Abstract
Ossifying fibroma (OF) is a rare, benign, fibro-osseous lesion of the jawbone characterised by replacement of the normal bone with fibrous tissue. The fibrous tissue shows varying amount of calcified structures resembling bone and/or cementum. The central variant of OF is rare, and shows predilection for mandible among the jawbone. Although it is classified as fibro-osseous lesion, it clinically behaves as a benign tumour and can grow to large size, causing bony swelling and facial asymmetry. This paper reports a case of large central OF of mandible in a 40-year-old male patient. The lesion was treated by segmental resection of mandible. Reconstruction of the surgical defect was done using avascular fibula bone graft. Role of three-dimensional printing of jaw and its benefits in surgical planning and reconstruction are also highlighted.
Keywords: oral and maxillofacial surgery, dentistry and oral medicine
Background
Central ossifying fibroma (OF) is fibro-osseous lesion in which the normal architecture of jawbone is replaced by varying amount of fibrous connective tissue and bone/cementum like material. Although histologically proven OF has been documented in other craniofacial and long bones, 90% of the cases involve the jawbone.1 Among the jawbones, OF has been predominantly reported in mandible, accounting for 70%–90% of cases.2 Although WHO classifies OF as a fibro-osseous lesion, it clinically behaves like a benign bone neoplasm.3 Clinically, central OF in its initial stages of growth is asymptomatic and may get discovered accidently on screening radiograph. However, as the lesion increases in size, it causes swelling, facial asymmetry, paresthesia and pain due to destruction of normal architecture of the jawbone. Radiographically, OF shows variable presentation, ranging from radiolucent to mixed to radiopaque lesion, depending on stage of its maturation. While the radiolucent appearance typically mimics a jaw cyst, mixed and radiopaque presentation is indicative of a fibro-osseous lesion. Treatment of OF is surgery, that varies from simple curettage or enucleation for smaller lesion to resection for larger pathology.4 This paper reports a case of OF in a 40-year-old male patient. The lesion involved considerable part of left hemimandible and was successfully treated by surgical resection. The surgical defect was reconstructed with avascular fibula bone graft to restore form and continuity of the mandible. Three-dimensional (3D) printed model of patient’s jawbone aided in surgical resection and reconstruction.
Case presentation
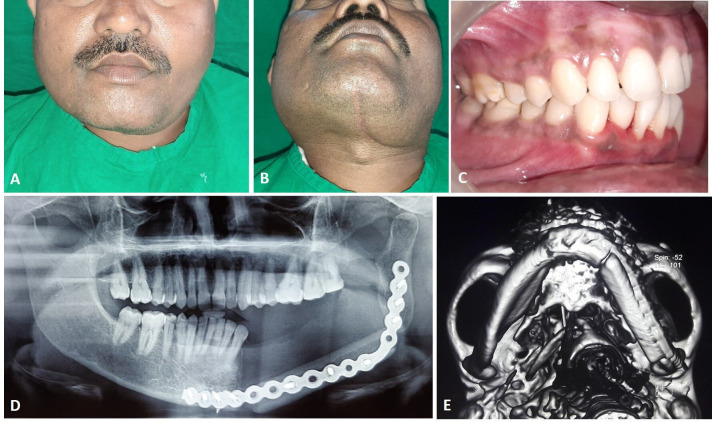
A 40-year-old male patient reported to oral and maxillofacial surgery clinic with complaint of painless swelling over the left lower third of the face for the past 2 months. There was no history of previous trauma in the region. Medical history of the patient was unremarkable. The patient did not have any associated paresthesia. Extra-oral examination showed diffuse swelling over left side of the lower third of face in the region of angle of mandible (figure 1A). The swelling was firm and non-tender on palpation. The overlying skin appeared normal, with no local rise in temperature. Intraoral examination showed diffuse swelling over left buccal vestibule in the molar region, extending posteriorly to the ramus of mandible. The left mandibular second molar was partially erupted and the third molar was clinically absent (figure 1B).
Figure 1.
Photographs showing (A) diffuse swelling over left lower part of the face, (B) intraoral swelling and (C) orthopantomogram showing mixed lesion involving the left mandible.
