Abstract
Background.
The enteric nervous system contains multiple classes of neurons, distinguishable by morphology, immunohistochemical markers and projections; however specific combinations differ between species. Here, types of enteric neurons in human colon, were characterised immunohistochemically, using retrograde tracing combined with multiple labelling immunohistochemistry, focussing on non-motor neurons.
Methods.
The fluorescent carbocyanine tracer, DiI, was applied to the myenteric plexus in ex-vivo preparations, filling neurons projecting within the plexus. Limits of projection lengths of motor neurons were established, allowing them to be excluded from the analysis. Long ascending and descending interneurons were then distinguished by labelling for discriminating immunohistochemical markers; calbindin, calretinin, enkephalin, 5-hydroxytryptamine, nitric oxide synthase and substance P. These results were combined with a previous published study in which nitric oxide synthase and choline acetyltransferase immunoreactivities were established.
Key Results.
Long ascending neurons (with projections longer than 8mm, which excludes more than 95% motor neurons) formed 4 types, in descending order of abundance, defined by immunoreactivity for: (i) ChAT+/ENK+ (ii) ChAT+/ENK+/SP+ (iii) ChAT+/Calb+ and (iv) ChAT+/ENK+/Calb+ . Long descending neurons, up to 70mm long also formed at least 4 types, distinguished by immunoreactivity for (i) NOS+ cells (without ChAT) (ii) ChAT+/NOS+ (iii) ChAT+/Calret+ and (iv) ChAT+/5HT+ cells (with or without NOS).
Conclusions & Inferences.
Long interneurons, which do not innervate muscularis externa, are likely to coordinate neural activity over distances of many centimetres along the colon. Characterising their neurochemical coding provides a basis for understanding their roles, investigating their connectivity and building a comprehensive account of human colonic enteric neurons.
Keywords: human colon, interneurons, cholinergic, myenteric plexus
INTRODUCTION
The enteric nervous system comprises several hundred million neurons with cell bodies within the wall of the gut, which interconnect to form intrinsic neural circuits[1, 2]. These pathways powerfully influence motility, secretion and blood flow. Lying outside the blood brain barrier, enteric neurons represent a potential target for drugs to modify disorders of gut function or electrical stimulation for therapeutic benefit. Current understanding of the ENS is largely derived from studies in laboratory animals. While many principles of organisation of the ENS in animal tissues apply to human colon[1], striking differences have also been identified. It is important therefore, wherever possible, to study human tissue directly. The availability of surgical specimens of human gastrointestinal tract, particularly from the large intestine, make this a viable possibility.
It is well established that a number of different functional classes of neurons are present in the myenteric and submucous plexuses. These include inhibitory and excitatory motor neurons, primary afferent neurons, interneurons that project up and down the gut, secretomotor neurons and vasomotor neurons. Human colonic motor neurons have been identified by retrograde labelling combined with immunohistochemistry.These studies have shown that both longitudinal and circular muscle motor neurons have relatively short polarised projections in the longitudinal axis of the gut, with >95% of excitatory motor neurons projecting less than 8mm and 95% of inhibitory motor neurons projecting less than 18mm down the colon[3–5]. Despite this, human colonic motor activity is coordinated over distances of many centimetres both in vivo[6] and ex vivo[7]. This suggests the existence of enteric neural pathways with longer projections than motor neurons. Such pathways have been demonstrated previously, using retrograde labelling ex vivo combined with immunohistochemical localisation of some markers[5, 8]. Using these methods, long descending neurons were shown to project up to 68mm and long ascending neurons projected up to 38mm[4]. Nearly all long ascending neurons were immunoreactive for choline acetyltransferase (ChAT), whereas only about half of long descending neurons were cholinergic[8] In addition to choline acetyltransferase, expression of immunoreactivity for nitric oxide synthase (NOS) [8], substance P, calretinin and vasoactive intestinal polypeptide (VIP) in long pathways have also been reported[5]. Here, we have focussed on the patterns of expression of a larger range of immunohistochemical markers which can be used to distinguish different types of neurons within these cholinergic and non-cholinergic ascending and descending pathways in human colon. These include calbindin, calretinin, enkephalin, 5-hydroxytryptamine and substance P, together with replication of studies of nitric oxide synthase. Results from these markers indicate that both ascending and descending long pathways comprise multiple immunohistochemical types.
MATERIALS AND METHODS
Results were obtained from colonic tissue from 36 patients (8 ascending colons, 6 transverse colons, 22 descending colons, 19 male/17 female, age 65 years ± 13 years; mean ± standard deviation) who underwent elective colorectal surgery (anterior resection or right hemicolectomy) for non-obstructing large bowel cancer. A total of 47 preparations from these 36 patients provided the data for this study. Preparations from an additional 14 patients were studied but were discarded due to poor uptake of DiI (<10 cells filled), contamination of cells filled from underlying tissues (especially from circular muscle), infections of the culture medium or poor quality immunohistochemistry. All patients gave prior informed consent for use of colonic tissue for research purposes (Southern Adelaide Clinical Human Research Ethics approval number 207.17). Tissue was collected from operating theatres and placed in room temperature oxygenated modified Krebs solution (NaCl; 118mM, KCl; 4.8mM, CaCl2; 2.5mM, MgSO4; 1.2mM, NaHCO3; 25mM, NaH2PO4; 1.0mM, glucose; 11mM, bubbled with 95% O2, 5% CO2, pH; 7.4) and transported immediately to the laboratory. The tissue was then pinned out in a Sylgard-lined petri dish (Dow Corning, Midland, MI) and the mucosa and submucosa were removed by sharp dissection. In some cases, strips of circular muscle were removed near the oral or anal ends of the tissue to expose the myenteric plexus. The preparation was then turned over and the serosa was dissected to remove loose connective tissue and fat. It was then washed repeatedly in sterile Krebs solution. The preparation was then cut to size and pinned out in a sterilised Sylgard-lined petri dish. From 1–3 small glass beads (500μm diameter), coated with the carbocyanine dye (Honig and Hume 1989) 1,1’-didodecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (referred to as DiI) (D-383, Invitrogen-Molecular Probes, ThermoFisher Scientific), were then pressed onto the target tissue (either the surface of the circular muscle or ganglia or longitudinally-running internodal strands of the myenteric plexus).
The preparation was then rinsed again in sterile Krebs solution, immersed in culture medium (DME/F12 (Sigma) with 10% v/v foetal bovine serum, 100 i.u./ml penicillin, 100μg/ml streptomycin, 20μg/ml gentamycin, 2.5μg/ml amphotericin, 1μM nicardipine and 1μM hyoscine) and incubated at 37˚C in 5% CO2 for 4–5 days. The petri dish was placed on an orbital mixer in the incubator in order to ensure mixing of the culture medium. Preparations to be stained for neuropeptide immunoreactivity were exposed to 83μM colchicine for the final 24 hours of the culture period. Preparation to be tested for 5-HT-immunoreactivity were treated for 30 minutes with the monoamine oxidase inhibitor pargyline (50 μM) followed by 10μM 5-hydroxytryptamine mesylate added to the bath. This loaded nerve cell bodies which otherwise are hard to detect.[9]
Tissues were then rinsed in phosphate buffered saline (PBS) and re-pinned in another Sylgard-lined petri dish under maximal tension, then immersion-fixed in 4% paraformaldehyde fixative for 36–48 hours (4% formaldehyde in 0.1M phosphate buffer, pH 7.2). Prior to fixation, the DiI-coated beads were removed. Preparations were then further dissected to remove as much of the circular muscle layer as possible. They were then rinsed in PBS then immersed in carbonate/bicarbonate buffered glycerol (pH8.6) for 2–3 days at room temperature which permeabilised the tissue for antibody penetration without dissolving the DiI. The preparation was then mounted in fresh carbonate/bicarbonate buffered glycerol and viewed under epifluorescence to determine whether DiI-labelling was adequate. If so, it was unmounted, rinsed in PBS then covered in a mixture of primary antisera dissolved in hypertonic PBS at room temperature. Preparations were incubated for 3–4 days in primary antibodies, then in a mixture of secondary antisera for another 24 hours. Primary antibodies are described in Supplementary Table 1. Preparations were then rinsed in PBS and equilibrated with carbonate/bicarbonate-buffered glycerol (pH8.6) and mounted.
Preparations were viewed on an Olympus IX71 microscope fitted with epifluorescence and appropriate filter sets (Chroma Corporation). Images were taken by a CoolSNAP ES digital camera (Roper Scientific, Photometerics, Tucson, AZ, USA) using analySIS 5.0 software (Soft Imaging System, Gulfview Heights, South Australia, Australia). The entire preparation was systematically scanned to locate all DiI-labelled nerve cell bodies with a 10x or higher power objective. Cells were photographed with 10–40x objectives. Image processing was restricted to brightness and contrast adjustments, cropping and formation of photomontages using Adobe Photoshop (CS6, Adobe Systems Inc., San Jose, CA). The stage of the epifluorescence microscope was fitted with Two Mitutoyo linear scales (1 μm resolution), connected to a Mitutoyo 2D- ALC Decoder (ALC-3701, Mitutoyo Corporation, Japan). Output via an RS232/USB connector was directed to a personal computer and coordinates were downloaded directly into Microsoft Office Excel via Bill Redirect Software (http://www.billproduction.com) together with an identifying keypress. Data were then plotted as smoothed line segments (for outlines) or symbols (for neurons) in the spreadsheet software. This system allowed the coordinates (with 1μm resolution) of DiI-filled cells to be recorded as scatter plots of large preparations, while viewing each cell body (with its immunohistochemical markers) at medium to high power. The Y coordinate was used to create the longitudinal distribution of immunohistochemically-labelled, DiI-filled cells, rounded to the nearest millimeter to provide the bins in the histograms of figures 1–8. Preparations used to map interneurons were on average 18mm wide. Few DiI-filled nerve cell bodies were located within 2mm of the edges of the preparations, suggesting that the full circumferential distribution was filled.
