Abstract
Objective
The aim of this study was to assess the association between air pollutant exposure and interstitial lung disease (ILD) in patients with connective tissue diseases (CTDs).
Setting
A nationwide, population-based, matched case–control study in Taiwan.
Participants
Using the 1997–2013 Taiwanese National Health Insurance Research Database, we identified patients with newly diagnosed CTD during 2001–2013, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), dermatomyositis (DMtis)/polymyositis (PM) and primary Sjögren’s syndrome (pSS).
Primary and secondary outcome measures
Patients with newly diagnosed ILD during 2012–2013 were identified as ILD cases, and selected patients with CTD without ILD matching (1:4) the CTD cases for CTD diagnosis, age, gender, disease duration and year of ILD diagnosis date were identified as non-ILD controls. Data of hourly level of air pollutants 1 year before the index date were obtained from the Taiwan Environmental Protection Agency. The association between ILD and air pollutant exposure was evaluated using logistic regression analysis shown as adjusted ORs (aORs) with 95% CIs after adjusting for potential confounders.
Results
We identified 505 newly diagnosed CTD-ILD patients, including 82 with SLE, 210 with RA, 47 with SSc, 44 with DMtis/PM and 122 with pSS. Ozone (O3) exposure (per 10 ppb) was associated with a decreased ILD risk in patients with CTD (aOR, 0.51; 95% CI, 0.33 to 0.79) after adjusting for potential confounders.
Conclusions
A previously unrecognised inverse correlation was found between O3 exposure and ILD in patients with RA and SSc. Further studies are warranted to explore the underlying mechanisms.
Keywords: epidemiology, rheumatology, thoracic medicine
Strengths and limitations of this study.
This population-based study was conducted on a relatively large sample size, which can be generalised to the national level.
The study used two nationwide databases to address the association between exposure to air pollutants and the development of interstitial lung disease in patients with connective tissue disease.
The selection bias of the present population-based study using claim data is minimal.
Given the nature of the secondary data, the analysis misses some crucial variables, such as disease activity and laboratory data.
Background
Interstitial lung disease (ILD) is characterised by progressive inflammation and fibrosis, and accumulating evidence has demonstrated an association between exposure to air pollutants and the development and disease course of ILD.1 2 Autoimmune rheumatic diseases show a strong correlated with ILD, including connective tissue disease-associated ILD (CTD-ILD) as well as interstitial pneumonia with autoimmune features; furthermore, the development of CTD-ILD has been reported to be an important cause of morbidity and mortality in patients with CTD.3 4 A recent Taiwanese study reported that exposure to air pollutants, primarily nitrogen dioxide (NO2), was associated with incident systemic lupus erythematosus (SLE).5 Given that exposure to air pollutants has been implicated in ILD and CTD, there is a need to investigate the association between exposure to air pollutants and CTD-ILD.
Among the distinct air pollutants, ozone (O3) is generated through chemical reactions, the so-called quenching reaction, among pollutants, primary oxides of nitrogen, in the presence of sunlight.6 Exposure to O3 was implicated with an increased Th2 response through enhancing the type 2 innate lymphoid cell (ILC)-associated pathway in airway cells, and Th1 response appears to be dominant in ILD.7 8 Intriguingly, previous studies have reported a positive association between exposure to O3 and the risk of acute exacerbation as well as poor pulmonary function in patients with idiopathic pulmonary fibrosis (IPF).9 10 However, few studies have shown that O3 exposure might have an inverse correlation with incident ILDs, and the quenching effect as well as dysregulated T cell response by O3 might possibly underlie this intriguing correlation.2 11 12 The aforementioned evidence indicates the complex association between exposure to air pollutants, particularly O3, and ILD. Moreover, evidence of the impacts of exposure to air pollutants on incident ILD in patients with CTD is still lacking. Therefore, there is a crucial need to address the impact of exposure to air pollutants on the development of ILD among patients with CTD. The Taiwanese National Health Insurance Research Database (NHIRD) has facilitated population-based epidemiological studies. Therefore, in the present study, we aimed to conduct a population-based case–control study to explore the association between ILD development and exposure to air pollutants in patients with CTDs, including SLE, rheumatoid arthritis (RA), systemic sclerosis (SSc), dermatomyositis (DMtis)/polymyositis (PM) and primary Sjögren’s syndrome (pSS), using the NHIRD.
Methods
Informed consent was waived as all the data used for analyses were deidentified.
Study design
This research was a nationwide, population-based, matched case–control study.
Data source
Taiwan had launched a single-payer, compulsory National Health Insurance programme in 1995, with nationwide coverage of up to 99.6% of Taiwan’s population in 2015.13 The NHIRD contains all-inclusive claims data regarding the information on registration, demographic characteristics, residence, medication prescription, diagnosis, examinations, procedures, surgeries, medical expenditure, outpatient services, inpatient services and medication prescription. The NHIRD also registered all patients with major illnesses such as CTDs and malignancies in the catastrophic illness registry in case the catastrophic illness-related diagnoses were validated by two independent specialists through a detailed review of patients’ original medical records. A catastrophic illness certificate is then issued to these patients, who are then exempt from expenses for medical services. In the present study, we used multiple files, including registration file, ambulatory file, inpatient file and catastrophic illness registry file, in the NHIRD from 1997 to 2013. The accuracy of the claims data from the NHIRD has been improved by regularly auditing the original medical records. The NHIRD was managed by the National Health Research Institute and was released for research purpose after the encryption of personal information.
