Abstract
Background:
The nonlesional skin of atopic dermatitis (AD) children with peanut allergy (PA) is associated with increased transepidermal water loss (TEWL), low urocanic (UCA) and pyroglutamic (PCA) acids (filaggrin [FLG] breakdown products), and reduced ratio of esterified ω-hydroxy fatty acid sphingosine ceramides (EOS-CER) to non-hydroxy fatty acid sphingosine ceramides (NS-CER) in the skin. The skin barrier of PA without AD (AD−PA+) subjects has not been studied.
Objective:
To explore whether AD−PA+ is associated with skin barrier abnormalities.
Methods:
33 participants were enrolled including 13 AD−PA+, 9 AD+PA+, and 11 non-atopic participants (NA).
Results:
The content of PCA in the stratum corneum (SC) of AD−PA+ subjects was significantly reduced in comparison to NA (Median: 67 vs 97 μg/mg protein; p=0.028). The ratio between cis- and trans-UCA significantly decreased from being the highest in NA group (1.62) to the lowest in AD+PA+ group (0.07, p<0.001 vs NA; p=0.006 vs AD−PA+ group), with AD−PA+ group having intermediate cis/trans-UCA ratio (1.17, p=0.024 vs NA group). The TEWL in AD−PA+ subjects did not differ from NA skin. Interestingly, AD−PA+ subjects had increased EOS/NS-CER ratio vs NA (1.9 vs 1.3; p=0.008), while AD+PA+ group had decreased proportion of EOS-CER (0.8, p=0.001 vs AD−PA+ group).
Conclusion:
Our data demonstrate that, irrespective of AD, PA is associated with decreased skin cis-UCA and PCA content. An increase in skin EOS/NS-CER ratio separates AD−PA+ from AD+PA+ and NA groups.
Keywords: atopic dermatitis, food allergy, skin barrier, peanut allergy
Capsule Summary
Skin in peanut allergy (PA) without atopic dermatitis (AD) is associated with low levels of filaggrin (FLG) breakdown products. Increased long chain lipids distinguish this group from PA with AD and normal skin.
INTRODUCTION
Food allergy (FA) affects nearly 7% of children and is associated with major health burdens (1). Peanut allergy (PA) is the most common form of FA and is thought to be associated with skin barrier dysfunction. Skin barrier abnormalities allow allergen penetration through the skin, immune cell sensitization, and systemic immunoglobulin E (IgE) responses to allergens (2,3). This scenario has been most convincingly demonstrated in filaggrin (FLG) deficient mice (4) and humans with atopic dermatitis (AD) (5). Risk factors for individuals who have concurrent AD with PA (AD+PA+) include early onset of AD, severity of AD, and duration of AD (6,7). Importantly, a recent study reported that early intervention in the treatment of AD reduces the occurrence of PA (8). This suggests that early detection of infants with skin barrier abnormalities may represent a window of opportunity for prevention of PA and other forms of FA.
There is also a subset of children who do not not have any history of AD but develop peanut allergy (AD−PA+) (9). It is not known if AD−PA+ subjects have a normal skin barrier. Recently, we have presented evidence that AD+PA+ children can be assigned to a distinct endotype and separated from children who have AD but not PA (AD+PA−) by a combination of parameters that include transepidermal water loss (TEWL), decreased skin urocanic acid (UCA) (surrogate marker for low FLG expression), increased type 2 immune activation, and reduced long chained esterified ω-hydroxy fatty acid sphingosine ceramides (EOS-CER) expression (10). Here we present evidence that the skin of AD−PA+ individuals has a distinct profile of polar and lipid components and that AD−PA+ subjects represent a distinct endotype different from AD+PA− or AD+PA+ subjects. Altogether, our findings provide a strong support for the hypothesis that the PA, with or without AD, is associated with an imbalance of stratum corneum (SC) lipid and protein components known to facilitate sensitization to allergens through the skin.
METHODS
Study design
In this article, we report our observations from a prospective, clinical mechanistic study approved by The National Jewish Health Institutional Review Board. Written informed consent was provided by the parent/legal guardian and written assent was provided by the participant, as applicable, before participation. Importantly, all laboratory data were analyzed without knowledge of study participant diagnostic group in order to eliminate any investigator bias. Endpoints measured in this study included TEWL area under the curve (TEWL AUC) assessed in nonlesional skin prior to skin tape stripping (STS) and repeated after 5, 10, 15, and 20 STS. STS samples were also assessed for FLG breakdown products and lipid profiles.
Study participants
A total of 33 participants were enrolled including 13 AD−PA+ participants, 9 AD+PA+ participants, and 11 non-atopic (NA) participants. The summary of study subjects clinical characteristics is shown in Table E1. NA controls were defined as those without a personal history of atopic diseases and negative skin prick tests to common foods and aeroallergens. All participants in the AD−PA+ and AD+PA+ groups had a history of immediate clinical reactions to peanut and a positive skin prick wheal size to peanut of 8 mm or greater (Table E1). Peanut wheal size was significantly greater in AD+PA+ subjects as compared to AD−PA− subjects; no significant difference in peanut specific IgE was observed. The AD−PA+ group had no history of previous skin rash. The AD+PA+ had AD as described in reference 10. Peanut-specific IgE was detected in serum samples from both AD−PA+ and AD+PA+ groups.