Figure 1:
Ascending motor neurons to the circular muscle, subdivided by calbindin and calretinin immunoreactivities (n=4). DiI was applied to circular muscle near the oral end of the preparation (origin of axes – A) to ensure that ascending projections were preserved; this means that the distribution of neurons with descending projections may have been truncated. Note that there are few motor neurons with ascending projections longer than 12mm; in fact, 95% of all ascending motor neurons lie within 8mm of the DiI application site (origin of the axes). Of ascending motor neurons, 83% lacked both calbindin and calretinin immunoreactivities (B – small square in the scatter plot), 15% were calbindin immunoreactive but few were immunoreactive for calretinin.
Data are provided with n referring to the number of patients. The regions of colon of samples are listed; results from ascending, transverse and descending colon were pooled. Proportions of cells are given as percentages: an overall percentage is provided for all cells pooled from all preparations and patients. We also report, where appropriate, the average percentage per patient, along with 95% confidence intervals. These were calculated on a patient-by-patient basis to provide an indication of variability between individuals. Lastly, we also calculated percentages across all filled neurons (combining data from all preparations) to provide an overall proportion. These are shown in Supplementary Tables 2–11.
RESULTS
Eight studies are described below, combining data from a total of 48 preparations, taken from ascending, transverse, descending and sigmoid colon. To test for regional differences, preparations were grouped into proximal colon (ascending and transverse) and distal colon (descending and sigmoid). Longitudinal distributions of DiI-filled neurons with combinations of immunoreactivity were then compared between proximal and distal colon. No obvious differences were observed in any study so data from all regions was pooled. It should be noted that there were small numbers of proximal colon preparations (usually two or fewer) in most of the 8 studies, so we cannot entirely exclude the possibility that differences exist in neuronal populations between regions.
Ascending circular muscle motor neurons: calbindin and calretinin.
The first aim was to characterise the lengths of projections of both ascending and descending circular muscle motor neurons in the longitudinal axis of the human colon. DiI was applied to the circular muscle either at the oral end of the tissue or towards the anal end in order to ensure that the full extent of either ascending or descending projections was preserved. For ascending motor neurons, the presence of calbindin and calretinin immunoreactivities was tested (see Figure 1 and Supplementary Table 2). In 5 preparations from 4 patients (1 ascending colon, 1 transverse colon, 2 descending colon, n=4, 4 males, average age 54: 36–70) a total of 328 neurons were labelled by DiI, of which 257 had ascending projections to the DiI application site. Ascending projections were generally short – over 95% of ascending neurons lay within 8mm of the DiI application site. Most ascending motor neurons were non-immunoreactive for both calbindin and calretinin (averaged across subjects 83.4%; 95% CI=8.7%, n=4). A total of 47 ascending motor neurons (averaged across subjects; 18.3%; 95% CI=9.7%, n=4) were immunoreactive for calbindin, and 10 neurons (averaging across subjects 3.9%; 95% CI=2.3%, n=4) were immunoreactive for calretinin.
The distribution of circular muscle motor neurons with descending projections was slightly truncated due to the length of the preparation (see Fig1 and Supplementary Table 3). Nevertheless, the results show that of 71 neurons with descending projections, most (55/71 – averaged across subjects 75.8%; 95%CI=11.6%, n=4) were labelled by neither calbindin nor calretinin antisera; 4 cells were immunoreactive for calbindin without calretinin, 6 neurons were immunoreactive for calretinin without calbindin and 6 for calretinin with calbindin. Thus, just 16/71 descending motor neurons were immunoreactive for one or other of these calcium binding proteins (averaged across subjects 24.2%; 95% CI=11.6%, n=4).
Descending circular muscle motor neurons: calbindin and enkephalin.
The lengths of projection of descending circular muscle motor neurons were analysed in 5 preparations labelled with calbindin and enkephalin antisera. Here DiI was applied to circular muscle (at origin of axes - see Figure 2) at least 40mm from the oral end of the preparation in order to ensure that descending motor pathways were intact. The 5 preparations consisted of 1 ascending colon, 4 descending colons (N=5, 3 male, average age 58: 42–90). A total of 235 motor neurons were labelled: of these 69 had descending projections and 95% of these were located within 18mm of the DiI application site. Of these 69 cells, 17 were immunoreactive for calbindin (averaging across subjects 24.3%; 95%CI=10.6%, n=5) and none was enkephalin-immunoreactive (Supplementary Table 4).
Figure 2:
Descending motor neurons to the circular muscle, subdivided by calbindin and enkephalin immunoreactivities (n=5). DiI was applied on the circular muscle (at the origin of the axes) near the anal end of the preparation to ensure that descending projections were intact (A). 95% of all descending motor neurons lie within 18mm of the DiI application site (origin of the axes). Calbindin was present in sub-populations of both ascending (excitatory) and descending (inhibitory) motor neurons (D), but Enkephalin immunoreactivity was sparse overall and absent from descending motor neurons (C).
We were also able to characterize the immunoreactivity of 166 ascending circular muscle motor neurons in these preparations (Supplementary Table 5). Just 1 ascending motor neuron was immunoreactive for leu-enkephalin. A total of 34 ascending motor neurons were immunoreactive for calbindin (average across subjects 20.3%; 95% CI = 6.0%, n=5).
The projections of longitudinal muscle motor neurons have recently been characterized in human colon[10]. Combining this report with the studies described above, at least 95% of descending motor neurons have projections less than 18mm long. Similarly, more than 95% of ascending motor neurons have projections less than 8mm long. These cutoffs were therefore used in the remainder of the study to characterize long, non-motor (ie: interneuronal) populations with minimal contamination from motor neuron populations.
Ascending interneurons: calbindin and calretinin.
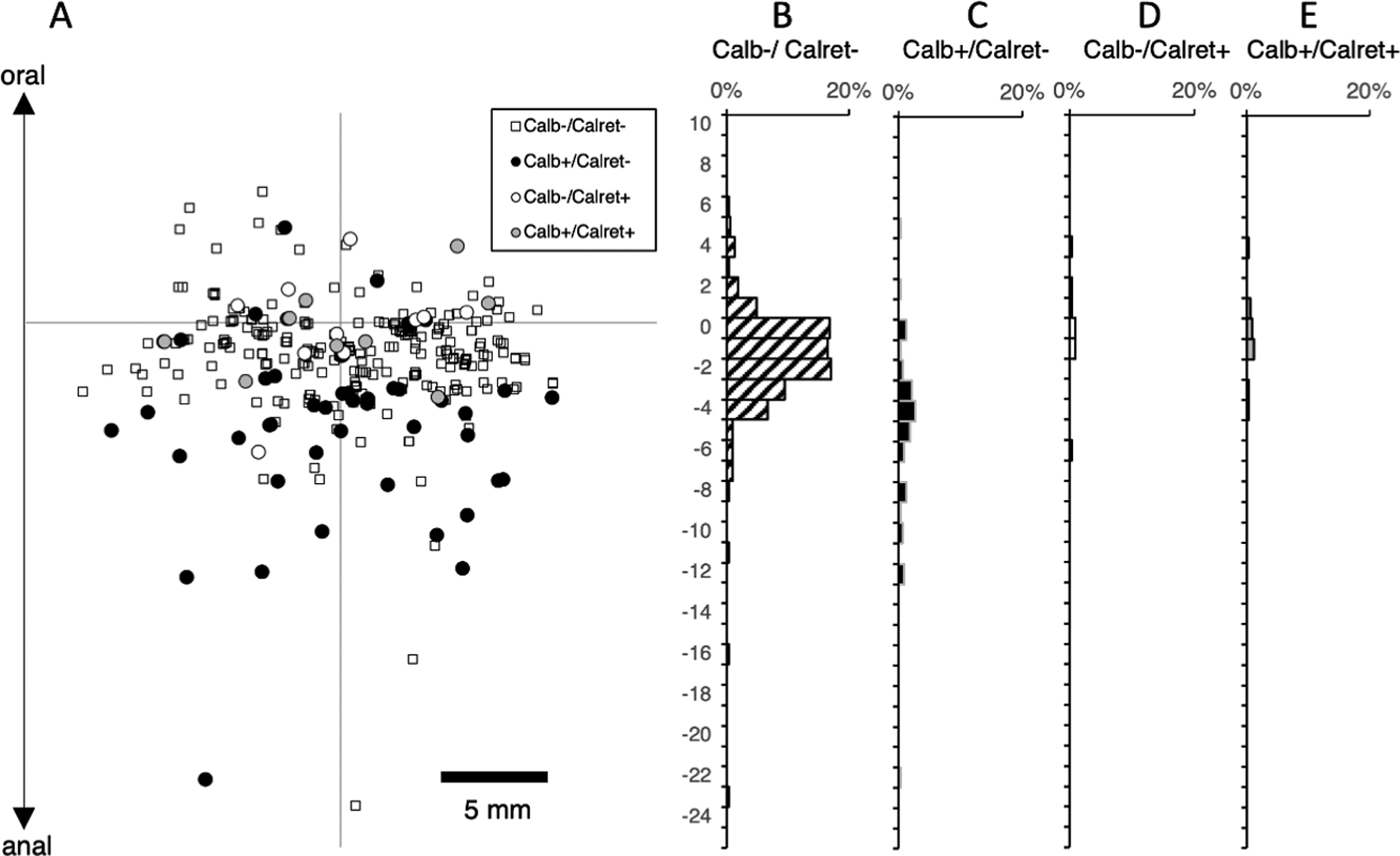
We then characterized ascending interneurons by applying DiI directly to the myenteric plexus, near the oral end of the preparation (to ensure that ascending pathways were intact). The first set of preparations was labelled with calbindin and calretinin antisera (Figure 3). A total of 962 neurons were labelled by DiI in 6 preparations (6 male patients, average age 58 years, range: 36 – 85, 2 transverse colons, 4 descending colons). Of these a total of 164 had orally-directed projections longer than 8mm and hence were likely to be ascending interneurons. Of these, just one cell was calretinin-immunoreactive (see Supplementary Table 6). A total of 54 neurons (out of 164; average across subjects 37.4%; 95%CI=17.9%, n=6)) were immunoreactive for calbindin (with or without calretinin). The remaining cells lacked both immunohistochemical markers (110 neurons: average across subjects; 62.6%; 95% CI=17.9%). Thus, calbindin is a marker for a significant subset of ascending interneurons in human colon, but calretinin is rarely expressed in long ascending interneurons. While colchicine distorts the fine details of cell morphology, the occasional DiI-filled neurons that were adequately filled appeared uni-axonal, including 22/25 calbindin-immunoreactive neurons (n=5). It is likely that collateral branches off the single parent axon supply varicose terminals in multiple myenteric ganglia that they pass through.