Identification of patients with CTD from the entire population in Taiwan
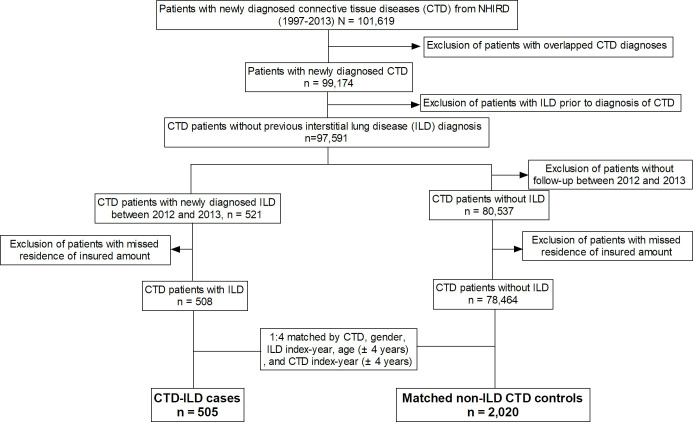
We used the 1997–2013 NHIRD to identify patients with CTDs who were registered in the catastrophic illness registry between 2001 and 2013 for newly diagnosed CTDs, including SLE (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 710.0), RA (ICD-9-CM codes 714.0 and 714.30–714.33), SSc (ICD-9-CM code 710.1), DMtis (ICD-9-CM code 710.3), PM (ICD-9-CM code 710.4) or pSS (ICD-9-CM code, 710.2). The date of CTD diagnosis was defined as the date of initial application for a catastrophic illness certificate for the corresponding CTD. From patients with CTD, we included those who did not have overlapping CTD diagnoses and those who did not have any ambulatory or inpatient visit with a diagnosis of ILD (ICD-9 code 515 and 516.36) or idiopathic interstitial pneumonia before the time of CTD diagnosis as the CTD cohort (figure 1).
Figure 1.
Flow chart of subject enrolment. NHIRD, National Health Insurance Research Database.
Identification of ILD cases from the CTD cohort
We identified those who had a new diagnosis of ILD (ICD-9 code 515 and 516.36) after the CTD diagnosis date during 2012–2013 as ILD cases. The index date was defined as the date of first ambulatory or inpatient visit with a diagnosis of ILD.
Selection of matched non-ILD controls from the CTD cohort
From the CTD cohort, we randomly selected those who never had a diagnosis of ILD matching (1:4) the ILD cases for diagnosis of CTDs, sex, age (±4 years), disease duration (±4 years) and the year of index date as non-ILD controls.
Measurement of exposure to air pollutants
The hourly level of air pollutants across from 60 air quality monitoring stations were used to calculate the mean level of exposed air pollutants, including particulate matter <2.5 µm in size (PM2.5), particulate matter <10 µm in size (PM10), NO2, carbon monoxide, sulphur dioxide (SO2) and O3, 1 year prior to the index date.14 The ambient air pollutant concentrations at each residential location were estimated using a spatio-temporal model built via a deep-learning approach.15 In brief, we used graph convolutional neural network to estimate the level of air pollutants at each residential locations, and the ambient level of air pollutants at 374 residential locations across Taiwan was estimated based on the data of three air quality monitoring stations near the location.
Potential confounders
The factors that may affect the association between exposure to air pollutants and incident ILD were taken into account as the confounder in the regression to estimate the impact of air pollutant on incident ILD in patients with CTD. Potential confounders that were adjusted for in the multivariable logistic regression model included age, gender, disease duration, Charlson Comorbidity Index (CCI) without the chronic pulmonary disease, diagnosis with chronic obstructive pulmonary disease (COPD)/asthma, use of biological disease-modifying antirheumatic drugs (bDMARDs), use of conventional synthetic DMARDs (csDMARDs), use of immunosuppressants, glucocorticoid (GC) dose (average daily prednisolone equivalent dose), urbanisation level of the patient’s residence and the level of payroll-related insured amount. The presence of comorbidity was defined as the presence of one or more inpatient visits or at least three ambulatory visits with a corresponding ICD-9-CM code within 1 year before the index date. The CCI revised by Deyo et al was applied to analyse the general comorbid medical condition.16 In Taiwan, the available bDMARDs before 31 December 2013 were anti-tumour necrosis factor (anti-TNF, including etanercept, adalimumab and golimumab), tocilizumab and rituximab. The csDMARDs included hydroxychloroquine (HCQ), sulfasalazine, methotrexate (MTX) and leflunomide (LEF). Immunosuppressants included cyclophosphamide (CP), ciclosporin (CSA), azathioprine (AZA) and mycophenolate mofetil/mycophenolic acid. Given that socioeconomic status might confound the association between air pollutant exposure and pulmonary diseases, we measured the socioeconomic status of each participant based on the urbanisation-level and payroll-related insured amount.17 The urbanisation level of the patient’s residence was categorised into four clusters based on population density (people/km2), population ratio of elderly subjects aged >65 years, population ratio of subjects with educational levels of college or above, population ratio of agricultural workers and the number of physicians/100 000 subjects.18 Payroll-related insured amount was transformed into categorical variable with four levels based on quantiles.
Statistical analyses
Data are represented as the number of patients (%) for categorical variables and either mean±SD for continuous variables. Categorical variables were compared using the χ2 test or the Fisher’s exact test, and continuous variables were compared using the t-test. Variables were considered as candidates for inclusion in the multivariable model if the associated univariate p value was lower than 0.20.19
The association between the risk of ILD development and the exposure to air pollutants was examined using a multivariable conditional logistic regression analysis after adjusting for age, gender, CCI, urbanisation level, level of payroll-related insured amount and medications for CTD and is represented as adjusted OR (aOR) with 95% CIs. All data were analysed using the statistical software V.9.3 (SAS Institute). A p<0.05 was considered as statistically significant.
Patient and public involvement
This research was performed without patient involvement. Patients were not invited with regards to design of study, measurement of outcome and interpretation of results.