Skin Tape Strip (STS) collection
D-Squame tape strips (22 mm diameter, CuDerm) were collected from the upper extremity of each subject. Skin lesions were in the region of the antecubital fossa of all AD subjects. Nonlesional skin, which had a normal clinical appearance and no visible excoriations, was obtained 5 cm from the skin lesion. In all subjects without AD (AD− PA+ and NA), the nonlesional STS was just below the antecubital fossa. The D-Squame pressure instrument D500 was used to apply all tape strips with equivalent pressure (e.g. 225 g/cm2). On application of the first tape disc, four marks were placed around the disc with a pen so that subsequent discs could be applied to the same location. Each tape disc was applied to a designated surface area of a D-Squame Disc Storage Card (CuDerm) and then placed into a plastic pouch and stored at −80°C until STS analysis was conducted for FLG breakdown products, lipids, and by electron microscopy.
Skin barrier assessments
TEWL was assessed using the AquaFlux AF200 (Biox). All skin barrier measurements for TEWL AUC were made on nonlesional, non-flexural, non-sun exposed skin from the upper extremity in the region of the antecubital fossa. The baseline lesional TEWL was also assessed for AD participants. All TEWL measurements were made in temperature and climate-controlled conditions. SC integrity of nonlesional skin was measured by TEWL, at the baseline before tape stripping and after 5, 10, 15, and 20 tape strips. TEWL AUC was calculated as previously described (10).
FLG skin measurements
FLG breakdown products, cis/trans-urocanic acid (total UCA) and pyrrolidone carboxylic acid (PCA), also known as pyroglutamic acid, were quantified via a liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) approach on a Sciex 6500QTRAP mass spectrometer coupled with a Shimadzu Nexera X2 UHPLC system as previously described (10). Briefly, SC from STS #15-16 was removed by scraping STS in 2 ml water-methanol (9:1, v/v) solution in a Petri dish with a rubber cell scraper. Floating SC particles were carefully transferred into the glass screw cap tubes. Petri dishes were washed twice with 1 ml methanol, which was combined with primary SC suspension. Final SC particle suspension was further subjected to a modified Bligh and Dyer extraction (11). A known amount of U-[13C,15N]proline was added at this step to ensure absolute quantitation of targeted molecules. Extraction was performed overnight by adding 0.25 ml chloroform, then phase separation was achieved by adding 2.0 ml chloroform and 0.45 ml 2% formic acid, followed by intensive vortexing and centrifugation (2,000g x 10 min). After centrifugation, the bottom chloroform phase was collected and kept for lipid analyses while the upper water-methanol phase was re-extracted by adding 2.25 ml chloroform, followed by additional intensive vortexing and centrifugation. At the end, the upper water-methanol phase was carefully collected, dried under a nitrogen stream, redissolved in methanol/water (1:1, v/v), and subjected to the LC-ESI-MS/MS analysis. The chloroform layers were combined and processed for lipid analyses. The protein interface was subjected to hydrolysis with 1N NaOH at 80°C for 3 hours, then neutralized with 1N HCl. The sample protein content was measured using a DC Protein Assay kit (Bio-Rad) with bovine serum albumin (BSA) as a protein standard.
LC separation of cis/trans-UCA, PCA, and proline was achieved using an Acquity UPLC BEH Amide (2.1 × 100 mm, 1.7 pm particle size) column using a gradient from acetonitrile (Solvent A) to methanol:water:formic acid (65:35:0.5, with 5 mM ammonium formate) (Solvent B) and the following elution program: hold at 5%B until 0.5 min, then linear increase to 20%B at 1 min, then increase to 60%B at 3 min, hold at 60%B until 4.1 min, then decrease to 5%B by 4.5 min, and hold at 5%B until 5 min. All amino acids were detected in positive ion mode using the following transitions: mass to charge (m/z) 139.1 > m/z 121.1 (UCA), m/z 130.2 > m/z 83.9 (PCA), and m/z 122.1 > m/z 75.0 (U-[13C,15N]proline). Exact quantitation of PCA and cis/trans-UCA was achieved by creating standard curves of responses of variable amounts of analytes versus a fixed amount of the internal standard (U-[13C,15N]proline). Authentic standards of cis-UCA, trans-UCA, and PCA were from MilliporeSigma (Burlington, MA); U-[13C,15N]-proline was from Cambridge Isotope Laboratories (Tewksbury, MA).
