Abstract
Background
As robot-assisted equipment is continuously being used in orthopaedic surgery, the past few decades have seen an increase in the usage of robotics for total knee arthroplasty (TKA). Thus, the purpose of the present study is to investigate the differences between robotic TKA and nonrobotic TKA on perioperative and postoperative complications and opioid consumption.
Methods
An administrative database was queried from 2010 to Q2 of 2017 for primary TKAs performed via robot-assisted surgery vs non–robot-assisted surgery. Systemic and joint complications and average morphine milligram equivalents were collected and compared with statistical analysis.
Results
Patients in the nonrobotic TKA cohort had higher levels of prosthetic revision at 1-year after discharge (P < .05) and higher levels of manipulation under anesthesia at 90 days and 1-year after discharge (P < .05). Furthermore, those in the nonrobotic TKA cohort had increased occurrences of deep vein thrombosis, altered mental status, pulmonary embolism, anemia, acute renal failure, cerebrovascular event, pneumonia, respiratory failure, and urinary tract infection during the inpatient hospital stay (all P < .05) and at 90 days after discharge (all P < .05). All of these categories remained statistically increased at the 90-days postdischarge date, except pneumonia and stroke. Patients in the nonrobotic TKA cohort had higher levels of average morphine milligram equivalents consumption at all time periods measured (P < .001).
Conclusions
In the present study, the use of robotics for TKA found lower revision rates, lower incidences of manipulation under anesthesia, decreased occurrence of systemic complications, and lower opiate consumption for postoperative pain management. Future studies should look to further examine the long-term outcomes for patients undergoing robot-assisted TKA.
Level of Evidence
Level III.
Keywords: Total knee arthroplasty, Robotics, Complications, Opioid consumption
Introduction
Total knee arthroplasty (TKA) is one of the most commonly performed procedures by orthopaedic surgeons treating end-stage knee osteoarthritis, with recent studies projecting an 85% increase in primary TKAs performed in the United States by 2030 [1] and a 78%-182% increase in revision TKAs [2]. This growth can largely be attributed to the success rate and long-term survivorship documented in TKA, with a greater than 90% long-term survivorship at both 10 and 15 years postoperatively [[3], [4], [5], [6]]. Patients undergoing TKA often experience positive clinical and functional outcomes, with patient-reported outcome measures indicating patient satisfaction to be around 70%-93% [[7], [8], [9], [10], [11]]. Since the introduction of TKA as a surgical option for end-stage knee osteoarthritis, the past few decades have seen advances in knee replacement technology such as different implant designs and material, computed tomography–based and magnetic resonance imaging–based cutting guides, enhanced recovery programs, patient-specific implants, and computer navigation [[12], [13], [14], [15], [16]]. Toward the end of the 21st century, advancements in surgical technology introduced robot-assisted surgery platforms into the operating room.
The first documented use of a robotic surgical arm,PUMA(Programmable Universal Manipulation Arm, Unimation, Danbury, CT), was in 1985 while performing a neurosurgical biopsy [17]. The subsequent decades saw improvements in robotic technology, ultimately culminating in the first FDA-approved robotic surgical system called the da Vinci Surgery System (Intuitive Surgical, Sunnyvale, CA) for general laparoscopic surgery [17]. Robotic surgical systems were introduced into orthopaedic surgery in the later 1980s. ROBODOC (Integrated Surgical Systems, Davis, CA), which was initially developed for total hip arthroplasty procedures [18,19], has been used worldwide in performing more than 15,000 TKAs [19,20]. Since the arrival of ROBODOC for use in total joint arthroplasty (TJA), other robot-assisted surgical systems such as CASPAR (Universal Robot Systems, Ortho, Germany), ACROBOT (Acrobot Company Ltd, Imperial College London, United Kingdom), and MAKO (Stryker Corporation, Kalamazoo, MI) have been developed and used in TJA procedures [[21], [22], [23]]. Today, many of the current platforms include robotic arm–assisted, robot-guided cutting jigs, and robotic milling systems that use an active, semiactive, or passive control system [24].
