Abstract
In pancreatic cancer, imaging plays an essential role in surveillance, diagnosis, resectability evaluation, and treatment response evaluation. Pancreatic cancer surveillance in high-risk individuals has been attempted using endoscopic ultrasound (EUS) or magnetic resonance imaging (MRI). Imaging diagnosis and resectability evaluation are the most important factors influencing treatment decisions, where computed tomography (CT) is the preferred modality. EUS, MRI, and positron emission tomography play a complementary role to CT. Treatment response evaluation is of increasing clinical importance, especially in patients undergoing neoadjuvant therapy. This review aimed to comprehensively review the role of imaging in relation to the current treatment strategy for pancreatic cancer, including surveillance, diagnosis, evaluation of resectability and treatment response, and prediction of prognosis.
Keywords: Pancreatic cancer, Imaging, Diagnosis, Resectability, Response evaluation, Prognosis
INTRODUCTION
Pancreatic cancer is the fifth most common cause of cancer-related deaths in South Korea, and the fourth leading cause of cancer-related deaths in the United States and Europe (1,2,3,4). Surgical resection is considered to be the only potentially curative treatment for pancreatic cancer. The majority of pancreatic cancer patients are diagnosed in locally advanced or metastatic status. Only 15% to 20% of pancreatic cancer patients are candidates for surgical resection (5).
The role of imaging has been evolving in line with the development of pancreatic cancer treatment, and imaging plays a crucial role in the screening, diagnosis, preoperative staging, postoperative surveillance, and treatment response evaluation of pancreatic cancer. This review focused on the latest treatment strategies for pancreatic cancer, as well as the role, limitations, and the future direction of imaging.
Current Treatment Strategy for Pancreatic Cancer
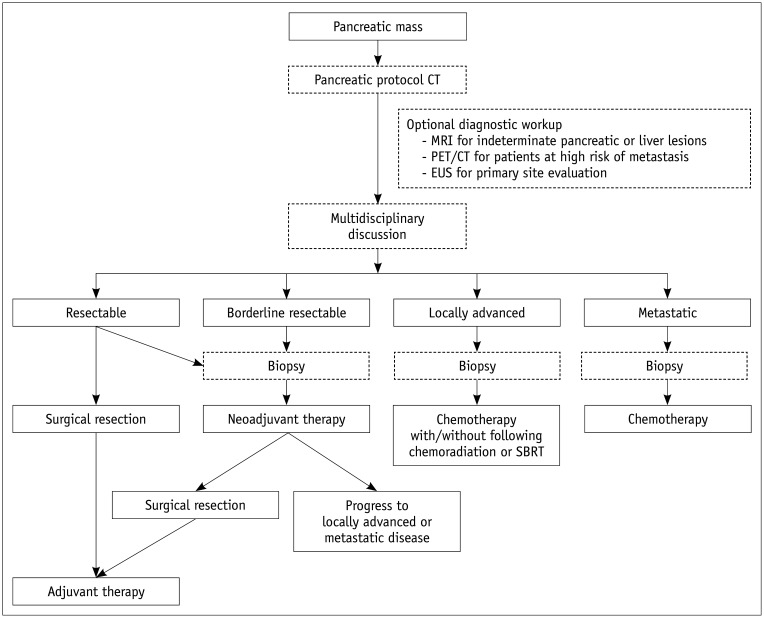
Pancreatic cancer is divided into four categories according to the local tumor extent and the presence of disseminated disease (Fig. 1). Treatment options vary for each category as follows (5,6,7):
Fig. 1. Treatment strategy for pancreatic cancer.
CT = computed tomography, EUS = endoscopic ultrasound, MRI = magnetic resonance imaging, PET = positron emission tomography, SBRT = stereotactic body radiation therapy
1) Resectable: tumors with a high probability of margin-negative resection
2) Borderline resectable: tumors that are involved with nearby structures and are neither resectable nor clearly unresectable with a high chance of an positive microscopic margin (R1) resection
3) Locally advanced: tumors that are involved with nearby structures to an extent that renders them unresectable despite the absence of evidence of metastatic disease
4) Metastatic: tumors that have disseminated.
Margin-negative (R0) resection of localized pancreatic cancer is considered as the only potentially curative treatment. The 5-year survival rate is approximately 18–24% when a R0 resection is achieved (8). R0 resection is defined by the absence of tumor cells within 1 mm of the surgical margin. Otherwise, the margin status is defined as a R1 or positive macroscopic margin (R2) (9,10). Patients categorized with either R1 or R2 margins in surgical resection show poor 5-year survival rates of 8–11%, similar to locally advanced disease (9,11,12,13,14). Patients who are considered to have high probability of R0 resection in radiologic evaluation are classified as “resectable,” and may be candidates for upfront surgery. To improve the survival of R0 resection patients, various adjuvant chemotherapeutic or chemoradiotherapeutic regimens have been attempted. According to two recent clinical trials, patients treated with combination chemotherapies, including modified 5-FU/leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX) or gemcitabine + capecitabine, showed significantly better overall survival than those treated with gemcitabine monotherapy (15,16). In all of the patients who underwent upfront surgical resection, adjuvant therapy is recommended, and the abovementioned combination chemotherapies are preferred in patients with good performance status (17,18).
Localized pancreatic cancers with a low likelihood of R0 resection can be divided into borderline resectable and locally advanced disease. Borderline resectable pancreatic cancer indicates tumors that are potentially downstaged and resectable upon favorable response to neoadjuvant therapy. Neoadjuvant therapy can increase the R0 resection rate in subsequent surgical resection, treat micrometastasis at an earlier stage, and provide an observation period to exclude pancreatic cancer showing rapid progression and poor response to therapy. Chemotherapy or chemoradiation therapies are more likely to be tolerated in the preoperative stage than in the postoperative stage (14,19). Two meta-analyses illustrated that approximately one-third of the borderline resectable pancreatic cancers could be completely resected, and the 5-year survival rate of those cases was promising (> 20%) (20,21). Chemotherapy with or without subsequent chemoradiation is commonly used in neoadjuvant therapy (22). However, no specific regimen is recommended due to limited evidence (5).
In unresectable pancreatic cancers, including locally advanced and metastatic disease, systemic chemotherapy is commonly employed. There are numerous options for a chemotherapeutic regimen. FOLFIRINOX/modified FOLFIRINOX, gemcitabine + nab-bound paclitaxel, and gemcitabine + cisplatin are the preferred regimens for patients with good performance status, while gemcitabine, capecitabine, and 5-FU monotherapy are the preferred regimens for patients with poor performance status. Chemoradiation or stereotactic body radiation therapy may be added for definitive treatment in locally advanced disease, and for palliative measures in metastatic disease.
Conventional Role of Imaging
Surveillance of Pancreatic Cancer
Surveillance is not recommended for asymptomatic general populations. In general populations, in which the incidence of pancreatic cancer is low (lifetime risk < 1.3%), the yield of surveillance is also low.
High-risk individuals (> 5% lifetime risk of pancreatic cancer) could be potential candidates for pancreatic cancer surveillance. High-risk individuals include 1) first-degree relatives (FDRs) of patients with pancreatic cancer from a familial pancreatic cancer kindred with at least two affected FDRs; 2) patients with Peutz-Jeghers syndrome; and 3) p16, BRCA2, and hereditary non-polyposis colorectal cancer mutation carriers with ≥ 1 affected FDRs (23). The detection of T1N0M0 pancreatic cancer that could be treated with R0 resection and high-grade dysplastic lesions should be the goal of surveillance. As screening modalities, endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI) are preferred. These imaging modalities have excellent sensitivity for small pancreatic lesions and do not use ionizing radiation. A few studies compared the diagnostic accuracy of EUS and MRI in a surveillance setting (24,25), and showed that EUS is more accurate in the detection of small solid lesions (24). However, MRI is more sensitive in the detection of cystic lesions and main pancreatic duct communication, allowing the diagnosis of intraductal papillary mucinous neoplasm, which is considered to be a precancerous lesion (24,26).
Imaging Diagnosis of Pancreatic Cancer
For imaging diagnosis of pancreatic cancer, diverse imaging modalities, including transabdominal ultrasound (US), computed tomography (CT), MRI and magnetic resonance cholangiopancreatography (MRCP), positron emission tomography (PET), and EUS are commonly used. The characteristics of these imaging modalities are summarized in Table 1.
