. 2012;26(3):385–92. [DOI] [PubMed] [Google Scholar]
- 35.Leyro TM, Vujanovic AA, Bonn-Miller MO. Examining associations between cognitive-affective vulnerability and HIV symptom severity, perceived barriers to treatment adherence, and viral load among HIV-positive adults. International journal of behavioral medicine. 2015;22(1):139–48. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr. Social ties and susceptibility to the common cold. Jama. 1997;277(24):1940–4. [PubMed] [Google Scholar]
- 37.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature neuroscience. 2011;14(2):163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology. 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PROMIS. PROMIS social isolation: a brief guide to the PROMIS social isolation [Available from: https://www.assessmentcenter.net/documents/PROMIS%20Social%20Isolation%20Scoring%20Manual.pdf
- 40.Yehia BR, French B, Fleishman JA, Metlay JP, Berry SA, Korthuis PT, Agwu AL, Gebo KA, HIV Research Network. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. J Acquir Immune Defic Syndr. 2014;65(3):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. The Journal of clinical investigation. 2013;123(3):958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthén L, Muthén B. Mplus User’s Guide, 8th ed Los Angeles, CA: 2017. [Available from: https://www.statmodel.com/download/usersguide/MplusUserGuideVer_8.pdf [Google Scholar]
- 43.Hooper D, Coughlan J, Mullen MJA. Structural equation modelling: Guidelines for determining model fit. 2008:2. [Google Scholar]
- 44.Kline R. Hypothesis testing In: Kenny D, Little T, editors. Principles and practice of structural equation modeling. New York, NY: Guilford Press; 2011. p. 193–210. [Google Scholar]
- 45.Aidala AA, Lee G, Abramson DM, Messeri P, Siegler A. Housing need, housing assistance, and connection to HIV medical care. AIDS and behavior. 2007;11(6 Suppl):101–15. [DOI] [PubMed] [Google Scholar]
- 46.Warren-Jeanpiere L, Dillaway H, Hamilton P, Young M, Goparaju L. Taking it one day at a time: African American women aging with HIV and co-morbidities. AIDS patient care and STDs. 2014;28(7):372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valtorta NK, Moore DC, Barron L, Stow D, Hanratty B. Older Adults’ Social Relationships and Health Care Utilization: A Systematic Review. Am J Public Health. 2018;108(4):e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones D, Cook R, Rodriguez A, Waldrop-Valverde D. Personal HIV knowledge, appointment adherence and HIV outcomes. AIDS and behavior. 2013;17(1):242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simeone C, Shapiro B, Lum PJ. Integrated HIV care is associated with improved engagement in treatment in an urban methadone clinic. Addict Sci Clin Pract. 2017;12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