Results
Study subjects with CTDs
A total of 505 patients with CTD-ILD were included in this study, consisting of 82 with SLE, 210 with RA, 47 with SSc, 44 with DMtis/PM and 122 with pSS. A total of 2020 patients were selected as matched non-ILD CTD controls. As shown in table 1, patients with CTD-ILD and the non-ILD CTD controls had similar distributions of age, gender and disease duration. Compared with the non-ILD controls, patients with ILD had a higher CCI (1.8±1.5 vs 1.4±1.4, p<0.01), were more likely to have COPD (25.0% vs 8.0%, p<0.01), received a higher dose of GC (5.1±8.5 vs 2.5±4.1 mg/day, prednisolone equivalent dose) and were more likely to use MTX (30.5% vs 22.4%, p<0.01), LEF (8.7% vs 5.1%, p<0.01), HCQ (61.0% vs 52.4%, p<0.01), CSA (5.5% vs 2.4%, p<0.01), AZA (13.3% vs 9.5%, p=0.01), CP (5.3% vs 1.5%, p<0.01) and anti-TNF (8.3% vs 4.7%, p<0.01). The socioeconomic status, including the urbanisation level and the level of payroll-related insured amount, tended to be similar between patients with ILD and the non-ILD controls. Regarding the exposure to air pollutants, patients with ILD had a slightly lower average exposure to PM2.5 (3.0±0.6 vs 3.1±0.7 µg/m3, p<0.01), PM10 (5.1±1.1 vs 5.4±1.2 µg/m3, p<0.01), SO2 (0.3±0.1 vs 0.4±0.1 ppb, p<0.01) and O3 (2.7±0.3 vs 2.8±0.3 ppb, p<0.01) (table 1). Altogether, these data showed that patients with CTD-ILD used a higher dose of GC; had greater proportions of using csDMARDs, immunosuppressants and anti-TNF and were exposed to lower levels of air pollutants, primarily PM2.5, PM10, SO2 and O3, than the non-ILD controls (see details in online supplemental dataset).
Table 1.
Characteristics of enrolled subjects with ILDs and matched non-ILD controls
| Variable | Non-ILD (n=2020) | ILD (n=505) | P value |
| Basic data | |||
| Age, years | 59.4±14.0 | 60.1±14.7 | 0.30 |
| Gender, female | 1520 (75.2) | 380 (75.2) | 1.00 |
| Disease duration, years | 6.9±5.1 | 6.7±5.7 | 0.45 |
| CCI | 1.4±1.4 | 1.8±1.5 | <0.01 |
| CCI without pulmonary disease | 1.3±1.5 | 1.5±1.4 | 0.01 |
| COPD | 161 (8.0) | 126 (25.0) | <0.01 |
| Asthma | 25 (1.2) | 12 (2.4) | 0.06 |
| Urbanisation | |||
| Level 1 | 588 (29.1) | 165 (32.7) | 0.48 |
| Level 2 | 634 (31.4) | 152 (30.1) | |
| Level 3 | 317 (15.7) | 74 (14.7) | |
| Level 4 | 481 (23.8) | 114 (22.6) | |
| Payroll-related insured amount, New Taiwan Dollars | |||
| ≤15 840 | 615 (30.4) | 160 (31.7) | 0.94 |
| 15 841–20 100 | 393 (19.5) | 96 (19.0) | |
| 20 100–27 600 | 523 (25.9) | 126 (25.0) | |
| ≥27 600 | 489 (24.2) | 123 (24.4) | |
| Medication | |||
| csDMARDs | |||
| Methotrexate | 452 (22.4) | 154 (30.5) | <0.01 |
| Sulfasalazine | 369 (18.3) | 105 (20.8) | 0.19 |
| Leflunomide | 103 (5.1) | 44 (8.7) | <0.01 |
| Hydroxychloroquine | 1058 (52.4) | 308 (61.0) | <0.01 |
| Ciclosporin | 49 (2.4) | 28 (5.5) | <0.01 |
| Azathioprine | 191 (9.5) | 67 (13.3) | 0.01 |
| Cyclophosphamide | 31 (1.5) | 27 (5.3) | <0.01 |
| Mycophenolate mofetil | 20 (1.0) | 6 (1.2) | 0.69 |
| Glucocorticoid | 1284 (63.6) | 408 (80.8) | <0.01 |
| Prednisolone equivalent, mg/day* | 2.5±4.1 | 5.1±8.5 | <0.01 |
| bDMARDs | |||
| Anti-TNF | 95 (4.7) | 42 (8.3) | <0.01 |
| Etanercept | 57 (2.8) | 25 (5.0) | 0.02 |
| Adalimumab | 36 (1.8) | 18 (3.6) | 0.01 |
| Golimumab | 3 (0.1) | 0 (0.0) | 0.39 |
| Tocilizumab | 1 (0.05) | 2 (0.4) | 0.04 |
| Rituximab | 16 (0.8) | 10 (2.0) | 0.02 |
| Air pollutant levels | |||
| PM2.5 (µg/m3) | 3.1±0.7 | 3.0±0.6 | <0.01 |
| PM10 (µg/m3) | 5.4±1.2 | 5.1±1.1 | <0.01 |
| SO2 (ppb) | 0.4±0.1 | 0.3±0.1 | <0.01 |
| NO2 (ppb) | 1.8±0.6 | 1.8±0.5 | 0.71 |
| CO (ppm) | 0.5±0.2 | 0.6±0.2 | 0.42 |
| O3 (ppb) | 2.8±0.3 | 2.7±0.3 | <0.01 |
Data are presented as mean±SD and N (%).
*Prednisolone equivalent.
bDMARDs, biological disease-modifying antirheumatic drugs; CCI, Charlson Comorbidity Index; CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; csDMARDs, conventional synthetic DMARDs; ILD, interstitial lung disease; MPA, mycophenolic acid; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter <10 μm; PM2.5, particulate matter <2.5 μm; SO2, sulphur dioxide; TNF, tumour necrosis factor.
bmjopen-2020-041405supp001.csv (369.1KB, csv)
Association of the risk of ILD development with comorbidity and socioeconomic status
As depicted in table 2, CCI without the pulmonary disease (aOR, 1.56; 95% CI 1.13 to 2.16) and COPD (aOR, 3.60; 95% CI 2.68 to 4.82) were significantly associated with a higher risk of developing ILD in patients with CTD. In subgroup analyses according to CTD, the positive association between COPD and ILD remained significant in patients with RA, SLE, pSS and DMtis/PM, but not in patients with SSc (tables 3–5).
