Abstract
The neuronal protein α-synuclein (αS) is central to the pathogenesis of Parkinson’s disease and other progressive brain diseases such as Lewy body dementia and multi-system atrophy. These diseases, collectively referred to as ‘synucleinopathies’, have long been considered purely proteinopathies: diseases characterized by the misfolding of a protein into small and large aggregates mainly consisting of that protein (in this case: α-synuclein). However, recent morphological insights into Lewy bodies, the hallmark neuropathology of human synucleinopathies, suggests these lesions are also rich in vesicles and other membranous organelles. Moreover, αS physiology and pathology are both strongly associated with various aspects of intracellular vesicle trafficking and lipid biology. αS physiologically binds to synaptic and other small vesicles, and several functions of αS in regulating vesicle biology have been proposed. Familial PD-linked αS excess and missense mutations have been shown to impair vesicle trafficking and alter lipid homeostasis. On the other hand, vesicle trafficking and lipid-related genes have emerged as Parkinson’s risk factors, suggesting a bidirectional relationship. The answer to the question “Does abnormal αS accumulation cause impaired vesicle trafficking and lipid dyshomeostasis or is αS aggregation the consequence of such impairments?” may be “both”. Here, we review current knowledge of the αS-lipid and αS-veside trafficking interplay, with a special focus on Parkinson’s disease and Lewy body dementia.
Keywords: Parkinson’s disease, synucleinopathy, alpha-synuclein, vesicle trafficking, lipids, protein aggregation
1. Membranous organelles and other lipid moieties may be an integral part of Lewy bodies
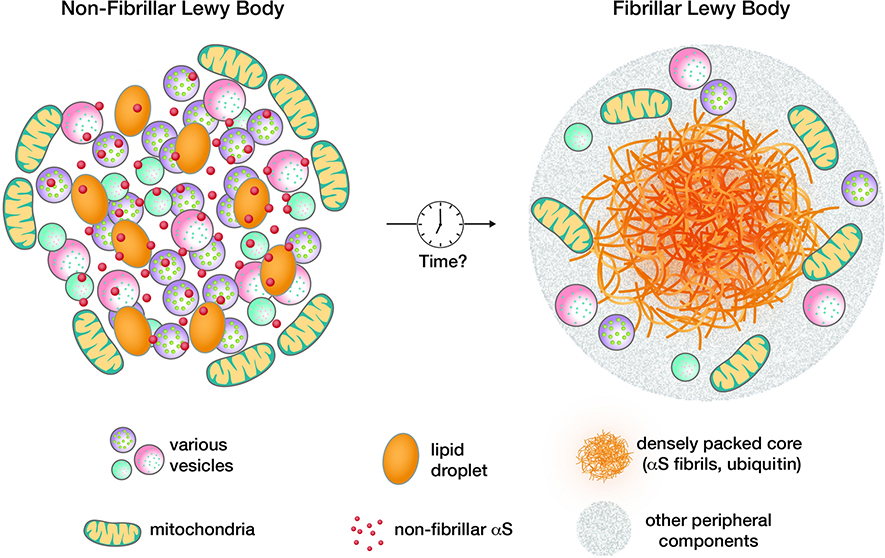
The 14 kDa cytoplasmic protein α-synuclein (αS) is strongly linked to Parkinson’s disease (PD), PD dementia, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and even some forms of Alzheimer’s disease. Large intraneuronal aggregates, Lewy bodies (LBs) and Lewy neurites (LNs) in somata and neurites, respectively, are the defining cytopathology of synucleinopathies. The exception is MSA, which is largely characterized by the accumulation of αS in the cytoplasm of oligodendrocytes, the myelin-producing cells of the brain. A provocative recent study, mostly based on correlative light and electron microscopy, postulated that LBs are largely composed of lipids, membrane fragments and membranous organelles such as vesicles and mitochondria. The study confirmed αS to be a major component of the lesions, but the protein was detected to a high degree in a non-fibrillar state (the authors acknowledged, however, that the technique used may have missed fibrillar αS to some extent) [112]. This notion was in agreement with certain observations from earlier decades [47, 88] but it challenged the widely accepted view of synucleinopathies as protein misfolding diseases whose key pathogenic lesion is the fibrillar LB [121]. It will be interesting to see how these seemingly disparate descriptions of LBs can be reconciled. One possible scenario would be that non-fibrillar LBs (Fig. 1, left half) are precursors to classical fibrillary LBs (Fig. 1, right half). This would be consistent with the idea of αS membrane interaction being a nucleation event in the aggregation of the αS protein [49]. It could be that the non-fibrillar, membrane-rich inclusions are what neuropathologists have referred to as pale bodies [52]. Another explanation would be that certain brain regions develop principally fibrillary while others develop principally non-fibrillar LBs. In any event, the prospect that at least some (perhaps many) LBs may be largely composed of clusters of vesicles, lipid droplets, membranes and mitochondria rather than solely fibrillar αS offers a potential sea change in the way we conceptualize PD pathogenesis. This new recognition may provide an opportunity to integrate various genetic and experimental data on the interplay of αS with different membranous components in neurons. In the subsequent sections, we will focus first on the vesicle trafficking machinery (with a special emphasis on that within synapses) and then on lipid pathways, aware that there is overlap between these aspects of αS biology and pathobiology. The genes and pathways that we will discuss are typically studied and interpreted in a neuronal context, but relevance for the oligodendroglial inclusion formation in MSA cannot be excluded (and has even been postulated in some cases). We will not specifically address lipid-related mechanisms from an MSA perspective, which we believe deserves a separate review (relevant previous reviews include [10, 145]). As one example, it has been proposed that the absolute levels of myelin lipids are altered in MSA, triggering an instability of the extremely lipid-rich myelin of oligodendrocytes [42].
Fig. 1:
Contrasting non-fibrillar, lipid-rich (left) and ‘classical’, fibrillar (right) LBs. Non-fibrillar LBs might be precursors of fibrillar LBs.
2. αS affects the trafficking of synaptic vesicles
Synaptic vesicle release
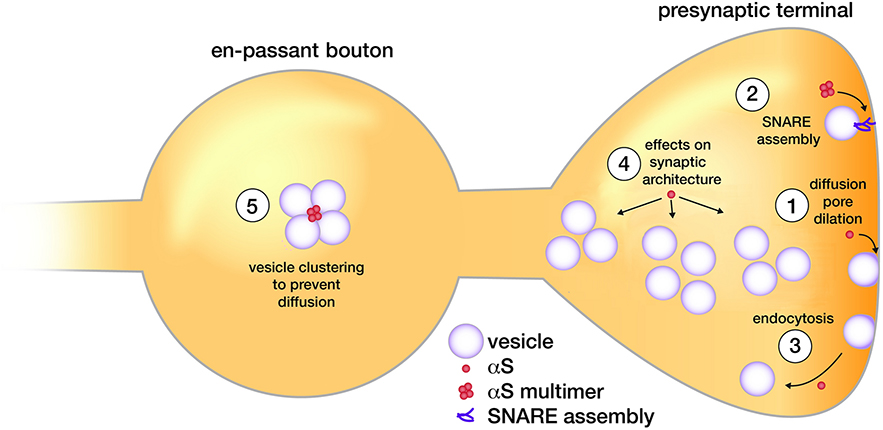
Physiologically, αS is localized in considerable part to presynaptic terminals in the brain [51, 77] and in cultured rodent neurons as they mature [144]. This localization may in part be driven by a relatively strong interaction between αS and synaptic vesicles: biophysical studies have demonstrated that due to their small size, synaptic vesicles possess the optimal degree of substantial membrane curvature needed to promote αS binding [60]. The exact function of αS at the synapse, and at synaptic vesicles in particular, is still under investigation. Several lines of evidence point to a regulatory function in synaptic vesicle release. A detailed study in cultured rodent neurons suggests that the absence of all synuclein homologs (αS, β-synuclein and γ-synuclein triple knockout) slows down cargo release from exocytotic vesicles [74]. Conversely, mild overexpression of αS was found to accelerate the kinetics of individual exocytotic events, thereby promoting cargo discharge. The observations led to the conclusion that synuclein promotes dilation of the exocytotic fusion pore in a gene-dose-dependent fashion ([74]; Fig. 2–1). The authors speculate that these findings help reconcile seemingly contradictory reports on synuclein effects on transmitter release. Fusion pore dilation could affect more slowly released neuromodulators such as dopamine and peptides, while the release of, e.g., glutamate would be less dependent on pore size. Indeed, loss of synuclein may have little effect on glutamate release [15, 55]. In contrast, the release of dopamine seems to be affected by alterations of synuclein levels (e.g., [66, 110]). This distinction between the effects of αS alterations on certain neurotransmitters but not others may have great functional import. Complexity is added by the notion that excess o r mutant αS may pathologically inhibit exocytosis, distinct from the physiological fusion-pore promoting effect of endogenous or mildly overexpressed αS [74]. Indeed, the overexpression in PC12 and chromaffin cells was shown to impair catecholamine release by interfering with a late step in exocytosis [66]. Moreover, in-vivo data indicating that mice lacking both αS and yS exhibit increased striatal dopamine release and excessive dopaminergic-like behavior [110] will need to be reconciled with the data by Logan et al., which are based on neuronal cultures [74].
Fig. 2:
Proposed roles for αS at synapses. See main text for details.
Molecular Mechanisms
None of the studies cited above has proposed a detailed molecular mechanism by which αS may affect synaptic vesicle biology. In a separate study, however, it was suggested that αS increases the number of assembled SNARE complexes by acting as a chaperone that directly interacts with Synaptobrevin-2 (VAMP-2) [15]. Interestingly, this chaperoning activity was later assigned to a previously unrecognized native multimeric form of αS ([13]; Fig. 2–2), and more recently the functional cooperation of αS and VAMP-2 in synaptic vesicle recycling was described [126]. Increased SNARE complex formation (mediated by αS) would be expected to strengthen the force that drives fusion pore dilation and hence, promote cargo release [114]. However, overexpression of synuclein was also proposed to inhibit the total extent of synaptic vesicle exocytosis (by inhibiting synaptic vesicle reclustering) [85]. And while a synuclein triple knockout had maximal effects on exocytotic fusion pore dilation [74], the VAMP-2/aS interaction was mapped to an amino-acid stretch of the αS C-terminus that is not conserved in βS or γS [15]. Moreover, it was proposed that αS can affect membrane fusion in vitro and in vivo through its direct effects on the lipid bilayer [37, 90], without the need for an indirect mechanism via SNARE proteins. A parallel study concluded that both membrane and SNARE interaction may be necessary for αS function [38], and the search for the definitive molecular mechanism of αS function(s) at the synapse continues.
Beyond Exocytosis
While the studies discussed thus far largely focused on exocytosis, it was also demonstrated that expression of αS may promote clathrin-dependent endocytosis [8]. Further, synucleins may regulate the kinetics of synaptic vesicle endocytosis ([131]; Fig. 2–3). Rather than being involved in the maintenance of vesicle trafficking, synucleins were proposed to have multiple effects on presynaptic architecture and the distribution of different pools of synaptic vesicles (e.g., [2, 132]; Fig. 2–4). Lastly, it has been suggested that αS acts on vesicles by ‘clustering’ them, thereby attenuating the kinetics of their recycling and preventing free dispersal between neighboring en passant boutons [137]. Like the possible chaperoning of SNARE protein assembly, this function was assigned to a native multimeric form of αS (Fig. 2–5) (discussed below). These observations about normal αS function raise the question of whether excess wild-type αS (e.g., in αS duplication/triplication PD patients) could help explain the recently recognized presence of multiple vesicular profiles in LBs.