Table 1. Characteristics of the Imaging Modalities Used for Diagnosis of Pancreatic Cancer.
| Imaging Modalities | Strengths | Weaknesses | Diagnostic Role in Imaging | Diagnostic Performance for Diagnosis of Pancreas Cancer |
|---|---|---|---|---|
| US | Accessibility | Sonographic window could be limited; operator dependent | Initial assessment of pancreatic lesion | Sensitivity 68–95% Specificity 50–100% |
| CT | High temporal and spatial resolution; wide anatomic coverage | Inappropriate for surveillance due to ionizing radiation; limited diagnostic performance in small pancreatic, hepatic, and peritoneal lesions | Primary imaging modality of pancreas cancer diagnosis, resectability evaluation, response evaluation | Sensitivity 89–91% Specificity 85–90% |
| MRI | Superior soft-tissue contrast; visualization of pancreatic and biliary duct abnormality | Lower spatial resolution; motion artifact | Adjunct diagnostic tool of equivocal pancreatic lesion and small hepatic lesion | Sensitivity 84–93% Specificity 82–89% |
| EUS | High spatial resolution; could be coupled with fine needle aspiration biopsy | Invasive; operator dependent | Adjunct diagnostic tool of equivocal pancreatic lesion Histologic diagnosis | Sensitivity 89–91% Specificity 81–86% |
| 18FDG PET/CT | Majority of pancreas cancer show increased 18FDG uptake; useful in evaluation of lymph node and distant metastasis | Difficult to distinguish between pancreatic cancer and pancreatitis | Evaluation of lymph node and distant metastasis | Sensitivity 89–91% Specificity 70–72% |
CT = computed tomography, EUS = endoscopic ultrasound, MRI = magnetic resonance imaging, PET = positron emission tomography, US = ultrasound, 18FDG = 18fluorine-2-fluoro-2-deoxy-D-glucose
Transabdominal Ultrasound
US is commonly used for initial imaging evaluation in asymptomatic or symptomatic patients. It is non-invasive, relatively inexpensive, and easily accessible. Pancreatic cancer often appears as a distinct or infiltrative hypoechoic focal pancreatic lesion, commonly accompanied by dilatation of the main pancreatic duct or bile duct. In conventional US, most focal pancreatic lesions exhibit hypoechogenicity; therefore, it is difficult to distinguish between pancreatic cancer and other focal pancreatic lesions. The diagnosis of pancreatic cancer in transabdominal US is highly dependent on the operator's technique, patient's body habitus, as well as the location and size of the tumor. The sensitivity and specificity of transabdominal US are considerably variable, ranging 68–95% and 50–100%, respectively (27,28,29). The limited diagnostic performance of US limits its role in the initial evaluation and lesion detection; therefore, US is rarely used for diagnosis, resectability evaluation, and response evaluation of pancreatic cancer.
Computed Tomography
CT shows excellent temporal and spatial resolution as well as wide anatomic coverage. It is recommended as the primary imaging modality for resectability evaluation according to the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines (5,7). The use of CT for treatment decision-making should include a thin (preferably submillimeter) and continuous section and ≤ 3 mm reconstruction, multiplanar reformation including the coronal plane, and maximal intensity projection or three-dimensional (3D) volumetric thick section images for vascular evaluation. For a proper evaluation of pancreatic lesions and adjacent vascular structures, both the pancreatic phase (40–50 seconds from intravenous contrast injection) and venous phase (65–70 seconds) should be included (6,7). If the CT images do not conform to the pancreatic protocol, re-examination using a high-quality pancreatic protocol CT is recommended for precise evaluation of tumor staging (30). Since pancreatic cancer can show rapid progression and dissemination, imaging evaluation should be performed within a month of definitive treatment (31).
Pancreatic cancer is usually seen as a mass lesion that exhibits hypoenhancement compared to the adjacent parenchyma in the pancreatic phase. It may cause interruption and upstream dilatation of the pancreatic or bile duct, abutment or encasement of adjacent vascular structures, direct invasion of adjacent organs, and regional lymph node enlargement. In meta-analyses, CT has shown sensitivity of 89–91% and specificity of 85–90% for the diagnosis of pancreatic cancer (32,33,34). Liver, peritoneum, and distant lymph nodes are the most common metastatic sites. Approximately 5% of pancreatic cancer may exhibit isoattenuation in both the pancreatic parenchymal and venous phases (35,36). In addition, CT shows low diagnostic accuracy for small liver, peritoneal, or lymph node metastasis (37,38,39).
Magnetic Resonance Imaging and Magnetic Resonance Cholangiopancreatography
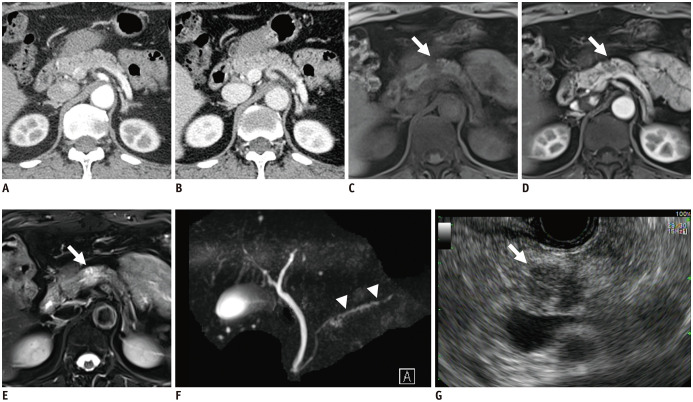
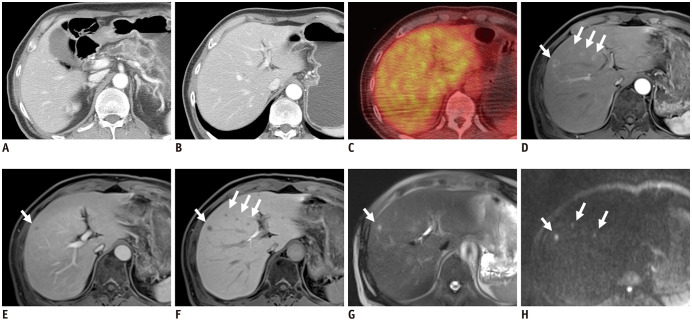
MRI for pancreatic cancer evaluation is recommended to include the following sequences: T2-weighted fast spine-cho; T1-weighted in-and-out of phase gradient-echo; T2-weighted fat-suppressed fast spin-echo; diffusion weighted imaging (DWI); 3D T1-weighted fat-suppressed gradient-echo dynamic images including precontrast, pancreatic, venous, and equilibrium phases; and T2-weighted MRCP sequences (7). The availability of various sequences and the superior soft-tissue contrast of MRI could assist in the detection and characterization of small, subtle, cystic, or isoattenuating pancreatic lesions and small liver lesions (Fig. 2). MRCP can non-invasively visualize abnormalities of the entire pancreatic and bile duct, including anatomic variations and obstructive dilatation. With these advantages, MRI is used as a problem-solving tool for indeterminate pancreatic lesions (especially small or isoattenuating tumors) or small liver lesions. MRI also shows some disadvantages, such as lower spatial resolution, vulnerability to motion artifacts, and limited multiplanar reformation capability. With its own advantages and disadvantages, MRI has shown similar diagnostic performance as CT. In meta-analyses, MRI has shown sensitivity of 84–93% and specificity of 82–89% for the diagnosis of pancreatic cancer (32,33,34,40). In candidates for upfront surgery, MRI with DWI can detect hepatic metastasis in about 1.5–2.3% of patients with no hepatic lesions on CT, and about 10.5–13.6% of those with indeterminate liver lesions on CT (41). Particularly, MRI with hepatobiliary contrast using gadoxetic acid demonstrates higher sensitivity than CT (85% vs. 69%) and higher accuracy for differentiating between metastasis and hepatic microabscess (37,42). With increased sensitivity for liver metastasis, an additional MRI may change the results of resectability assessments in a significant number of patients (14.4%) (Fig. 3) (43).
Fig. 2. Isoattenuating pancreatic cancer on CT.
A 55-year-old female patient was referred for a small pancreatic lesion that was detected in transabdominal ultrasound (image was not available).