Figure 3:
Ascending interneurons with calbindin and calretinin immunoreactivities. The DiI application site was on the myenteric plexus at the origin of the axes (A). The region where motor neurons were abundant is shown in grey and was excluded from the analysis which concentrated on ascending interneurons located more than 8mm aboral to the application site. Immunohistochemical labelling for calbindin and calretinin was combined with DiI tracing. Calbindin immunoreactive neurons accounted for just under one third of ascending interneurons (32.9% - C). In contrast, calretinin was only present in one cell with a long (>8mm) ascending projection (E) and thus is not a marker for ascending interneurons in the human colon. Most ascending interneurons lacked both calbindin and calretinin (B – small white squares).
Ascending interneurons: substance P and Enkephalin.
In another set of 8 preparations (5 male patients; 3 transverse colons; 5 descending colon, average age; 68 years, range 56–75) a total of 1213 neurons were labelled by DiI applied to the myenteric plexus. Of these, 211 neurons had projections longer than 8mm and were thus classified as long ascending interneurons. These were tested for immunoreactivity for substance P and leu-enkephalin (Supplementary Table 7). A total of 132 neurons (out of 211, averaging across subjects 62.5%; 95% CI=18.2%, n=8) were immunoreactive for enkephalin and a subset of these (45 neurons of the 132) were SP-immunoreactive (see Figure 4). In other words, the majority of ascending interneurons were immunoreactive for ENK, and approximately one third of these were also immunoreactive for SP. Strikingly, very few long ascending neurons were SP-immunoreactive without enkephalin (just 2 cells of 211). Thus, substance P is a marker for a subset of enkephalin-immunoreactive ascending interneurons. In the area from 0–8mm aboral to the DiI application site, where ascending circular and longitudinal muscle motor neurons are prevalent, SP immunoreactive neurons without enkephalin were abundant (see Figure 4A), consistent with a previous study of circular muscle motor neurons[5].
Figure 4:
Ascending interneurons labelled with Substance P (SP) and leu-enkephalin (ENK) immunoreactivity. Filled neurons located more than 8mm aboral to the DiI application site on the myenteric plexus (at the origin of axes - A) were considered ascending interneurons (below the greyed area - A). 211 neurons with long ascending projections (>8mm) were labelled: of these 132 (62.6%) were immunoreactive for ENK (C&E) and about one third of the ENK-immunoreactive neurons were also immunoreactive for SP (E). Thus, SP is largely confined to a subset of the ENK-immunoreactive ascending interneurons. Within 8mm of the DiI application site, where motor neuron populations are numerous, there are many neurons with SP which lack ENK (black circles D) and neurons that lacked both ENK and SP (B – small white squares). The micrographs in F show a typical DiI-filled ascending interneuron immunoreactive for both SP and ENK located 14.8mm aboral to the DiI application site.
Ascending interneurons: calbindin and enkephalin.
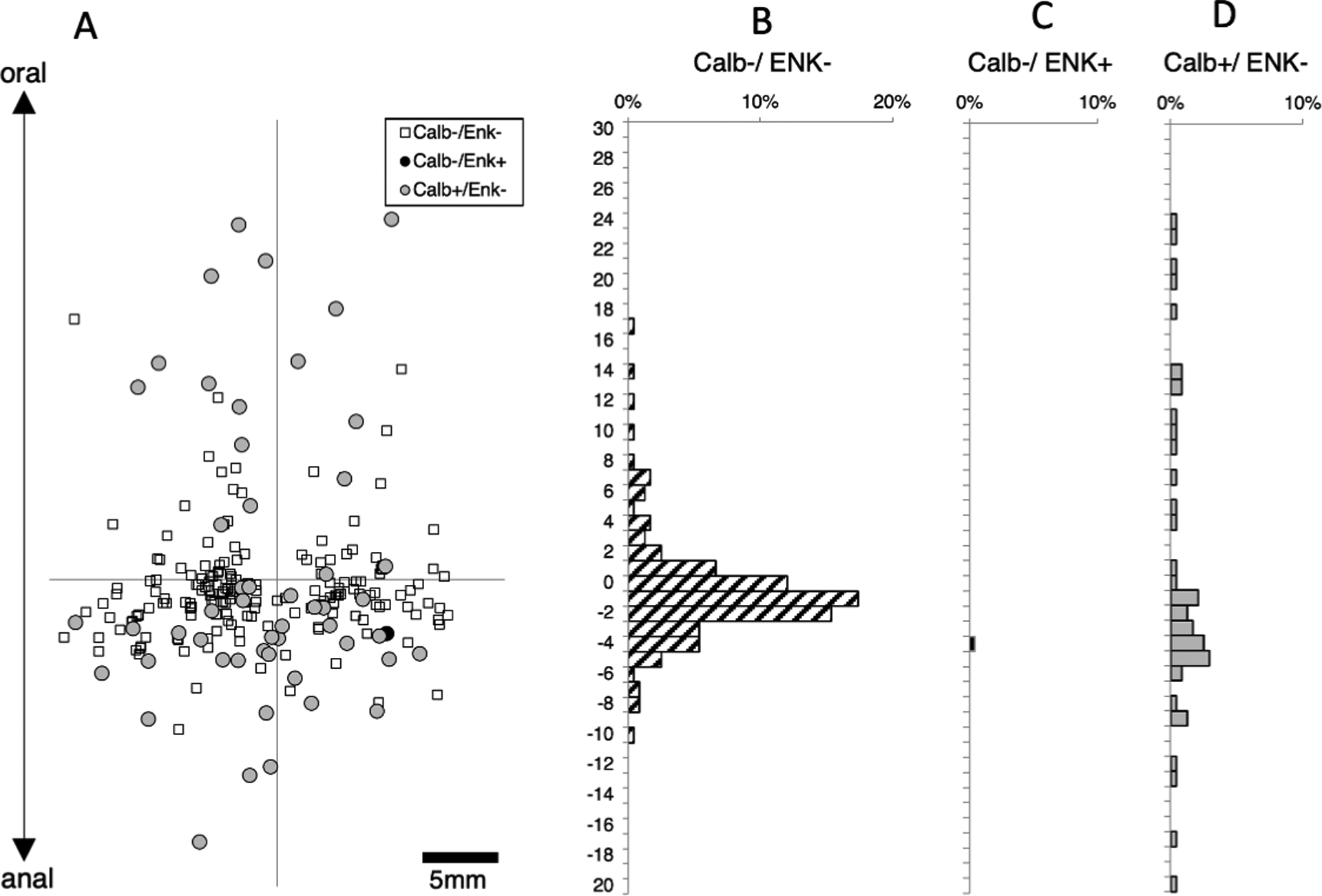
Data from figures 3 and 4 suggest that enkephalin and calbindin immunoreactivities may account for most ascending interneurons. To test this, another set of 6 preparations was set up (1 male; 2 ascending colons, 1 transverse colon, 3 descending colons, average age 70 years, range: 53 – 83). DiI was applied to the myenteric plexus near the oral end of the preparation. A total of 903 neurons were labelled in the 6 preparations and 371 of these were located more than 8mm anal to the DiI application site (see Figure 5). Of these 371 putative long ascending interneurons, just 33 lacked both enkephalin and calbindin immunoreactivities (averaging across subjects 10.6%; 95%CI=4.8%, n=6). Enkephalin immunoreactivity was present in 246 of 371 cells (averaging across subjects 71.5% of cells; 95%CI=9.5%, n=6). Therefore, enkephalin is major marker of ascending interneurons (with or without calbindin) – the remainder of ascending interneurons mostly have calbindin immunoreactivity without enkephalin. Calbindin (with or without enkephalin) was present in 32.9% of cells, averaging across subjects; 95%CI=11.8%, n=6) of long ascending neurons (see Supplementary Table 8).