Orthopantomogram of the jawbone revealed mixed radiopaque-radiolucent lesion over left mandible extending from left mandibular second premolar to the ramus region. The lesion was well defined with a sclerotic border and had central area of trabeculations radiating to the periphery (figure 1C). The above clinical and imaging findings were suggestive of a clinically benign and radiologically mixed lesion of the jawbone. The differential diagnosis included: fibrous dysplasia, calcifying epithelial odontogenic tumour and central OF.
Investigations
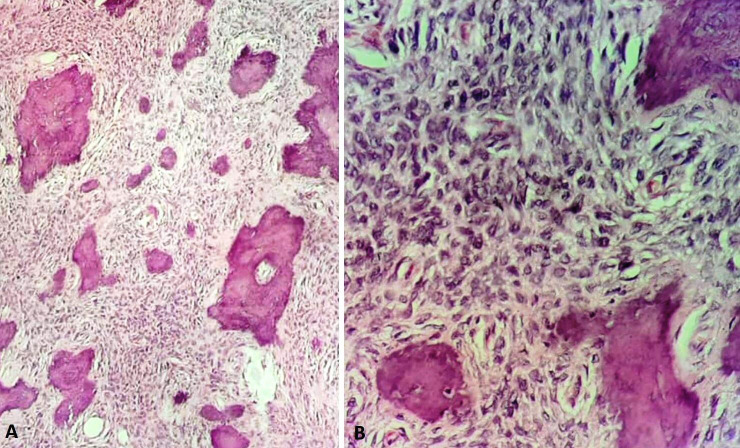
Incision biopsy from the lesion was planned and executed under local anaesthesia via intraoral approach. The histological evaluation of the specimen showed islands of trabeculae of immature bone dispersed in highly cellular fibrous connective tissue matrix (figure 2). Based on the microscopic examination findings, final histological diagnosis of central OF was made.
Figure 2.
Photomicrographs (H&E stain) showing islands of trabeculae of immature bone dispersed in highly cellular fibrous connective tissue matrix (A) 10× magnification and (B) 40× magnification.
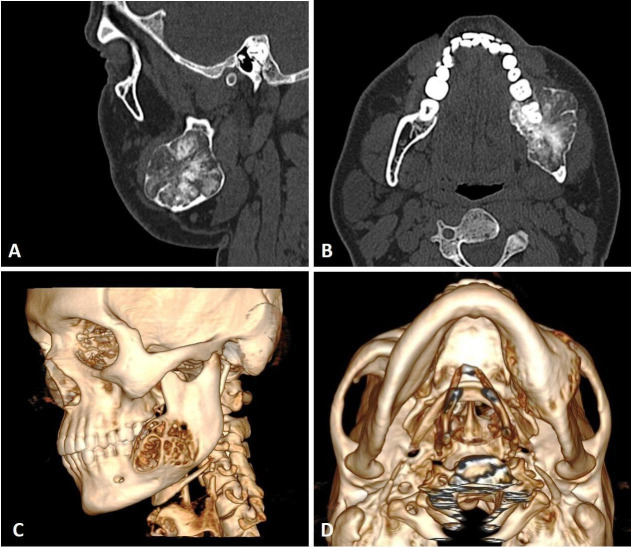
To further study the nature and extent of the lesion, patient was subjected to CT scan. Sectional images of CT scan showed expansile lesion involving the left mandible, with expansion and thinning of lingual and buccal cortices and involvement of lower border of the mandible. The boundary of the lesion was well demarcated. It consisted of multiple areas of calcification within the lytic lesion (figure 3A, B). Multiple areas of perforation over buccal and lingual cortex were noted (figure 3C, D).
Figure 3.
CT scan showing expansile lesion involving the left mandible, with multiple areas of calcification within the lytic lesion, (A) coronal section, (B) axial section and (C, D) three-dimensional formatted images.