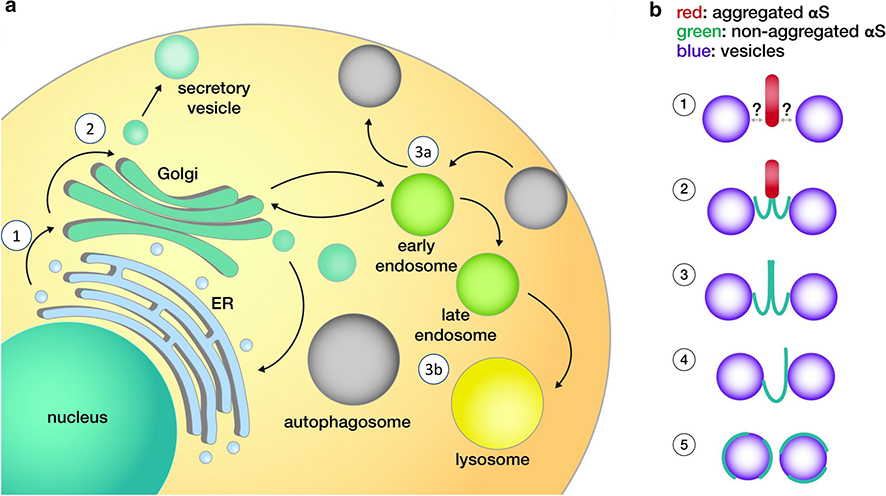
Fig. 3:
a. Proposed sites of αS interactions for non-synaptic membrane vesicles. b. Possible modes of aberrant αS-membrane interactions. See main text for key to circled numbers.
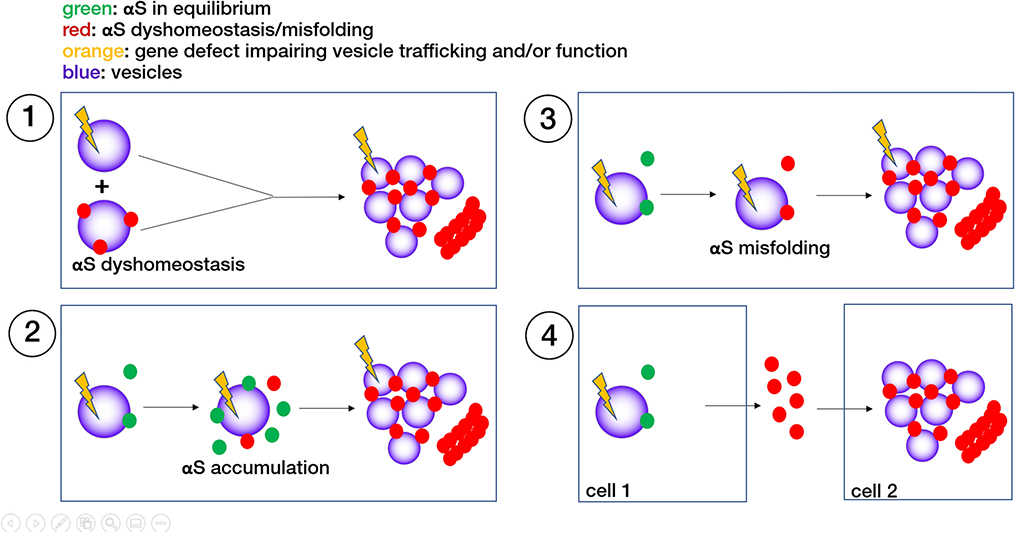
Fig. 4:
Different scenarios of how aberrant trafficking or composition of cellular vesicles may cause or aggravate αS dyshomeostasis, contributing to PD pathogenesis. The end point in all cases are αS membrane-rich and or fibrillar aggregates. See main text for further details.
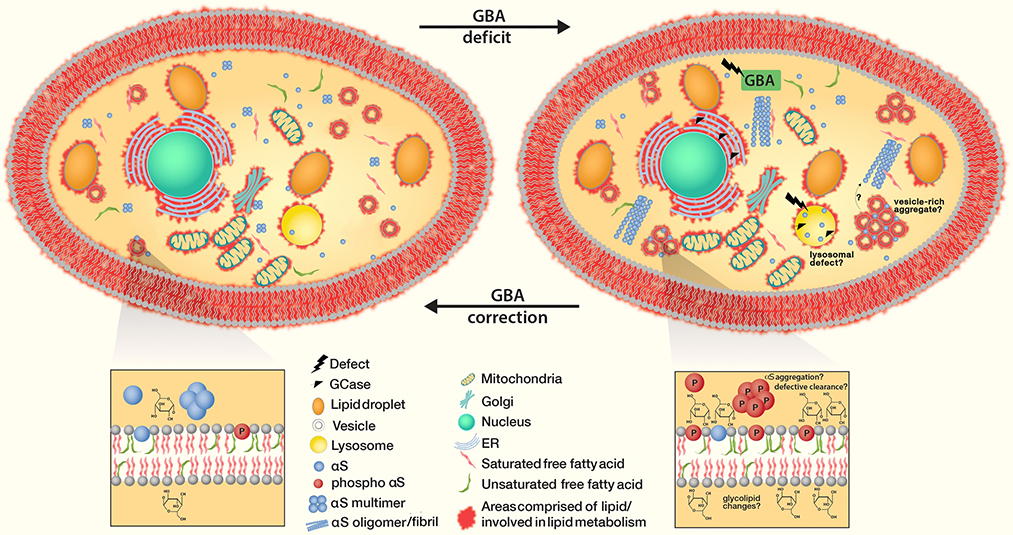
Fig. 5:
Correcting GBA deficits to equilibrium restores physiologic GCase and repairs the GCase lysosomal pathway. It further prevents αS accumulation at membranes, accumulation of αS monomers as well as (downstream) oligomer formation/fibrillar aggregation.
Relevance for PD
All in all, αS may physiologically promote dilation of the fusion pore [74], while an inhibition of exocytosis may require synuclein overexpression [55, 110], supporting a pathological role for too much αS. Thus, αS synaptic function versus dysfunction is likely a matter of αS concentration and conformation. One must also keep in mind that αS aggregation will deplete cells of the functional form of αS to some extent, leading to a partial loss of function. However, αS KO mice [2] and even αS, βS and γS triple KO mice [55] do not develop clear-cut PD-like phenotypes. This suggests that synuclein loss of physiological function may not be central to PD pathogenesis. Alternatively, processes during development could compensate for its KO, while gradual loss of αS function in the adult brain may well contribute to PD pathology [25]. And yet, beyond the question of loss- or gain-of-function, it is not even clear whether αS-mediated synaptic trafficking dysfunction is a major driver of neurodegeneration in PD [9]. A common pathophysiological hypothesis for PD is that the physical loss of dopaminergic neurons depletes dopamine in the striatum, causing the motor symptoms bradykinesia, tremor, rigidity and postural instability, but it has also been proposed that a dysfunction of still-existing nerve cells triggers these events (reviewed in [107]).
3. aS affects the trafficking of non-synaptic vesicles
ER-to-Golgi (Fig. 3a-1)
While synaptic vesicle membranes may offer favorable geometry for αS-membrane binding, αS interactions with other cellular vesicles, other organelles or even cytoskeletal components have been reported [9]. One of the earliest characterizations of αS reports its synaptic localization in the hippocampal region, but in cortical layers II and III, the authors found αS protein to be ‘clearly localized around cell bodies, probably in the Golgi area’ [77], indicating that the exact subcellular locale of αS might be neuron-type specific. In this context, the first and rate-limiting step in the secretory pathway, ER-to-Golgi transport, has been suggested to be affected by αS when expressed in yeast (an organism that does not possess endogenous αS) [93]. A screen in αS-expressing yeast revealed membrane-trafficking proteins as suppressors of αS cytotoxicity [27]. The hits included Ypt1p/Rab1, involved in ER/Golgi vesicle tethering. Follow-up studies confirmed impaired ER-to-Golgi transport upon αS overexpression in rat kidney cells, HeLa cells, neuroendocrine PC12 cells, and dopaminergic SH-SY5Y cells [73, 129, 143]. A locale for αS activity consistent with these observations would be post-ER budding, perhaps via interfering with Rab- and SNARE-dependent COPII vesicle tethering and/or fusion [53, 129].
Intra- and post-Golgi (Fig. 3a-2)
Excess/ectopic αS has also been suggested to disrupt intra-Golgi and post-Golgi secretory trafficking. In this context, αS has been proposed to interact with Rab8 (Golgi), Rab3a (post-Golgi), and Rab5 (early endosomes) in αS A30P transgenic mice [30]. In addition, overexpression of Rab11 (recycling endosomes) as well as Rab8 and Rab3a rescued αS toxicity in different models [11, 27, 53, 148]. Interestingly, αS-mediated trafficking defects (in yeast and beyond) seem to be efficiently rescued by Ykt6 [27, 129], an R-SNARE that can participate in multiple fusogenic SNARE complexes. This is consistent with excess or mutant αS impairing subcellular trafficking at several different levels. An important secretory cargo in dopaminergic neurons is the dopamine transporter, and it was shown that this protein may accumulate in the early secretory pathway upon αS over-expression, hindering dopamine uptake [91]. Considerable overexpression of αS in cultured mammalian cell lines was shown to even disrupt the Golgi apparatus, causing ‘Golgi fragmentation’ and cytotoxicity [54]. The causative αS species were described as ‘prefibrillar’ aggregates, but the exact nature of these assemblies and the molecular mechanisms behind the Golgi fragmentation remained elusive. A study in cultured neurons described impaired vesicle trafficking, Golgi fragmentation and neuritic degeneration as the consequences of αS interfering with the microtubule network (actin filaments and microtubule- independent trafficking remained unaffected) [67]. Within the degenerating neurites, the authors found numerous ‘spherical co-aggregates’ of tubulins and αS. However, besides in PD, Golgi fragmentation can be observed in other neurodegenerative diseases [138] and may not be considered an aS-specific effect.
Endolysosomal (Fig. 3a-3)
The role of the endolysosomal system in αS toxicity might be bidirectional: αS has been proposed to affect this system, but the clearance of αS may in turn depend upon the endosome-lysosome pathway (e.g. [3]), and Rab11a might play a role in the process [11]. αS expression in yeast interferes with endosomal trafficking [118], while αS aggregates in cultured neurons were suggested to impair the transport of Rab7/TrkB receptor-containing endosomes and autophagosomes [135]. Increased αS expression was shown to disrupt various vesicle transport events in both yeast and A53T iPSC-derived patient neurons, and rescue via activation of the E3 ubiquitin ligase NEDD4 (RSP5 in yeast) was reported [22, 127]. Lysosomal dysfunction induced by αS excess was shown to occur through disruptions in protein trafficking in models of human midbrain synucleinopathy [79]. Changes in endolysosomal enzyme activities have been observed in PD cerebrospinal fluid [40]. Aggregated or over-expressed αS was suggested to inhibit macroautophagy, causing lack of clearance and additional αS accumulation [143]. Related to that, impaired degradation of mutant αS by chaperone-mediated autophagy was reported [29]. Loss-of-function mutations of the lysosomal lipid-degrading enzyme glucocerebrosidase (GCase) is linked to both PD and Gaucher’s disease (see section 5), and the underlying mechanisms include impaired lysosomal-enzyme targeting and protein degradation; thus, lipid-modulating therapeutic targets and strategies might be effective for both disorders [79].