Several studies have shown improved accuracy in implant positioning and limb alignment with the use of robotic arms in TKA procedures [[25], [26], [27], [28], [29], [30]]. However, potential concerns associated with using robotic arms for TKA include increased costs, increased surgical time, and no guarantee of improved accuracy or decreased postoperative complications [[30], [31], [32], [33]]. Despite contrasting views and evidence in regard to robotics in TKA, utilization of robot arm–assisted TKA has been rapidly growing, with a reported 6.8% increase in usage between 2005 and 2014 [34].
With the rise in the number of robotic TKAs performed in the United States and mixed data on its impact on clinical outcomes, there remains a need for continued research to examine the outcomes in patients undergoing TKA with robot-assisted equipment. The purpose of this study was to quantify and compare the rates of postoperative complications and opiate consumption in patients after robot-assisted TKA vs conventional TKA with a large nationwide database.
Material and methods
Patient records were queried from PearlDiver (PearlDiver Inc., Fort Wayne, IN), a commercially available administrative claims database, using the International Classification of Disease, Ninth Revision and Tenth Revision (ICD-9 andICD-10) codes. This study used the MKnee data set that contains the medical records of approximately 1 million patients from 2007 to Q2 of 2018 from various provider groups around the country. Institutional review board exemption was granted for this study because the provided data were deidentified and compliant with the Health Insurance Portability and Accountability Act.
A retrospective cohort design was used to compare between patients who underwent TKA via robot-assisted surgery and patients who underwent TKA via non–robot-assisted surgery. Patients who had undergone TKA were identified using the ICD-9 and ICD-10 procedural codes. Exclusion criteria included patients receiving arthroplasty for pathologic or traumatic fractures, as well as miscoded revisions. Patients were placed into the ‘robotic TKA’ cohort if they had received a primary TKA via robot-assisted surgery, whereas patients were placed into the “nonrobotic TKA” cohort if they received a primary TKA via conventional surgery. Only patients who underwent primary TKA between 2010 and Q2 of 2017 were included to ensure a minimum 1-year follow-up in the database for all included patients. To ensure that only robot-assisted surgeries were examined, only codes that defined robot-assisted surgery were included. These codes are separate and different from the codes used to define computer-assisted or patient-specific cutting guides, which were not included in this study. The ICD codes that defined the study cohorts are provided in Appendix Table A1.
Each cohort was queried for basic demographic information, clinical characteristics, and hospital course data such as age, sex, hospital region, body mass index (BMI), length of stay (LOS), 90-day readmission rate, Charlson Comorbidity Index (CCI), and comorbidities. In addition, data were queried to measure the trends of robot-assisted TKA usage during the examined study period. Specific comorbidities queried included tobacco use, rheumatoid arthritis, liver disease, congestive heart failure, cardiac disease (ischemic heart disease, coronary artery disease, and pulmonary artery disease), chronic obstructive pulmonary disease, chronic kidney disease, history of alcohol use, and preoperative anemia.
Incidences of perioperative and postoperative systemic and joint complications were queried for the 2 patient cohorts. Systemic complications were examined during the surgical encounter before discharge and at 90 days after discharge. Systemic complications queried included cerebrovascular event (stroke, nontraumatic hemorrhage, occlusion of cerebral arteries), altered mental status (AMS), anemia (after hemorrhagic, iron deficiency from blood loss), acute renal failure (ARF), myocardial infarction, pneumonia, deep vein thrombosis (DVT), pulmonary embolism (PE), urinary tract infection (UTI), and respiratory failure (RF). The codes used to define systemic complications are provided in Appendix Table A2.
Postoperative joint complications were examined at both 90 days after discharge and 1 year after discharge. Joint complications queried included prosthetic joint infection (PJI), periprosthetic fracture, prosthetic knee dislocation, prosthetic revision, aseptic loosening, and manipulation under anesthesia (MUA). PJI was defined by procedural codes that indicated a surgical intervention for a deep joint infection to exclude superficial wound complications that would have been included in diagnosis codes for PJI. The codes used to define joint complications are provided in Appendix Table A3.