Treatment
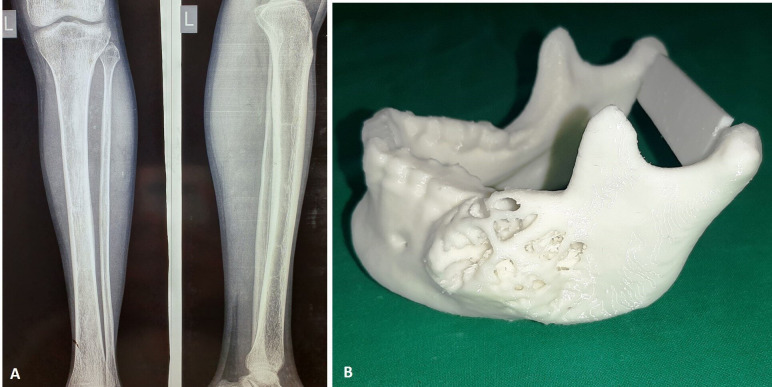
Based on the findings of histopathological examination and CT, surgical removal of the lesion was planned under general anaesthesia. The surgical options of management using either enucleation or resection were discussed. Due to the large size of the lesion, multiple areas of cortical perforation and involvement of the lower border of mandible, surgical resection was chosen in consultation with patient and his relatives. Reconstruction of the defect was planned with fibula bone graft. Anteroposterior and lateral view radiograph of the lower left limb was taken for preoperative assessment of fibula bone, to rule out any pathologies, fractures or disorders in bone mineralisation (figure 4A).
Figure 4.
(A) Radiograph of left lower limb and (B) three-dimensional printed anatomical model of the jawbone.
High-resolution CT scans (0.50 mm cuts) of mandible and fibula were submitted for fabrication of 3D printed anatomical model. The 3D anatomical model was used to evaluate the size and extent of pathology and for patient education (figure 4B). The 3D model was then used for mock surgery (video 1). Osteotomy cuts on mandible were marked on the 3D model, for surgical excision of the lesion with a safe margin. The titanium reconstruction plate was pre-bent and adapted using the 3D model as a template.
Video 1.
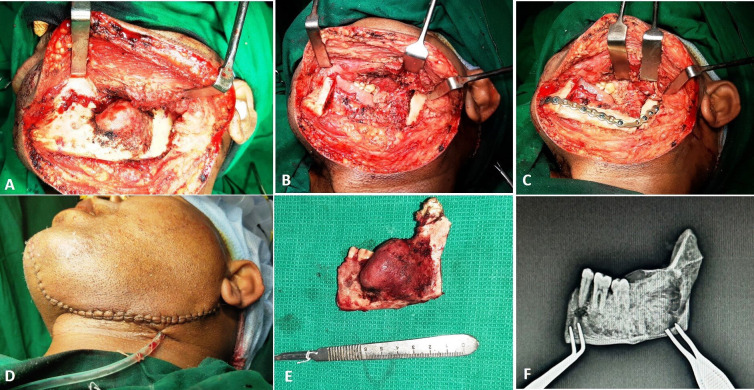
The surgical procedure was then executed under general anaesthesia. Extended submandibular incision was used to approach the lesion (figure 5A). The osteotomy cuts were placed as planned and executed during the mock surgery and the lesion was excised in-toto (figure 5B). Fibula bone graft was harvested and shaped to reconstruct the surgical defect. The pre-adapted titanium plate was used to fix the bone graft to the native mandible (figure 5C). Closure was done in layers (figure 5D). The excised mandible (figure 5E) was intraoperatively subjected to imaging, for radiological assessment of bone specimen margins, which was found to be adequate (figure 5F).
Figure 5.
Intraoperative images showing: (A) surgical exposure, (B) resection, (C) reconstruction, (D) closure, (E) excised specimen and (F) intraoperative imaging of resected specimen.