Analysis of SC lipids
STS processing for lipid extraction
The bottom chloroform layer from the extraction procedure was used for lipid analysis by mass spectrometry. A fixed amount of the internal standard (N-palmitoyl-D-erythro-sphingosine [d7], D7-ceramide) was added at the beginning of the extraction process. D7-ceramide as well as other standards of NS-ceramides (N-16:0-, N-18:0-, N-20:0-, N-24:1-, and N-24:0-D-erythrosphingosines) were from Avanti Polar Lipids, Inc (Alabaster, AL). Data were normalized to the total amount of hydrolyzed protein, determined as described above.
Lipid analysis by targeted lipid chromatography-tandem mass spectrometry
EOS-CER and NS-CER were identified and quantified using a targeted LC-ESI-MS/MS approach on a Sciex 6500QTRAP mass spectrometer coupled with a Shimadzu Nexera X2 UHPLC system as previously described (12). All molecules were detected in positive ions mode. EOS-CER and NS-CER were detected as a transition from molecular ions to the m/z 264, m/z 292, and m/z 320 as our work has identified all three sphingoid bases (C18-, C20-, and C22-sphingosine) being present in human skin ceramides. Chromatography was performed on an Ascentis Express RP-Amide 2.7 pm 2.1 × 50 mm column using gradient elution from methanol:water:formic acid (65:35:0.5, 5mM ammonium formate) to methanol:chloroform:water:formic acid (90:10:0.5:0.5, 5 mM ammonium formate). Absolute amounts of NS-CER were determined in a quantitative and semi-quantitative way by using correction factors from standard curves that were created using variable amounts of N-14:0-24:0 ceramides with C18-sphingosine as a base versus a fixed amount of D7-ceramide. Correction factors for molecular species for which there are no available standards were used with best possible approximation to the closest available molecular species of ceramide standards. Absolute amounts of EOS-CER were determined in a semi-quantitative way by using a correction factor from a standard curve created using variable amounts of N-24:0-D-erythro-sphingosine (24:0-CER) versus D7-ceramide.
Analysis of protein-bound ceramides by targeted lipid chromatography-tandem mass spectrometry
Protein-bound ceramides were extracted from neutralized protein hydrolysates using Bligh and Dyer extraction (11). D7-ceramide internal standard was added again before initiation of extraction to allow semi-quantitative estimation of ω-hydroxy ceramides that formed upon basic hydrolysis of proteins. Separation of ω-hydroxy ceramides was achieved using same chromatography conditions as described above. The identification of ω-hydroxy ceramides was performed against the authentic standard of ceramide 1 (d18:1/26:0/18:1) N-[26-oleoyloxy hexacosanoyl]-D-erythro-sphingosine) (Avanti Polar Lipids, Inc) subjected to the same hydrolysis procedure as protein, thus providing the standard of ω-hydroxy N-26:0-sphingosine (ω-hydroxy ceramide).
Statistical analysis
To compare differences in the demographic characteristics between groups, chi-square tests were used for categorical variables and Mann-Whitney or Kruskal-Wallis tests were used for two group or three group comparisons, respectively. Comparisons in FLG breakdown products, lipid ratios, free- and protein-bound EOS-CER between the three study groups were done using one-way ANOVA tests with multiple comparisons. P<0.05 was considered statistically significant.
RESULTS
Skin TEWL measurements
TEWL was measured in nonlesional skin at the baseline before STS and after 5, 10, 15, and 20 STS. At the baseline, TEWL was similar for all subjects. AD+PA+ subjects were found to have impaired skin barrier as demonstrated by progressively increasing TEWL with consecutive STS (Fig 1, A). However, AD−PA+ subjects did not demonstrate an impaired skin barrier as measured by the TEWL following 20 STS (Fig 1, A, red line). This is consistent with the lack of visible skin abnormalities and no personal history of AD in these subjects. Integration of TEWL measurements over all 20 STS layers (TEWL AUC) also did not reveal TEWL difference between NA and AD−PA+ groups (Fig 1, B).
Figure 1. TEWL and TEWL AUC after sequential STS by group.
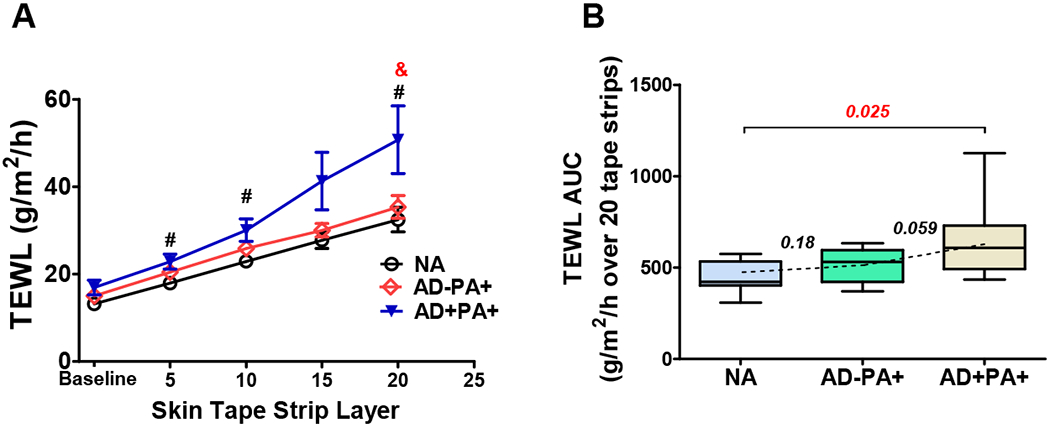
(A) TEWL measurements at the baseline and after 5, 10, 15, and 20 STS. The line figure represents Mean±SE for TEWL measurements at each STS layer (black line represents NA, red line represents AD−PA+, and blue line represents AD+PA+ groups). #,& - significant differences (p<0.05) between AD+PA+ and NA (#) or between AD+PA+ and AD−PA+ (&). (B) TEWL AUC box plots: here and in all subsequent figures, the box margins are the 25-75%-interquartile range (50% of the observations), whisker lines are minimal and maximal observations, and the annotations are the p values from one-way ANOVA with the multiple comparison analysis.