Figure 5:
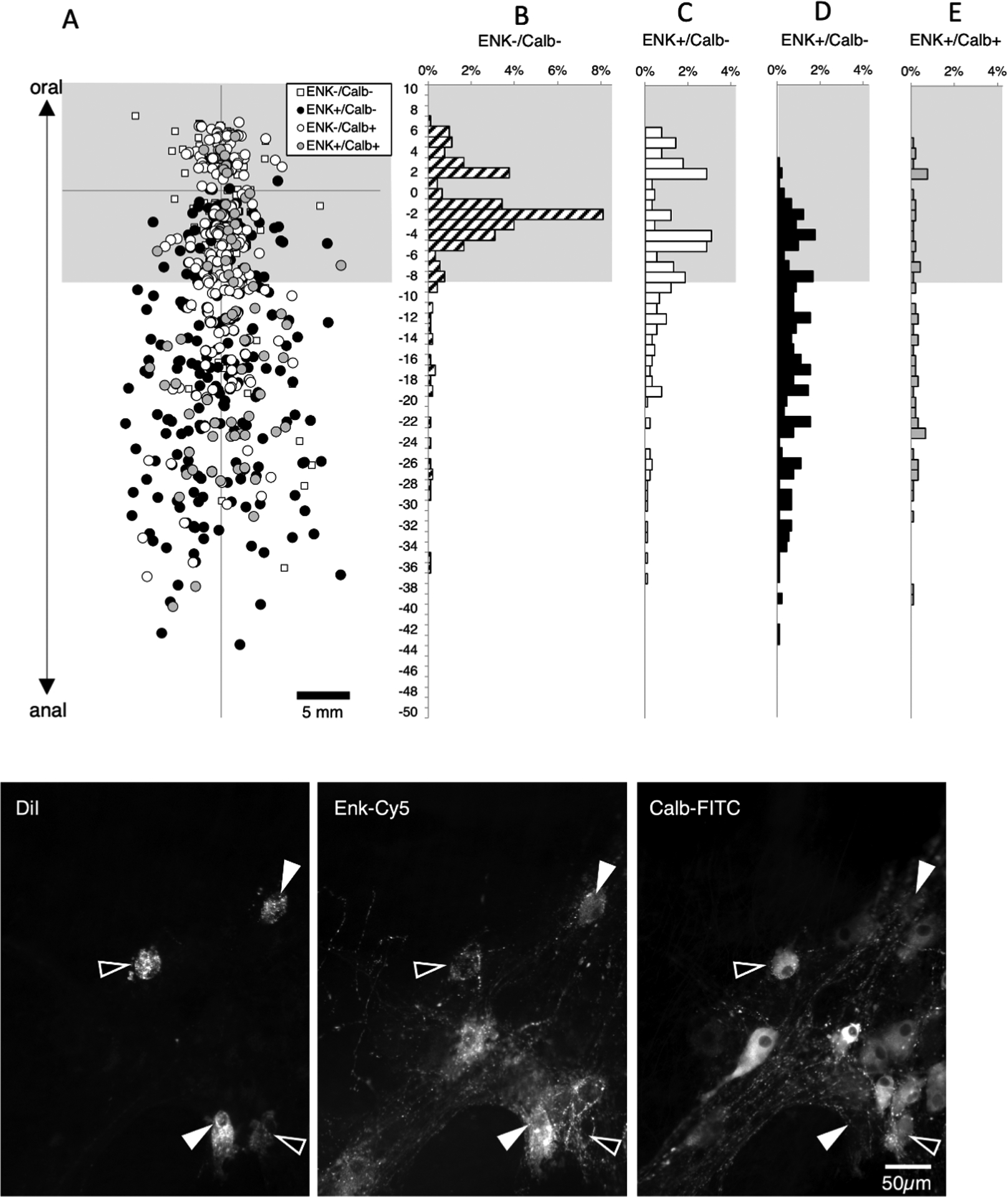
Ascending interneurons with enkephalin and/or calbindin immunoreactitivies. DiI was applied to the myenteric plexus at the origin of the axes and each symbol (A) represents one DiI-labelled nerve cell body pooled from 6 specimens. Cells within 8mm of the DiI application site (greyed area) were not analysed as these contain a mixture of populations. Histograms (B-E) show the distribution of neurons with immunoreactivity for enkephalin and/or calbindin. Note that in the area occupied by ascending interneurons (below the greyed area), large numbers of neurons were immunoreactive for enkephalin, with or without calbindin (Figure 5D&E); a few cells were immunoreactive for calbindin without enkephalin (Figure 5C and relatively few cells (33 cells of 371; 8.9% of ascending interneurons) lacked both of these markers (Figure 5B – small white squares in A). Micrographs in F show three DiI-filled neurons, located 11.7mm aboral to the DiI application site; two were immunoreactive for calbindin but not ENK (open arrowheads) and two that were immunoreactive for ENK without calbindin (solid arrowheads).
Descending interneurons: calbindin and enkephalin
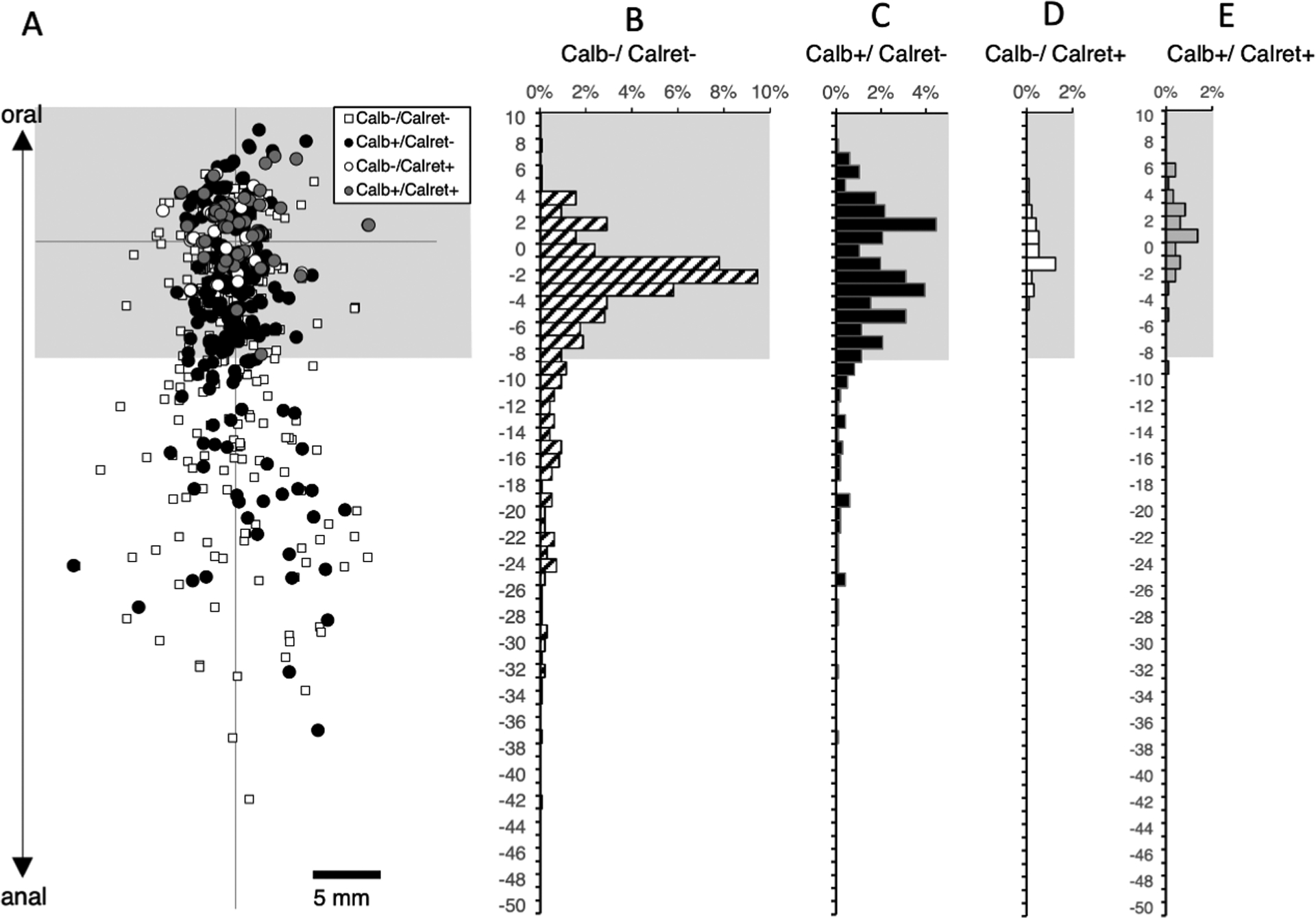
We then tested whether enkephalin was present in long descending interneurons. DiI was applied to the myenteric plexus near the anal border of the tissue, ensuring the myenteric neurons with long descending projections were preserved. We restricted analysis to neurons with aboral projections in the myenteric plexus more than 18mm long, to limit contamination by motor neurons. A total of 1992 neurons were DiI-filled in 6 preparations (4 male, 6 descending colons, average age 59, range 42 – 86). Of these, 450 neurons were located more than 18mm oral to the application site (Figure 6). Projections up to 60mm long were identified. Among this interneuron-enriched population, the majority were immunoreactive for calbindin (344/450 cells, averaging across subjects 74.3%; 95% CI=8.3%) Figure 6). A total of 23 cells (among the 450 long descending interneurons) were immunoreactive for enkephalin (5.1%). This is a striking contrast to ascending interneuronal populations where enkephalin immunoreactivity was detected in a large majority (>70%) of neurons and calbindin was found in a minority (see Figure 5). A total of 105 cells (averaging 25.6% across all patients, 95% CI=8.2%) lacked both calbindin and enkephalin immunoreactivities amongst the 450 DiI-labelled long descending interneurons (Supplementary Table 9).