Both pancreatic (A) and venous phases (B) in CT showed no demonstrable lesion in the pancreas or significant pancreatic duct dilatation. MRI showed an approximately 1.5-cm focal pancreatic lesion (white arrows) with hypointensity in the T1-weighted image (C), hypoenhancement in the pancreatic phase (D), and moderate hyperintensity in the T2-weighted image (E). Magnetic resonance cholangiopancreatography (F) showed minimal pancreatic duct dilatation distal to the pancreatic mass, suggesting duct involvement (white arrowheads). The mass was seen as a hypoechoic mass in EUS (G).
Fig. 3. Detection of small liver metastasis in MRI.
A 65-year-old male patient was admitted for chronic pancreatitis and pancreatic body cancer.
Since there was no apparent vascular invasion in CT (A) and no demonstrable metastatic lesion in CT (B) and PET/CT (C), the pancreatic lesion was considered to be resectable. However, MRI showed multiple small hepatic lesions (white arrows) with peripheral enhancement in the pancreatic phase (D), hypointensity in the venous phase (E), decreased uptake in the hepatobiliary phase (F), hyperintensity in the T2-weighted image (G), and high signal intensity in the diffusion weighted image (b = 800) (H), suggesting liver metastases.
Pancreatic cancer shows hypointensity in precontrast T1-weighted images. In T2-weighted images with or without fat suppression, the signal intensity of pancreatic cancer is variable (44). After contrast enhancement, the tumor usually shows hypoenhancement in the pancreatic phase, and occasionally delayed enhancement in the equilibrium phase. Since the majority of pancreatic cancers show restricted diffusion, DWI could help detect pancreatic cancer (45,46). However, pancreatitis could appear as restricted diffusion, which is often indistinguishable from pancreatic cancer on DWI. In addition, DWI has poor spatial resolution and is vulnerable to artifacts caused by motion or bowel gas (47). Pancreatic cancer also often exhibits upstream pancreatic duct dilatation or cutoff on MRCP or T2-weighted imaging.
Positron Emission Tomography
18Fluorine-2-fluoro-2-deoxy-D-glucose (18FDG) is the most widely used radiotracer in PET scans. As a glucose analogue, 18FDG allows in vivo imaging of glycolytic activity, which is usually elevated in solid tumors, including pancreatic cancer. Both KRAS mutation, which is observed in most (> 90%) pancreatic cancers, and a hypoxic microenvironment increase 18FDG uptake by upregulating HK2 and GLUT1 expression. Since focal pancreatitis can also exhibit increased 18FDG uptake, it is difficult to distinguish between pancreatic cancer and focal pancreatitis (48). The potential additional benefits of 18FDG-PET or 18FDG-PET/CT over pancreatic CT in the diagnosis of pancreatic cancer remain debatable (34,49,50,51). 18FDG-PET/CT shows a sensitivity of 89–91% and specificity of 70–72% for diagnosis of pancreatic cancer (33,34). PET/CT covers the entire body and is beneficial for finding distant metastases. It may also be useful in lymph node staging (51,52). The NCCN guidelines recommend that PET/CT should not be a substitute for pancreatic CT or MRI; however, it could be combined with CT or MRI as an adjunct modality in patients at high risk of metastatic disease, such as those with borderline resectable disease, markedly elevated CA19-9 levels, large primary tumors, large regional lymph nodes, and a very symptomatic presentation (7).
Endoscopic Ultrasound
Because of its high spatial resolution, EUS can be used to obtain additional information for pancreatic cancers when the pancreatic lesion is equivocal in CT or when there is questionable blood vessel or lymph node involvement (53,54,55). However, the operator dependence of EUS and anatomic variations of the celiac and superior mesenteric arteries can limit the diagnostic capability of EUS. EUS shows sensitivity of 89–91% and specificity of 81–86% for the diagnosis of pancreatic cancer (33,34). However, the inclusion of EUS as a routine imaging tool for a resectability evaluations remains controversial. EUS is included as a routine imaging tool in the ESMO guidelines, but not in the NCCN guidelines. The primary goal of EUS is to perform pathologic diagnosis using fine-needle aspiration (FNA).
Histologic Diagnosis of Pancreatic Cancer
For patients with resectable disease, preoperative histologic confirmation is not mandatory, and resection should not be delayed when clinical suspicion of pancreatic cancer is high (7). However, biopsy should be performed for patients with unresectable disease before initiating neoadjuvant or systemic chemotherapy with or without radiation. FNA using EUS is commonly performed for pathologic diagnosis. According to a recent meta-analysis, EUS-guided FNA has a sensitivity of 91% and specificity of 97% (56). Owing to the shorter penetration depth of EUS-guided FNA, in comparison with percutaneous CT or US-guided FNA, the diagnostic yield is similar, and the probability of postprocedural complications and peritoneal seeding is low (57,58). Even in patients with resectable disease, FNA was not significantly associated with increased mortality, suggesting that FNA can be safely performed without having a significant impact on the patient's clinical course (59). If EUS-guided FNA for a tumor is not possible, other methods such as brushing cytology with cholangiography, CT- or US-guided percutaneous biopsy, and laparoscopic biopsy could be performed as alternatives. Percutaneous biopsy is contraindicated in potentially resectable pancreatic cancer, due to the risk of tumor seeding (5).
Recently, the importance of genetic profiling of tumors has been gradually emphasized. In the recently updated NCCN guidelines, genetic profiling of tumor tissue was strongly recommended (17). Genetic profiling may require additional tissue sampling; however, it could provide clinically relevant information. Targeted DNA sequencing and analysis using biopsied tissue could be performed without delaying routine diagnostic workup for pancreatic cancer, which could identify potentially actionable targets in 17–26% of patients (60,61).
Staging of Pancreatic Cancer
For pathologic staging, tumor-node-metastasis (TNM) staging developed by the American Joint Committee on Cancer is commonly used. Recently, TNM staging was updated to the 8th edition (62). In the 8th edition, T stage was changed to be based on the tumor size, and the extrapancreatic extension and resectable status were removed from the definition of T stage (Table 2). Regional lymph node metastasis was subdivided into N1 and N2, according to the number of metastatic lymph nodes. With the changed definitions of T and N stages, the 8th edition provides better reproducibility and improved prognostic accuracy compared to the 7th edition (63,64,65,66). Particularly, the newly introduced N2 stage is highly prognostic, emphasizing the importance of nodal staging (63,64).
Table 2. Pathologic Tumor-Node-Metastasis Staging System of the AJCC.
| T Category | AJCC 7th Edition | AJCC 8th Edition | |
|---|---|---|---|
| T Criteria | T Criteria | Changes in 8th Edition | |
| TX | Primary tumor cannot be assessed | Primary tumor cannot be assessed | |
| T0 | No evidence of primary tumor | No evidence of primary tumor | |
| Tis | Carcinoma in situ, including PanIN with high-grade dysplasia | Carcinoma in situ, including PanIN, IPMN, ITPN, and MCN with high-grade dysplasia | IPMN, ITPN, and MCN with high grade dysplasia were added to this category |
| T1 | Tumor limited to the pancreas, 2 cm or less in greatest dimension | Tumor ≤ 2 cm in greatest dimension T1a: tumor ≤ 0.5 cm in greatest dimension T1b: tumor > 0.5 cm and < 1 cm in greatest dimension T1c: tumor 1–2 cm in greatest dimension |
T1 were subcategorized into T1a, T1b, and T1c based on size |
| T2 | Tumor limited to the pancreas, more than 2 cm in greatest dimension | Tumor > 2 cm and ≤ 4 cm in greatest dimension | Definitions of T2, T3 were based on size |
| T3 | Tumor extends beyond the pancreas, but without involvement of the celiac axis or the SMA | Tumor > 4 cm in greatest dimension | Extrapancreatic extension was removed from the criteria |
| T4 | Tumor involves the celiac axis or the SMA (unresectable primary tumor) | Tumor involves celiac axis, SMA, and/or CHA, regardless of size | Resectability was removed from the definition |
| N Category | N Criteria | N Criteria | |
| NX | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed | |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis | |
| N1 | Regional lymph node metastasis | Metastasis in one to three regional lymph nodes | Regional lymph node positivity was divided to N1 and N2 based on the number of metastatic lymph nodes |
| N2 | Metastasis in four or more regional lymph nodes | ||
| M Category | M Criteria | M Criteria | |
| M0 | No distant metastasis | No distant metastasis | |
| M1 | Distant metastasis | Distant metastasis | |
| Prognostic Stage Groups | Criteria | Criteria | |
| 0 | Tis N0 M0 | Tis N0 M0 | |
| IA | T1 N0 M0 | T1 N0 M0 | |
| IB | T2 N0 M0 | T2 N0 M0 | |
| IIA | T3 N0 M0 | T3 N0 M0 | |
| IIB | T1 N1 M0 T2 N1 M0 T3 N1 M0 |
T1 N1 M0 T2 N1 M0 T3 N1 M0 |
|
| III | T4 (any N) M0 | T1 N2 M0 T2 N2 M0 T3 N2 M0 T4 (any N) M0 |
T1–3 N2 M0 pancreatic cancers were added to stage III |
| IV | (Any T) (any N) M1 | (Any T) (any N) M1 | |
AJCC = American Joint Committee on Cancer, CHA = common hepatic artery, IPMN = intraductal papillary mucinous neoplasm, ITPN = intraductal tubulopapillary neoplasm, MCN = mucinous cystic noeplasm, PanIN = pancreatic intraepithelial neoplasia, SMA = superior mesenteric artery
The treatment strategy for pancreatic cancer is determined by the resectability status, and pathologic staging is only possible in resected pancreatic cancers; therefore, the clinical utility of pathologic TNM stage is limited.