Skin FLG breakdown products
It has previously been demonstrated that breakdown of FLG protein gives rise to skin components of Natural Moisturizing Factor (NMF), urocanic acid (UCA) and pyroglutamic acid (PCA) (13). The trans-UCA isoform is the direct product of histidine transformation, but under UV-light trans-UCA is converted to cis-UCA isoform. A previous study demonstrated a significant decrease in UCA and PCA levels in the skin of AD+PA+ subjects (10). In this study, this observation was confirmed: we found a significant decrease in the content of both total UCA and PCA in AD+PA+ subjects as compared to NA subjects (Fig 2, A and B). Surprisingly, the skin of AD−PA+ subjects also revealed the following trends of a decrease in UCA content (Fig 2, A) and a significant decrease in PCA content (Fig 2, B). Moreover, the individual analysis of the content of cis-UCA and trans-UCA in the skin revealed a noteworthy phenomenon. The ratio between cis- and trans- isoforms of UCA dramatically declined in AD+PA+ skin samples, and was also significantly decreased in the skin of AD−PA+ subjects as compared to NA subjects (Fig 2, C). Interestingly, the content of trans-UCA was not influenced by either AD or PA, but its conversion to cis-UCA was progressively affected in AD−PA+ and AD+PA+ subjects (Fig 2, D). This observation is especially important in view of previous data that demonstrated the immunosuppressive properties of cis-UCA but not trans-UCA (14–16). Our data suggests that the skin of AD−PA+ people has diminished amounts of FLG protein, given the decreased levels of FLG breakdown products, UCA and PCA, in the skin of these subjects. Although the study groups were somewhat imbalanced by gender (Table E1), sex did not have any effect on the skin tape measurements (data not shown).
Figure 2. FLG breakdown products in nonlesional skin.
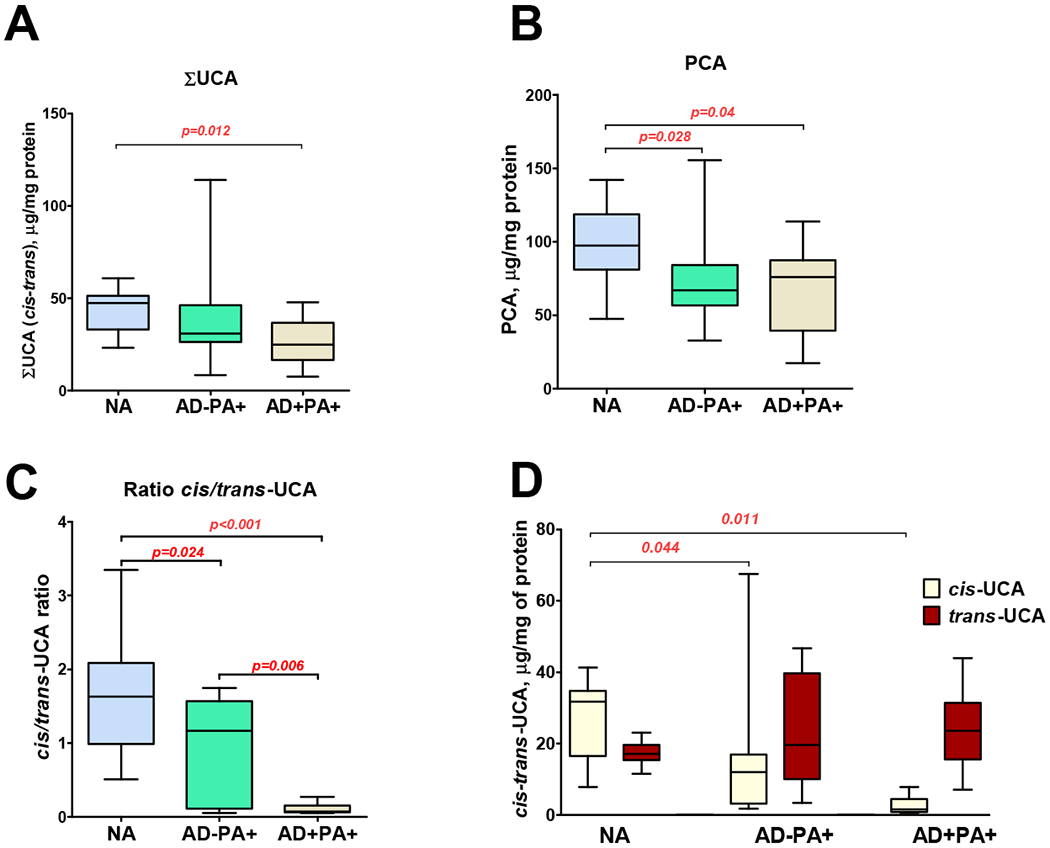
Comparisons between groups for FLG breakdown products, total UCA (A), PCA (B), cis/trans-UCA ratio (C) and cis- and trans-UCA, were all assessed at skin tapes 15 and 16 collected from nonlesional skin areas. (D) Cis-UCA and trans-UCA levels in NA, AD−PA+, and AD+PA+ subjects.