Figure 6:
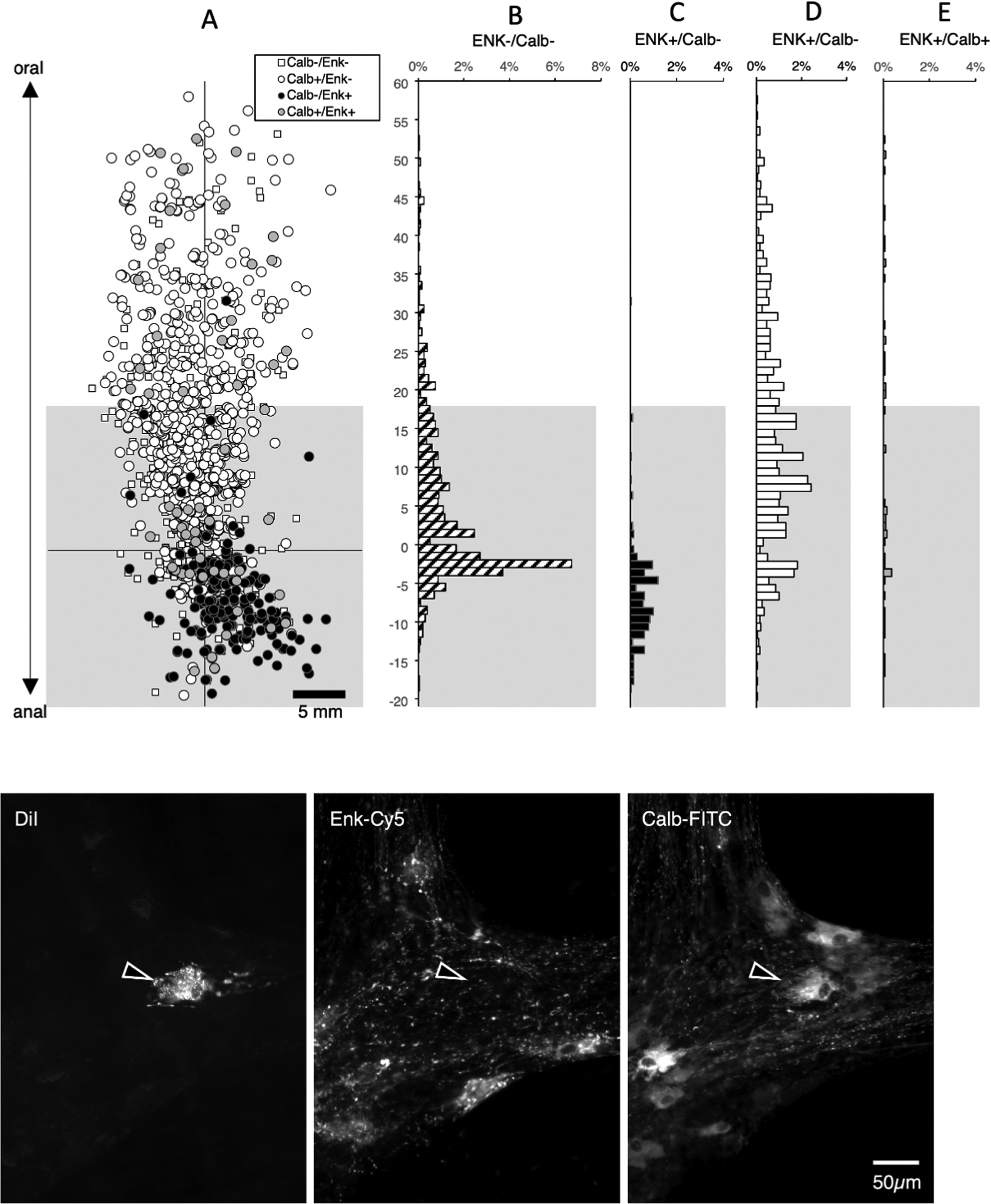
Descending interneurons with enkephalin and/or calbindin immunoreactitivities. DiI was applied to the myenteric plexus near the anal end of specimens (n=6). A total of 1992 neurons were DiI-filled; of these 422 were located more than 18mm oral to the application site (above the greyed-out area) and hence were likely to be descending interneurons (A). The majority of these interneurons was immunoreactive for calbindin (326 cells or 77.3% - D). A total of 22 cells among the 422 interneurons were immunoreactive for enkephalin (5.2% - C+E) in sharp contrast to ascending interneuronal populations where enkephalin immunoreactivity was detected in the majority of neurons and calbindin was found in a minority (see Figure 5). A further 95 cells (22.5% of all interneurons) lacked both calbindin and enkephalin immunoreactivities (B- small white squares in A). Micrographs in F show a single DiI-filled cell, located 56.2mm oral to the DiI application site that was immunoreactive for calbindin but not ENK.
Descending interneurons: 5-HT and nitric oxide synthase (NOS)
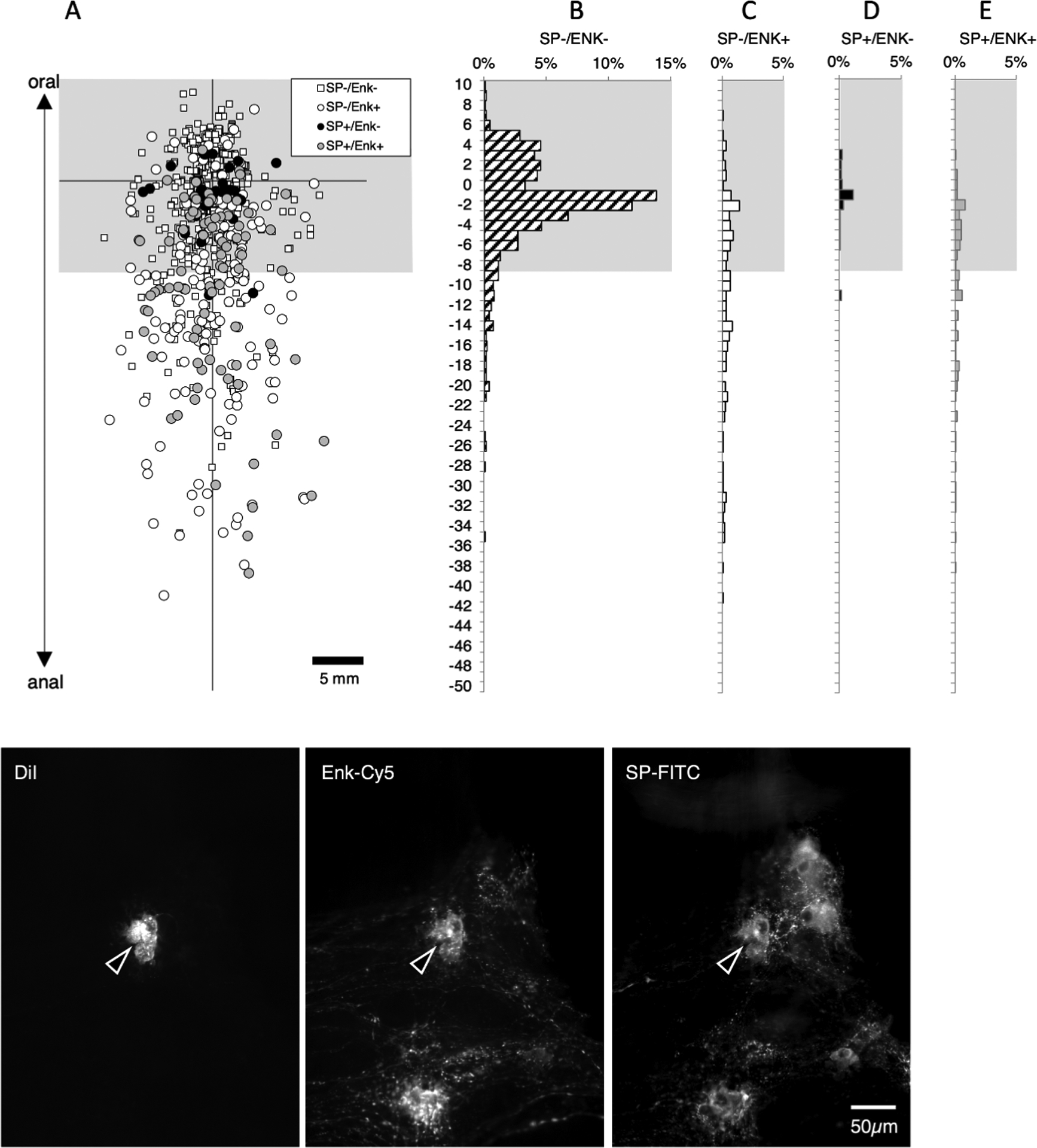
In another set of 5 preparations with DiI placed to label long descending pathways (2 male, 2 ascending colons; 3 descending colons, average age 72, range 60–90), we tested other markers likely to be present in descending interneurons: namely 5-HT and NOS (see supplementary table 10). In these studies, 3 closely-spaced DiI-coated beads were applied to the myenteric plexus to maximise filling of sparse 5-HT immunoreactive neurons; a total of 3111 neurons were DiI-labelled (n=5). Of these, 1117 were located more than 18mm oral to the application site (see Figure 7). NOS immunoreactivity was present in 675 neurons without 5-HT (averaging across subjects 59.5%; 95% CI=2.0%, n=5) and another 52 cells had both NOS and 5HT-immunoreactivities (average across all 5 patients 5.0%, 95% CI=1.4%). Thus, NOS was overall a marker for a total of 65.1% of neurons in this enriched interneuronal population. 5-HT without NOS was detected in 40 long descending neurons (3.6%) and the remainder of the 1117 interneurons (350 neurons) lacked both NOS and 5-HT (averaging across preparations: 32.3% 95%CI=1.8%, n=5). Thus, 5-HT immunoreactivity was detected in a total of 8.2% of the long descending interneuron population and NOS immunoreactivity in 65.1% of all long descending interneurons.One feature of 5-HT neurons was very striking (see Figure 7); 5-HT-immunoreactive neurons showed a very precise polarity, none of them displayed any orally-directed polarity.