Resectability Evaluation
The resectability of pancreatic cancer plays a pivotal role in deciding the treatment strategy. Localized pancreatic cancers can be categorized as resectable pancreatic cancers that are candidates for upfront surgical resection, borderline resectable pancreatic cancers that could be candidates for surgical resection upon favorable response to neoadjuvant therapy, and locally advanced pancreas cancers in which surgical resection is difficult to attempt and chemotherapy and/or radiation therapy are preferred. Several resectability criteria have been proposed (Table 3), and they share key anatomic structures for determining resectability, including the celiac artery, common hepatic artery (CHA), superior mesenteric artery (SMA), superior mesenteric vein (SMV), and portal vein (PV) (6,7,14,67). Detailed assessment of vascular contact or involvement should be performed, including abutment (tumor involvement of ≤ 180° of the vascular circumference), encasement (tumor involvement of > 180° of the vascular circumference), deformity, occlusion, and thrombosis (bland or tumor) (Fig. 4).
Table 3. Comparison of Resectability Criteria for Pancreatic Cancer without Distant Metastasis.
| Resectability Status | Resectability Criteria | |||
|---|---|---|---|---|
| MD Anderson (14) | AHPBA/SSAT/SSO (6) | Alliance (60) | NCCN (7) | |
| Celiac artery | ||||
| Resectable | No involvement | No involvement | No involvement | No involvement |
| Borderline | Short-segment abutment or encasement | Abutment (≤ 180°) | Abutment (≤ 180°) (Body/tail only) encasement (> 180°) without aorta nor gastroduodenal artery, reconstructable with modified Appleby procedure |
|
| Locally advanced | Encasement and no technical option for reconstruction | Any involvement | Encasement (> 180°) | (Head/uncinate only) encasement (> 180°) (Body/tail only) encasement (> 180°), surgically unreconstructable |
| CHA | ||||
| Resectable | No involvement | No involvement | No involvement | No involvement |
| Borderline | Short-segment abutment or encasement | Abutment (≤ 180°) or gastroduodenal artery encasement up to hepatic artery | Any surgically reconstructable involvement | Any involvement without celiac axis or CHA bifurcation |
| Locally advanced | Encasement and no technical option for reconstruction | Encasement (> 180°) | Surgically unreconstructable involvement | Any involvement with celiac axis or CHA bifurcation |
| SMV | ||||
| Resectable | No involvement | No involvement | No involvement | No involvement |
| Borderline | Abutment (≤ 180°) | Abutment (≤ 180°) | Abutment (≤ 180°) | Abutment (≤ 180°) |
| Locally advanced | Encasement (> 180°) | Encasement (> 180°) | Encasement (> 180°) | Encasement (> 180°) |
| SMV/PV | ||||
| Resectable | Patent | No abutment, distortion, tumor thrombus, or encasement | No involvement or abutment (≤ 180°) | No involvement or abutment (≤ 180°), without vein contour irregularity |
| Borderline | Short-segmental occlusion and surgically reconstructable | Any surgically reconstructable involvement | Any surgically reconstructable involvement | Encasement (> 180°), or abutment (≤ 180°) with venous contour irregularity or thrombosis, but surgically reconstructable |
| Locally advanced | Occluded and no technical option for reconstruction | Surgically unreconstructable involvement | Surgically unreconstructable involvement | Surgically unreconstructable involvement or occlusion |
AHPBA = American Hepato-Pancreato-Biliary Association, NCCN = National Comprehensive Cancer Network, PV = portal vein, SMV = superior mesenteric vein, SSAT = Society for Surgery of the Alimentary Tract, SSO = Society of Surgical Oncology
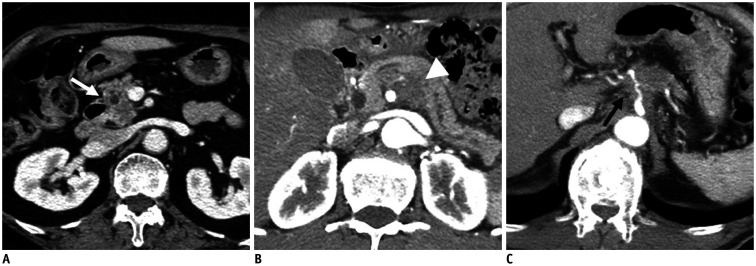
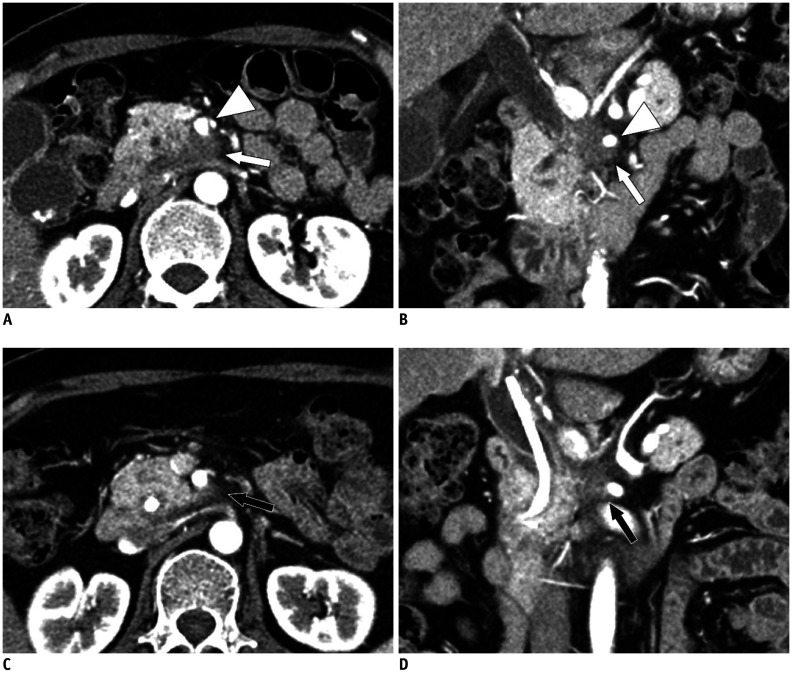
Fig. 4. Resectability of pancreatic cancer determined by the National Comprehensive Cancer Network criteria.
A. Approximately 2-cm resectable pancreatic cancer (white arrow) confined to the pancreas showing no vascular involvement. B. Approximately 2.5-cm borderline resectable pancreatic cancer (white arrowhead) exhibiting superior mesenteric artery contact of less than 180°. C. Approximately 3.7-cm locally advanced pancreatic cancer (black arrow) showing encasement of the celiac artery and proximal common hepatic artery.
The main difference between guidelines is related to the inclusion of surgical reconstructability of artery and vein in the determination of borderline resectability. For SMV/PV, in all guidelines, surgically reconstructable involvement of pancreatic cancer was used as a criterion for borderline resectability. For CHA, the MD Anderson and Alliance for Clinical Trials in Oncology group criteria use surgically reconstructable involvement as a criterion for borderline resectability; however, the NCCN criteria use celiac axis or CHA-celiac bifurcation involvement as a criterion. For the celiac axis, surgically reconstructable involvement is a criterion for borderline resectability in MD Anderson criteria. In contrast, NCCN criteria describe the cases of borderline resectability in detail, according to tumor location. For SMA, all guidelines use the criteria for tumor abutment rather than surgical reconstructability in determining borderline resectability.