Table 2.
Crude and adjusted ORs (aORs) for the association between ILD and variables in patients with CTD
| OR (95% CI) | aOR (95% CI) | |
| Comorbidity | ||
| CCI without pulmonary disease | 2.04 (1.52 to 2.74) | 1.56 (1.13 to 2.16)** |
| COPD | 4.21 (3.19 to 5.55) | 3.60 (2.68 to 4.82)** |
| Urbanisation | ||
| Level 1 | Reference | |
| Level 2 | 0.86 (0.67 to 1.09) | |
| Level 3 | 0.83 (0.61 to 1.13) | |
| Level 4 | 0.85 (0.65 to 1.10) | |
| Payroll-related insured amount, New Taiwan Dollars | ||
| ≤15 840 | Reference | |
| 15 841–20 100 | 0.94 (0.70 to 1.25) | |
| 20 100–27 600 | 0.92 (0.71 to 1.21) | |
| ≥27 600 | 0.96 (0.71 to 1.30) | |
| Medication | ||
| Methotrexate | 1.75 (1.35 to 2.25) | 1.41 (1.06 to 1.89)* |
| Sulfalazine | 1.22 (0.93 to 1.61) | 0.84 (0.62 to 1.14) |
| Leflunomide | 1.85 (1.26 to 2.72) | 1.47 (0.96 to 2.25) |
| Hydroxychloroquine | 1.47 (1.19 to 1.80) | 1.18 (0.93 to 1.48) |
| Immunosuppressants | 2.05 (1.55 to 2.70) | 1.35 (0.99 to 1.85) |
| Steroid†, mg/day | 1.12 (1.09 to 1.14) | 1.09 (1.06 to 1.11)** |
| Anti-TNF | 1.99 (1.33 to 2.99) | 1.25 (0.79 to 1.97) |
| Tocilizumab | 8.00 (0.73 to 88.23) | 7.81 (0.56 to 109.85) |
| Rituximab | 2.50 (1.14 to 5.51) | 1.55 (0.64 to 3.74) |
| Air pollutants | ||
| PM2.5 (per 10 µg/m3) | 0.67 (0.58 to 0.78) | 0.72 (0.47 to 1.09) |
| PM10 (per 10 µg/m3) | 0.80 (0.74 to 0.88) | 1.06 (0.83 to 1.37) |
| SO2 (per 10 ppb) | 0.19 (0.08 to 0.49) | 0.40 (0.12 to 1.30) |
| NO2 (per 10 ppb) | 1.03 (0.87 to 1.23) | |
| CO (per 1 ppm) | 1.19 (0.78 to 1.80) | |
| O3 (per 10 ppb) | 0.50 (0.35 to 0.71) | 0.51 (0.33 to 0.79)** |
*P<0.05, **p<0.001.
†Prednisolone equivalent.
CCI, Charlson comorbidity index; CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; ILD, interstitial lung disease; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter <10 μm; PM2.5, particulate matter <2.5 μm; SO2, sulphur dioxide; TNF, tumour necrosis factor.
Table 3.
Crude and adjusted ORs (aORs) for the association between the risk of ILD development and variables in patients with rheumatoid arthritis
| Variable | Univariable | Multivariable |
| OR (95% CI) | aOR (95% CI) | |
| Comorbidity | ||
| CCI without pulmonary disease | 3.49 (1.67 to 7.30) | 1.70 (0.77 to 3.71) |
| COPD | 2.94 (1.98 to 4.36) | 2.35 (1.54 to 3.59)** |
| Urbanisation | ||
| Level 1 | Reference | |
| Level 2 | 1.06 (0.71 to 1.57) | |
| Level 3 | 0.87 (0.54 to 1.40) | |
| Level 4 | 0.88 (0.58 to 1.32) | |
| Payroll-related insured amount, New Taiwan Dollars | ||
| ≤15 840 | Reference | |
| 15 841–20 100 | 1.10 (0.72 to 1.68) | |
| 20 100–27 600 | 0.88 (0.59 to 1.30) | |
| ≥27 600 | 0.95 (0.57 to 1.57) | |
| Medication | ||
| Methotrexate | 1.66 (1.22 to 2.26) | 1.28 (0.91 to 1.82) |
| Sulfasalazine | 1.16 (0.86 to 1.58) | |
| Leflunomide | 1.94 (1.30 to 2.91) | 1.35 (0.85 to 2.15) |
| Hydroxychloroquine | 1.73 (1.25 to 2.38) | 1.36 (0.95 to 1.94) |
| Immunosuppressants | 2.68 (1.60 to 4.50) | 1.53 (0.86 to 2.73) |
| Steroid†(†), mg/day | 1.15 (1.10 to 1.21) | 1.11 (1.05 to 1.17)** |
| Anti-TNF | 2.11 (1.39 to 3.21) | 1.31 (0.82 to 2.09) |
| Tocilizumab | 8.00 (0.73 to 88.23) | 11.19 (0.75 to 166.66) |
| Rituximab | 2.67 (1.20 to 5.94) | 1.67 (0.69 to 4.02) |
| Air pollutants | ||
| PM2.5 (per 10 µg/m3) | 0.77 (0.61 to 0.97) | 0.97 (0.50 to 1.89) |
| PM10 (per 10 µg/m3) | 0.86 (0.76 to 0.97) | 0.89 (0.62 to 1.29) |
| SO2 (per 10 ppb) | 0.52 (0.13 to 2.03) | |
| NO2 (per 10 ppb) | 0.98 (0.75 to 1.28) | |
| CO (per 1 ppm) | 0.96 (0.48 to 1.92) | |
| O3 (per 10 ppb) | 0.70 (0.41 to 1.20) | 0.69 (0.37 to 1.29) |
***p<0.001.
†Prednisolone equivalent.