Skin lipids
One characteristic of ceramides, and all lipids in AD skin, is the overall shortening of their fatty acid chain length, which is a result of type 2 immune activation in the AD skin (12). Here we performed targeted lipidomic analysis of STS layers 15-16 in NA, AD−PA+ and AD+PA+ subjects to determine if this phenomenon is recapitulated in AD−PA+ subjects. Remarkably, AD−PA+ subjects did not show evidence of any changes in the chain length of sphingosine-linked fatty acids, as shown by the absolute amount of three major molecular species of NS-ceramides with palmitic (16:0), lignoceric (24:0), and behenic (26:0) fatty acids. In contrast, AD+PA+ subjects demonstrated a clear shift towards a prevalence of short-chained NS-ceramide with palmitic acid (Fig 3, A). Furthermore, the calculated ratio between the short-chain N-16:0-sphingosine ceramide (16:0-NS-CER) and the sum of the long-chain N-24:0- and N-26:0-sphingosine ceramides (24:0-, 26:0-NS-ceramides) confirmed that the AD+PA+ group is clearly distinct from AD−PA+ and NA groups (Fig 3, B), while AD−PA+ group did not differ from NA subjects for this parameter.
Figure 3. Major NS-ceramides in nonlesional skin.
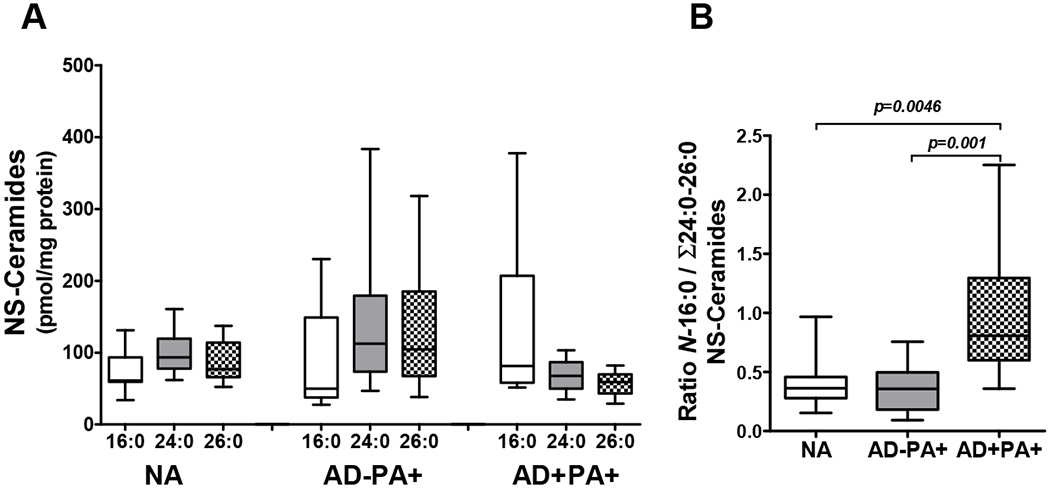
(A) Comparisons between groups for major NS-ceramide molecular species (C18-sphinosine with amide-linked 16:0, 24:0, and 26:0 fatty acids) and (B) the ratio between N-16:0- and the sum of N-24:0- and N-26:0-NS-ceramides. Ceramide levels were assessed at STS 15-16. Data for three major species of NS-ceramides (N-16:0-, N-24:0, and N-26:0-sphingosines) are presented.