Although the definition of resectability is debatable, both the NCCN and ESMO guidelines currently recommend the use of NCCN resectability criteria, which is adapted from a consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association (8,68). When resectability was evaluated by NCCN guidelines, the R0 resection rate in upfront surgery was reported to be 73%, 55%, and 16% in resectable, borderline resectable, and locally advanced status, respectively (69).
The resectability of pancreatic cancer should be determined through a multidisciplinary consultation, ideally including diagnostic imaging, interventional endoscopy, medical oncology, radiation oncology, surgery, pathology, geriatric medicine, and palliative care (7). For proper communication among experts in various fields, the use of a structured reporting form is recommended for radiologic reporting (68).
In a meta-analysis performed in 2005, sensitivity and specificity of helical CT for resectability were 81% and 82%, respectively, while those of MRI were 82% and 78%, respectively (32). A prospective study published in 2004 also showed that helical CT has a superior diagnostic performance (sensitivity and specificity of 76% and 97%, respectively) compared to MRI (57% and 90%) and EUS (23% and 100%) (70). In these reports, most of the CTs were helical CT, not multidetector CT, with limited 3D reformatting capability, and MRI showed low spatial resolution with two-dimensional T1 sequences (≥ 5 mm section thickness). More recently, several studies compared the diagnostic performance for resectability between multidetector CT and MRI with a 3D T1 sequence for dynamic phases. They showed similar diagnostic performance of multidetector CT (sensitivity and specificity of 87–88% and 63–86%, respectively) and MRI (sensitivity and specificity of 83–93% and 50–75%, respectively). Although MRI shows similar diagnostic performance as that of CT, CT is preferred over MRI due to the limited availability and high cost of MRI.
A problem with resectability evaluations using the current imaging modalities is the debate in interobserver agreements. One study reported a very high interobserver agreement on NCCN criteria (71), while another study demonstrated low interobserver agreement even with experienced radiologists, particularly for borderline resectable cases (72).
Surveillance after Surgical Resection
The value of postoperative surveillance in pancreatic cancer is controversial. Although routine surveillance imaging studies would increase medical costs, there is no firm evidence of routine surveillance increasing survival rates (73,74,75). Although carbohydrate antigen 19-9 (CA19-9) is the most widely used serum biomarker for postoperative surveillance, CA19-9 surveillance lacks evidence for survival benefits (76). The ESMO guideline does not recommend routine imaging studies for postoperative surveillance. In contrast, the NCCN guideline recommends chest CT, abdominal and pelvic CT or MRI, and serum CA19-9 examination every 3–6 months for 2 years after surgery and then every 6–12 months.
Unsolved or Debated Issues Related to Imaging in Pancreatic Cancer
Resectability Evaluation in Patients Receiving Neoadjuvant Treatment
When evaluating images of patients undergoing chemotherapy or chemoradiation with the aim of neoadjuvant therapy, the radiologist should evaluate both the progression and regression of the disease. Assessment of an increasing tumor extent or newly occurring metastatic lesions is important, as the lesion in such cases often becomes unresectable and the treatment strategy must be changed.
Radiologic evaluation of tumor regression is known to be more difficult than tumor progression in pancreatic cancer, especially after neoadjuvant therapy. Radiologic response does not accurately reflect pathological tumor regression (77). Neoadjuvant therapy induces necrosis, edema, inflammation, and fibrosis of the tumor, interfering with the radiologic evaluation of tumor regression (77,78). Therefore, neoadjuvant therapy decreases the accuracy of CT scans in determining resectability. Overestimation of the remaining tumor size and vascular invasion is commonly known to occur (79). Thus, changes in tumor size are not well-associated with resectability after neoadjuvant therapy (80).
In a retrospective study, among 122 borderline resectable patients who underwent neoadjuvant therapy, only a small proportion demonstrated a radiologic complete response (0%) or partial response (12%), while the majority showed stable disease (69%). Radiologic downstaging from borderline resectable to resectable status was only observed in one (0.8%) patient. Despite the limited radiologic response, 66% (85/129) of the patients underwent surgical resection, and R0 resection was achieved in 95% (81/85) of patients who underwent surgery. Tumor response evaluated by the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 was not associated with overall survival (81). Another study showed that the majority of patients who underwent neoadjuvant therapy and surgical resection continued to exhibit a locally advanced or borderline resectable stage (70%) on CT. However, R0 resection was possible in 92% of cases (82).
To overcome the limitations of radiologic response evaluation, several alternative assessment methods have been investigated (83,84,85). A recent prospective study suggested that partial regression of tumor contact with vascular structures on pancreatic CT indicates a high likelihood of R0 resection and suitability for surgical exploration (86). A perivascular halo in post-neoadjuvant therapy CT could also be a sign of regression of tumor-vascular contact and the possibility of R0 resection (87). Increased tumor attenuation in the pancreatic and venous phases of post-neoadjuvant CT, compared to pre-neoadjuvant CT, was most likely attributed to increased fibrosis and was associated with R0 resection (88). However, changes in tumor attenuation require prospective validation, as other studies have shown contradictory results (86,89).
Recent studies have indicated that quantitative radiomic analysis is promising for predicting histologic tumor response. In patients with appropriate histologic response to chemoradiation, a decreased mean CT number, skewness, and increased kurtosis have been observed in posttreatment unenhanced CT (90).
Perfusion or diffusion parameters could also be used to predict resectability after neoadjuvant treatment. A high value of the volume transfer constant in pretreatment CT or MRI was significantly correlated with radiologic tumor response (91,92). Preoperative or postoperative apparent diffusion coefficient (ADC) values were significantly associated with R0 resection (93,94). An increased ADC in post-chemoradiation MRI compared to that in pre-chemoradiation MRI was associated with a histopathological response of pancreatic cancer, suggesting that ADC is a potential biomarker for pathological response (94). A small prospective study showed an association between the metabolic response in PET/CT (≥ 30% decreased 18FDG uptake after neoadjuvant therapy) and histologic tumor regression (95). Another study also showed that better pathologic response is expected in a metabolic responder (pretreatment standardized uptake value [SUV] ≥ 4.7 and ≥ 46% decreased 18FDG uptake after neoadjuvant therapy) (96).
These novel imaging parameters, including radiomics, perfusion, diffusion, and metabolic imaging, demonstrate promising results; however, there is limited evidence for predicting R0 resection before surgical resection.
Response Evaluation in Locally Advanced Pancreatic Cancer
In patients with unresectable disease, the World Health Organization (WHO) guidelines or RECIST version 1.0 or 1.1 are widely used for evaluation of response to chemotherapy and/or chemoradiation. In recent phase III clinical trials comparing combined chemotherapy regimens (FOLFIRINOX or gemcitabine + nab-paclitaxel) and gemcitabine monotherapy, RECIST 1.0 was used for response assessment (97,98). In these studies, progression-free survival increased along with the overall survival in the combined chemotherapy group, suggesting that progression defined by RECIST 1.0 has clinical relevance. However, evaluation of imaging response in unresectable disease is associated with the same problems as those encountered in cases undergoing neoadjuvant therapy, including difficulties in size measurement in infiltrative or irregular tumors as well as inaccuracies in the assessment of tumor regression (Fig. 5). In a consensus statement from the National Cancer Institute clinical trials planning meeting on pancreatic cancer treatment, tumor shrinkage assessed by either WHO or RECIST was not recommended as a primary endpoint of clinical trials, as they are poor surrogates for overall survival (99).
Fig. 5. Pancreatic cancer showing partial response after a long period of chemotherapy.
A 55-year-old female was diagnosed with pancreatic cancer in the uncinate process.
A, B. The pancreatic phase of initial CT showed an infiltrative hypoenhancing mass lesion (white arrows) involving the uncinate process and retroperitoneal margin, as well as encasing superior mesenteric artery (white arrowheads) and its jejunal branches, suggesting locally advanced tumor. C, D. After approximately 2 years of FOLFIRINOX chemotherapy, the lesion (black arrows) showed a reduction in size and extent of vascular involvement. It was apparent that the tumor became smaller with chemotherapy, but it was difficult to determine exactly how much of the viable tumor remained. The patient underwent pancreaticoduodenectomy, and margin negative (R0) resection was achieved without resection of vessels.