Outcome and follow-up
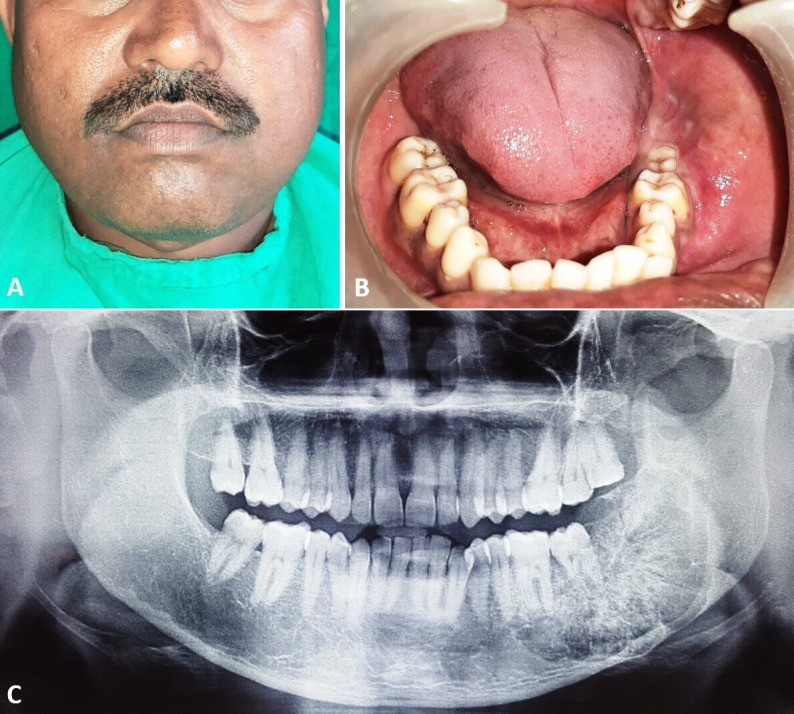
The patient had uneventful postoperative period and was kept on regular follow-up visits. At 2-year follow-up the patient showed satisfactory healing with no clinical evidence of recurrence. The facial symmetry was acceptable with stable occlusion and normal mouth opening (figure 6A–C). An orthopantomogram and CT scan showed the fibula bone graft maintaining the continuity of the mandible, with no evidence of bone resorption and recurrence (figure 6D, E).
Figure 6.
Two-year postoperative images showing: (A) facial appearance, (B) neck scar, (C) stable dental occlusion, (D) orthopantomogram and (E) three-dimensional CT scan showing continuity of lower border of the mandible.
Discussion
Central OF is a fibro-osseous lesion in which the normal bone is replaced by fibrous tissue, containing variable amount of calcified structure. The calcified structure histologically resembles bone or cementum. The amount of calcification depends on the stage and maturation of the lesion. WHO in 1972 classified OF in two subtypes: OF and cementifying fibroma, depending on the nature of the calcified tissue within the fibrous stroma. However, the subclassification is of academic interest, as the biological behaviour of the two variants are identical. In 1992, WHO revised the classification and gave a single terminology of cemento-OF.5 Further in 2005, the term cemento-OF was replaced by OF.6 Other less commonly used terminology for OF described in literature include: osteo-fibrous dysplasia, non-osteogenic fibroma, osteo-fibroma, fibro-osteoma and benign fibro-osseous lesion of periodontal ligament origin.1
Although the etiopathogenesis of OF is not definitely known, it is believed to develop from the pluripotent mesenchymal cells of the periodontal ligament of tooth.7 This is supported by the fact that the periodontal ligament cells have shown the ability to produce cementum and osteoid like material, which is a characteristic feature of OF.8 Both trauma and genetic mutation have been studied as possible agents for initiation of OF.9 10 OF shows predilection for the lower jaw, with 70%–90% cases reported in mandible.2 In the mandible, it is invariably seen in tooth bearing areas which show higher rates of bone and cementum induction.1 This further supports the odontogenic origin of OF. Posterior region in the premolar-molar area is more commonly involved than the anterior part of jaw. OF is commonly seen in second to fourth decade of life, with women being involved five times more as compared with men.11 12 In the present case, OF involved the left mandible, in premolar-molar region in a 40-year-old male patient. No previous history of trauma was reported. No family history of similar jaw lesion was reported, thus ruling out possible genetic aetiology in the present case.