EOS-CER are uniquely expressed in the skin and are critical for skin barrier function, including water retention in the skin. EOS-CER are present in the skin not only in free form, but they are also precursors to the oxidized and protein-bound ceramides (17,18). It was previously reported that the relative proportion of free EOS-CER within all ceramides is diminished in the skin of AD patients (19–21). In the current study, our semi-quantitative targeted EOS-CER analysis has shown that protein-normalized content of free and protein bound EOS-CER is decreased in AD+PA+ STS as compared to STS from NA subjects (Fig 4, A–C). On the contrary, both free and protein-bound EOS-CER were significantly increased in the SC of AD−PA+ subjects relative to both NA and AD+PA+ groups (Fig 4, A–C). Furthermore, the proportion of EOS-CER was significantly increased in the skin of AD−PA+ subjects in comparison to the skin of both AD+PA+ and NA subjects, as shown by the ratio of total EOS/NS-CER in the STS analysis (Fig 4, D). These data suggest that the skin of AD−PA+ people has increased biosynthesis of EOS-CER as a potential mechanism to compensate for the loss of FLG and its degradation products. This compensatory increase in EOS-CER may account for lack of skin TEWL changes in AD−PA+ subjects.
Figure 4. Free EOS-ceramides and protein-bound ω-hydroxy ceramides are increased in stratum corneum of peanut allergic subjects.
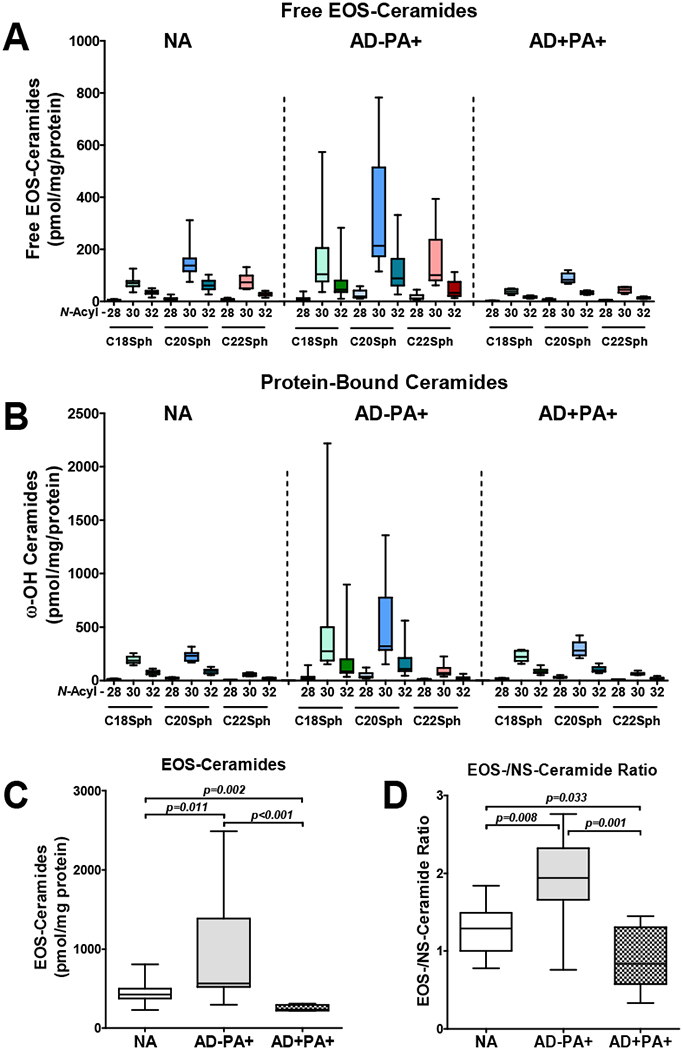
Free EOS-ceramides (A,C) and protein-bound ω-hydroxy ceramides that originate from EOS-ceramides (B) are increased in absolute amounts, as well as in relative to NS-ceramide proportion (D) in the skin of subjects with allergy to peanuts in comparison to healthy and atopic and peanut allergic subjects. Numbers (28, 20, 32) shown on X axes represent the chain length of saturated omega-hydroxy fatty acids N-acylating corresponding C18-C22-sphingosines.
DISCUSSION
Our current findings provide evidence that the stratum corneum of children with allergy to peanut is not normal and bears a distinctive epidermal signature. While the exact causation link between observed abnormalities in stratum corneum composition and the development of food allergy is yet to be defined, our data support the hypothesis that skin barrier abnormalities, i.e. low FLG, may facilitate the onset of PA irrespective of a past or present history of AD. This hypothesis is most strongly supported by animal model observations that the epicutaneous application of protein allergens to nonlesional skin of FLG-deficient mice results in the activation of type 2 cytokine production and generation of protein-specific IgE and IgG1 immunoglobulins (4). It has been further suggested that genetic or environmental factors lead to an impaired skin barrier with activation of skin proteases that facilitate allergen penetration (5, 22). Until now, there has been no information about the normal appearing skin of PA patients, specifically whether it is associated with impaired skin barrier function. In our study, we demonstrate that a routine measure of skin barrier function such as TEWL does not reveal skin impairment in PA only people (Fig 1), similiar to the lack of such demonstration in AD subjects without food allergy (10). This is consistent with the lack of AD in such individuals, since increased TEWL has primarily been associated with AD codiagnozed with allergy to multiple foods.