Molecular Mechanisms
All these observations suggest as a potential trigger for pathology that excess αS accumulates in neuronal somata, thus interacting with vesicles that might physiologically be ‘synuclein-free’ [138]. Indeed, it is unclear at present whether αS has a discrete normal function in the somatic compartment of neurons; virtually all the cited studies in this section address αS pathobiology. To our knowledge, a vesicle trafficking defect in cell bodies of αS KO mice or cell lines has not been reported, and yet the occurrence of high αS levels in erythrocytes strongly suggests that αS has physiological functions which go beyond its synaptic role [9]. In addition, most of the studies we cite assume or postulate that αS ‘aggregation’ (often poorly characterized) drives the observed effects. However, the interaction between αS and vesicles is mediated by the specific formation of amphipathic α-helices, and aggregated αS (i.e., dimeric and greater) is unlikely to preserve this fold (see next section).
4. What form of αS may lead to aberrant vesicle trafficking?
Different models of αS-membrane interaction
Vesicle trafficking defects in PD are unlikely to be caused by LBs per se. Rather, LBs are hypothesized to be the end products of prolonged αS-vesicle trafficking defects. The above-mentioned rodent and cellular models of the effects of mutant or excess wt αS were not reported to have large ‘Lewy-like’ filamentous αS aggregates. One alternative scenario would be the formation of smaller aggregates. Axonal αS aggregates have been suggested to impede the transport of endosomal vesicles via a Rab7-mediated mechanism (see section 3), distinct from simply filling the axonal cytoplasm or inhibiting all axonal transport [135]. Presynaptic αS ‘micro-aggregates’ were observed upon in vivo multiphoton imaging of mice mildly overexpressing αS-GFP, even in young (1, 3, 6 month old) animals [122]. Presynaptic αS ‘micro-aggregation’ and deficits in neurotransmitter release were also linked in transgenic mice expressing the aggregation-prone truncated αS 1–120 species [50, 81]. It should be borne in mind that the interaction between normal αS and certain vesicles is apparently mediated by the formation of amphipathic αS helices. These helices can ‘sense’ the curvature of small vesicles and have a much lower binding affinity for larger vesicles and organelle membranes such as the ER or plasma membrane [100]. It is unclear how aggregated αS would be able to sustain such specific interactions with vesicles to interfere with their trafficking (Fig. 3B-1). Specific vesicle targeting of misfolded αS may be possible if any of the aggregated material still contains at least a portion of helical αS (Fig. 3B-2; green indicates helical αS). Relevant in this context could be a recent publication describing aberrant lipid association of aggregated αS in a specific subset of neurotransmitter-containing, secretogranin-II-positive, large dense-core vesicles [12]. Another possibility would be that the principal αS species that interferes with vesicle trafficking is not at all aggregated but helical. In this context, it has been reported that membrane-associated multimeric (and presumably helical) wt αS helps regulate vesicle homeostasis by normally tethering vesicles in synaptic boutons [137]. An excess of this tethering activity of multimeric αS - due to increased αS levels - could lead to aberrant clusters of vesicles (Fig. 3B-3), causing a local ‘traffic jam’ [6]. However, in another study, αS helices were stabilized at vesicle membranes by strategic point mutations, which resulted in the formation of cytoplasmic aggregates of vesicles, lipid droplets, and αS [36]. The mutant αS that accumulated in these inclusions was characterized as largely monomeric by intact-cell crosslinking and YFP complementation, raising the question of how monomeric αS may confer vesicle clustering. One explanation could be a proposed ‘double anchor’ mechanism (Fig. 3B-4), in which the N-terminus of an αS monomer binds to one vesicle, while another stretch closer to the C-terminus binds to another vesicle [48]. However, a direct or indirect bridging of vesicles via αS monomers does not seem necessary for stalling vesicles, and theoretical scenarios exist in which excess αS helical monomers (but not multimers) can impair vesicle biology (Fig. 3B-5). It has for example been shown that monomeric αS can induce membrane curvature [139], and an excess of this curve-inducing activity could interfere with normal vesicle movement. In another possible scenario, excess ‘coating’ of vesicles with monomeric αS helices under pathological conditions could interfere with key events in the trafficking of vesicles such as their interaction with the cytoskeleton.
Insights from αS genetics
It is tempting to postulate that excess membrane binding of αS is the starting point for αS interfering with vesicle trafficking. The ‘toxic species’ could either be an excess of the normal membrane-binding αS helix, or it could be an aggregate of non-helical monomers that is triggered by excess membrane binding via ‘primary nucleation’ by lipids [49]. Two- to threefold overexpression of wt αS from gene duplication or triplication leads to aggressive familial forms of PD and/or DLB [18, 117]. Excess wt αS might be expected to distribute to both the aqueous cytoplasm and membranes, so the disease-conferring effects of duplication/triplication could be explained by increased αS at membranes. Similarly, the fPD-causing αS E46K mutation binds to membranes in excess [95, 103, 150]. This effect can be amplified by placing analogous E→K mutations in the two adjacent αS repeat motifs (creating the “3K” mutant), thereby leading to a stepwise increase in monomer levels, abnormal monomer-membrane interaction, cytotoxicity and vesicle-rich inclusions [35]. However, this relatively simple model is challenged by other fPD-causing αS mutations. While H50Q [4] and A53T [98] may also increase membrane binding, A30P [65] and G51D [68] increase the fraction of total αS in the cytosol [33]. Nonetheless, these all cause PD and presumably all develop vesicle/αS-rich Lewy bodies.
Two distinct pathways to αS toxicity?
αS genetics indeed suggests that two routes to aberrant αS behavior in cells may exist: one that is initiated by excess membrane interaction (E46K-like), and one that instead starts with cytosolic accumulation (A30P- and G51D-like) [33]. Perturbing vesicle trafficking via the membrane-associated pathway seems plausible, whereas in the case of the cytosolic pathway, this link is less obvious. And yet, PD caused by A30P [109] and G51D [61] on the one hand and that caused by E46K [150] on the other are both characterized by αS aggregation and the formation of LBs and LNs (see also Table 1 in [99]). This is consistent with experimental data implicating both excess [49] and reduced [14] membrane interaction in αS aggregation. Interestingly, both reduced [14] and increased [34] membrane interaction via strategic mutagenesis has been shown to elevate αS monomers at the expense of native multimers, consistent with the evidence that monomer accumulation occurs with all fPD-causing αS mutants [35]. In light of vesicle/organelle/lipid accumulations in Lewy bodies [112], it would be very interesting to contrast in detail A30P or G51D Parkinson brains to those of patients with E46K, αS duplication/triplication or sporadic PD. Would this comparison reveal that A30P and G51D is more characterized by fibrillar LBs, whereas E46K displays more lipid-rich LBs? Would sporadic PD cases exhibit both kinds of LBs or could they be divided into more fibrillar and more lipid-rich cases? And lastly: would the membrane-associated pathway compromise neuronal functions by a different mechanism (‘lipotoxicity’) than the cytosolic pathway (‘proteotoxicity’)?
Table 1:
Select PD-relevant genes involved in vesicle trafficking and/or lipid homeostasis. Dark green indicates more direct aspects, light green more indirect aspects.
| Gene/Protein ([reference] | Connection to vesicle trafficking/function | Connection to lipid homeostasis |
|---|---|---|
| SNCA/αS [see section 1] | - binds to (small) vesicles - KD and OE affect synaptic trafficking - OE affects somatic trafficking |
binds to membrane lipids may bind to free FAs may affect lipid metabolism |
| GBA/GCase [79] | important for lysosome function | key enzyme in glycolipid catabolism |
| SCARB2/LIMP2 [84, 116] | chaperone for GBA trafficking | Indirect effect on lipids via GBA |
| LRRK2 [94, 154] | regulates endocytic/lysosomal/ER-Golgi trafficking | may affect LD biology |
| RAB29/RAB7-L [56] | endosomal Rab GTPase | may affect LD biology |
| RAB39B [142] | Endosomal/lysosomal/Golgi Rab GTPase | may affect LD biology |
| VPS35 [153] | retromer subunit; affects endosomal sorting | may affect LD biology |
| VPS13C [84] | endosomal sorting | may affect LD biology |
| ATP6AP2 [64] | ATPase cation transporter, lysosome | may affect lysosomal steps of lipid biology |
| ATP13A2 [101] | ATPase cation transporter, lysosome | may affect lysosomal steps of lipid biology |
| SYT11/Synaptotagmin-11 [1] | regulates lyso/autophagosome fusion; exocytosis | may affect LD biology |
| SYNJ1/Synaptojanin-1 [92] | regulates synaptic endocytosis | phospholipase; affects lipid headgroups |
| DNAJC6/Auxilin-1 [43] | neuron-specific clathrin-uncoating chaperone | ? |
| PLA2G6 [83] | indirect effects via membrane lipid composition | phospholipase; affects lipid headgroups |
| SREBF-1 [41] | indirect effects via membrane lipid composition | transcription factor; regulates sterol synthesis |
| ELOVL7/FA-Elongase 7 [17, 69] | indirect effects via membrane lipid composition | affects chain length of membrane lipids |
| DGKQ [20, 84] | indirect effects via membrane lipid composition | diacylglycerol kinase; affects lipid headgroups |
Can non-aggregated αS be cytotoxic?
Studies on αS pathology have classically assigned any detrimental effect of αS to its aggregation (often ill-defined), and the latter may include αS effects on vesicle trafficking. As discussed earlier, normal amphipathic αS helices interact with vesicles [32, 36], while aggregated αS should lose this interaction, causing at least a partial loss of function. Many reported effects of αS overexpression, however, seem more consistent with a gain-of-toxic-function. An excess of the proposed functions for normal, non-aggregated αS (generation of membrane curvature [139]; vesicle tethering [108, 137]) could lead to phenotypes similar to the newly-recognized ultrastructure of LBs [112].. Aggregation into large αS lesions in this and other scenarios may be a secondary process, and by temporarily limiting the amount of free αS that can affect vesicle membranes, the aggregation process could even be protective for neurons to some degree. This idea may be analogous to the theory that amyloid plaques in Alzheimer’s cortex temporarily sequester (‘lock up’) otherwise diffusible cytotoxic oligomers. In this context, an early yeast-centered study on αS toxicity seems relevant: for a large number of random αS point mutants, their fibrillization rates in vitro and their yeast toxicity in vivo did not correlate, suggesting that fibrillization is not necessary for aS-induced yeast toxicity [134]. The authors concluded that αS cytotoxicity in yeast is caused by the protein binding to membranes at levels sufficient to non - specifically disrupt membrane homeostasis. Subsequent studies helped further support this concept of membrane-mediated toxicity: wt human αS expression in yeast (which lack αS) led to abnormal vesicle clustering/aggregation [53, 119] and associated vesicle trafficking defects in yeast and beyond [27]. ‘Amyloid’ (fibrillar αS) is typically not apparent in the yeast model (see also review by Jarosz and Khurana [59]), even though at least one study also observed fibrillar aggregates upon αS expression in yeast [128]. All in all, it seems possible that an exaggeration of the normal αS vesicle binding (e.g., by excess αS amphipathic helix formation) may have adverse effects on vesicle biology in the absence of ‘classical’ proteinaceous αS aggregation, and studies on cytotoxicity in PD should not ignore this possibility.