CCI, Charlson Comorbidity Index; CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter <10 μm; PM2.5, particulate matter <2.5 μm; SO2, sulphur dioxide; TNF, tumour necrosis factor.
Table 4.
Crude and adjusted ORs (aORs) for the association between the risk of ILD development and variables in patients with systemic lupus erythematosus and primary Sjögren’s syndrome
| Variable | Sytemic lupus erythematosus | Primary Sjögren’s syndrome | ||
| Univariable | Multivariable | Univariable | Multivariable | |
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Comorbidity | ||||
| CCI without pulmonary disease | 2.87 (0.86 to 9.65) | 1.64 (0.40 to 6.66) | 1.47 (0.98 to 2.22) | 1.36 (0.87 to 2.14) |
| COPD | 7.83 (3.54 to 17.29) | 10.52 (3.97 to 27.89)** | 7.14 (3.85 to 13.24) | 5.99 (3.04 to 11.78)** |
| Urbanisation | ||||
| Level 1 | Reference | Reference | Reference | |
| Level 2 | 0.51 (0.27 to 0.94) | 0.69 (0.27 to 1.78) | 0.81 (0.50 to 1.31) | |
| Level 3 | 0.59 (0.28 to 1.23) | 0.79 (0.26 to 2.44) | 1.24 (0.68 to 2.25) | |
| Level 4 | 0.74 (0.37 to 1.47) | 1.81 (0.48 to 6.88) | 0.90 (0.53 to 1.55) | |
| Payroll-related insured amount, New Taiwan Dollars | ||||
| ≤15 840 | Reference | Reference | ||
| 15 841–28 800 | 0.61 (0.26 to 1.39) | 1.18 (0.66 to 2.10) | ||
| 28 801–45 800 | 0.93 (0.46 to 1.88) | 1.02 (0.58 to 1.78) | ||
| ≥45 801 | 1.07 (0.52 to 2.20) | 0.86 (0.46 to 1.59) | ||
| Medication | ||||
| Methotrexate | 0.69 (0.19 to 2.44) | 0.81 (0.29 to 2.26) | ||
| Sulfasalazine | 3.04 (0.99 to 9.26) | 3.19 (0.81 to 12.56) | 0.96 (0.41 to 2.25) | |
| Leflunomide | <0.01 (<0.01–>99) | 1.33 (0.14 to 12.82) | ||
| Hydroxychloroquine | 1.27 (0.75 to 2.13) | 1.26 (0.82 to 1.95) | ||
| Immunosuppressants | 1.47 (0.89 to 2.45) | 1.06 (0.55 to 2.05) | 3.39 (1.62 to 7.07) | 1.84 (0.78 to 4.34) |
| Steroid†, mg/day | 1.09 (1.05 to 1.14) | 1.09 (1.03 to 1.15)** | 1.17 (1.09 to 1.25) | 1.11 (1.03 to 1.18)** |
| Air pollutants | ||||
| PM2.5 (per 10 µg/m3) | 0.59 (0.41 to 0.86) | 0.23 (0.07 to 0.73)* | 0.61 (0.44 to 0.84) | 0.73 (0.30 to 1.76) |
| PM10 (per 10 µg/m3) | 0.79 (0.64 to 0.98) | 1.96 (0.98 to 3.89) | 0.76 (0.63 to 0.91) | 1.04 (0.61 to 1.75) |
| SO2 (per 10 ppb) | 0.13 (0.01 to 1.30) | 0.41 (0.01 to 15.74) | 0.08 (0.01 to 0.61) | 0.10 (0.01 to 1.41) |
| NO2 (per 10 ppb) | 1.41 (0.93 to 2.15) | 0.98 (0.15 to 6.57) | 0.99 (0.70 to 1.40) | |
| CO (per 1 ppm) | 2.45 (0.96 to 6.20) | 0.94 (0.03 to 26.11) | 1.34 (0.59 to 3.04) | |
| O3 (per 10 ppb) | 0.23 (0.09 to 0.58) | 0.06 (0.01 to 0.43)** | 0.65 (0.31 to 1.34) | |
*p<0.05, **p<0.005.
†Prednisolone equivalent.
CCI, Charlson Comorbidity Index; CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; NA, not available; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter <10 μm; PM2.5, particulate matter <2.5 μm; SO2, sulphur dioxide; TNF, tumour necrosis factor.
Table 5.