The clinical presentation of OF depends on the stage of growth of the lesion. Smaller lesions are asymptomatic and may get discovered on routine radiographic examination. The reported incidence of patients presenting with OF discovered as incidental finding is variable. An old case series of OF published in 1977, in Japanese population showed almost all the cases presented with symptom of swelling and/or pain.13 In contrast, over half of the patients in the American population during the same time period were symptom-free, and got diagnosed incidentally.14 A recent case series of OF from Hong Kong showed that majority of the cases were diagnosed as incidental finding.15 This variation possibly indicates the difference in the dental awareness and oral healthcare availability at different time periods and geographic locations. Discovery of a lesion as an incidental finding implies that patients are visiting the oral healthcare facility more frequently for other reasons, where the lesions that are not yet big enough to produce symptoms, gets diagnosed serendipitously. Swelling, followed by pain was the most common clinical presentation of OF in a retrospective review of 25 Indian patients over a period of 10 years.1 The other clinical features associated with OF include: paresthesia, pus discharge, tooth mobility and exfoliation of tooth.1 11 15 In the present case the lesion was symptomatic and clinically presented as a diffuse facial swelling along with an intraoral swelling associated with partially erupted mandibular molar.
The radiological finding in OF varies from complete radiolucency, to radiolucent lesion with varying amount of radiopacity, to complete radiopacity. The extent of radiopacity seems to be associated with maturation of the lesion. In a review of 18 cases of OF by Sciubba et al, 56% of patients (mean age of 30 years) showed complete radiolucency.11 In contrast, review of 24 cases of OF by MacDonald-Jankowski had complete radiolucency in only 13% of case (mean age of 38 years).15 The appearance of complete radiolucency associated with younger age suggests that opacification in OF increases with age.16
OF in its early stages is small in size and usually completely radiolucent in appearance on radiograph. Such presentation can resemble and needs to be differentiated from periapical tooth pathology, central giant cell granuloma and ameloblastoma.1 As the tumour matures and increases in size, it presents as radiolucent lesion with variable areas of opacity and should be differentiated from mixed jawbone lesions like fibrous dysplasia, calcifying epithelial odontogenic tumour/cyst, adenomatoid odontogenic tumour and condensing osteitis.1 17 Mature and longstanding lesions are complete radiopaque and resemble odontome, mature cemento-osseous dysplasia, osteoblastoma and osteosarcoma on radiograph.1 18 Radiographically, OF presents with well-defined sclerotic borders and is predominantly unilocular. Multilocular presentation in OF is observed in 20% cases.16 Expansion, thinning and perforation of buccal and lingual cortex, and involvement of the lower border of mandible is associated with large lesions, as seen in the present case. Radiographic presentation of the reported case was of a unilocular mixed lesion with a well-defined sclerotic border. Association with partial tooth eruption and missing third molar, though infrequent, was seen in our case.
OF with mixed appearance should be differentiated from fibrous dysplasia. While OF has well-defined margins, fibrous dysplasia has indistinct borders which tend to blend with the surrounding normal bone. OF shows concentric bone expansion around a definite epicentre resulting in altered bone morphology, tooth displacement and resorption. In contrast, fibrous dysplasia results in minimum alteration of bone morphology and rarely causes tooth resorption.3 The major differentiating feature of OF from calcifying epithelial odontogenic cyst, calcifying epithelial odontogenic tumour and adenomatoid odontogenic tumour is presence of multifocal calcification and association of one or more missing teeth with these lesions.19 Condensing osteitis is a localised reactive lesion presenting as sclerosis around the apices of teeth with pulpitis or pulpal necrosis. Unlike OF, imaging findings of condensing osteitis is characterised by a periapical, poorly bordered, non-expansile radiopacity associated with carious tooth.20 Other radiopaque lesions like osteoma and odontoma must be differentiated from OF. Odontomas are the most common odontogenic tumour and radiologically appear as conglomerate of enamel and dentine, resembling multiple toothlike structures.21 Osteomas are non-tooth related lesions which present as jawbone radiopacities.22 Osteomas may arise either from medullary bone (central) or cortex (peripheral) and present as expansile bony growths. At imaging, they present as non-tooth-related, expansile, sclerotic masses and can clinically and radiologically mimic OF.23 24
Final diagnosis of OF is by histological evaluation of incision or excision biopsy specimen. OF shows variable degree of calcifications within hypercellular stroma of fibrous connective tissue. Degree of calcifications increases with maturation of the lesion and stages of bone and cementum deposition. However, histological differentiation of calcified deposits as bone or cementum is difficult. Although biochemical studies including ultrastructure analysis have been used to differentiate between bone and cementum, it is of little clinical significance as both variety of lesion shows similar biological behaviour.25
Treatment of OF is surgery. The extent of surgery varies from curettage, enucleation to more radical excision of the lesion. The choice of modality of the surgical management depends on the nature of lesion, size and location.1 Smaller lesions with definite margin between the tumour mass and the surrounding normal bone are managed by enucleation. Whereas, ill-defined lesions where its borders with normal bone are indistinct are treated by curettage. Resection is more ablative option and should be reserved for lesions with large size, involvement or proximity to the lower border of mandible, thinning and/or perforation of buccal/lingual cortex and lesion with recurrence. In the present case, although the lesion was well defined, resection was chosen for management of the pathology due to its large size and involvement of the lower border of mandible. Also, with the presence of multiple areas of cortical plate, perforation would have made enucleation difficult and less efficient in terms of complete removal of the pathology.