5. Genetic PD risk factors in trafficking pathways
What we have discussed so far has addressed the question of how changes in αS (mutant; excess wt) might alter vesicle trafficking. On the other hand, several known and emerging PD-related genes such as RAB7/PARK16 [56], VPS35/PARK17 [153], VPS 13C/PARK23 [84], SYNJ1/PARK20 [92], SYT11 [1], LRRK2/PARK8 [94, 154] and SCARB2 [84, 116] are primarily implicated in vesicle trafficking (reviewed in [1]; see also Table 1), but mutations or polymorphisms can lead to PD. It is not yet clear whether the PD pathology associated with these mutations (especially the rare ones) involves ‘typical’ LB-like αS accumulation/aggregation, but LRRK2 PD pathology and other emerging evidence suggests that the ‘other direction’ could be true: primary aberrant vesicle trafficking causes secondary αS accumulation and aggregation. The mechanisms, however, are not obvious, and several possible scenarios exist (Fig. 4). (1) Additive effects: age-related αS accumulation and the aforementioned PD-linked genetic defects may both compromise vesicle trafficking, eventually causing neuronal dysfunction and death. This possibility is consistent with a two-hit scenario, in which the gene defect constitutes a ‘first hit’ and age-related αS accumulation a ‘second hit’ (Fig. 4–1). (2) Failure of αS degradation: the above-mentioned PD risk genes may directly or indirectly affect the degradation of cellular αS, e.g. in the endolysosomal pathway. The accumulating αS may further compromise vesicle homeostasis, and αS aggregates may induce neuronal dysfunction and death via proteotoxicity (Fig. 4–2). (3) Effects on αS folding homeostasis. Genetic defects in vesicle trafficking pathways may change the membrane lipid landscape of cells due to changes in the size, shape, composition, subcellular localization or abundance of certain vesicle species or even other membranous organelles. This in turn may affect αS folding homeostasis, e.g., the ratios of soluble:insoluble, multimeric:monomeric and/or somatic:neuritic αS. αS in such a dyshomeostasis may form proteinaceous and vesicle-rich aggregates. αS may get trapped in the soma, causing detriment as outlined in section 3 (Fig. 4–3). (4) Non-cell-autonomous mechanisms (‘pathogenic spread’). Genetic defects in vesicle trafficking, i.e., in endo- and exocytosis, may cause aberrant cellular release and reuptake of αS. The aberrant release of misfolded αS species may induce pathology in nearby cells. Conversely, the uptake of misfolded αS from the extracellular space may become more detrimental if vesicular transport and endolysosomal degradation are already impaired (Fig. 4–4). It should be noted that essentially all these possibilities, with the exception of (1), can be considered ‘vicious-cycle’ scenarios, because accumulation or misfolding of αS could further aggravate vesicle trafficking defects. We currently lack a detailed understanding of the normal biology of most PD-risk genes. The proteins in question have a variety of assigned functions that can go beyond vesicle trafficking, but a considerable overlap with cellular lipid homeostasis exists (see section 7 below). This uncertainty will be exemplified for one of the most complex and enigmatic proteins in PD research, LRRK2.
LRRK2
Roles for Leucine-Rich Repeat Kinase 2 (LRRK2/PARK8; [94, 154]) in ER export and secretory trafficking (reviewed in [138]) as well as in lysosomal autophagic degradation and synaptic vesicle trafficking have been proposed (reviewed in [1]). Many but not all cases of LRRK2 PD are characterized by LB formation [99]. Further complexity is added by the fact that LRRK2 may not be very abundant in neurons but more highly expressed in other brain cells such as microglia [104]. This distribution does not necessarily reflect the relative contribution of the respective cell types to PD pathology, but it is possible that LRRK2’s interplay with αS is not cell-autonomous. Accordingly, LRRK2 has been proposed to be involved in the clearance of extracellular aggregated proteins [104]. While we do not favor one theory of LRRK2 function over the other, it seems premature to link LRRK2 to a certain step in vesicle trafficking. Below (section 6), we will also discuss a possible involvement of LRRK2 in cellular lipid homeostasis, which in turn may affect vesicle homeostasis.
GBA and others
The loss of function of another PD-related gene, Glucocerebrosidase (GSA, GCase), has been proposed to cause lysosomal dysfunction (see section 6) but not necessarily alter the trafficking of small vesicles [79]. Similarly, ATP6AP2 [64] and ATP13A2, genes that are both linked to PD, have been implicated in lysosomal function rather than vesicle trafficking [101]. This raises the question of to what extent these and some of the other PD-risk genes mentioned above act through vesicle function independent of actual vesicle trafficking. It may be impossible to clearly separate these two aspects, and the term ‘vesicle trafficking’ can be understood to include aspects of vesicle function.
6. Lipid pathways may cause synucleinopathy by altering vesicle trafficking
Upstream of αS
Earlier, we discussed cis factors that increase αS interactions with membranes: certain point mutations (but not others) as well as elevated total wt αS levels lead to an increase in membrane-associated αS; this increased membrane interaction will likely interfere with vesicle trafficking. As far as trans factors are concerned, vesicle membrane composition can affect αS-membrane interactions. It is well established in vitro that small vesicles with pronounced lipid packaging defects attract αS; packaging defects are promoted by high curvature and lipid desaturation (increased membrane fluidity) [90]. Similarly, lipid head groups have been identified as a determinant of αS-membrane interactions [102]. Accordingly, diverse changes in cellular membrane composition, be they in lipid head groups or in fatty acyl side chains, might affect αS-membrane interactions. The existing literature suggests: (1) fatty acyl saturation reduces αS binding to membranes [90]; (2) negative charges in lipid head groups enhance αS-membrane binding [124]; and (3) decreased length of fatty acyl chains may also reduce αS membrane binding [58]. In this context, it is interesting that genome-wide association studies (GWAS) have identified fatty acid (FA) elongase 7 (ELOVL7) as a PD risk factor [17, 69]. By controlling fatty acyl chain length, this enzyme may indeed affect αS membrane binding, but work is needed to elucidate the details. The phospholipase PLA2G6, has been suggested to impact PD risk [83]. Phospholipase D (PLD) expression and activity have been observed to be reduced in DLB patient brain tissue; αS is increased in the same samples [5]. Phospholipases act on membrane phospholipids and hydrolyze acyl and phosphate esters, thereby modifying lipid head groups and potentially also cellular free FA homeostasis. Synaptojanin-1 (SYNJ1), an inositol-phosphatase important for synaptic activity, has been identified as responsible for some cases of early onset atypical PD [92]. GWAS have also highlighted the diacylglycerol kinase DGKQ (which generates phosphatidic acid from diglyceride) as a PD risk factor [20, 84, 115, 116, 152]. Changes in cellular phospholipid content likely impacts αS membrane interactions and thereby alters vesicle trafficking. Most prominently, mutations in glucocerebrosidase (GBA), a glycolipid metabolism gene, are now proven to augment PD risk [23, 87]. Related to this, SCARB2, encoding LIMP2 (important for GCase activity), has also been identified as a GWAS risk factor [84, 116]. The complex role of GBA and its cofactors in PD pathogenesis is addressed below. In summary, the mode of αS interaction with membranes makes it appear plausible that primary (inherited) membrane alterations could alter vesicle trafficking by recruiting or repelling αS.
Downstream of αS
Wt αS overexpression or αS E46K expression was shown to result in excess FA levels, requiring storage in neutral form to prevent cellular toxicity [44]. Excess FA is shuttled through the neutral lipid pathway to form TG and stored as lipid droplets, during which a build-up of DG can occur. Genetic studies showed that DG accumulation can exacerbate trafficking defects in a yeast model of αS toxicity [44]. In this scenario, αS causes a change in cellular lipid content, possibly via altered gene transcription, which then in turn affects vesicle trafficking. Consistent with a prominent role of DG buildup in the ER, a unique proteomic approach identified the phosphatidate phosphatase, Pah1, a regulator of DG and PA content at the ER membrane as a target for reducing αS toxicity. This study concludes that inhibiting DG production could be a therapeutic strategy for PD [120].
A ‘bi-directional’ mechanism?
GBA
As mentioned above, mutations in GBA increase PD risk [23, 87], and SCARB2, responsible for production of LIMP2 (which functions in lysosome and endosome maintenance), is a PD risk factor by GWAS [84, 116]. Certain GBA mutations lower the enzyme’s hydrolase activity in lysosomes, resulting in insufficient GCase enzymatic function and resultant neurodegeneration, either via changing cellular sphingolipid/ceramide homeostasis [79], by direct connection between GlcCer accumulation and αS homeostasis (e.g., causing αS conformational changes), or through autophagic-lysosomal malfunctions [106]. GlcCer build-up may also trigger the stress response ERAD pathway [106]. Ultimately, Gaucher’s and PD may be intimately linked via a bidirectional loop system whereby insufficient GCase alters lysosome function, GlcCer accumulates resulting in αS buildup and conformational change (e.g., abnormal αS oligomer formation) and this disrupts trafficking of GCase to lysosomes [79]. Moreover, αS buildup can disrupt ER/Golgi trafficking, further contributing to lysosome dysfunction [106]; reviewed in [146]. Abnormal αS oligomers may in turn reduce lysosomal GCase activity; hence αS oligomers are further stabilized [79] (Fig. 5). Loss-of-function mutations in GBA decrease physiological αS conformation (i.e., lower α-helical tetramers and related multimers), and genetic transfection of functional GCase or miglustat drug treatment (blocking a synthetic enzyme for glycosphingolipids) restores physiological αS tetramer:monomer ratios and decreases cytotoxicity [62]. The above suggests a complex connection between GCase, αS multimeric assembly, and both lysosomal and ER/Golgi trafficking. We favor a scenario in which glycolipid alterations are the key detrimental event in the pathogenic cascade, likely by triggering abnormal alpha-synuclein conformations.
7. Lipid pathways may cause synucleinopathy independent of vesicle trafficking
Lipid dyshomeostasis and lipid droplet biology
A systematic PD GWAS data analysis highlighted lipid homeostasis as the conjoining factor among many PD-relevant processes [63]. Certain aspects of the underlying αS biology may be independent of vesicle trafficking, e.g., effects on lipid droplets (LDs), which are central organelles in the regulation and storage of cellular lipid. Seipin, an integral membrane protein at endoplasmic reticulum/LD contact sites, is a lipid homeostatic gene important to LD synthesis and maintenance [19, 136]. Seipin has been reported to be differentially expressed in PD vs. control postmortem brain tissue [39, 71]. Based on gene ontology analysis, the biological process of FA β-oxidation was reported to be overrepresented in the nigral proteome of a PD patient [71]. Sterol changes have also been connected to PD, e.g. the sterol regulatory element-1 binding transcription factor SREBF-1, a regulator of sterol synthesis, is a PD risk factor in some GWAS [41]. In this context, several studies have proposed statins as a PD treatment, but their benefit has been controversial [16]. Rab proteins have been identified as substrates for LRRK2 kinase activity [123, 149], and one of these, Rab10, impacts LD formation, with Rab10 and LRRK2 knockout models both accumulating lipid droplets [70, 86]. VPS35, encoding a component of the retromer trafficking complex, has been associated with late-onset, autosomal dominant PD [140, 153] and is connected to multiple other PD-associated genes. First, the phospholipase PLA2G6, binds to VPS35 and VPS26 and promotes recycling of both proteins and lipids. A lack of PLA2G6 impairs the vesicle trafficking function of VPS and thereby generates ceramide accumulation, inducing a feedback loop that alters membrane fluidity, retromer and neuronal processes [72]. Second, the PD-associated mutation, D620N, in VPS35 augments the kinasing of RAB10 by LRRK2 [82], thus connecting three prominent PD-associated genes with lipid metabolism. However, it is challenging to separate these effects on general lipid biology from effects specifically on vesicle trafficking (see sections 2–6). Nonetheless, even clear-cut fPD genes such as LRRK2 may be linked to effects in LD biology and cellular lipid composition. In addition, αS has been shown to interact with lipid droplets [24], with free polyunsaturated FAs [113] and in particular with free arachidonic acid [31], underlining the potential of aS-lipid interactions that are not immediately linked to vesicle trafficking.