Crude and adjusted ORs for the association between the risk of ILD development and variables in patients with systemic sclerosis and dermatomyositis/polymyositis
| Variable | Systemic sclerosis | Dermatomyositis/ polymyositis | ||
| Univariable | Multivariable | Univariable | Multivariable | |
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Comorbidity | ||||
| CCI without pulmonary disease | 0.39 (0.11 to 1.46) | 5.51 (1.11 to 27.26)* | 1.55 (0.68 to 3.54) | |
| COPD | 0.80 (0.33 to 1.91) | 2.80 (0.97 to 8.07) | 4.19 (1.38 to 12.71) | 6.73 (1.39 to 32.51)* |
| Urbanisation | ||||
| Level 1 | Reference | Reference | Reference | |
| Level 2 | 1.02 (0.47 to 2.20) | 1.53 (0.56 to 4.18) | 0.90 (0.39 to 2.08) | |
| Level 3 | 0.39 (0.11 to 1.46) | 0.73 (0.15 to 3.68) | 0.74 (0.26 to 2.13) | |
| Level 4 | 0.80 (0.33 to 1.91) | 1.97 (0.51 to 7.57) | 0.84 (0.31 to 2.27) | |
| Payroll-related insured amount, New Taiwan Dollars | ||||
| ≤15 840 | Reference | Reference | Reference | |
| 15 841–28 800 | 1.01 (0.36 to 2.79) | 0.36 (0.12 to 1.11) | 0.39 (0.09 to 1.61) | |
| 28 801–45 800 | 1.19 (0.45 to 3.12) | 0.66 (0.25 to 1.77) | 0.32 (0.09 to 1.16) | |
| ≥45 801 | 1.14 (0.42 to 3.14) | 0.74 (0.30 to 1.86) | 0.24 (0.06 to 0.94)* | |
| Medication | ||||
| Methotrexate | 1.00 (0.24 to 4.11) | 5.70 (2.74 to 11.88) | 7.55 (2.77 to 20.62)** | |
| Sulfasalazine | <0.01 <0.01–>99) | 12.00 (1.25 to 115.36) | 2.31 (0.14 to 36.92) | |
| Leflunomide | 2.00 (0.18 to 22.06) | >99(<0.01–>99) | ||
| Hydroxychloroquine | 0.87 (0.44 to 1.72) | 2.21 (1.12 to 4.39) | 0.95 (0.38 to 2.38) | |
| Immunosuppressants | 2.11 (1.01 to 4.44) | 1.05 (0.40 to 2.74) | 1.52 (0.79 to 2.95) | |
| Steroid†, mg/day | 1.16 (1.07 to 1.25) | 1.12 (1.02 to 1.23)* | 1.07 (1.02 to 1.11) | 1.06 (1.004 to 1.12)* |
| Air pollutants | ||||
| PM2.5 (per 10 µg/m3) | 0.69 (0.41 to 1.18) | 1.14 (0.27 to 4.73) | 0.57 (0.34 to 0.95) | 0.31 (0.05 to 1.80) |
| PM10 (per 10 µg/m3) | 0.77 (0.57 to 1.04) | 0.91 (0.35 to 2.35) | 0.72 (0.54 to 0.97) | 1.34 (0.47 to 3.79) |
| SO2 (per 10 ppb) | 0.06 (0.00 to 2.27) | 0.12 (<0.01 to 16.34) | 0.07 (0.00 to 1.99) | 2.82 (0.01 to 574.01) |
| NO2 (per 10 ppb) | 0.95 (0.53 to 1.70) | 0.90 (0.50 to 1.62) | ||
| CO (per 1 ppm) | 0.96 (0.24 to 3.86) | 0.51 (0.11 to 2.43) | ||
| O3 (per 10 ppb) | 0.28 (0.08 to 1.02) | 0.16 (0.02 to 1.30) | 0.30 (0.08 to 1.11) | 0.36 (0.06 to 2.40) |
*p<0.05, **p<0.005.
†Prednisolone equivalent.
CCI, Charlson comorbidity index; CO, carbon monoxide; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; NA, not available; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter <10 μm; PM2.5, particulate matter <2.5 μm; SO2, sulphur dioxide; TNF, tumour necrosis factor.
Association between medications for CTD and the risk of ILD development
As shown in table 2, a positive association can be found between prednisolone equivalent dose (mg/day) and ILD risk (aOR 1.09, 95% CI, 1.06 to 1.11) in all patients with CTD. The positive association between GC dose and ILD risk remained statistically significant in all of the subgroups of CTD (tables 3–5). Regarding the use of DMARDs, we observed a positive association of ILD risk with MTX use among all patients with CTD (table 2). However, subgroup analyses of CTD revealed that the positive association between MTX use and ILD risk remained statistically significant only in patients with DMtis/PM (tables 3–5).
Association between exposure to air pollutants and ILD development
We then evaluated the factors associated with ILD development in patients with various CTDs. Exposure to O3 (aOR, 0.51; 95% CI, 0.33 to 0.79) was found to have an inverse association with the risk of ILD development after adjusting for potential confounders. As shown in tables 3–5, the subgroup analyses based on CTD revealed that the protective effect of O3 against ILD risk was consistently present in patients with SLE, but did not reach statistical significance in patients with the other CTDs.
Discussion
The association between O3 exposure and ILD development currently remains elusive, and evidence regarding air pollutant exposure and CTD-ILD is extremely sparse despite the increasing awareness of CTD-ILD. In this population-based case–control study, we found that O3 exposure was inversely associated with the development of ILD in patients with CTD after adjusting for potential confounders, including concomitant medications and socioeconomic status. The finding highlights the previously unrecognised association between exposure to air pollutants, particularly O3, and the development of CTD-ILD.
Although there is increasing evidence to implicate exposure to air pollutants in the development of ILD, current evidence remains elusive due to the varied definition for ILDs and the distinct air pollutants.20 Rice et al conducted a community-dwelling population-based study in Framingham and reported that higher long-term exposure to elemental carbon, an indicator of traffic pollution, was associated with the incidence and progression of interstitial lung abnormalities (ILAs); however, they found no association between average levels of PM2.5 and incident ILAs.12 In detail, unlike the positive association found between elemental carbon (OR 1.27, 95% CI 1.04 to 1.55) as well as PM2.5 (OR 1.02, 95% CI 0.85 to 1.23) and ILAs, an inverse association was found between O3 (OR 0.91, 95% CI 0.78 to 1.06) and ILAs. Similarly, Sack C et al investigated 2671 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) Lung study and reported that exposure to ambient NOX was associated with a higher prevalence of ILAs, but the association was not found with exposure to PM2.5 and O3.2 Remarkably, consistent with our finding and the result of the study of Rice et al, there was a significant inverse association between exposure to O3 and incident ILAs (OR 0.30, 95% CI 0.10 to 0.93) in never-smokers. Furthermore, one delicate Italian study also found the trend of the inverse association between O3 exposure and the incidence rate of IPF.11 These three studies and our findings in patients with CTDs found the consistent but previously unrecognised inverse association between exposure to O3 and incident ILDs.