Early lesion of OF is well defined and encapsulated. However, after reaching a size of more than 2–3 cm it is seen to infiltrate beyond its margins, into the surrounding bone.1 The resection margins in OF, should not be more than 5 mm into the healthy bone, as the tumour in its advanced stage does not infiltrate more than 1–2 mm beyond its radiological margin.26 It is important to check for adequacy of margins of the excised specimen intraoperatively, to reduce the risk of residual tumour and subsequent recurrence of the disease. Development of high-resolution imaging and navigational systems which are based on CT or MRI with microscope-based navigation, allows excellent evaluation of resected margins intraoperatively.27 However, these systems are expensive and are available only at higher centres. A simple technique of two-dimensional (2D) imaging of the resected bone specimen was used to assess the adequacy of bone margins in the present case. The technique is simple, inexpensive and gives a fair idea of the width of normal bone beyond the pathological margins.28 This present case highlights this simple and economical method of intraoperative assessment of bone margin using 2D imaging of the resected tumour.
Reconstruction of the surgical defect after excision of the pathology is essential to restore form and function of mandible. Although free fibula remains the gold standard for mandible reconstruction, avascular bone grafts from fibula, rib and iliac bone can be used to reconstruct mandibular defect less than 6–10 cm in size.29 Avascular fibula bone graft used in present case satisfactorily bridged the surgical defect and maintained the contour of mandible. Three-dimensional printed anatomical model of mandible was used in the present case to aid in preoperative assessment of size and shaping of the bone graft to adequately fit the surgical defect. Preoperative bending of the reconstruction plate was carried out on the anatomical model, thus improving accuracy of reconstruction and saving intraoperative time. Arrival of low-cost printers in market in the recent years has lowered the cost and popularised the use of 3D printed anatomical models in maxillofacial reconstruction surgery.30 Use of 3D models improves preoperative planning. It helps to visualise the lesion more realistically and act as aid for patient education and counselling. Mock surgery can be carried on these 3D models thus providing training for the procedure and pre-shaping of bone plate. It also aids in reconstruction surgery thus improving efficiency, treatment-outcome and reduce operating time.
Wide variations have been reported in the recurrence rate of OF in English literature ranging from 0% to 80%.1 16 Various factors which affect the recurrence rate of OF include size and maturation of lesion, age of the patient, type of surgical treatment and follow-up period. Liu et al reported recurrence rate of 26.3% in lesions with well-defined boundaries, while poorly defined lesions recurred in 50% cases.31 Risk of recurrence is highest after curettage and has been reported to be as high as 85%, while wide resection results in least risk of recurrence, with reported range of 0%–4%.31 In the present case, the patient showed satisfactory healing with no clinical evidence of recurrence at 2-year follow-up. An orthopantomogram and CT scan showed maintenance of the continuity of the mandible, with no evidence of bone resorption and recurrence.
This paper reports a case of OF of mandible in an adult male patient. The paper also highlights the role of 3D printed model in facilitating preoperative evaluation of the complex nature of the jaw defect and intraoperative assistance in its reconstruction. The surgical defect in the present case was reconstructed with avascular fibula bone graft to restore the form and continuity of the mandible. Avascular fibula although contraindicated for long span defects, is an effective alternative when the segment to be reconstructed in small to moderate in size (less than 6–10 cm).