To objectively measure pain management load between the 2 cohorts, morphine milligram equivalents (MME) were calculated in and queried directly from the database. The evaluation captured patients who had an opioid claim (a) between discharge and 90 days, (b) a subsequent claim between 90 days and 6 months, and (c) another subsequent claim between 6 months and 1 year. The average cumulative MME for each of these 3 time periods was queried directly from the database. To ensure MME levels were tied to the initial primary TKA, patients who received general anesthesia within the 1-year follow-up were excluded to account for potential opioid use associated with additional procedures. Furthermore, because preoperative opioid use has been shown to affect postoperative opioid use, patients with preoperative opioid use were excluded. The following Uniform System of Classification (USC) codes were used to identify opioid claims: USC-02211, USC-02212, USC-02214, USC-02221, USC-02222, USC-02231.
All data analyses were performed using the R statistical software (R Project for Statistical Computing, Vienna, Austria) integrated within PearlDiver with an α level set to 0.05. Multivariable logistic regression adjusting for patient sex, age, CCI, BMI, and the presence of the comorbidities tobacco use and diabetes mellitus were used to calculate odds ratios (ORs) with corresponding 95% confidence intervals (CIs) for rates of joint and systemic complications between the 2 cohorts. Demographic, MME, and clinical characteristics were compared using chi-square analysis for categorical variables and Welch’s t-test for continuous variables.
Results
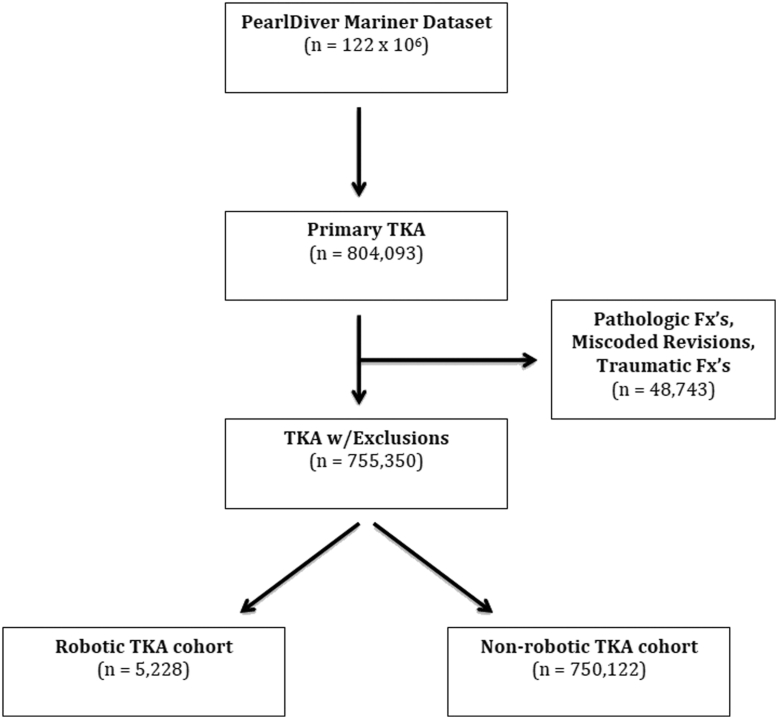
Between 2010 and Q2 of 2017 in the PearlDiver database, a total of 804,093 primary TKA procedures were performed. This number decreased to 755,350 after adjusting for exclusion criteria. Of this total, 5228 patients received a primary TKA via robot-assisted surgery and 750,122 received a primary TKA via non–robot-assisted surgery (Fig. 1). As demonstrated by the data (Table 1), a greater proportion of patients in the nonrobotic TKA cohort were female (63.13% vs 55.11%, P < .001), were between the age of 65 and 79 years (57.71% vs 50.34%, P < .001), classified as morbidly obese (53.40% vs 32.34%, P < .001), and had a higher average burden of comorbidities (1.38 vs 1.06, P < .001). In addition (Table 2), those in the nonrobotic TKA cohort had an increased occurrence of 90-day readmissions (6.53% vs 5.01%, P < .001). On the contrary, a greater proportion of patients in the robotic-TKA cohort were male (44.89% vs 36.87%, P < .001), younger than the age of 65 years (49.66% vs 42.29%, P < .001), had BMI classifications of less than 30 and between 30 and 40 (BMI<30: 19.26% vs 4.51%, P < .001; BMI 30-40: 48.40% vs 41.10%, P < .001), and had a longer hospital LOS (4.38 vs 3.00, P < .001). At the start of the examined study period (2010), the total number of robotic TKAs performed represented just 0.18% of all primary TKAs, but by the end of the study period (Q2 of 2017), this number increased to 1.5% of all primary TKAs.