Figure 7:
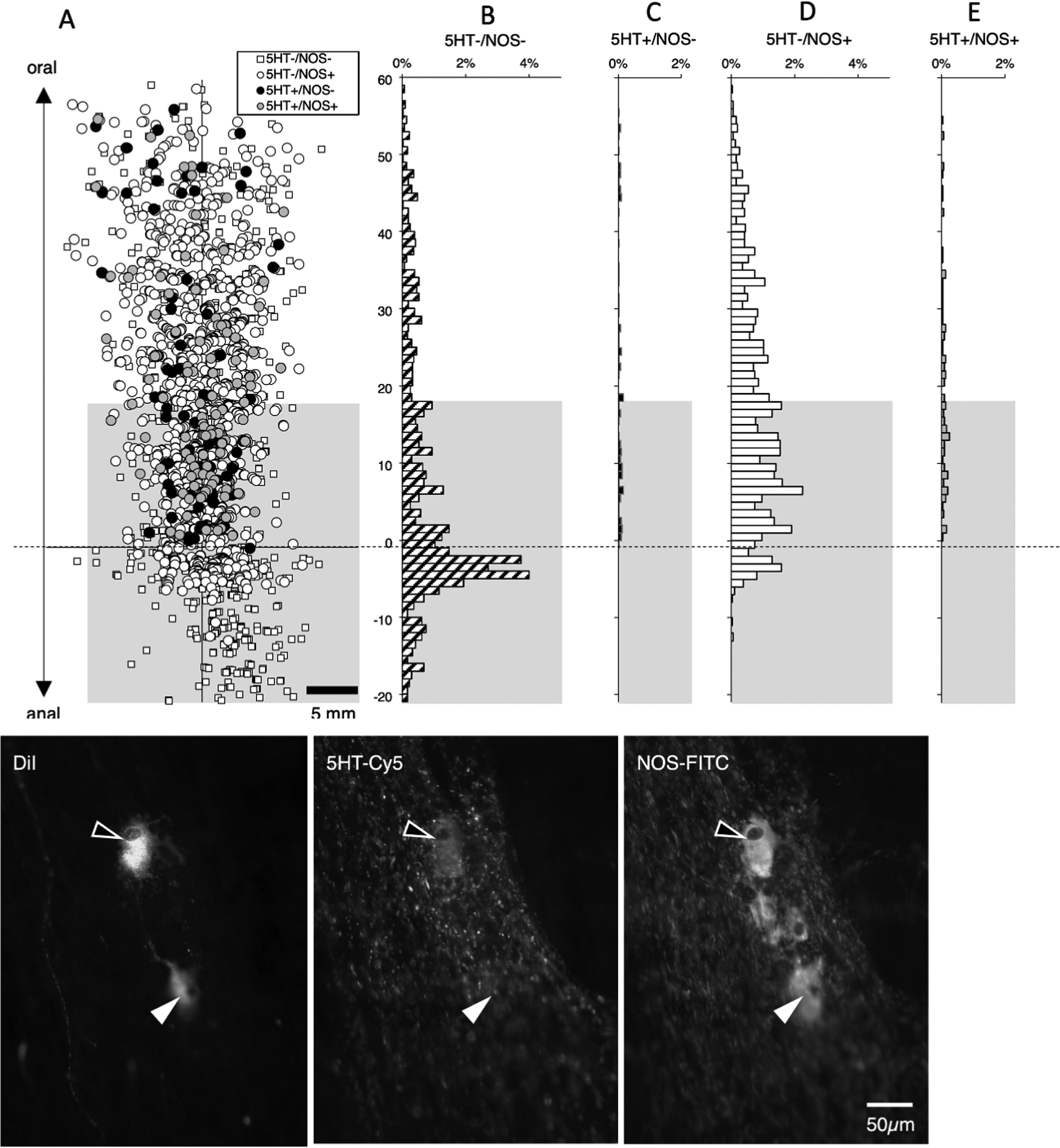
Descending interneurons with 5-HT and/or NOS immunoreactitivies. DiI was applied to the myenteric plexus near the anal end of specimens (n=5). A total of 3111 neurons were DiI-filled; of these 1117 were located more than 18mm oral to the application site (above the greyed-out area - A) and hence were likely to be descending interneurons. Of the interneurons, 675 were immunoreactive for NOS without 5HT (60.4% - D) and another 52 cells were both NOS and 5HT-immunoreactive (4.7% - E). There were 40 interneurons with 5HT immunoreactivity without NOS (3.6% - C) and the remainder of the 1117 interneurons (350 neurons; 31.4%) lacked both NOS and 5HT (B – corresponding to small white squares in A). The results indicate that a majority of descending interneurons are immunoreactive for NOS (61.5%) and that 5-HT interneurons account for 8.3% of all descending interneurons. It is very clear that none of the 5-HT immunoreactive neurons had an orally-directed projection (see C and E) whereas some NOS-immunoreactive neurons did project orally for short distances (see D). Micrographs in F show two DiI-filled cells (29.3mm oral to the DiI application site) both of which were immunoreactive for NOS, while one (open arrowhead) was also immunoreactive for 5-HT.
Descending interneurons: calretinin and nitric oxide synthase (NOS)
We labelled another set of 6 preparations with DiI applied to the myenteric plexus near the aboral end of the preparations, combined with calretinin and NOS immunoreactivity (2 male, 2 ascending colons; 4 descending colons, average age 64.5, range 38–83). A total of 1288 neurons were DiI-labelled (n=6); of these, 329 were located more than 18mm oral to the DiI application site (see Supplementary Table 11). Of this descending interneuron-enriched pool, the majority of these cells were immunoreactive for NOS without calretinin (205 cells; average across subjects 60.3%; 95%CI=10.1%) and there was a small population of NOS with calretinin (12 cells; (averaging across subjects 3.7%; 95%CI=3.5%, n=6). Thus, NOS (with or without calretinin) was calculated across subjects to be present overall in 66.0% of long descending neurons (compared to 65.1% in the previous series of experiments – see Figure 7). Calretinin without NOS was present in 78 of 329 cells, (average across subjects 25.6%; 95% CI=11.5%, n=6). The precise polarity of calretinin immunoreactive neurons is shown in Figure 8 – almost no calretinin-immunoreactive neurons have ascending projections. The remaining 31 neurons (of 329 averaging across subjects 10.4%; 95% CI=3.4%) lacked both NOS and calretinin. Thus, NOS and calretinin are present in close to 90% of long descending interneurons in the human colon.
Figure 8:
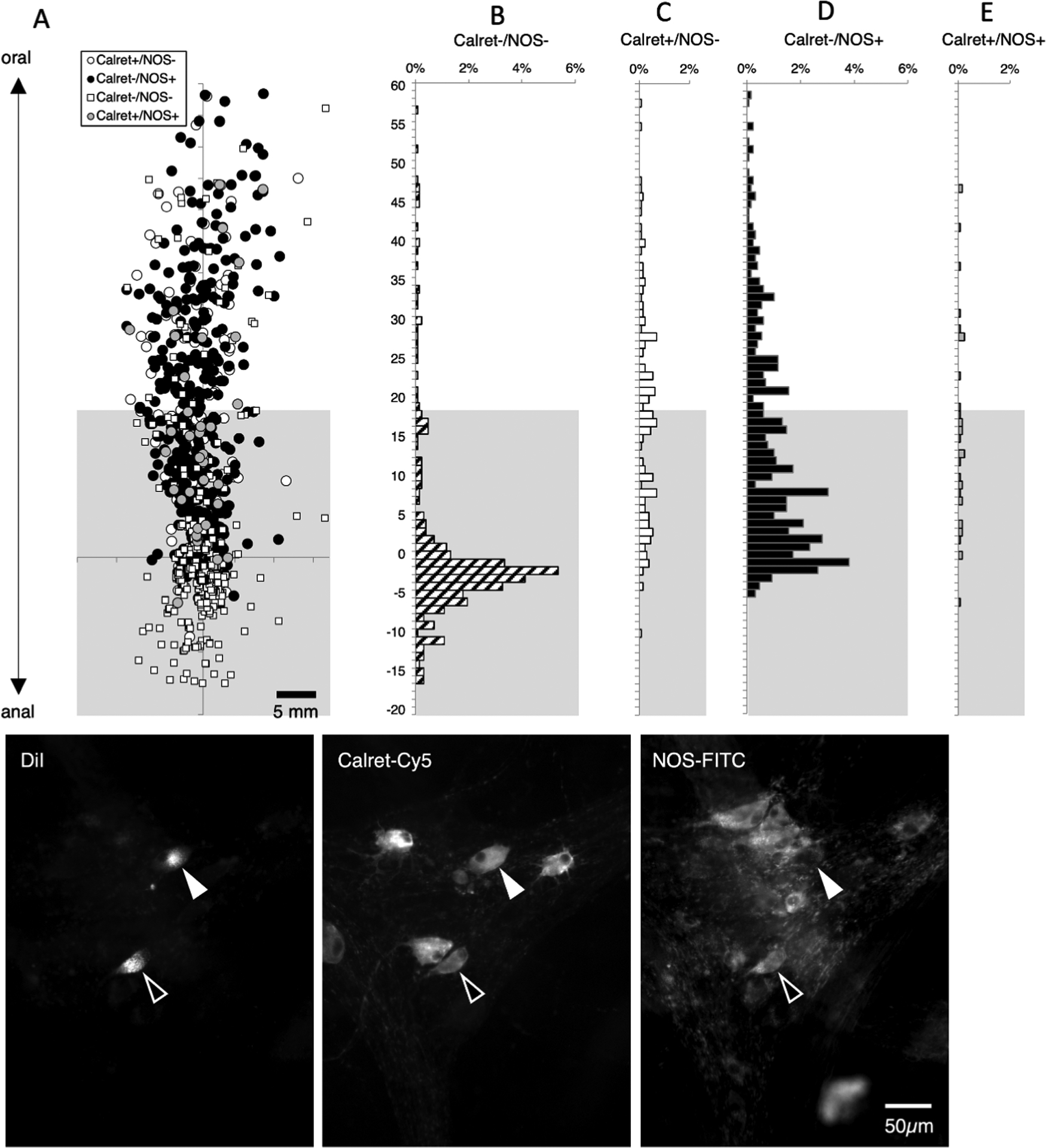
Descending interneurons – immunoreactivity for calretinin and NOS. DiI was applied to the myenteric plexus, towards the anal end of the preparations and DiI-filled neurons were mapped with their immunohistochemical coding. The greyed area marks where a mixture of motor neurons and interneurons are located (based on lengths of projections); above this is the area more than 18mm oral to the DiI application site where descending interneurons predominate (A). A total of 1288 neurons were labelled with DiI; of these, 329 were located more than 18mm oral to the DiI application site. As expected, the majority of these cells were immunoreactive for NOS without Calretinin (205 of 329, 62.3% - D) or NOS with calretinin (12 cells; 3.6% E). A number of descending interneurons were immunoreactive for calretinin without NOS (78 of 329 cells, 23.7%. C). The remaining 10.3% of interneurons lacked both calretinin and NOS immunoreactivities (B – corresponding to small white squares in A). Micrographs in F show 2 DiI-filled nerve cell bodies located 28.0mm oral to the DiI application site, both of which were immunoreactive for calretinin and one also co-localised NOS immunoreactivity (open arrow).