αS aggregation
Excess membrane binding of αS has been proposed to be the starting point of αS aggregation via ‘primary nucleatin’ [49]. On the other hand, a molecular pathway to αS aggregation has been defined that is based on deficient αS membrane binding and accumulation of monomeric αS in the cytoplasm [14]. Feeding cells with unsaturated FAs has been shown to increase αS membrane binding and promote αS inclusion formation as well as S129 phosphorylation, a marker of αS dyshomeostasis [58]. GCase dysfunction (as occurs in Gaucher’s disease and PD) may also alter the homeostasis of αS folding: it can shift native αS tetramers to excess αS monomers [62], which have been proposed to be the starting point for αS aggregation [7, 35]. These examples signify that changes in lipid composition which affect aS-membrane interactions can influence αS biology independent of trafficking.
Is it possible to separate lipid homeostasis and vesicle trafficking?
Vesicle trafficking affects lipids, lipids affect trafficking, and proteins involved in one process can directly or indirectly influence the other, or have a dual role by directly affecting both. In Table 1 we contrast vesicle trafficking and non-trafficking effects of select ‘PD genes’.
8. Lipid profiles may be altered in PD tissues and biological fluids
Patient plasma, CSF and brain tissue
Lipidomic analyses of PD patient samples generally converge on a sense that lipids are altered in PD patients (plasma, CSF, brains) relative to controls. While details on all published studies is beyond the scope of this review, we do note in summary that changes have been reported in brain tissue and body fluids of some of the most abundant lipid classes, namely, phosphotidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphotidylserine (PS). Decreases in specific polyunsaturated PC species were noted in PD frontal cortex [147] and visual cortex, as were some shorter chain and saturated species and lysophosphotidylcholines [21]. PE, which in the brain accounts for approximately 45% of phospholipids [130], was found to be decreased in patient brains at early clinical stages of PD (Hoehn and Yahr stages I and II) [57]. A reduction in several unsaturated PE species was observed in the visual cortex of PD patients compared with controls [21]. Similar to PC, PE and PI classes were also lower specifically in the substantia nigra of male PD patients relative to controls [111]. Although not among the foremost changes in the brain, increased PI 36:1, 38:3 and 40:4 in PD vs. control and decreased PI 38:5 were observed in PD patient amygdala and visual cortex relative to controls [21]. Specific PS species were reported to be increased in PD frontal cortex, namely PS with 36:1,36:2 and 38:3 fatty acyl sidechains [75]. There are many other lipidomic and metabolomic studies of patient postmortem brains, CSF and plasma samples (beyond the scope of this review). The majority find lipids and fatty acids (FAs) to be significantly different between PD patients and controls. One such example is an untargeted metabolomic study that noted the profile of plasma and CSF of PD patients indicates perturbations in the glycerophospholipid and sphingolipid classes (among others) relative to controls [125]. Interestingly, another study [141] of PD CSF identified several FAs to be modified, as indicated by increases in decanoic, quinic, valerenic, arachidonic, dihomo-Y-linolenic, and 10-hydroxydecanoic acids. There is still some controversy in the field as to such changes relating to classes, species, abundance and degree of unsaturation, as variability appears to be high and may be dependent on sample type, time point, sample treatment and PD stage, not to mention the rigor of the quantitative methods.
PD-relevant models
Although beyond the scope of this review, lipid and FA analyses have also underscored changes in lipid types and abundance in many PD cellular and animal models. Given the above, we have been exploring the role of lipids/FA in establishment and progression of PD/synucleinopathies (i.e., does the changing lipid landscape during aging impact PD). In a previous review [45], we detailed the connections between human genetics and patient samples and PD-relevant models, focusing primarily on phospholipids.
Biological significance
Lipid changes in PD-relevant settings can be in response to αS pathology or promoting αS pathology, and bidirectional relationships seem plausible. Some of the studies presented may be confounded by cell loss and the presence of dead or dying cells in PD brain tissue. In any event, the suitability of one or more lipid alterations as a biomarker will need to be explored further.
9. What factors in the αS, lipid and trafficking interchange could be druggable?
LRRK2
Growing evidence that an in increase in LRRK2 kinase activity plays an important role in the LRRK2-linked form of PD suggests that decreasing its kinase activity could become a PD treatment. LRRK2 drug development has progressed over the past decade and selective kinase inhibitors have yielded positive initial results in preclinical studies and early-stage clinical trials. Side effects in peripheral tissues appear manageable, but a complete understanding of LRRK2 pathobiology, its function in trafficking and the mechanisms of action of LRRK2 inhibitors lie ahead (reviewed in [151]).
GBA
The discovery of GBA-related PD triggered a search for the pathogenic mechanisms through which decreased GCase function may affect PD pathogenesis. Targeting the GCase-lysosomal pathway emerged as a rational approach to find neuroprotective drugs in PD [105]. Attempted enzyme replacement therapy for Gaucher’s disease neither prevented PD development nor did it modify symptoms or progression, possibly due to a lack of passage of the exogenous enzyme across the blood-brain barrier [76]. An alternative therapy for Gaucher’s patients uses miglustat, an iminosugar that inhibits the biosynthesis of glycolipids that are substrates for GBA and accumulate pathologically in glycosphingolipidoses [28]. Miglustat can cross the blood–brain barrier, but no clear data are available as to how miglustat may affect parkinsonism in Gaucher’s patients [76]. For therapeutic purposes, ‘correcting’ GCase levels would restore physiologic GCase, reestablish the GCase lysosomal pathway and prevent αS accumulation at membranes as well as its misfolding and potential aggregation (Fig 6).
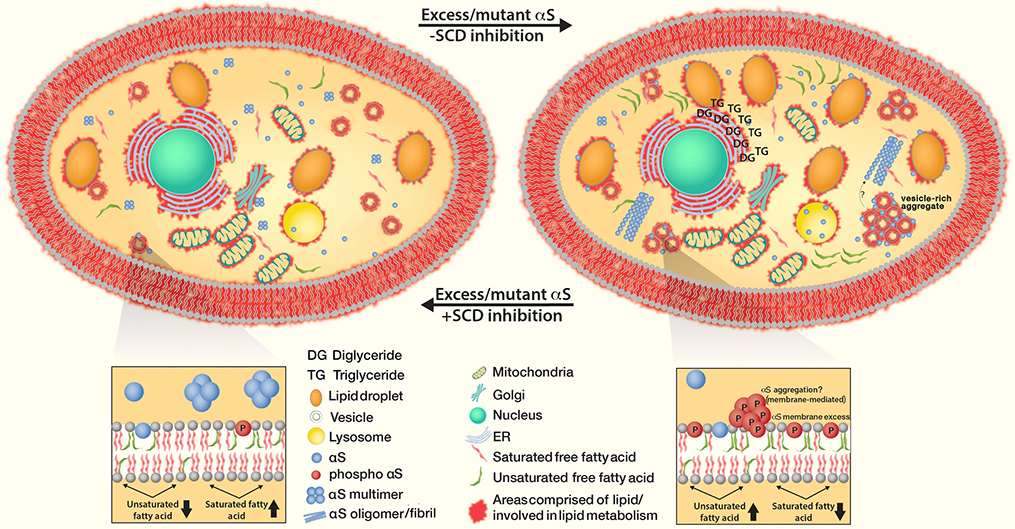
Fig. 6 :
SCD inhibition restores αS-Induced diglyceride (DG) accumulation in the ER, trafficking defects as well as increased triglycerides (TG) and lipid droplets. The treatment reduces clusters of vesicles when excess αS monomers accumulate and form cytoplasmic inclusions (as well as αS fibrillization that might be downstream). Decreased SCD activity prevents membrane defects, decreases αS phosphorylation and restores intact equilibria between αS monomers vs. physiological helical tetramers as well as cytosolic vs. membrane-associated αS.
Stearoyl CoA Desaturase (SCD)
SCD is special among the targets listed here because the gene itself has not been implicated in genetic forms of PD. However, the key product of SCD, oleic acid (18:1), was found to be upregulated under PD-relevant conditions: elevated oleic acid (18:1) levels (and palmitoleic acid (16:1) to a lesser degree) were observed in αS-expressing yeast; increases in unsaturated FAs were identified in E46K hαS mice; increases in DG and TG for storage of excess FA were found in E46K and αS triplication iPSC-derived neurons. SCD inhibition was shown to prevent αS wt toxicity in yeast, worm and rodent neurons [44]. Independently, SCD was identified as a PD-relevant target in a viability screen of yeast expressing αS [133]. Another group later showed that SCD inhibition in a C. elegans PD model alters PD-relevant pathology [78]. SCD was identified in an unbiased phenotypic screen of αS and reported to reduce αS inclusion formation and αS pS129 levels; accordingly, αS multimer:monomer ratios and αS solubility improved upon SCD inhibition [58]. Now that SCD has emerged as an αS-relevant target in four independent studies, it will be important to establish its in vivo relevance in a mouse PD model such as the new αS 3K mouse [89]. Ideally, SCD inhibition would decrease FA unsaturation, in turn altering membrane fluidity such that αS interactions are reduced, phosphorylated αS is decreased and physiologic αS tetramer:monomer ratio is restored (see Fig. 6). An attractive approach could be to specifically target SCD5, a brain-specific isoform in the human genome; this strategy would bypass peripheral side effects.
αS conformation
Several publications have come forward with the idea of targeting the αS protein itself. This is remarkable because a protein without enzymatic activity is often considered ‘undruggable’. The porphyrin phthalocyanine tetrasulfonate was proposed to directly bind to vesicle-bound αS, thereby stabilizing its α-helical conformation and delaying pathogenic misfolding and aggregation of monomers [46]. Nortriptyline, in contrast, was proposed to reconfigure the soluble monomeric state of αS, preventing aggregation, toxicity, and possibly also membrane binding [26]. Subsequently, the aminosterol squalamine was shown to inhibit the putative lipid-induced initiation of αS aggregation, while the related compound trodusquemine inhibited both this process and fibril-dependent secondary pathways in the aggregation [96, 97]. It is possible that other targets may act in part via the conformation of αS in an indirect fashion. SCD inhibition [58] and restoration of GBA function [62] were both shown to increase αS multimer:monomer ratios..
Others
A small molecule pharmacological chaperone has been proposed to increase the stability of the retromer complex. While aimed at APP processing, this study suggests that such small molecules might also increase retromer function in PD-relevant settings [80]. The promise of phospholipases and other lipid-related enzymes involved in PD remains to be tested.
10. Conclusions
The common view of synucleinopathy pathogenesis is that the cells fall victim to ‘proteinopathy’: ‘natively unfolded’ αS misfolds into β-sheet-rich fibrillar aggregates, which then interfere with vital cellular functions and overwhelm the cellular protein degradation systems. However, there is accumulating evidence that not just proteins, and αS in particular, but also lipids are central to PD-pathogenesis. To summarize briefly: (1) genetics: more and more PD-related genes have been linked to lipid and membrane trafficking pathways (Table 1); (2) patient samples: state-of-the art characterization of LBs has shown them to be rich in lipids and membranous organelles (section 1), and body fluids of control vs. PD subjects show differences in lipid content (section 7); (3) the characteristics of the αS protein: αS is a membrane-binding and potentially also a FA-binding protein (section 4); αS excess may alter lipid pathways (section 3).