In the nab-paclitaxel + gemcitabine phase III trial, metabolic response defined by decreased SUV on 18FDG-PET/CT was more frequently observed than radiologic response, and metabolic response was associated with longer overall survival (100).
Predicting Prognosis in Patients with Upfront Surgery
According to the current guidelines, resectable pancreatic cancers are recommended for upfront surgery (5,7,18). However, resectable pancreatic cancers with high-risk features, such as high serum CA19-9 levels, large primary tumors, large regional lymph nodes, as well as excessive weight loss and extreme pain, could also be candidates for neoadjuvant therapy (7). Recently, the role of neoadjuvant strategy has been gradually increasing. In a recent meta-analysis, surgery after neoadjuvant therapy was reported to improve overall survival compared to adjuvant chemotherapy after upfront surgery (101). To date, no large-scale phase III trial has been published; however, small prospective trials showed better survival using neoadjuvant strategies (102). If we could perform preoperative survival stratification of the candidates for upfront surgery, patients with predicted poor prognosis may be good candidates for neoadjuvant strategies. Imaging studies are expected to play an important role in the survival stratification and assessment of resectability. However, only a limited number of studies are being performed on the imaging prognostic biomarker.
Pancreatic cancers with irregular rim-like enhancement and a relatively hypovascular central area on dynamic MRI showed poor differentiation and frequent tumor necrosis, as well as poorer disease-free survival and overall survival (103). Similarly, several studies demonstrated that lower enhancement of pancreatic cancer in the venous phase of CT was associated with poor overall survival (104,105,106). A CT texture analysis revealed that low average attenuation and standard deviation in the pancreatic phase image was associated with poor disease-free survival (107). Poorly enhancing areas of pancreatic cancer correspond to necrotic or fibrotic areas, which contribute to the aggressive nature of the disease (108).
DWI is another imaging method that reflects the fibrotic stromal component of pancreatic cancer (109,110). However, the prognostic significance of ADC is variable; some studies showed strong association between low ADC values and poor overall survival, while others demonstrated no significant association (103,111,112,113). Recently, the low ADC value of the upstream pancreas was reported to be significantly associated with overall survival after curative resection, suggesting an association between inflammation and pancreatic cancer progression (111).
18FDG-PET/CT can also be used to predict the postsurgical outcome of pancreatic cancer. Several studies have reported that the metabolic tumor volume, total lesion glycolysis, or maximum SUV (SUVmax) were associated with disease-free survival or overall survival. In particular, pancreatic cancers with high SUVmax values showed consistently poor overall survival, although the cutoff point was different among studies (114,115,116,117).
Summary
In pancreatic cancer, imaging plays an essential role in the surveillance, diagnosis, resectability evaluation, and response evaluation. With the development of therapeutic strategies for pancreatic cancer, the role of imaging has been gradually changing. Surveillance of pancreatic cancer should be performed only in high-risk individuals, and MRI and EUS are the preferred imaging modalities. CT is primarily used for imaging diagnosis and resectability evaluation of pancreatic cancers, and MRI, PET-CT, and EUS could be optionally used at the radiologist's discretion. It is not accurate to evaluate the regression of pancreatic cancer in imaging after chemotherapy or chemoradiation therapy, and tumor shrinkage in imaging is a poor surrogate for overall survival of pancreatic cancer. Although innovative evaluation methods using new radiologic criteria (i.e., perfusion imaging, radiomics, DWI, PET/CT, etc.) have been proposed, there is insufficient evidence for their clinical usefulness. Post-surgical outcome prediction in resectable pancreatic cancers, treatment response evaluation, and prognosis prediction of unresectable pancreatic cancers are problems that remain unsolved.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann Oncol. 2019;30:781–787. doi: 10.1093/annonc/mdz051. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Eeurope: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–430. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 6.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 7.Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert JW, Wolpin B, Clancy T, Wang J, Mamon H, Shinagare AB, et al. Borderline resectable pancreatic cancer: conceptual evolution and current approach to image-based classification. Ann Oncol. 2017;28:2067–2076. doi: 10.1093/annonc/mdx180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 10.Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surgs. 2008;207:510–519. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. doi: 10.1097/00000658-200112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. Folfirinox or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 17.Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17:603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 18.Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek C, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:2082–2088. doi: 10.1200/JCO.19.00946. [DOI] [PubMed] [Google Scholar]
- 19.Mornex F, Girard N, Delpero JR, Partensky C. Radiochemotherapy in the management of pancreatic cancer—Part I: neoadjuvant treatment. Semin Radiat Oncol. 2005;15:226–234. doi: 10.1016/j.semradonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Gillen S, Schuster T, Meyer Zum, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assifi MM, Lu X, Eibl G, Reber HA, Li G, Hines OJ. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery. 2011;150:466–473. doi: 10.1016/j.surg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VW, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059–2069. doi: 10.1007/s11605-011-1659-7. [DOI] [PubMed] [Google Scholar]
- 23.US Preventive Services Task Force. Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. JAMA. 2019;322:438–444. doi: 10.1001/jama.2019.10232. [DOI] [PubMed] [Google Scholar]
- 24.Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505–1513. doi: 10.1136/gutjnl-2014-308008. [DOI] [PubMed] [Google Scholar]
- 25.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capurso G, Signoretti M, Valente R, Arnelo U, Lohr M, Poley JW, et al. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc. 2015;7:833–842. doi: 10.4253/wjge.v7.i9.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlson BM, Ekbom A, Lindgren PG, Källskog V, Rastad J. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology. 1999;213:107–111. doi: 10.1148/radiology.213.1.r99oc25107. [DOI] [PubMed] [Google Scholar]
- 28.Maringhini A, Ciambra M, Raimondo M, Baccelliere P, Grasso R, Dardanoni G, et al. Clinical presentation and ultrasonography in the diagnosis of pancreatic cancer. Pancreas. 1993;8:146–150. doi: 10.1097/00006676-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Xu MM, Sethi A. Imaging of the pancreas. Gastroenterol Clin North Am. 2016;45:101–116. doi: 10.1016/j.gtc.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Walters DM, Lapar DJ, de Lange EE, Sarti M, Stokes JB, Adams RB, et al. Pancreas-protocol imaging at a high-volume center leads to improved preoperative staging of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2011;18:2764–2771. doi: 10.1245/s10434-011-1693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raman SP, Reddy S, Weiss MJ, Manos LL, Cameron JL, Zheng L, et al. Impact of the time interval between MDCT imaging and surgery on the accuracy of identifying metastatic disease in patients with pancreatic cancer. AJR Am J Roentgenol. 2015;204:W37–W42. doi: 10.2214/AJR.13.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, et al. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438–445. doi: 10.1097/01.rct.0000164513.23407.b3. [DOI] [PubMed] [Google Scholar]
- 33.Treadwell JR, Zafar HM, Mitchell MD, Tipton K, Teitelbaum U, Jue J. Imaging tests for the diagnosis and staging of pancreatic adenocarcinoma: a meta-analysis. Pancreas. 2016;45:789–795. doi: 10.1097/MPA.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 34.Toft J, Hadden WJ, Laurence JM, Lam V, Yuen L, Janssen A, et al. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: a systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol. 2017;92:17–23. doi: 10.1016/j.ejrad.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB., Jr Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764–768. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Park SH, Yu ES, Kim MH, Kim J, Byun JH, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. 2010;257:87–96. doi: 10.1148/radiol.10100015. [DOI] [PubMed] [Google Scholar]