Figure 1.
Flow diagram of patients included in the study. Fx's, fractures.
Table 1.
Demographics and clinical characteristic comparisons for robotic and nonrobotic TKA groups.
| Demographic variable | Non–robot-assisted primary TKA (n = 750,122) n (%) | Robot-assisted primary TKA (n = 5228) n (%) | P |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 473,585 (63.13) | 2881 (55.11) | <.001b |
| Age, n (%) | |||
| <65 | 317,197 (42.29) | 2596 (49.66) | <.001b |
| BMIa, n (%) | |||
| <30 | 5509 (4.51) | 181 (19.26) | <.001b |
| 30-40 | 51,478 (42.10) | 455 (48.40) | <.001b |
| ≥40 | 65,295 (53.40) | 304 (32.34) | <.001b |
| CCI, mean ± SD | 1.38 ± 1.82 | 1.06 ± 1.61 | <.001 |
| Specific comorbidities, n (%) | |||
| Tobacco use | 115,242 (15.36) | 724 (13.85) | .003 |
| Rheumatoid arthritis | 35,438 (4.72) | 189 (3.62) | <.001b |
| Liver disease | 49,874 (6.65) | 363 (6.94) | .410 |
| Congestive heart failure | 47,216 (6.29) | 245 (4.69) | <.001b |
| Cardiac disease | 184,182 (24.55) | 1171 (22.40) | <.001b |
| COPD | 170,196 (22.69) | 1175 (22.48) | .725 |
| Chronic kidney disease | 55,300 (7.37) | 300 (5.74) | <.001b |
| History of alcohol use | 14,488 (1.93) | 113 (2.16) | .249 |
| Preoperative anemia | 143,398 (19.12) | 985 (18.84) | .626 |
SD, standard deviation; COPD, chronic obstructive pulmonary disease.
BMI data were only available for 18% of the patients in the robotic TKA cohort and 16% of the patients in the nonrobotic TKA cohort.
Bolded entries refer to complications that are statistically significant.
Table 2.
Comparison of LOS and the 90-d readmission rate for robotic and nonrobotic TKA groups.
| Hospital course variable | Non–robot-assisted primary TKA (n = 750,122) n (%) | Robot-assisted primary TKA (n = 5228) n (%) | P |
|---|---|---|---|
| LOS, mean ± SD | 3.00 ± 1.73 | 4.38 ± 2.50 | <.001a |
| 90-day readmission rate, n (%) | 49,012 (6.53) | 265 (5.01) | <.001a |
SD, standard deviation.
Bolded entries refer to complications that are statistically significant.
In terms of joint complications examined, those in the nonrobotic TKA cohort had significantly higher risks of prosthetic revision at 1 year after discharge (OR: 1.21, 95% CI: 1.03-1.43), MUA at 90 days after discharge (OR: 2.50, 95% CI: 1.96-3.28), and MUA at 1 year after discharge (OR: 2.18, 95% CI: 1.78-2.71) as compared with those in the robotic TKA cohort. All other joint complications examined (prosthetic knee dislocation, periprosthetic fracture, aseptic loosening, and PJI) did not reach statistical significance at both 90 days after discharge and 1 year after discharge (Table 3).