It can be argued that lipid alterations in synucleinopathies may trigger toxic αS β-sheet aggregation but aren’t toxic themselves, a scenario that can be called ‘lipid-induced proteinopathy’. The genetics are generally in line with this concept because (as far as one can tell) certain lipid- and trafficking-related genes seem to cause classical PD symptoms and αS brain pathology. And yet we emphasize in this review the relevance of the ‘other direction’, i.e. ‘protein-induced lipotoxity’, which we believe has been underestimated. This assumption is based in considerable part on the new insights into the nature of LBs ([112]; section 1): αS neuropathology in PD may be rich in lipids and vesicular organelles. Fibrillar aggregates were also observed, but at a lower frequency than traditionally thought (roughly 20% of all LBs contained fibrils).
Compared to proteotoxicity, the toxicity that stems from impaired vesicle trafficking is relatively self-evident: a cell that attempts to deliver cargo while the respective vesicles get ‘stuck’ may indeed suffer. However, it is not clear if protein-induced lipotoxity is largely caused by impaired vesicle trafficking (sections 2, 3 and 6). Trafficking-independent mechanisms may exist (section 7). And there may be bidirectional scenarios in which lipid alterations impair vesicle trafficking, and altered trafficking impairs lipid homeostasis (sections 6 and 7).
Assuming that both lipid-induced proteotoxicity and protein-induced lipotoxicity are relevant, PD and related human synucleinopathies may simultaneously be proteinopathies and lipidopathies. A vicious cycle of dyshomeostasis in protein folding and lipid metabolism might be triggered by early and subtle changes in either lipid or protein handling; the initial alteration may differ from case to case [45]. ‘Vicious cycle’ scenarios have been proposed for αS dyshomeostasis caused by GBA dysfunction [79] and oleic acid excess [44], which makes drug development efforts around GBA and SCD (the rate-limiting enzyme producing oleic acid and palmitoleic acid) attractive (section 10). Since the unexpected ultrastructure of LBs [112] is central for many aspects of what has been discussed here, it will be of critical importance to not only independently confirm the lipid membrane-rich nature of LBs, but to also extend the analysis to specific sub-forms of PD. For example, it could be very telling to compare the exact appearance of LBs in different fPD αS mutations, since two ways of LB formation may exist (recently reviewed in [33]): one via excess membrane binding of monomers (E46K, A53T) and one via excess accumulation of soluble monomers in the cytosol (A30P, G51D). Similarly, the morphology of LBs in sporadic vs. GBA vs. LRRK2 PD as well as the pathology of rarer lipid-related and non-lipid-related mutations would be very interesting to study. These studies should also include a detailed analysis of different brain regions, most importantly cortex vs. midbrain. Also, a similar detailed morphological characterization of αS inclusions in MSA would be desirable.
If αS-induced ‘lipidopathy’ and ‘vesiculopathy’ both occur, the question as to which form of αS causes neuronal injury cannot be ignored. The interplay between αS and vesicle membranes is specific and intimately linked to αS’s ability to form α-helices at membranes (section 4). β-Sheet aggregates of αS should lose this preference for vesicles. Several possible scenarios are discussed in section 4, but we predict that more and more studies will show that non-amyloid, non-aggregated excess αS can be associated with harm inside neurons. In this context, it would be important to better understand αS function (section 2), to be able to assess if an excess of normal αS function might already be a key contributor to αS pathology. It is remarkable that one of the proposed functions of αS is vesicle clustering [108, 137]. The newly described features of LBs [112] are consistent with excess vesicle clustering and potentially an excess normal function of αS. However, other functions of αS have been proposed at the synapse (section 2), while the occurrence of somatic αS is typically discussed as an ‘accident’ that may be the starting point of aggregation (section 3).
Genetic and other considerations (sections 5,6,7) suggest an intertwined relationship between ER, Golgi, endosomal/lysosomal function/trafficking and lipid content in terms of lipid equilibrium and αS homeostasis. Membrane lipid alterations and membrane fluidity can impact αS:membrane interactions and vesicle trafficking, resulting in PD-relevant phenotypes, and we have outlined several bidirectional scenarios. We hope to understand much more about this complex interchange over time. At the same time, we believe that new therapeutic strategies such as SCD inhibition will show promise for PD and DLB treatment even before the riddles of lipid dyshomeostasis in these diseases are solved.
Acknowledgements
We are grateful to Gina Dove and Renee Brathwaite for administrative support. We thank Tom DiCesare for the final illustrations. The αS-related work of our groups is supported by NIH grants NS099328 (to UD) and NS083845 (to DS) and a grant by the Michael J Fox Foundation (to SF).
Footnotes
Competing Interests. DS is a director and consultant to Prothena Biosciences. The other authors declare no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Abeliovich A, Gitler AD (2016) Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539:207–216. doi: 10.1038/nature20414 [DOI] [PubMed] [Google Scholar]
- 2.Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239–252 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJA, Cooper JM (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiology of Disease 42:360–367. doi: 10.1016/j.nbd.2011.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, et al. (2013) Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord 28:811–813. doi: 10.1002/mds.25421 [DOI] [PubMed] [Google Scholar]
- 5.Bae E-J, Lee H-J, Jang Y-H, Michael S, Masliah E, Min DS, Lee S-J (2014) Phospholipase D1 regulates autophagic flux and clearance of α-synuclein aggregates. Cell Death Differ 21:1132–1141. doi: 10.1038/cdd.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels T (2019) A traffic jam leads to Lewy bodies. Nat Neurosci 22:1043–1045. doi: 10.1038/s41593-019-0435-y [DOI] [PubMed] [Google Scholar]
- 7.Bartels T, Choi JG, Selkoe DJ (2011) α-synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477:107–110. doi: 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R (2009) Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic 10:218–234. doi: 10.1111/j.1600-0854.2008.00853.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendor JT, Logan TP, Edwards RH (2013) The function of α-synuclein. Neuron 79:1044–1066. doi: 10.1016/j.neuron.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleasel JM, Wong JH, Halliday GM, Kim WS (2014) Lipid dysfunction and pathogenesis of multiple system atrophy. Acta Neuropathol Commun 2:15. doi: 10.1186/2051-5960-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breda C, Nugent ML, Estranero JG, Kyriacou CP, Outeiro TF, Steinert JR, Giorgini F (2015) Rab11 modulates α-synuclein-mediated defects in synaptic transmission and behaviour. Human Molecular Genetics 24:1077–1091. doi: 10.1093/hmg/ddu521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brekk OR, Moskites A, Isacson O, Hallett PJ (2018) Lipid-dependent deposition of alpha-synuclein and Tau on neuronal Secretogranin II-positive vesicular membranes with age. Sci Rep 8:15207. doi: 10.1038/s41598-018-33474-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burre J, Sharma M, Sudhof TC (2014) alpha-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A 111:E4274–83. doi: 10.1073/pnas.1416598111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burré J, Sharma M, Südhof TC (2015) Definition of a Molecular Pathway Mediating α-synuclein Neurotoxicity. J Neurosci 35:5221–5232. doi: 10.1523/JNEUROSCI.4650-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667. doi: 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bykov K, Yoshida K, Weisskopf MG, Gagne JJ (2017) Confounding of the association between statins and Parkinson disease: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 26:294–300. doi: 10.1002/pds.4079 [DOI] [PubMed] [Google Scholar]
- 17.Chang D, Nalls MA, Hallgrfmsdottir IB, Hunkapiller J, van der Brug M, Cai F, et al. (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 49:1511–1516. doi: 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chartier-Harlin M-C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1 [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Goodman JM (2017) The collaborative work of droplet assembly. Biochim Biophys Acta 1862:1205–1211. doi: 10.1016/j.bbalip.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YP, Song W, Huang R, Chen K, Zhao B, Li J, et al. (2013) GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson’s disease in a Chinese population. J Clin Neurosci 20:880–3. doi: 10.1016/j.jocn.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 21.Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, et al. (2011) Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 6:e17299. doi: 10.1371/journal.pone.0017299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, et al. (2013) Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science 342:983–987. doi: 10.1126/science.1245296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark LN, Ross BM, Wang Y, Mejia-Santana H, Harris J, Louis ED, et al. (2007) Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology 69:1270–1277. doi: 10.1212/01.wnl.0000276989.17578.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL (2002) Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J Biol Chem 277:6344–6352. doi: 10.1074/jbc.M108414200 [DOI] [PubMed] [Google Scholar]
- 25.Collier TJ, Redmond DE, Steece-Collier K, Lipton JW, Manfredsson FP (2016) Is Alpha-synuclein Loss-of-Function a Contributor to Parkinsonian Pathology? Evidence from Non-human Primates. Front Neurosci 10:12. doi: 10.3389/fnins.2016.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collier TJ, Srivastava KR, Justman C, Grammatopoulous T, Hutter-Paier B, Prokesch M, et al. (2017) Nortriptyline inhibits aggregation and neurotoxicity of alpha-synuclein by enhancing reconfiguration of the monomeric form. Neurobiol Dis 106:191–204. doi: 10.1016/j.nbd.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313:324–328. doi: 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox TM, Aerts JMFG, Andria G, Beck M, Belmatoug N, Bembi B, et al. (2003) The role of the iminosugar N-butyldeoxynojirimycin (miglustat) in the management of type I (non-neuronopathic) Gaucher disease: a position statement. J Inherit Metab Dis 26:513–526. doi: 10.1023/a:1025902113005 [DOI] [PubMed] [Google Scholar]
- 29.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295. doi: 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- 30.Dalfó E, Gómez-Isla T, Rosa JL, Nieto Bodelón M, Cuadrado Tejedor M, Barrachina M, et al. (2004) Abnormal alpha-synuclein interactions with Rab proteins in alpha-synuclein A30P transgenic mice. J Neuropathol Exp Neurol 63:302–313. doi: 10.1093/jnen/63.4.302 [DOI] [PubMed] [Google Scholar]
- 31.Darios F, Ruipérez V, López I, Villanueva J, Gutierrez LM, Davletov B (2010) Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep 11:528–533. doi: 10.1038/embor.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273:9443–9449 [DOI] [PubMed] [Google Scholar]
- 33.Dettmer U (2018) Rationally Designed Variants of α-synuclein Illuminate Its in vivo Structural Properties in Health and Disease. Front Neurosci 12:623. doi: 10.3389/fnins.2018.00623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dettmer U, Newman AJ, von Saucken VE, Bartels T, Selkoe D (2015) KTKEGV repeat motifs are key mediators of normal α-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity. Proc Natl Acad Sci USA 112:9596–9601. doi: 10.1073/pnas.1505953112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, et al. (2015) Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun 6:7314. doi: 10.1038/ncomms8314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dettmer U, Ramalingam N, von Saucken VE, Kim T-E, Newman AJ, Terry-Kantor E, et al. (2017) Loss of native α-synuclein multimerization by strategically mutating its amphipathic helix causes abnormal vesicle interactions in neuronal cells. Hum Mol Genet 26:3466–3481. doi: 10.1093/hmg/ddx227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeWitt DC, Rhoades E (2013) α-synuclein can inhibit SNARE-mediated vesicle fusion through direct interactions with lipid bilayers. Biochemistry 52:2385–2387. doi: 10.1021/bi4002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, et al. (2013) Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife 2:e00592. doi: 10.7554/eLife.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dijk KD, Berendse HW, Drukarch B, Fratantoni SA, Pham TV, Piersma SR, et al. (2012) The proteome of the locus ceruleus in Parkinson’s disease: relevance to pathogenesis. Brain Pathol 22:485–98. doi: 10.1111/j.1750-3639.2011.00540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dijk KD, Persichetti E, Chiasserini D, Eusebi P, Beccari T, Calabresi P, et al. (2013) Changes in endolysosomal enzyme activities in cerebrospinal fluid of patients with Parkinson’s disease: CSF Endolysosomal Enzymes in PD. Mov Disord 28:747–754. doi: 10.1002/mds.25495 [DOI] [PubMed] [Google Scholar]