- 37.Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, et al. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology. 2011;260:446–453. doi: 10.1148/radiol.11103548. [DOI] [PubMed] [Google Scholar]
- 38.Roche CJ, Hughes ML, Garvey CJ, Campbell F, White DA, Jones L, et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR Am J Roentgenol. 2003;180:475–480. doi: 10.2214/ajr.180.2.1800475. [DOI] [PubMed] [Google Scholar]
- 39.Dirisamer A, Schima W, Heinisch M, Weber M, Lehner HP, Haller J, et al. Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur J Radiol. 2009;69:536–541. doi: 10.1016/j.ejrad.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Chen FM, Ni JM, Zhang ZY, Zhang L, Li B, Jiang CJ. Presurgical evaluation of pancreatic cancer: a comprehensive imaging comparison of CT versus MRI. AJR Am J Roentgenol. 2016;206:526–535. doi: 10.2214/AJR.15.15236. [DOI] [PubMed] [Google Scholar]
- 41.Jeon SK, Lee JM, Joo I, Lee DH, Ahn SJ, Woo H, et al. Magnetic resonance with diffusion-weighted imaging improves assessment of focal liver lesions in patients with potentially resectable pancreatic cancer on CT. Eur Radiol. 2018;28:3484–3493. doi: 10.1007/s00330-017-5258-1. [DOI] [PubMed] [Google Scholar]
- 42.Choi SY, Kim YK, Min JH, Cha DI, Jeong WK, Lee WJ. The value of gadoxetic acid-enhanced MRI for differentiation between hepatic microabscesses and metastases in patients with periampullary cancer. Eur Radiol. 2017;27:4383–4393. doi: 10.1007/s00330-017-4782-3. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Park MS, Lee JY, Han K, Chung YE, Choi JY, et al. Incremental role of pancreatic magnetic resonance imaging after staging computed tomography to evaluate patients with pancreatic ductal adenocarcinoma. Cancer Res Treat. 2019;51:24–33. doi: 10.4143/crt.2017.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamm EP, Bhosale PR, Vikram R, de Almeida Marcal LP, Balachandran A. Imaging of pancreatic ductal adenocarcinoma: state of the art. World J Radiol. 2013;5:98–105. doi: 10.4329/wjr.v5.i3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007;188:409–414. doi: 10.2214/AJR.05.1918. [DOI] [PubMed] [Google Scholar]
- 46.Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, et al. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481–483. doi: 10.1007/s00261-007-9192-6. [DOI] [PubMed] [Google Scholar]
- 47.Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, Tateyama A, et al. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology. 2012;263:732–740. doi: 10.1148/radiol.12111222. [DOI] [PubMed] [Google Scholar]
- 48.Kato K, Nihashi T, Ikeda M, Abe S, Iwano S, Itoh S, et al. Limited efficacy of 18F-FDG PET/CT for differentiation between metastasis-free pancreatic cancer and mass-forming pancreatitis. Clin Nucl Med. 2013;38:417–421. doi: 10.1097/RLU.0b013e3182817d9d. [DOI] [PubMed] [Google Scholar]
- 49.Rijkers AP, Valkema R, Duivenvoorden HJ, van Eijck CH. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol. 2014;40:794–804. doi: 10.1016/j.ejso.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto I, Shirakawa S, Shinzeki M, Asari S, Goto T, Ajiki T, et al. 18-fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clinl Gastroenterol Hepatol. 2013;11:712–718. doi: 10.1016/j.cgh.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 51.Ghaneh P, Hanson R, Titman A, Lancaster G, Plumpton C, Lloyd-Williams H, et al. PET-PANC: multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol Assess. 2018;22:1–114. doi: 10.3310/hta22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844–850. doi: 10.1111/j.1572-0241.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 54.Nawaz H, Fan CY, Kloke J, Khalid A, McGrath K, Landsittel D, et al. Performance characteristics of endoscopic ultrasound in the staging of pancreatic cancer: a meta-analysis. JOP. 2013;14:484–497. doi: 10.6092/1590-8577/1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- 56.Banafea O, Mghanga FP, Zhao J, Zhao R, Zhu L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: a meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016;16:108. doi: 10.1186/s12876-016-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okasha HH, Naga MI, Esmat S, Naguib M, Hassanein M, Hassani M, et al. Endoscopic ultrasound-guided fine needle aspiration versus percutaneous ultrasound-guided fine needle aspiration in diagnosis of focal pancreatic masses. Endosc Ultrasound. 2013;2:190–193. doi: 10.4103/2303-9027.121239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–695. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 59.Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105–1110. doi: 10.1136/gutjnl-2014-307475. [DOI] [PubMed] [Google Scholar]
- 60.Singhi AD, George B, Greenbowe JR, Chung J, Suh J, Maitra A, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology. 2019;156:2242–2253.e4. doi: 10.1053/j.gastro.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Lowery MA, Jordan EJ, Basturk O, Ptashkin RN, Zehir A, Berger MF, et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: potential actionability and correlation with clinical phenotype. Clin Cancer Res. 2017;23:6094–6100. doi: 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- 62.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. Chicago: Springer; 2017. [Google Scholar]
- 63.Schlitter AM, Jesinghaus M, Jäger C, Konukiewitz B, Muckenhuber A, Demir IE, et al. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer. 2017;84:121–129. doi: 10.1016/j.ejca.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 64.van Roessel S, Kasumova GG, Verheij J, Najarian RM, Maggino L, de Pastena M, et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018;153:e183617. doi: 10.1001/jamasurg.2018.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185–191. doi: 10.1097/SLA.0000000000001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol. 2017;24:2023–2030. doi: 10.1245/s10434-017-5810-x. [DOI] [PubMed] [Google Scholar]
- 67.Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the American Pancreatic Association. Radiology. 2014;270:248–260. doi: 10.1148/radiol.13131184. [DOI] [PubMed] [Google Scholar]
- 69.Hong SB, Lee SS, Kim JH, Kim HJ, Byun JH, Hong SM, et al. Pancreatic cancer CT: prediction of resectability according to NCCN criteria. Radiology. 2018;289:710–718. doi: 10.1148/radiol.2018180628. [DOI] [PubMed] [Google Scholar]
- 70.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 71.Loizou L, Duran CV, Axelsson E, Andersson M, Keussen I, Strinnholm J, et al. Radiological assessment of local resectability status in patients with pancreatic cancer: interreader agreement and reader performance in two different classification systems. Eur J Radiol. 2018;106:69–76. doi: 10.1016/j.ejrad.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Joo I, Lee JM, Lee ES, Son JY, Lee DH, Ahn SJ, et al. Preoperative CT classification of the resectability of pancreatic cancer: interobserver agreement. Radiology. 2019;293:343–349. doi: 10.1148/radiol.2019190422. [DOI] [PubMed] [Google Scholar]
- 73.Witkowski ER, Smith JK, Ragulin-Coyne E, Ng SC, Shah SA, Tseng JF. Is it worth looking? Abdominal imaging after pancreatic cancer resection: a national study. J Gastrointest Surg. 2012;16:121–128. doi: 10.1007/s11605-011-1699-z. [DOI] [PubMed] [Google Scholar]
- 74.Sheffield KM, Crowell KT, Lin YL, Djukom C, Goodwin JS, Riall TS. Surveillance of pancreatic cancer patients after surgical resection. Ann Surg Oncol. 2012;19:1670–1677. doi: 10.1245/s10434-011-2152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tzeng CW, Abbott DE, Cantor SB, Fleming JB, Lee JE, Pisters PW, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol. 2013;20:2197–2203. doi: 10.1245/s10434-013-2889-6. [DOI] [PubMed] [Google Scholar]
- 76.Daamen LA, Groot VP, Heerkens HD, Intven MPW, van Santvoort HC, Molenaar IQ. Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford) 2018;20:297–304. doi: 10.1016/j.hpb.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 77.White RR, Paulson EK, Freed KS, Keogan MT, Hurwitz HI, Lee C, et al. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J Gastrointest Surg. 2001;5:626–633. doi: 10.1016/s1091-255x(01)80105-0. [DOI] [PubMed] [Google Scholar]
- 78.Sasson AR, Wetherington RW, Hoffman JP, Ross EA, Cooper H, Meropol NJ, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: analysis of histopathology and outcome. Int J Gastrointest Cancer. 2003;34:121–128. doi: 10.1385/IJGC:34:2-3:121. [DOI] [PubMed] [Google Scholar]