Our current study of the skin components that control skin pH and moisturization (FLG breakdown products: PCA and UCA) and provide hydrophobic barrier (lipids) has identified abnormalities in the skin composition of AD−PA+ subjects (Figs 2–4). These changes have never been previously observed when studying the skin of AD subjects with or without FA (10,12). What separates the skin of peanut-allergic people who do not have AD from those who do is the lack of particular changes in lipid chain length that are driven by type 2 cytokines in AD (12) and the increase in the proportion of ultra long-chain EOS-CER versus much shorter NS-CER (Fig 4 and references 10,12). Increased levels of EOS-CER are likely a compensatory response to an impaired FLG expression in the skin of AD−PA+ patients. It is not known what drives the decline in FLG expression in the skin of AD−PA+ patients (as measured by the content of its breakdown products PCA and UCA). Aside from FLG null mutations and type 2 cytokine regulation of FLG, there are many environmental causes of low FLG in the skin, including detergents, pollution, immune cytokines, and stress (23).
Our current study has revealed another phenomenon that has not been previously described in the literature. We have observed a unique decline in trans- to cis-UCA conversion in the skin of not only AD subjects with PA, but also in people with PA without AD (Fig 2, D). This observation has, potentially, a very important consequence for the development of allergic responses in the skin, as cis-isoform of UCA and not its trans-isoform possesses immunosuppressive properties through the ability to bind to serotonin 5-HT2A receptor (24). It is known that trans-UCA is initially formed from histidine, an amino acid that is enriched in FLG. Then it is converted to cis-UCA by UV-light (14–16). A recent study published in The Journal of Allergy and Clinical Immunology demonstrated that UV-light is more beneficial than vitamin D supplementation as an eczema prevention strategy (25), suggesting that along with skin vitamin D production, UV-light regulates cis-UCA levels in the skin, an additional immunoregulatory substance in the skin. Reduced levels of cis-UCA observed in the skin of PA subjects in this study suggests that they may be less exposed to UV-light, or there are additional factor(s) that are involved in trans- to cis-UCA conversion in the skin that are yet to be identified.
FLG is critical for skin barrier function not only due to its importance as a major source of NMF for the skin and the involvement of its breakdown product, cis-UCA, in local skin immunosuppression, but also by functioning as a core for the assembly of skin proteo-lipid complexes during corneocyte maturation (17,18). It is logical to expect that proper SC lamellar structure formation will be affected if the lamellar complexes assembly is impaired due to FLG insufficiency, even if the expression of other proteins, lipid components, and their binding to proteins are not affected. However, we did not find clear indications of the disturbance of lamellar structures in SC STS samples from AD−PA+ subjects (data not shown). Further investigation of a larger cohort of AD−PA+ subjects is required to characterize the skin epithelial barrier in AD−PA+ subjects.
We have recently described the mechanism driven by type 2 cytokines, IL-4 and IL-13, that shortens lipid chain length in the skin of people with AD (12). Clearly, lipids in the skin of people with PA only, but no AD, do not bear the signature of being affected by type 2 cytokines (Fig 3). Surprisingly, we have observed an increase in the proportion of EOS-CER (free and protein-bound) in the skin of these people (Fig 4). This could be a sign of a potential, yet to be identified, compensatory mechanism for the lack of FLG expression that is observed only without hyperactivation of type 2 immune responses. This upregulation of EOS-CER biosynthesis might be responsible for the lack of abnormalities in skin TEWL in AD−PA+ subjects (Fig 1). However, this might not be enough to protect from allergen penetration through the skin that has decreased content of FLG as measured by skin content of NMF (Fig 2).
In summary, the current study demonstrates that individuals with PA have reduced expression of immunosuppressive cis-UCA and PCA, the products of FLG breakdown, irrespective of AD, but the subtype of AD−PA+ can be distinguished from AD+PA+ by a unique SC lipid signature. Further development of STS methodology and mass spectrometric analyses of SC components has the potential to become a clinical application to identify people at risk for developing PA and AD in early childhood. The results from this study may provide future approaches, using skin tape stripping, to monitor epidermal changes during atopic match progression and its responses to targeted prevention therapies.
Supplementary Material
Clinical Implications.
Food allergy, without overt atopic dermatitis, is associated with skin barrier dysfunction and may require targeted therapy to prevent food allergy.
ACKNOWLEDGEMENTS
The authors are grateful to Nicole Meiklejohn for her outstanding efforts in assisting with the editing and preparation of this manuscript.
Funding sources
This work was supported by the NIH/NCATS Colorado CTSA grant (number UL1 TR002535), the Atopic Dermatitis Research Network Leadership Center grant (1UM1AI151958-01), the Atopic Dermatitis Research Network Clinical Research Center grant (1U01AI152037-01), and The Edelstein Family Chair for Pediatric Allergy at National Jewish Health.