We then re-examined the small proportion (~10%) of DiI-filled descending interneurons that were immunoreactive for neither NOS nor Calretinin. A total of 31 NOS-/Calret- cells in 5 preparations were identified and their coordinates recorded. The preparations were then re-stained with calbindin antisera, followed by Cy5-coupled secondary antisera. Of these 31 neurons, 24 were immunoreactive for calbindin (77.4%); the remaining 7 lacked NOS, calretinin and calbindin immunoreactivities. This suggests that the majority of descending interneurons that lack both NOS and calretinin are immunoreactive for calbindin. Furthermore these 3 markers account for over 95% of all long descending interneurons.
DISCUSSION
Enteric motor neurons
Motor neurons were not the primary focus of this project, but initial studies were essential to establish their projections and immunoreactivity for markers later used to define long descending and ascending interneuronal pathways. Circular muscle motor neurons in human colon have previously been reported to have relatively short projections – up to 12mm for ascending (mostly excitatory neurons) and 20mm for descending neurones (mostly inhibitory motor neurons)[4, 5]; these findings were confirmed here. Longitudinal muscle motor neurons in human colon have even shorter projections [10]. For this reason, we used cut-offs of 18mm on the oral side and 8mm aborally, which exclude at least 95% of motor neurons when DiI was applied to the myenteric plexus. Our results on motor neuron populations confirmed that calretinin is expressed in very few circular muscle motor neurons[5] whereas another calcium binding protein, calbindin, was detected in 15–20% of both ascending and descending motor neurons in the present study. Enkephalin immunoreactivity was also shown to be sparse in circular muscle motor neurons.
Long ascending pathways
When DiI was applied to the myenteric plexus of human colon, it labelled cell bodies up to 43mm aborally (ie: with orally-directed axons). This is slightly longer than the 30 – 33mm previously reported[4, 5, 8] and may reflect the full extent of orally-directed pathways. In a previous study it was reported that more than 90% of long ascending neurons in human colonic myenteric plexus were cholinergic[8]. Here, we have shown that nearly all (>90%) of these cholinergic long ascending interneurons are immunoreactive for enkephalin and/or calbindin. Within the enkephalin immunoreactive population (making up 62.5 – 71.5% of all long ascending neurons), about one third are also immunoreactive for tachykinins. This combination of markers was also reported in ascending interneurons in the guinea pig small intestine.[11] Our results also showed that enkephalin immunoreactivity is sparse in long descending interneurons (<5%), this means that a high proportion of enkephalin-immunoreactive varicose axons in enteric ganglia are likely to arise from ascending interneurons. This may have functional significance, since there are many dense “baskets” of enkephalin-immunoreactive varicosities in colonic myenteric ganglia. These are likely to reflect strong synaptic connections of ascending interneurons onto unknown target neurons. Whether there are any additional populations of local enkephalin-containing interneurons with short projections (<8mm long) is currently unknown. It has previously been suggested that a proportion of enkephalin-immunoreactive myenteric neurons in human colon may project orally and function as either ascending interneurons or excitatory motor neurons. Furthermore, a subset of these cells are also immunoreactive for tachykinins[12, 13]. Coexistence of excitatory transmitters is common in the enteric nervous system, with tachykinins abundant in cholinergic neurons[1, 14–17]. Many of these neurons are also immunoreactive for enkephalin[14, 17, 18]. Enkephalin causes inhibition of enteric nerve fibres[19] and presynaptic inhibition of synaptic transmission[20] and acts as a brake on excitatory transmission during repetitive firing[21]. Endogenous opioids, including enkephalins, may modestly modulate ascending excitatory motor reflexes[22] and peristaltic motor patterns in small intestine[23, 24]. The presence of enkephalin-immunoreactivity in ascending interneuronal pathways is consistent with these roles.
The other major marker in long ascending neurons was calbindin; this marker was present in 32.9 – 37.4% of these neurons. A previous study concluded that calbindin is present in 25.2% of all myenteric neurons in human colon and that it coexists, in a minority of neurons, with calretinin, VIP or somatostatin. The study concluded that calbindin is expressed by a number of different types of human colonic myenteric neurons and, similar to this study, that it was not a marker for a specific class of neurons[25]. This is of contrast to the guinea pig ileum where calbindin immunoreactivity was first characterised in the enteric nervous system[12]. In this laboratory animal’s small intestine, calbindin is confined to a subset of Dogiel type II neurons in the myenteric plexus[12, 13]
These results point to differences in the “chemical coding” of enteric neurons between species and reinforce the importance of studying human neurons directly, when possible[1]. For example, calretinin is a marker of ascending cholinergic interneurons in the extensively studied guinea pig small intestine[11, 26], guinea pig distal colon[27, 28], guinea pig proximal colon[29] but is only present in locally-projecting neurons in mouse colon[30]. In the present study, calretinin was confined to neurons with long descending and local projections. Putting together previous reports with the present study, the data suggest that there are 4 classes of ascending interneurons (i) ChAT+/ENK+/SP+ (ii) ChAT+/ENK+ and (iii) ChAT+/ENK+/Calb+ and (iv) ChAT+/Calb+. This is comparable to the guinea pig distal colon where multiple ascending interneurons have been distinguished[27].
Long descending pathways
It has previously been reported that descending pathways in human colon are longer than ascending, that descending neurons are more numerous[4] and there are more classes/types than for ascending neurons[8]. The most abundant single marker for myenteric neurons with long descending projections was the calcium-binding protein calbindin, which was present in 74.3% of cells. Since NOS was expressed in 65.3–66.0% of descending interneurons, there must be considerable coexistence of these two markers. This was not tested directly in the present study. Within the mixed population of long descending neurons, it is likely that many neurons are also immunoreactive for vasoactive intestinal polypeptide (VIP) which co-exists with NOS in many neurons in human colon[5, 31], although this was also not specifically tested.
As well as NOS-immunoreactive neurons, an additional population with calretinin immunoreactivity, without NOS immunoreactivity, accounted for 25.6% of long descending neurons, making these two markers account for over 90% in total. Very limited overlap was detected between NOS and calretinin among descending interneurons, as reported previously[32] There is an additional population of 5-HT immunoreactive neurons which account for 8.1% (95%CI: 1.3%, n=5) of this population, however over half of them are NOS-immunoreactive. The overlap between 5-HT and calretinin has not been determined.
This raises the question of which descending interneurons are cholinergic. It has previously been reported that choline acetyltransferase (ChAT) immunohistochemical labelling is present in nearly half (49%) of descending interneurons in human colon and that over half of these cells were also immunoreactive for NOS[8]. Since descending motor neurons (which are mostly inhibitory motor neurons) are rarely immunoreactive for ChAT[3], it is likely that all ChAT+/NOS+ neurons are descending interneurons. A population of ChAT+/NOS+ cells has been reported by several groups studying human colon with multiple labelling immunohistochemistry; estimates range from 3–10% of all myenteric neurons[33–35]. Some of these are likely to correspond to the NOS-immunoreactive, 5-HT containing cells, since all 5-HT neurons in small intestine[18] and large intestine of guinea pig colon[27] are ChAT immunoreactive and 5-HT cells in these species all have long descending projections[36].
Putting this data together, the populations of long descending interneurons in the myenteric plexus of human large intestine make up at least 4 classes. There are (i) ChAT+/NOS+ cells (as previously defined) (ii) ChAT+/Calret+ (iii) ChAT+/5HT+ cells with or without NOS and (iv)NOS+ cells (without ChAT). Calbindin is also present in 74.3% of descending interneurons, including many with NOS, but the exact combinations cannot be ascertained from the studies presented here.
Lengths of projections and functional significance
The present study showed that long descending projections run for up to 70 mm down the colon, whereas long ascending neurons project for up to 43 mm present study. These lengths are unlikely to be due to limitations of the technique. Using identical techniques with DiI filling ex vivo in the guinea pig small intestine, descending projections up to 110mm have been reported, whereas ascending projections run for just 13mm[37]. Since human colonic neurons filled from the myenteric plexus can project further than the longest motor neurons, they must project to a target other than the muscularis externa. Most probably, the long descending and ascending myenteric neurons act as interneurons in polysynaptic polarised reflex pathways involved in propulsion and perhaps other functions. Some are likely to be involved in coordination of periodic motor complexes elicited by distension[7, 38, 39]; The varicose axons branching in myenteric ganglia that are immunoreactive for NOS, ChAT, calretinin, calbindin, enkephalin, substance P and 5-hydroxytryptamine may belong to these interneurons. However, it is possible that some of these neurons play other roles, since there have been several reports in laboratory animals of mechanosensitivity in unipolar enteric neurons with polarised projections[40, 41]. Lastly, it should be noted that the methods in this study would not identify classes of myenteric interneurons with shorter projections than motor neurons. This may well explain the paucity of Dogiel type II neurons, most of which have local projections, at least in the guinea pig[42, 43].