Studies have postulated that the quenching effect of O3 could possibly be responsible for the inverse association between exposure to O3 and incident ILDs.2 12 In fact, ground-level O3 is a secondary pollutant resulting from the photochemical reaction among traffic-related air pollutants, including NO2 and volatile organic compounds.6 A number of previous studies reported an inverse correlation between O3 level and traffic-related air pollutants, including NO2 and elemental carbon.2 11 12 However, some studies have reported that exposure to O3 and other air pollutants was positively associated with a deteriorated disease course in patients with ILD, including hospitalisation, poor lung function and exacerbation of ILD.9 10 21 The aforementioned discordant findings with regard to the impact of O3 exposure on ILDs reflect the complexity of ILD pathogenesis, which consists of initial insults resulting in the influx of inflammatory cells as well as alveolar epithelial damage and the subsequent deterioration caused by the recruitment and activation of pulmonary fibroblasts and myofibroblasts.22 Therefore, we postulate that O3 exposure may exert distinct effects, including the quenching effect, on the development and clinical deterioration of ILD and the present study further provides evidence regarding the impact of O3 exposure on the development of CTD-ILD.
The pathogenesis of CTD-ILD has been increasingly investigated in recent years, and the balance among T cells, including ILCs, has been identified to play an important role in the pathogenesis of CTD-ILD.23–25 Sendo et al, using Zym-treated SKG mice to simulate RA-ILD, observed an increase in pathogenic Th17 cells in the inflamed lung tissue of RA-ILD mice and that tofacitinib ameliorated the ILD.26 In another recent study, which analysed the cytokine profiles of 40 patients with PM/DMtis-ILD, Th1 cells were found to play a key role in the pathogenesis of PM/DM-ILD.27 Intriguingly, O3 has been implicated in a high Th2 response in airway cells by enhancing the type 2 ILC-associated pathway.7 28 Therefore, the O3-associated expansion of the Th2 pathway through ILCs may at least partly explain the potential protective effects of O3 on the development of ILD in patients with CTD by ameliorating Th17/Th1-associated signalling in the airway.
Intriguingly, smoking was reported to affect the association between O3 exposure and pulmonary diseases, including ILDs.2 Sack et al observed that O3 exerted a protective role in incident ILAs in never-smokers (OR 0.30, 95% CI 0.10 to 0.93) among participants in the MESA Lung study, whereas O3 tended to be a risk factor for ILAs in ever-smokers (OR 1.44, 95% CI 0.52 to 4.01).2 Smoking status is not recorded in the NHIRD; however, the majority of enrolled patients with CTD were females (74.9%), and there is a marked low prevalence of tobacco use among females in Taiwan (2.6%). Thus, we believe that the magnitude of the protective effect of O3 against the development of CTD-ILD might at least partly be attributed by the high proportion of females in the study population.29
There are some limitations in the present study. First, the NHIRD cannot provide laboratory data including titers of autoantibody; however, the medication data are comprehensive. In addition, the diagnoses of SLE, RA and SS were validated by at least two experienced and qualified rheumatologists by reviewing patients’ medical charts, laboratory findings and images to issue a catastrophic illness certificate. Similarly, the accuracy of ILD in the claim is also a concern. One recently published study aimed to validate claims-based algorithms for identification of ILD in patients with RA found that the accuracy of RA-ILD was high if the diagnosis was made by specialists.30 In the present study, we merely enrolled patients within the aforementioned catastrophic illness registry file. Therefore, the diagnoses of CTD and ILD were made by the rheumatologist, and the risk for misclassification should be at least partly mitigated. Second, the disease activity of CTD is not recorded, but we believe that we have adjusted for the essential CTD-associated medications, which were comprehensively in NHIRD. We believe the adjustment of medications should largely reflect the disease activity. Third, varied mechanisms may underlie distinct CTDs; however, patients with distinct CTDs might have similar profibrotic pathways in the development of ILD.31 Fourth, given the case–control design we merely claim the association, instead of causal inference, between exposure to air pollutants and incident ILDs in patients with CTD. Furthermore, we have conducted further analyses using a longer period (2 years) of air pollutant exposure, and the results were consistent with the finding in the present study using 1 year exposure to air pollutants (online supplemental table 1).
bmjopen-2020-041405supp002.pdf (95.4KB, pdf)
In conclusion, exposure to air pollutants is increasingly found to be associated with the development of a number of pulmonary diseases, including ILDs.
Recent evidence has demonstrated that O3 exposure appeared to have a negative association with the development of ILDs. In the present population-based case–control study, we found that exposure to O3 was inversely associated with incident CTD-ILDs among patients with CTD in Taiwan. Further studies are warranted to validate these findings and explore the underlying mechanisms.
Supplementary Material
Footnotes
J-CY and W-CC contributed equally.
Contributors: Conceived and designed the experiments: H-HC, W-CC, J-CY, Y-HC and D-YC. Acquired data: Y-MY, C-HL, J-CY and H-HC. Contributed materials/analysis tools: W-CC, Y-MY, J-CY and H-HC. Wrote the paper: H-HC, J-CY and W-CC.
Funding: Funding This study was supported in part by grants from Veterans General Hospitals and the University System of Taiwan Joint Research Program (VGHUST109-V2-2-1 and VGHUST109-V2-2-3).