- 41.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, et al. (2011) Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet 7:e1002141. doi: 10.1371/journal.pgen.1002141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Don AS, Hsiao J-HT, Bleasel JM, Couttas TA, Halliday GM, Kim WS (2014) Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta Neuropathol Commun 2:150. doi: 10.1186/s40478-014-0150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim Y-I, Zenvirt S, et al. (2012) A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS ONE 7:e36458. doi: 10.1371/journal.pone.0036458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fanning S, Haque A, Imberdis T, Baru V, Barrasa MI, Nuber S, et al. (2018) Lipidomic Analysis of α-Synuclein Neurotoxicity Identifies Stearoyl CoA Desaturase as a Target for Parkinson Treatment. Mol Cell. doi: 10.1016/j.molcel.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanning S, Selkoe D, Dettmer U (2020) Parkinson’s disease: proteinopathy or lipidopathy? NPJ Parkinsons Dis 6:3. doi: 10.1038/s41531-019-0103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonseca-Ornelas L, Eisbach SE, Paulat M, Giller K, Fernández CO, Outeiro TF, et al. (2014) Small molecule-mediated stabilization of vesicle-associated helical α-synuclein inhibits pathogenic misfolding and aggregation. Nat Commun 5:5857. doi: 10.1038/ncomms6857 [DOI] [PubMed] [Google Scholar]
- 47.Forno LS, Norville RL (1976) Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol 34:183–197 [DOI] [PubMed] [Google Scholar]
- 48.Fusco G, Pape T, Stephens AD, Mahou P, Costa AR, Kaminski CF, et al. (2016) Structural basis of synaptic vesicle assembly promoted by α-synuclein. Nat Commun 7:12563. doi: 10.1038/ncomms12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galvagnion C, Buell AK, Meisl G, Michaels TCT, Vendruscolo M, Knowles TPJ, Dobson CM (2015) Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat Chem Biol 11:229–234. doi: 10.1038/nchembio.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Reitbock P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, et al. (2010) SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 133:2032–2044. doi: 10.1093/brain/awq132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George JM, Jin H, Woods WS, Clayton DF (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15:361–372 [DOI] [PubMed] [Google Scholar]
- 52.Gibb WR, Scott T, Lees AJ (1991) Neuronal inclusions of Parkinson’s disease. Mov Disord 6:2–11. doi: 10.1002/mds.870060103 [DOI] [PubMed] [Google Scholar]
- 53.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, et al. (2008) The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA 105:145–150. doi: 10.1073/pnas.0710685105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gosavi N, Lee H-J, Lee JS, Patel S, Lee S-J (2002) Golgi Fragmentation Occurs in the Cells with Prefibrillar α-synuclein Aggregates and Precedes the Formation of Fibrillar Inclusion. J Biol Chem 277:48984–48992. doi: 10.1074/jbc.M208194200 [DOI] [PubMed] [Google Scholar]
- 55.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, et al. (2010) αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci USA 107:19573–19578. doi: 10.1073/pnas.1005005107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo X-Y, Chen Y-P, Song W, Zhao B, Cao B, Wei Q-Q, et al. (2014) An association analysis of the rs1572931 polymorphism of the RAB7L1 gene in Parkinson’s disease, amyotrophic lateral sclerosis and multiple system atrophy in China. Eur J Neurol 21:1337–1343. doi: 10.1111/ene.12490 [DOI] [PubMed] [Google Scholar]
- 57.Hattingen E, Magerkurth J, Pilatus U, Mozer A, Seifried C, Steinmetz H, et al. (2009) Phosphorus and proton magnetic resonance spectroscopy demonstrates mitochondrial dysfunction in early and advanced Parkinson’s disease. Brain 132:3285–3297. doi: 10.1093/brain/awp293 [DOI] [PubMed] [Google Scholar]
- 58.Imberdis T, Negri J, Ramalingam N, Terry-Kantor E, Ho GPH, Fanning S, et al. (2019) Cell models of lipid-rich α-synuclein aggregation validate known modifiers of α-synuclein biology and identify stearoyl-CoA desaturase. Proc Natl Acad Sci USA. doi: 10.1073/pnas.1903216116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarosz DF, Khurana V (2017) Specification of Physiologic and Disease States by Distinct Proteins and Protein Conformations. Cell 171:1001–1014. doi: 10.1016/j.cell.2017.10.047 [DOI] [PubMed] [Google Scholar]
- 60.Jensen MB, Bhatia VK, Jao CC, Rasmussen JE, Pedersen SL, Jensen KJ, et al. (2011) Membrane curvature sensing by amphipathic helices: a single liposome study using alpha-synuclein and annexin B12. J Biol Chem 286:42603–14. doi: 10.1074/jbc.M111.271130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, et al. (2013) α-synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol 125:753–769. doi: 10.1007/s00401-013-1096-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S, Yun SP, Lee S, Umanah GE, Bandaru VVR, Yin X, et al. (2018) GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc Natl Acad Sci USA 115:798–803. doi: 10.1073/pnas.1700465115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klemann C, Martens GJM, Sharma M, Martens MB, Isacson O, Gasser T, et al. (2017) Integrated molecular landscape of Parkinson’s disease. NPJ Parkinsons Dis 3:14. doi: 10.1038/s41531-017-0015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korvatska O, Strand NS, Berndt JD, Strovas T, Chen D-H, Leverenz JB, et al. (2013) Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum Mol Genet 22:3259–3268. doi: 10.1093/hmg/ddt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18:106–108. doi: 10.1038/ng0298-106 [DOI] [PubMed] [Google Scholar]
- 66.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, et al. (2006) Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci 26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee H-J, Khoshaghideh F, Lee S, Lee S-J (2006) Impairment of microtubule-dependent trafficking by overexpression of α-synuclein. European Journal of Neuroscience 24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x [DOI] [PubMed] [Google Scholar]
- 68.Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, et al. (2013) G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol 73:459–471. doi: 10.1002/ana.23894 [DOI] [PubMed] [Google Scholar]
- 69.Li G, Cui S, Du J, Liu J, Zhang P, Fu Y, et al. (2018) Association of GALC, ZNF184, IL1R2 and ELOVL7 With Parkinson’s Disease in Southern Chinese. Front Aging Neurosci 10:402. doi: 10.3389/fnagi.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z, Schulze RJ, Weller SG, Krueger EW, Schott MB, Zhang X, et al. (2016) A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets. Sci Adv 2:e1601470. doi: 10.1126/sciadv.1601470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Licker V, Turck N, Kovari E, Burkhardt K, Cote M, Surini-Demiri M, et al. (2014) Proteomic analysis of human substantia nigra identifies novel candidates involved in Parkinson’s disease pathogenesis. Proteomics 14:784–94. doi: 10.1002/pmic.201300342 [DOI] [PubMed] [Google Scholar]
- 72.Lin G, Lee P-T, Chen K, Mao D, Tan KL, Zuo Z, et al. (2018) Phospholipase PLA2G6, a Parkinsonism-Associated Gene, Affects Vps26 and Vps35, Retromer Function, and Ceramide Levels, Similar to α-Synuclein Gain. Cell Metab 28:605–618.e6. doi: 10.1016/j.cmet.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 73.Locascio JJ, Eberly S, Liao Z, Liu G, Hoesing AN, Duong K, et al. (2015) Association between α-synuclein blood transcripts and early, neuroimaging-supported Parkinson’s disease. Brain 138:2659–2671. doi: 10.1093/brain/awv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Logan T, Bendor J, Toupin C, Thorn K, Edwards RH (2017) α-synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 20:681–689. doi: 10.1038/nn.4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lou X, Kim J, Hawk BJY-K, (2017) α-synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking. Biochem J 474:2039–2049. doi: 10.1042/BCJ20170200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Markovic I, Kresojevic N, Kostic VS (2016) Glucocerebrosidase and parkinsonism: lessons to learn. J Neurol 263:1033–1044. doi: 10.1007/s00415-016-8085-4 [DOI] [PubMed] [Google Scholar]
- 77.Maroteaux L, Scheller RH (1991) The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res 11:335–343 [DOI] [PubMed] [Google Scholar]
- 78.Maulik M, Mitra S, Basmayor AM, Lu B, Taylor BE, Bult-Ito A (2019) Genetic Silencing of Fatty Acid Desaturases Modulates α-synuclein Toxicity and Neuronal Loss in Parkinson-Like Models of C. elegans. Front Aging Neurosci 11:207. doi: 10.3389/fnagi.2019.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazzulli JR, Xu Y-H, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146:37–52. doi: 10.1016/j.cell.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, et al. (2014) Pharmacological chaperones stabilize retromer to limit APP processing. Nat Chem Biol 10:443–449. doi: 10.1038/nchembio.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michell AW, Tofaris GK, Gossage H, Tyers P, Spillantini MG, Barker RA (2007) The effect of truncated human alpha-synuclein (1–120) on dopaminergic cells in a transgenic mouse model of Parkinson’s disease. Cell Transplant 16:461–474 [DOI] [PubMed] [Google Scholar]
- 82.Mir R, Tonelli F, Lis P, Macartney T, Polinski NK, Martinez TN, et al. (2018) The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem J 475:1861–1883. doi: 10.1042/BCJ20180248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan NV, Westaway SK, Morton JEV, Gregory A, Gissen P, Sonek S, et al. (2006) PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet 38:752–754. doi: 10.1038/ng1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46:989–93. doi: 10.1038/ng.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, et al. (2010) Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65:66–79. doi: 10.1016/j.neuron.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ness D, Ren Z, Gardai S, Sharpnack D, Johnson VJ, Brennan RJ, et al. (2013) Leucine-rich repeat kinase 2 (LRRK2)-deficient rats exhibit renal tubule injury and perturbations in metabolic and immunological homeostasis. PLoS ONE 8:e66164. doi: 10.1371/journal.pone.0066164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, Halter CA, et al. (2009) Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology 72:310–316. doi: 10.1212/01.wnl.0000327823.81237.d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nishimura M, Tomimoto H, Suenaga T, Nakamura S, Namba Y, Ikeda K, et al. (1994) Synaptophysin and chromogranin A immunoreactivities of Lewy bodies in Parkinson’s disease brains. Brain Res 634:339–344 [DOI] [PubMed] [Google Scholar]
- 89.Nuber S, Rajsombath M, Minakaki G, Winkler J, Müller CP, Ericsson M, et al. (2018) Abrogating Native α-Synuclein Tetramers in Mice Causes a L-DOPA-Responsive Motor Syndrome Closely Resembling Parkinson’s Disease. Neuron 100:75–90.e5. doi: 10.1016/j.neuron.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nuscher B, Kamp F, Mehnert T, Odoy S, Haass C, Kahle PJ, Beyer K (2004) Alpha-synuclein has a high affinity for packing defects in a bilayer membrane: a thermodynamics study. J Biol Chem 279:21966–75. doi: 10.1074/jbc.M401076200 [DOI] [PubMed] [Google Scholar]
- 91.Oaks AW, Marsh-Armstrong N, Jones JM, Credle JJ, Sidhu A (2013) Synucleins Antagonize Endoplasmic Reticulum Function to Modulate Dopamine Transporter Trafficking. PLoS ONE 8:e70872. doi: 10.1371/journal.pone.0070872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olgiati S, De Rosa A, Quadri M, Criscuolo C, Breedveld GJ, Picillo M, et al. (2014) PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics 15:183–188. doi: 10.1007/s10048-014-0406-0 [DOI] [PubMed] [Google Scholar]