The results of the present study, in combination with previous tracing studies, provide a systematic description of several major classes of enteric neurons in the human colon.
Supplementary Material
ACKNOWLEDGMENTS, FUNDING AND DISCLOSURES
This work was supported by NIH grant #1OT2OD24899-01 (NIH SPARC consortium grant) to Tache Y (UCLA) “Comprehensive Structural and Functional Mapping of Mammalian Colonic Nervous System”. We would like to thank Andy Roberts of SA Biomedical Engineering Department of Flinders Medical Centre for developing the software for the stage measurement system. We are extremely grateful to the patients who supported this study by giving consent for the use of their tissues for research; without their generosity, this work could not have been carried out.
Footnotes
Competing Interests
The authors have no competing interests
REFERENCES
- 1.Brookes SJH and Costa M, Cellular organisation of the mammalian enteric nervous system, in Innervation of the Gastrointestinal tract, Brookes SJH and Costa M, Editors. 2002, Harwood. [Google Scholar]
- 2.Furness JB, The enteric nervous system. 2006, Oxford: Blackwell. [Google Scholar]
- 3.Porter AJ, et al. , The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology, 1997. 113(6): p. 1916–23. [DOI] [PubMed] [Google Scholar]
- 4.Wattchow DA, Brookes SJ, and Costa M, The morphology and projections of retrogradely labeled myenteric neurons in the human intestine. Gastroenterology, 1995. 109(3): p. 866–75. [DOI] [PubMed] [Google Scholar]
- 5.Wattchow DA, et al. , The polarity of neurochemically defined myenteric neurons in the human colon. Gastroenterology, 1997. 113(2): p. 497–506. [DOI] [PubMed] [Google Scholar]
- 6.Dinning PG, et al. , Quantification of in vivo colonic motor patterns in healthy humans before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterology & Motility, 2014. 26(10): p. 1443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer NJ, et al. , Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? American Journal of Physiology - Gastrointestinal & Liver Physiology, 2012. 302(1): p. G34–43. [DOI] [PubMed] [Google Scholar]
- 8.Porter AJ, et al. , Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut, 2002. 51(1): p. 70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa M, et al. , Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their visualization and reactions to drug treatment. Neuroscience, 1982. 7(2): p. 351–63. [DOI] [PubMed] [Google Scholar]
- 10.Humenick A, et al. , Characterisation of projections of longitudinal muscle motor neurons in human colon. Neurogastroenterology & Motility, 2019. 31(10): p. e13685. [DOI] [PubMed] [Google Scholar]
- 11.Brookes SJ, et al. , Orally projecting interneurones in the guinea-pig small intestine. Journal of Physiology, 1997. 505(Pt 2): p. 473–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furness JB, et al. , Immunohistochemical evidence for the presence of calcium-binding proteins in enteric neurons. Cell & Tissue Research, 1988. 252(1): p. 79–87. [DOI] [PubMed] [Google Scholar]
- 13.Iyer V, et al. , Electrophysiology of guinea-pig myenteric neurons correlated with immunoreactivity for calcium binding proteins. J Auton Nerv Syst, 1988. 22(2): p. 141–50. [DOI] [PubMed] [Google Scholar]
- 14.Costa M, et al. , Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience, 1996. 75(3): p. 949–967. [DOI] [PubMed] [Google Scholar]
- 15.Schemann M, Schaaf C, and Mader M, Neurochemical Coding Of Enteric Neurons In the Guinea Pig Stomach. Journal of Comparative Neurology, 1995. 353(2): p. 161–178. [DOI] [PubMed] [Google Scholar]
- 16.Vanden Berghe P, et al. , Neurochemical coding of myenteric neurons in the guinea-pig antrum. Cell & Tissue Research, 1999. 297(1): p. 81–90. [DOI] [PubMed] [Google Scholar]
- 17.Pfannkuche H, et al. , Enkephalin-Immunoreactive Subpopulations In the Myenteric Plexus Of the Guinea-Pig Fundus Project Primarily to the Muscle and Not to the Mucosa. Cell & Tissue Research, 1998. 294(1): p. 45–55. [DOI] [PubMed] [Google Scholar]
- 18.Steele PA, Brookes SJ, and Costa M, Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience, 1991. 45(1): p. 227–39. [DOI] [PubMed] [Google Scholar]
- 19.Morita K and North RA, Opiates and enkephalin reduce the excitability of neuronal processes. Neuroscience, 1981. 6(10): p. 1943–51. [DOI] [PubMed] [Google Scholar]
- 20.Cherubini E, Morita K, and North RA, Opioid inhibition of synaptic transmission in the guinea-pig myenteric plexus. British Journal of Pharmacology, 1985. 85(4): p. 805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konishi S, Tsunoo A, and Otsuka M, Enkephalin as a transmitter for presynaptic inhibition in sympathetic ganglia. Nature, 1981. 294(5836): p. 80–2. [DOI] [PubMed] [Google Scholar]
- 22.Allescher HD, et al. , Modulatory effect of endogenous and exogenous opioids on the excitatory reflex pathway of the rat ileum. Neuropeptides. 34(1): p. 62–8. [DOI] [PubMed] [Google Scholar]
- 23.Shahbazian A, et al. , Involvement of mu- and kappa-, but not delta-, opioid receptors in the peristaltic motor depression caused by endogenous and exogenous opioids in the guinea-pig intestine. British Journal of Pharmacology, 2002. 135(3): p. 741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterman SA, Costa M, and Tonini M, Modulation of peristalsis in the guinea-pig isolated small intestine by exogenous and endogenous opioids. British Journal of Pharmacology, 1992. 106(4): p. 1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zetzmann K, et al. , Calbindin D28k-Immunoreactivity in Human Enteric Neurons. International Journal of Molecular Sciences, 2018. 19(1): p. 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brookes SJH, Steele PA, and Costa M, Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res, 1991. 263(3): p. 471–81. [DOI] [PubMed] [Google Scholar]
- 27.Lomax AE and Furness JB, Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell & Tissue Research, 2000. 302(1): p. 59–72. [DOI] [PubMed] [Google Scholar]
- 28.Lomax AE, et al. , Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. Journal of the Autonomic Nervous System, 1999. 76(1): p. 45–61. [DOI] [PubMed] [Google Scholar]
- 29.Messenger JP, Bornstein JC, and Furness JB, Electrophysiological and Morphological Classification Of Myenteric Neurons In the Proximal Colon Of the Guinea-Pig. Neuroscience, 1994. 60(1): p. 227–244. [DOI] [PubMed] [Google Scholar]
- 30.Sang Q, Williamson S, and Young HM, Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. Journal of Anatomy, 1997. 190(Pt 2): p. 209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brehmer A, Schrodl F, and Neuhuber W, Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochemistry & Cell Biology, 2006. 125(5): p. 557–65. [DOI] [PubMed] [Google Scholar]
- 32.Beuscher N, et al. , What neurons hide behind calretinin immunoreactivity in the human gut? Histochemistry & Cell Biology, 2014. 141(4): p. 393–405. [DOI] [PubMed] [Google Scholar]
- 33.Murphy EM, et al. , Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterology & Motility, 2007. 19(2): p. 126–34. [DOI] [PubMed] [Google Scholar]
- 34.Ng KS, et al. , Quantification and neurochemical coding of the myenteric plexus in humans: No regional variation between the distal colon and rectum. Neurogastroenterology & Motility, 2018. 30(3): p. 03. [DOI] [PubMed] [Google Scholar]
- 35.Wattchow D, et al. , Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterology & Motility, 2008. 20(12): p. 1298–305. [DOI] [PubMed] [Google Scholar]
- 36.Meedeniya AC, et al. , The projections of 5-hydroxytryptamine-accumulating neurones in the myenteric plexus of the small intestine of the guinea-pig. Cell & Tissue Research, 1998. 291(3): p. 375–84. [DOI] [PubMed] [Google Scholar]
- 37.Brookes SJH, Retrograde tracing of enteric neuronal pathways. Neurogastroenterology & Motility, 2001. 13(1): p. 1–18. [DOI] [PubMed] [Google Scholar]
- 38.Costa M, et al. , Identification of multiple distinct neurogenic motor patterns that can occur simultaneously in the guinea pig distal colon. American Journal of Physiology - Gastrointestinal & Liver Physiology, 2019. 316(1): p. G32–G44. [DOI] [PubMed] [Google Scholar]
- 39.Dinning PG, et al. , Neurogenic and myogenic motor patterns of rabbit proximal, mid, and distal colon. American Journal of Physiology - Gastrointestinal & Liver Physiology, 2012. 303(1): p. G83–92. [DOI] [PubMed] [Google Scholar]
- 40.Mazzuoli G and Schemann M, Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. Journal of Physiology, 2009. 587(Pt 19): p. 4681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer NJ and Smith TK, Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. Journal of Physiology, 2004. 558(Pt 2): p. 577–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bornstein JC, et al. , Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. J Comp Neurol, 1991. 314(3): p. 437–51. [DOI] [PubMed] [Google Scholar]
- 43.Song ZM, Brookes SJ, and Costa M, Identification of myenteric neurons which project to the mucosa of the guinea-pig small intestine. Neurosci Lett, 1991. 129(2): p. 294–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