Disclaimer: The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taiwan (IRB number: CE14149B-3).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All of the data and materials are provided in the manuscript and supplemental data.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Araki T, Putman RK, Hatabu H, et al. . Development and progression of interstitial lung abnormalities in the Framingham heart study. Am J Respir Crit Care Med 2016;194:1514–22. 10.1164/rccm.201512-2523OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sack C, Vedal S, Sheppard L, et al. . Air pollution and subclinical interstitial lung disease: the multi-ethnic study of atherosclerosis (MESA) air-lung study. Eur Respir J 2017;50:1700559. 10.1183/13993003.00559-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilfong EM, Lentz RJ, Guttentag A, et al. . Interstitial pneumonia with autoimmune features: an emerging challenge at the intersection of rheumatology and pulmonology. Arthritis Rheumatol 2018;70:1901–13. 10.1002/art.40679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet 2012;380:689–98. 10.1016/S0140-6736(12)61079-4 [DOI] [PubMed] [Google Scholar]
- 5.Tang K-T, Tsuang B-J, Ku K-C, et al. . Relationship between exposure to air pollutants and development of systemic autoimmune rheumatic diseases: a nationwide population-based case-control study. Ann Rheum Dis 2019;78:1288–91. 10.1136/annrheumdis-2019-215230 [DOI] [PubMed] [Google Scholar]
- 6.Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–42. 10.1016/S0140-6736(02)11274-8 [DOI] [PubMed] [Google Scholar]
- 7.Kumagai K, Lewandowski R, Jackson-Humbles DN, et al. . Ozone-Induced nasal type 2 immunity in mice is dependent on innate lymphoid cells. Am J Respir Cell Mol Biol 2016;54:782–91. 10.1165/rcmb.2015-0118OC [DOI] [PubMed] [Google Scholar]
- 8.Sumida A, Hasegawa Y, Okamoto M, et al. . TH1/TH2 immune response in lung fibroblasts in interstitial lung disease. Arch Med Res 2008;39:503–10. 10.1016/j.arcmed.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 9.Johannson KA, Vittinghoff E, Lee K, et al. . Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J 2014;43:1124–31. 10.1183/09031936.00122213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannson KA, Vittinghoff E, Morisset J, et al. . Air pollution exposure is associated with lower lung function, but not changes in lung function, in patients with idiopathic pulmonary fibrosis. Chest 2018;154:119–25. 10.1016/j.chest.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti S, Harari S, Caminati A, et al. . The association between air pollution and the incidence of idiopathic pulmonary fibrosis in northern Italy. Eur Respir J 2018;51:1700397. 10.1183/13993003.00397-2017 [DOI] [PubMed] [Google Scholar]
- 12.Rice MB, Li W, Schwartz J, et al. . Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: the Framingham heart study. Thorax 2019;74:1063–9. 10.1136/thoraxjnl-2018-212877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health and Welfare The National health insurance statistics 2015: National health insurance administration. Taiwan. [Google Scholar]
- 14.Environmental Protection Administration Taiwan air quality monitoring net work. Available: https://taqm.epa.gov.tw/taqm/en/b0201.aspx [Accessed 10 Oct 2019].
- 15.Qi Y, Li Q, Karimian H, et al. . A hybrid model for spatiotemporal forecasting of PM2.5 based on graph convolutional neural network and long short-term memory. Sci Total Environ 2019;664:1–10. 10.1016/j.scitotenv.2019.01.333 [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 17.Kloog I, Ridgway B, Koutrakis P, et al. . Long- and short-term exposure to PM2.5 and mortality: using novel exposure models. Epidemiology 2013;24:555–61. 10.1097/EDE.0b013e318294beaa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B-Y, Chen C-H, Chuang Y-C, et al. . Changes in the relationship between childhood asthma and ambient air pollution in Taiwan: Results from a nationwide survey repeated 5 years apart. Pediatr Allergy Immunol 2019;30:188–94. 10.1111/pai.12999 [DOI] [PubMed] [Google Scholar]
- 19.Bursac Z, Gauss CH, Williams DK, et al. . Purposeful selection of variables in logistic regression. Source Code Biol Med 2008;3:17. 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johannson KA, Balmes JR, Collard HR. Air pollution exposure: a novel environmental risk factor for interstitial lung disease? Chest 2015;147:1161–7. 10.1378/chest.14-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sesé L, Nunes H, Cottin V, et al. . Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax 2018;73:145–50. 10.1136/thoraxjnl-2017-209967 [DOI] [PubMed] [Google Scholar]
- 22.Hoyles RK, Derrett-Smith EC, Khan K, et al. . An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor β receptor. Am J Respir Crit Care Med 2011;183:249–61. 10.1164/rccm.201002-0279OC [DOI] [PubMed] [Google Scholar]
- 23.Mi S, Li Z, Yang H-Z, et al. . Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol 2011;187:3003–14. 10.4049/jimmunol.1004081 [DOI] [PubMed] [Google Scholar]
- 24.Desai O, Winkler J, Minasyan M, et al. . The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med 2018;5:43. 10.3389/fmed.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellanos JG, Longman RS. The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest 2019;129:2640–50. 10.1172/JCI124617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sendo S, Saegusa J, Yamada H, et al. . Tofacitinib facilitates the expansion of myeloid-derived suppressor cells and ameliorates interstitial lung disease in SKG mice. Arthritis Res Ther 2019;21:184. 10.1186/s13075-019-1963-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda S, Kotani T, Ishida T, et al. . Exploration of pathomechanism using comprehensive analysis of serum cytokines in polymyositis/dermatomyositis-interstitial lung disease. Rheumatology 2020;59:310–8. 10.1093/rheumatology/kez301 [DOI] [PubMed] [Google Scholar]
- 28.Flayer CH, Larson ED, Joseph A, et al. . Ozone-induced enhancement of airway hyperreactivity in rhesus macaques: effects of antioxidant treatment. J Allergy Clin Immunol 2020;145:312–23. 10.1016/j.jaci.2019.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taiwan tobacco control annual report 2018. health promotion administration, Ministry of health and welfare, Taiwan. Available: https://health99.hpa.gov.tw/media/public/pdf/22077.pdf [Accessed 10 Oct 2019].
- 30.Cho S-K, Doyle TJ, Lee H, et al. . Validation of claims-based algorithms to identify interstitial lung disease in patients with rheumatoid arthritis. Semin Arthritis Rheum 2020;50:592–7. 10.1016/j.semarthrit.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 31.Castelino FV, Varga J. Interstitial lung disease in connective tissue diseases: evolving concepts of pathogenesis and management. Arthritis Res Ther 2010;12:213. 10.1186/ar3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041405supp001.csv (369.1KB, csv)
bmjopen-2020-041405supp002.pdf (95.4KB, pdf)