Learning points.
The present report highlights a case of ossifying fibroma (OF) of mandible in an adult male patient, which presented as painless extraoral swelling. Other clinical presentation of OF include: pain, paresthesia, tooth displacement/mobility and root resorption.
The paper also advocates the use of three-dimensional printed anatomical models, which aids in patient education (about the extent, size and location of deformity), preoperative bending of reconstruction plate, determination of size and adaptation of the bone graft, thus saving intraoperative time and improving the surgical outcome.
The paper also highlights the use of intraoperative two-dimensional imaging of resected specimen as an affordable tool in initial screening of adequacy of resected jawbone margins.
Although vascular tissue transfer is gold standard for jawbone reconstruction, avascular fibula can be effectively used in short span mandibular defects, as described in the present case.
Footnotes
Contributors: KN, PP and NP were involved in case management. KN and AG were involved in manuscript writing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mohanty S, Gupta S, Kumar P, et al. Retrospective analysis of ossifying fibroma of jaw bones over a period of 10 years with literature review. J Maxillofac Oral Surg 2014;13:560–7. 10.1007/s12663-013-0545-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachou S, Terzakis G, Doundoulakis G, et al. Ossifying fibroma of the temporal bone. J Laryngol Otol 2001;115:654–6. 10.1258/0022215011908522 [DOI] [PubMed] [Google Scholar]
- 3.Swami AN, Kale LM, Mishra SS, et al. Central ossifying fibroma of mandible: a case report and review of literature. J Indian Acad Oral Med Radiol 2015;27:131–5. [Google Scholar]
- 4.Marx R, Stern D. Oral and maxillofacial pathology. Quintessence, Chicago 2003:789–79.
- 5.Kramer IR, Pindborg JJ, shear M. Histological typing of odontogenic tumours. 2nd edn Berlin: Springer, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Reichart PA, Philipsen HP, Sciubba JJ. The new classification of Head and Neck Tumours (WHO]—any changes? Oral Oncol 2006;42:757–8. 10.1016/j.oraloncology.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Regezi JA, Sciubba JJ. Oral pathology: clinico pathologic correlations. 3rd edn Philadelphia: W.B. Saunders, 1999: 357–60. [Google Scholar]
- 8.Bertolini F, Caradonna L, Bianchi B, et al. Multiple ossifying fibromas of the jaws: a case report. J Oral Maxillofac Surg 2002;60:225–9. 10.1053/joms.2002.29832 [DOI] [PubMed] [Google Scholar]
- 9.Ong AH, Siar CH. Cemento-ossifying fibroma with mandibular fracture. Case report in a young patient. Aust Dent J 1998;43:229–33. 10.1111/j.1834-7819.1998.tb00169.x [DOI] [PubMed] [Google Scholar]
- 10.Neville BW, Damm DD, Allen CM, et al. Bone pathology : Chi AC, Oral and maxillofacial pathology. 3rd edn Noida: Elsevier, 2009: 647. [Google Scholar]
- 11.Sciubba JJ, Younai F. Ossifying fibroma of the mandible and maxilla: review of 18 cases. J Oral Pathol Med 1989;18:315–21. 10.1111/j.1600-0714.1989.tb01559.x [DOI] [PubMed] [Google Scholar]
- 12.Mintz S, Velez I. Central ossifying fibroma: an analysis of 20 cases and review of the literature. Quintessence Int 2007;38:221–7. [PubMed] [Google Scholar]
- 13.Sakota Y. [Fibro-osseous lesions of the jaws. Part 1. Solitary lesions (author's transl)]. Kokubyo Gakkai Zasshi 1977;44:217–35. 10.5357/koubyou.44.217 [DOI] [PubMed] [Google Scholar]
- 14.Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. II. benign fibro-osseous lesions of periodontal ligament origin. Oral Surg Oral Med Oral Pathol 1973;35:340–50. 10.1016/0030-4220(73)90072-8 [DOI] [PubMed] [Google Scholar]