- 93.Outeiro TF, Lindquist S (2003) Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302:1772–5. doi: 10.1126/science.1090439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paisan-Rufz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44:595–600. doi: 10.1016/j.neuron.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 95.Perlmutter JD, Braun AR, Sachs JN (2009) Curvature dynamics of alpha-synuclein familial Parkinson disease mutants: molecular simulations of the micelle- and bilayer-bound forms. J Biol Chem 284:7177–7189. doi: 10.1074/jbc.M808895200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perni M, Flagmeier P, Limbocker R, Cascella R, Aprile FA, Galvagnion C, et al. (2018) Multistep Inhibition of α-synuclein Aggregation and Toxicity in Vitro and in Vivo by Trodusquemine. ACS Chem Biol 13:2308–2319. doi: 10.1021/acschembio.8b00466 [DOI] [PubMed] [Google Scholar]
- 97.Perni M, Galvagnion C, Maltsev A, Meisl G, Müller MBD, Challa PK, et al. (2017) A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity. Proc Natl Acad Sci USA 114:E1009–E1017. doi: 10.1073/pnas.1610586114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047 [DOI] [PubMed] [Google Scholar]
- 99.Poulopoulos M, Levy OA, Alcalay RN (2012) The neuropathology of genetic Parkinson’s disease. Mov Disord 27:831–842. doi: 10.1002/mds.24962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pranke IM, Morello V, Bigay J, Gibson K, Verbavatz JM, Antonny B, Jackson CL (2011) alpha-synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J Cell Biol 194:89–103. doi: 10.1083/jcb.201011118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP, et al. (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 38:1184–1191. doi: 10.1038/ng1884 [DOI] [PubMed] [Google Scholar]
- 102.Rhoades E, Ramlall TF, Webb WW, Eliezer D (2006) Quantification of alpha-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J 90:4692–700. doi: 10.1529/biophysj.105.079251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rovere M, Powers AE, Jiang H, Pitino JC, Fonseca-Ornelas L, Patel DS, et al. (2019) E46K-like α-synuclein mutants increase lipid interactions and disrupt membrane selectivity. J Biol Chem. doi: 10.1074/jbc.RA118.006551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schapansky J, Nardozzi JD, LaVoie MJ (2015) The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson’s disease. Neuroscience 302:74–88. doi: 10.1016/j.neuroscience.2014.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schapira AHV (2015) Glucocerebrosidase and Parkinson disease: Recent advances. Mol Cell Neurosci 66:37–42. doi: 10.1016/j.mcn.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schöndorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, et al. (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5:4028. doi: 10.1038/ncomms5028 [DOI] [PubMed] [Google Scholar]
- 107.Schulz-Schaeffer WJ (2010) The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120:131–143. doi: 10.1007/s00401-010-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scott D, Roy S (2012) α-synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci 32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seidel K, Schöls L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, et al. (2010) First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Annals of Neurology NA-NA. doi: 10.1002/ana.21966 [DOI] [PubMed] [Google Scholar]
- 110.Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R (2008) Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gammα-synuclein. Eur J Neurosci 27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seyfried TN, Choi H, Chevalier A, Hogan D, Akgoc Z, Schneider JS (2018) Sex-Related Abnormalities in Substantia Nigra Lipids in Parkinson’s Disease. ASN Neuro 10:1759091418781889. doi: 10.1177/1759091418781889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, et al. (2019) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099–1109. doi: 10.1038/s41593-019-0423-2 [DOI] [PubMed] [Google Scholar]
- 113.Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ (2003) The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 37:583–95 [DOI] [PubMed] [Google Scholar]
- 114.Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, et al. (2012) SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science 335:1355–1359. doi: 10.1126/science.1214984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simon-Sanchez J, van Hilten JJ, van de Warrenburg B, Post B, Berendse HW, Arepalli S, et al. (2011) Genome-wide association study confirms extant PD risk loci among the Dutch. Eur J Hum Genet 19:655–61. doi: 10.1038/ejhg.2010.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41:1308–12. doi: 10.1038/ng.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. (2003) alpha-synuclein locus triplication causes Parkinson’s disease. Science 302:841. doi: 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- 118.Soper JH, Kehm V, Burd CG, Bankaitis VA, Lee VM-Y (2011) Aggregation of α-synuclein in S. cerevisiae is Associated with Defects in Endosomal Trafficking and Phospholipid Biosynthesis. J Mol Neurosci 43:391–405. doi: 10.1007/s12031-010-9455-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, et al. (2008) Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae. Mol Biol Cell 19:1093–1103. doi: 10.1091/mbc.E07-08-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soste M, Charmpi K, Lampert F, Gerez JA, van Oostrum M, Malinovska L, et al. (2019) Proteomics-Based Monitoring of Pathway Activity Reveals that Blocking Diacylglycerol Biosynthesis Rescues from Alpha-Synuclein Toxicity. Cell Syst 9:309–320.e8. doi: 10.1016/j.cels.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. doi: 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spinelli KJ, Taylor JK, Osterberg VR, Churchill MJ, Pollock E, Moore C, et al. (2014) Presynaptic alpha-synuclein aggregation in a mouse model of Parkinson’s disease. J Neurosci 34:2037–2050. doi: 10.1523/JNEUROSCI.2581-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, et al. (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5:. doi: 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stockl M, Fischer P, Wanker E, Herrmann A (2008) Alpha-synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J Mol Biol 375:1394–1404. doi: 10.1016/j.jmb.2007.11.051 [DOI] [PubMed] [Google Scholar]
- 125.Stoessel D, Schulte C, Teixeira Dos Santos MC, Scheller D, Rebollo-Mesa I, Deuschle C, et al. (2018) Promising Metabolite Profiles in the Plasma and CSF of Early Clinical Parkinson’s Disease. Front Aging Neurosci 10:51. doi: 10.3389/fnagi.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun J, Wang L, Bao H, Premi S, Das U, Chapman ER, Roy S (2019) Functional cooperation of α-synuclein and VAMP2 in synaptic vesicle recycling. Proc Natl Acad Sci USA 116:11113–11115. doi: 10.1073/pnas.1903049116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tardiff DF, Jui NT, Khurana V, Tambe MA, Thompson ML, Chung CY, et al. (2013) Yeast reveal a “druggable” Rsp5/Nedd4 network that ameliorates α-synuclein toxicity in neurons. Science 342:979–983. doi: 10.1126/science.1245321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tenreiro S, Reimao-Pinto MM, Antas P, Rino J, Wawrzycka D, Macedo D, et al. (2014) Phosphorylation modulates clearance of alpha-synuclein inclusions in a yeast model of Parkinson’s disease. PLoS Genet 10:e1004302. doi: 10.1371/journal.pgen.1004302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC (2010) α-synuclein Delays Endoplasmic Reticulum (ER)-to-Golgi Transport in Mammalian Cells by Antagonizing ER/Golgi SNAREs. MBoC 21:1850–1863. doi: 10.1091/mbc.e09-09-0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vance JE (2008) Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 49:1377–1387. doi: 10.1194/jlr.R700020-JLR200 [DOI] [PubMed] [Google Scholar]
- 131.Vargas KJ, Makani S, Davis T, Westphal CH, Castillo PE, Chandra SS (2014) Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci 34:9364–9376. doi: 10.1523/JNEUROSCI.4787-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vargas KJ, Schrod N, Davis T, Fernandez-Busnadiego R, Taguchi YV, Laugks U, et al. (2017) Synucleins Have Multiple Effects on Presynaptic Architecture. Cell Rep 18:161–173. doi: 10.1016/j.celrep.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vincent BM, Tardiff DF, Piotrowski JS, Aron R, Lucas MC, Chung CY, et al. (2018) Inhibiting Stearoyl-CoA Desaturase Ameliorates α-synuclein Cytotoxicity. Cell Rep 25:2742–2754.e31. doi: 10.1016/j.celrep.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 134.Volles MJ, Lansbury PT (2007) Relationships between the sequence of alpha-synuclein and its membrane affinity, fibrillization propensity, and yeast toxicity. J Mol Biol 366:1510–1522. doi: 10.1016/j.jmb.2006.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM-Y (2014) Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. MBoC 25:4010–4023. doi: 10.1091/mbc.e14-02-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walther TC, Chung J, Farese RV (2017) Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol. doi: 10.1146/annurev-cellbio-100616-060608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S (2014) α-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol 24:2319–2326. doi: 10.1016/j.cub.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang T, Hay JC (2015) Alpha-synuclein Toxicity in the Early Secretory Pathway: How It Drives Neurodegeneration in Parkinsons Disease. Front Neurosci 9:. doi: 10.3389/fnins.2015.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Westphal CH, Chandra SS (2013) Monomeric synucleins generate membrane curvature. J Biol Chem 288:1829–1840. doi: 10.1074/jbc.M112.418871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams ET, Chen X, Moore DJ (2017) VPS35, the Retromer Complex and Parkinson’s Disease. J Parkinsons Dis 7:219–233. doi: 10.3233/JPD-161020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Willkommen D, Lucio M, Moritz F, Forcisi S, Kanawati B, Smirnov KS, et al. (2018) Metabolomic investigations in cerebrospinal fluid of Parkinson’s disease. PLoS ONE 13:e0208752. doi: 10.1371/journal.pone.0208752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilson GR, Sim JCH, McLean C, Giannandrea M, Galea CA, Riseley JR, et al. (2014) Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am J Hum Genet 95:729–735. doi: 10.1016/j.ajhg.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winslow AR, Chen C-W, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, et al. (2010) α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. The Journal of Cell Biology 190:1023–1037. doi: 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Withers GS, George JM, Banker GA, Clayton DF (1997) Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res 99:87–94. doi: 10.1016/s0165-3806(96)00210-6 [DOI] [PubMed] [Google Scholar]
- 145.Wong JH, Halliday GM, Kim WS (2014) Exploring myelin dysfunction in multiple system atrophy. Exp Neurobiol 23:337–344. doi: 10.5607/en.2014.23.4.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wong YC, Krainc D (2016) Lysosomal trafficking defects link Parkinson’s disease with Gaucher’s disease. Mov Disord 31:1610–1618. doi: 10.1002/mds.26802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wood PL, Tippireddy S, Feriante J, Woltjer RL (2018) Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 13:e0191815. doi: 10.1371/journal.pone.0191815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yin G, Lopes da Fonseca T, Eisbach SE, Anduaga AM, Breda C, Orcellet ML, et al. (2014) α-synuclein interacts with the switch region of Rab8a in a Ser129 phosphorylation-dependent manner. Neurobiology of Disease 70:149–161. doi: 10.1016/j.nbd.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 149.Yun HJ, Kim H, Ga I, Oh H, Ho DH, Kim J, et al. (2015) An early endosome regulator, Rab5b, is an LRRK2 kinase substrate. J Biochem 157:485–495. doi: 10.1093/jb/mvv005 [DOI] [PubMed] [Google Scholar]
- 150.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173. doi: 10.1002/ana.10795 [DOI] [PubMed] [Google Scholar]
- 151.Zhao Y, Dzamko N (2019) Recent Developments in LRRK2-Targeted Therapy for Parkinson’s Disease. Drugs 79:1037–1051. doi: 10.1007/s40265-019-01139-4 [DOI] [PubMed] [Google Scholar]
- 152.Zhu XC, Cao L, Tan MS, Jiang T, Wang HF, Lu H, et al. (2017) Association of Parkinson’s Disease GWAS-Linked Loci with Alzheimer’s Disease in Han Chinese. Mol Neurobiol 54:308–318. doi: 10.1007/s12035-015-9649-5 [DOI] [PubMed] [Google Scholar]
- 153.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, et al. (2011) A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet 89:168–175. doi: 10.1016/j.ajhg.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–607. doi: 10.1016/j.neuron.2004.11.005 [DOI] [PubMed] [Google Scholar]