Abbreviations
- AD
atopic dermatitis
- AD−PA+
peanut allergy without AD
- AD+PA+
peanut allergy with AD
- AD+PA−
AD without peanut allergy
- BSA
bovine serum albumin
- AUC
area under the curve
- EOS-CER
esterified ω-hydroxy fatty acid sphingosine ceramides
- FA
food allergy
- FLG
filaggrin
- IgE
immunoglobulin E
- LC-ESI-MS/MS
liquid chromatography electrospray ionization tandem mass spectrometry
- NA
non-atopic control
- NMF
natural moisturizing factor
- NS-CER
non-hydroxy fatty acid containing sphingosine ceramides
- PA
peanut allergy
- PCA
pyrrolidone carboxylic acid
- SC
stratum corneum
- STS
skin tape strip
- TEWL
transepidermal water loss
- UCA
urocanic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflicts to declare.
REFERENCES
- 1.QuickStats: Percentage of children aged <18 years with a food or digestive allergy in the past 12 months,* by age group - National Health Interview Survey, 2007-2018†. MMWR Morb Mortal Wkly Rep 2019;68:831 DOI: 10.15585/mmwr.mm6838a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tordesillas L, Goswami R, Benedé S, Grishina G, Dunkin D, Järvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest 2014;124:4965–75. DOI: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AR, Knaysi G, Wilson JM, Wisniewski JA. The skin as a route of allergen exposure: part I. Immune components and mechanisms. Curr Allergy Asthma Rep 2017;17:6 DOI: 10.1007/s11882-017-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009. September;124(3):485–93, 493.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker MT, Green JE, Ferrie RP, Queener AM, Kaplan MH, Cook-Mills JM. Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018; 141:1711–25. e9 DOI: 10.1016/j.jaci.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy 2015;45:255–64. DOI: 10.1111/cea.12406. [DOI] [PubMed] [Google Scholar]
- 7.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003;348:977–85. DOI: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 8.Miyaji Y, Yang L, Yamamoto-Hanada K, Narita M, Saito H, Ohya Y. Earlier aggressive treatment to shorten the duration of eczema in infants resulted in fewer food allergies at 2 years of age. J Allergy Clin Immunol Pract 2019. DOI: 10.1016/j.jaip.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Samady W, Warren C, Kohli S, Jain R, Bilaver L, Mancini AJ, Gupta R. The prevalence of atopic dermatitis in children with food allergy. Ann Allergy Asthma Immunol. 2019. June;122(6):656–657. e1 DOI: 10.1016/j.anai.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung DYM, Calatroni A, Zaramela LS, LeBeau PK, Dyjack N, Brar K, et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 2019;11 DOI: 10.1126/scitranslmed.aav2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7. DOI: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 12.Berdyshev E, Goleva E, Bronova I, Dyjack N, Rios C, Jung J, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018;3 DOI: 10.1172/jci.insight.98006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011. ;66:934–40. DOI: 10.1111/j.1398-9995.2010.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med 1983;158:84–98. DOI: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lappin MB, el-Ghorr A, Kimber I, Norval M. The role of cis-urocanic acid in UVB-induced immunosuppression. Adv Exp Med Biol 1995;378:211–3. DOI: 10.1007/978-1-4615-1971-3_47. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs NK, Norval M. Urocanic acid in the skin: a mixed blessing? J Invest Dermatol 2011;131:14–7. DOI: 10.1038/jid.2010.276. [DOI] [PubMed] [Google Scholar]
- 17.Elias PM, Gruber R, Crumrine D, Menon G, Williams ML, Wakefield JS, et al. Formation and functions of the corneocyte lipid envelope (CLE). Biochim Biophys Acta 2014;1841:314–8. DOI: 10.1016/j.bbalip.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005;6:328–40. DOI: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 19.Janssens M, van Smeden J, Gooris GS, Bras W, Portale G, Caspers PJ, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res 2012;53:2755–66. DOI: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol 2010;130:2511–4. DOI: 10.1038/jid.2010.161. [DOI] [PubMed] [Google Scholar]
- 21.van Smeden J, Janssens M, Kaye EC, Caspers PJ, Lavrijsen AP, Vreeken RJ, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp Dermatol 2014;23:45–52. DOI: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 22.Joensen UN, Jørgensen N, Meldgaard M, Frederiksen H, Andersson AM, Menné T, Johansen JD, Carlsen BC, Stender S, Szecsi PB, Skakkebæk NE, Rajpert-De Meyts E, Thyssen JP. Associations of filaggrin gene loss-of-function variants with urinary phthalate metabolites and testicular function in young Danish Men. Environ Health Perspect. 2014. April;122(4):345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest 2019;129:1463–74. DOI: 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, et al. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci U S A 2006;103:17420–5. DOI: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rueter K, Jones AP, Siafarikas A, Lim EM, Bear N, Noakes PS, et al. Direct infant UV light exposure is associated with eczema and immune development. J Allergy Clin Immunol 2019;143:1012–20. e2 DOI: 10.1016/j.jaci.2018.08.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