- 15.MacDonald-Jankowski DS, Li TK. Ossifying fibroma in a Hong Kong community: the clinical and radiological features and outcomes of treatment. Dentomaxillofac Radiol 2009;38:514–23. 10.1259/dmfr/51064053 [DOI] [PubMed] [Google Scholar]
- 16.MacDonald-Jankowski DS. Ossifying fibroma: a systematic review. Dentomaxillofac Radiol 2009;38:495–513. 10.1259/dmfr/70933621 [DOI] [PubMed] [Google Scholar]
- 17.Andrew D. Radiopacities of the jaws: interpretation and diagnosis. Prim Dent J 2018;7:31–7. 10.1308/205016818822610299 [DOI] [PubMed] [Google Scholar]
- 18.Avramidou F-M, Markou E, Lambrianidis T. Cross-Sectional study of the radiographic appearance of radiopaque lesions of the jawbones in a sample of Greek dental patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:e38–43. 10.1016/j.tripleo.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 19.Kerr AR, Phelan JA. Benign Lesions of the oral cavity : Greenberg MS, Glick M, Ship JA, Burket’s Oral Medicine. 11th edn New Delhi: BC Decker Inc, 2008: 143–5. [Google Scholar]
- 20.Abrahams JJ, Berger SB. Inflammatory disease of the jaw: appearance on reformatted CT scans. AJR Am J Roentgenol 1998;170:1085–91. 10.2214/ajr.170.4.9530065 [DOI] [PubMed] [Google Scholar]
- 21.Slootweg PJ. Lesions of the jaws. Histopathology 2009;54:401–18. 10.1111/j.1365-2559.2008.03097.x [DOI] [PubMed] [Google Scholar]
- 22.Nilesh K, Vande A, Reddy S. Central compact osteoma of mandibular condyle. BMJ Case Rep 2020;13:pii: e233082. 10.1136/bcr-2019-233082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilesh K, V Vande A, K Veerabhadrappa S. Solitary peripheral ivory osteoma of the mandible presenting with difficulty in deglutition: a case report. J Dent Res Dent Clin Dent Prospects 2017;11:56–60. 10.15171/joddd.2017.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilesh K, Bhujbal RB, Nayak AG. Solitary central osteoma of mandible in a geriatric patient: report and review. J Clin Exp Dent 2016;8:e219–22. 10.4317/jced.52792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojo MA, Omoregie OF, Altini M, et al. A Clinico-pathologic review of 56 cases of ossifying fibroma of the jaws with emphasis on the histomorphologic variations. Niger J Clin Pract 2014;17:619–23. 10.4103/1119-3077.141429 [DOI] [PubMed] [Google Scholar]
- 26.Commins DJ, Tolley NS, Milford CA. Fibrous dysplasia and ossifying fibroma of the paranasal sinuses. J Laryngol Otol 1998;112:964–8. 10.1017/S0022215100142203 [DOI] [PubMed] [Google Scholar]
- 27.Schaaf H, Streckbein P, Obert M, et al. High resolution imaging of craniofacial bone specimens by flat-panel volumetric computed tomography. J Craniomaxillofac Surg 2008;36:234–8. 10.1016/j.jcms.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 28.Nilesh K, Mukherji S. Intraoperative use of C-Arm machine to evaluate bone surgical margin of resected mandible specimen. International Journal of Health Sciences & Research 2015;5:338–40. [Google Scholar]
- 29.Devireddy SK, Senthil Murugan M, Kishore Kumar RV, et al. Evaluation of non-vascular fibula graft for mandibular reconstruction. J Maxillofac Oral Surg 2015;14:299–307. 10.1007/s12663-014-0657-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louvrier A, Marty P, Barrabé A, et al. How useful is 3D printing in maxillofacial surgery? J Stomatol Oral Maxillofac Surg 2017;118:206–12. 10.1016/j.jormas.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Shan X-F, Guo X-S, et al. Clinicopathological characteristics and prognosis of ossifying fibroma in the jaws of children: a retrospective study. J Cancer 2017;8:3592–7. 10.7150/jca.21556 [DOI] [PMC free article] [PubMed] [Google Scholar]