Table 3.
Comparison of joint complications for robotic and nonrobotic TKA groups.
| Joint complication | Non–robot-assisted primary TKA (n = 750,122) n (%) | Robot-assisted primary TKA (n = 5228) n (%) | OR (95% CI) |
|---|---|---|---|
| Prosthetic dislocation | |||
| 90 d | 159 (0.02) | 2 (0.04) | 0.56 (0.18-3.37) |
| 1 y | 248 (0.03) | 3 (0.06) | 0.58 (0.22-2.35) |
| Prosthetic joint infection | |||
| 90 d | 4637 (0.62) | 25 (0.48) | 1.27 (0.88-1.93) |
| 1 y | 7221 (0.96) | 39 (0.75) | 1.27 (0.94-1.78) |
| Periprosthetic fracture | |||
| 90 d | 300 (0.04) | 0 (0) | NA |
| 1 y | 676 (0.09) | 0 (0) | NA |
| Aseptic loosening | |||
| 90 d | 182 (0.02) | 2 (0.04) | 0.64 (0.21-3.89) |
| 1 y | 1212 (0.16) | 9 (0.17) | 0.93 (0.51-1.93) |
| Prosthetic revision | |||
| 90 d | 5489 (0.73) | 44 (0.84) | 0.95 (0.72-1.31) |
| 1 y | 25,060 (3.34) | 151 (2.89) | 1.21 (1.03-1.43)a |
| Manipulation under anesthesia | |||
| 90 d | 19,139 (2.55) | 59 (1.13) | 2.50 (1.96-3.28)a |
| 1 y | 25,059 (3.34) | 88 (1.68) | 2.18 (1.78-2.71)a |
Bolded entries refer to complications that are statistically significant.
For systemic complications examined during the inpatient hospital stay, patients in the nonrobotic TKA cohort had significantly higher occurrences of DVT (OR: 2.40, 95% CI: 1.50-4.18), AMS (OR: 2.40, 95% CI: 1.29-5.26), PE (OR: 3.76, 95% CI: 1.94-8.76), anemia (OR: 2.26, 95% CI: 2.08-2.46), ARF (OR: 2.56, 95% CI: 1.89-3.60), cerebrovascular event (OR: 1.80, 95% CI: 1.17-2.98), pneumonia (OR: 3.64, 95% CI: 1.88-8.49), RF (OR: 2.70, 95% CI: 1.69-4.70), and UTI (OR: 1.47, 95% CI: 1.25-1.75) (Table 4). In addition, patients in the nonrobotic TKA cohort at 90 days after discharge exhibited significantly higher rates of DVT (OR: 1.55, 95% CI: 1.28-1.90), AMS (OR: 1.44, 95% CI: 1.01-2.16), PE (OR: 2.16, 95% CI: 1.52-3.21), anemia (OR: 2.50, 95% CI: 2.12-2.98), ARF (OR: 1.71, 95% CI: 1.29-2.32), RF (OR: 1.89, 95% CI: 1.24-3.07), and UTI (OR: 1.47, 95% CI: 1.25-1.75) (Table 4).
Table 4.
Comparison of systemic complications for robotic and nonrobotic TKA groups.