- 79.Cassinotto C, Cortade J, Belleannée G, Lapuyade B, Terrebonne E, Vendrely V, et al. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol. 2013;82:589–593. doi: 10.1016/j.ejrad.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269:733–740. doi: 10.1097/SLA.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 81.Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 82.Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baliyan V, Kordbacheh H, Parakh A, Kambadakone A. Response assessment in pancreatic ductal adenocarcinoma: role of imaging. Abdom Radiol (NY) 2018;43:435–444. doi: 10.1007/s00261-017-1434-7. [DOI] [PubMed] [Google Scholar]
- 84.Cassinotto C, Sa-Cunha A, Trillaud H. Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer. Diagn Interv Imaging. 2016;97:1225–1232. doi: 10.1016/j.diii.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 85.Gassner EM, Poskaite P. Imaging response evaluation after novel neoadjuvant treatments of pancreatic cancer. European Surgery. 2019;51:146–152. [Google Scholar]
- 86.Cassinotto C, Mouries A, Lafourcade JP, Terrebonne E, Belleannée G, Blanc JF, et al. Locally advanced pancreatic adenocarcinoma: reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology. 2014;273:108–116. doi: 10.1148/radiol.14132914. [DOI] [PubMed] [Google Scholar]
- 87.Kim YE, Park MS, Hong HS, Kang CM, Choi JY, Lim JS, et al. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology. 2009;250:758–765. doi: 10.1148/radiol.2502080501. [DOI] [PubMed] [Google Scholar]
- 88.Marchegiani G, Todaro V, Boninsegna E, Negrelli R, Sureka B, Bonamini D, et al. Surgery after FOLFIRINOX treatment for locally advanced and borderline resectable pancreatic cancer: increase in tumour attenuation on CT correlates with R0 resection. Eur Radiol. 2018;28:4265–4273. doi: 10.1007/s00330-018-5410-6. [DOI] [PubMed] [Google Scholar]
- 89.Wagner M, Antunes C, Pietrasz D, Cassinotto C, Zappa M, Sa Cunha A, et al. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol. 2017;27:3104–3116. doi: 10.1007/s00330-016-4632-8. [DOI] [PubMed] [Google Scholar]
- 90.Chen X, Oshima K, Schott D, Wu H, Hall W, Song Y, et al. Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: an exploratory study. PLoS One. 2017;12:e0178961. doi: 10.1371/journal.pone.0178961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park MS, Klotz E, Kim MJ, Song SY, Park SW, Cha SW, et al. Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology. 2009;250:110–117. doi: 10.1148/radiol.2493080226. [DOI] [PubMed] [Google Scholar]
- 92.Akisik MF, Sandrasegaran K, Bu G, Lin C, Hutchins GD, Chiorean EG. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology. 2010;256:441–449. doi: 10.1148/radiol.10091733. [DOI] [PubMed] [Google Scholar]
- 93.Okada KI, Hirono S, Kawai M, Miyazawa M, Shimizu A, Kitahata Y, et al. Value of apparent diffusion coefficient prior to neoadjuvant therapy is a predictor of histologic response in patients with borderline resectable pancreatic carcinoma. J Hepatobiliary Pancreat Sci. 2017;24:161–168. doi: 10.1002/jhbp.430. [DOI] [PubMed] [Google Scholar]
- 94.Dalah E, Erickson B, Oshima K, Schott D, Hall WA, Paulson E, et al. Correlation of ADC with pathological treatment response for radiation therapy of pancreatic cancer. Transl Oncol. 2018;11:391–398. doi: 10.1016/j.tranon.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinrich S, Schäfer M, Weber A, Hany TF, Bhure U, Pestalozzi BC, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 96.Kittaka H, Takahashi H, Ohigashi H, Gotoh K, Yamada T, Tomita Y, et al. Role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg. 2013;37:169–178. doi: 10.1007/s00268-012-1775-x. [DOI] [PubMed] [Google Scholar]
- 97.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. ;The Groupe Tumeurs Digestives of Unicancer and the PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 99.Philip PA, Mooney M, Jaffe D, Eckhardt G, Moore M, Meropol N, et al. Consensus report of the National Cancer Institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol. 2009;27:5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramanathan RK, Goldstein D, Korn RL, Arena F, Moore M, Siena S, et al. Positron emission tomography response evaluation from a randomized phase III trial of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. Ann Oncol. 2016;27:648–653. doi: 10.1093/annonc/mdw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bradley A, Van Der. Upfront surgery versus neoadjuvant therapy for resectable pancreatic cancer: systematic review and bayesian network meta-analysis. Sci Rep. 2019;9:4354. doi: 10.1038/s41598-019-40951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raufi AG, Manji GA, Chabot JA, Bates SE. Neoadjuvant treatment for pancreatic cancer. Semin Oncol. 2019;46:19–27. doi: 10.1053/j.seminoncol.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Lee S, Kim SH, Park HK, Jang KT, Hwang JA, Kim S. Pancreatic ductal adenocarcinoma: rim enhancement at MR imaging predicts prognosis after curative resection. Radiology. 2018;288:456–466. doi: 10.1148/radiol.2018172331. [DOI] [PubMed] [Google Scholar]
- 104.Zhu L, Shi X, Xue H, Wu H, Chen G, Sun H, et al. CT imaging biomarkers predict clinical outcomes after pancreatic cancer surgery. Medicine (Baltimore) 2016;95:e2664. doi: 10.1097/MD.0000000000002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukukura Y, Takumi K, Higashi M, Shinchi H, Kamimura K, Yoneyama T, et al. Contrast-enhanced CT and diffusion-weighted MR imaging: performance as a prognostic factor in patients with pancreatic ductal adenocarcinoma. Eur J Radiol. 2014;83:612–619. doi: 10.1016/j.ejrad.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 106.Cassinotto C, Chong J, Zogopoulos G, Reinhold C, Chiche L, Lafourcade JP, et al. Resectable pancreatic adenocarcinoma: role of CT quantitative imaging biomarkers for predicting pathology and patient outcomes. Eur J Radiol. 2017;90:152–158. doi: 10.1016/j.ejrad.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 107.Yun G, Kim YH, Lee YJ, Kim B, Hwang JH, Choi DJ. Tumor heterogeneity of pancreas head cancer assessed by CT texture analysis: association with survival outcomes after curative resection. Sci Rep. 2018;8:7226. doi: 10.1038/s41598-018-25627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hattori Y, Gabata T, Zen Y, Mochizuki K, Kitagawa H, Matsui O. Poorly enhanced areas of pancreatic adenocarcinomas on late-phase dynamic computed tomography: comparison with pathological findings. Pancreas. 2010;39:1263–1270. doi: 10.1097/MPA.0b013e3181dbc583. [DOI] [PubMed] [Google Scholar]
- 109.Muraoka N, Uematsu H, Kimura H, Imamura Y, Fujiwara Y, Murakami M, et al. Apparent diffusion coefficient in pancreatic cancer: characterization and histopathological correlations. J Magn Reson Imaging. 2008;27:1302–1308. doi: 10.1002/jmri.21340. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Chen ZE, Nikolaidis P, McCarthy RJ, Merrick L, Sternick LA, et al. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging. 2011;33:136–142. doi: 10.1002/jmri.22414. [DOI] [PubMed] [Google Scholar]
- 111.Hayano K, Miura F, Wada K, Suzuki K, Takeshita K, Amano H, et al. Diffusion-weighted MR imaging of pancreatic cancer and inflammation: prognostic significance of pancreatic inflammation in pancreatic cancer patients. Pancreatology. 2016;16:121–126. doi: 10.1016/j.pan.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 112.Chen BB, Tien YW, Chang MC, Cheng MF, Chang YT, Yang SH, et al. Multiparametric PET/MR imaging biomarkers are associated with overall survival in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2018;45:1205–1217. doi: 10.1007/s00259-018-3960-0. [DOI] [PubMed] [Google Scholar]
- 113.Kurosawa J, Tawada K, Mikata R, Ishihara T, Tsuyuguchi T, Saito M, et al. Prognostic relevance of apparent diffusion coefficient obtained by diffusion-weighted MRI in pancreatic cancer. J Magn Reson Imaging. 2015;42:1532–1537. doi: 10.1002/jmri.24939. [DOI] [PubMed] [Google Scholar]
- 114.Choi HJ, Kang CM, Lee WJ, Song SY, Cho A, Yun M, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med J. 2013;54:1377–1383. doi: 10.3349/ymj.2013.54.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kitasato Y, Yasunaga M, Okuda K, Kinoshita H, Tanaka H, Okabe Y, et al. Maximum standardized uptake value on 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography and glucose transporter-1 expression correlates with survival in invasive ductal carcinoma of the pancreas. Pancreas. 2014;43:1060–1065. doi: 10.1097/MPA.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamamoto T, Sugiura T, Mizuno T, Okamura Y, Aramaki T, Endo M, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncoly. 2015;22:677–684. doi: 10.1245/s10434-014-4046-2. [DOI] [PubMed] [Google Scholar]
- 117.Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55:898–904. doi: 10.2967/jnumed.113.131847. [DOI] [PubMed] [Google Scholar]