| Systemic complication | Non–robot-assisted primary TKA (n = 750,122) n (%) | Robot-assisted primary TKA (n = 5228) n (%) | OR (95% CI) |
|---|---|---|---|
| Deep vein thrombosis | |||
| In-hospital | 5345 (0.71) | 15 (0.29) | 2.40 (1.50-4.18) |
| 90 d | 23,274 (3.10) | 101 (1.93) | 1.55 (1.28-1.90)a |
| Altered mental status | |||
| In-hospital | 3111 (0.41) | 8 (0.15) | 2.40 (1.29-5.26)a |
| 90 d | 6298 (0.84) | 27 (0.52) | 1.44 (1.01-2.16)a |
| Pulmonary embolism | |||
| In-hospital | 4026 (0.54) | 7 (0.13) | 3.76 (1.94-8.76)a |
| 90 d | 9203 (1.23) | 28 (0.54) | 2.16 (1.52-3.21)a |
| Anemia | |||
| In-hospital | 179,851 (23.98) | 613 (11.73) | 2.26 (2.08-2.46)a |
| 90 d | 49,227 (6.56) | 135 (2.58) | 2.50 (2.12-2.98) |
| Acute renal failure | |||
| In-hospital | 15,987 (2.13) | 38 (0.73) | 2.56 (1.89-3.60)a |
| 90 d | 13,049 (1.74) | 46 (0.88) | 1.71 (1.29-2.32)a |
| Myocardial infarction | |||
| In-hospital | 1620 (0.22) | 5 (0.10) | 1.95 (0.90-5.45) |
| 90 d | 3104 (0.41) | 14 (0.27) | 1.38 (0.85-2.46) |
| Cerebrovascular event | |||
| In-hospital | 5310 (0.71) | 18 (0.34) | 1.80 (1.17-2.98)a |
| 90 d | 10,827 (1.44) | 64 (1.22) | 1.03 (0.81-1.33) |
| Pneumonia | |||
| In-hospital | 4086 (0.54) | 7 (0.13) | 3.64 (1.88-8.49)a |
| 90 d | 9261 (1.23) | 46 (0.88) | 1.25 (0.94-1.69) |
| Respiratory failure | |||
| In-hospital | 6914 (0.92) | 15 (0.29) | 2.70 (1.69-4.70)a |
| 90 d | 6022 (0.80) | 19 (0.36) | 1.89 (1.24-3.07)a |
| Urinary tract infection | |||
| In-hospital | 13,477 (1.80) | 43 (0.82) | 1.85 (1.39-2.54)a |
| 90 d | 34,869 (4.65) | 143 (2.74) | 1.47 (1.25-1.75)a |
NA, not applicable.
Bolded entries refer to complications that are statistically significant.
Opioid prescription claims for patients in the robotic TKA cohort was available for 690 of the 5228 at the 90-day evaluation, 63 of the 5228 at the 6-month evaluation, and 17 of the 5228 at the 1-year evaluation. For patients in the nonrobotic TKA cohort, opioid prescription claims were available for 104,611 of the 750,122 at the 90-day evaluation, 17,660 of the 750,122 at the 6-month evaluation, and 8572 of the 750,122 at the 1-year evaluation. At the 90-day MME evaluation, 6-month MME evaluation, and 1-year MME evaluation, patients in the nonrobotic TKA cohort had significantly higher levels of MME consumption than those in the robotic TKA cohort (90 days: 1150 vs 873, P < .001; 6 months: 2898 vs 1837, P < .001; 1 year: 6203 vs 3578, P < .001) (Table 5).
Table 5.
Comparison of MME results for robotic and nonrobotic TKA groups.
| Average total morphine milligram equivalents (MME)b | Non–robot-assisted primary TKA (n = 750,122) | Robot-assisted primary TKA (n = 5228) | P |
|---|---|---|---|
| 90 d (mg) | 1150 | 873 | <.001a |
| 6 mo (mg) | 2898 | 1837 | <.001a |
| 1 y (mg) | 6203 | 3578 | <.001a |
bPharmaceutical data for patients in the robotic TKA cohort were only available for 690 of the 5228 at the 90-d evaluation, 63 of the 5228 at the 6-mo evaluation, and 17 of the 5228 patients at the 1-y evaluation. Pharmaceutical data for patients in the nonrobotic TKA cohort were only available for 104,611 of the 750,122 at the 90-d evaluation, 17,660 of the 750,122 at the 6-mo evaluation, and 8575 of the 750,122 patients at the 1-y evaluation.
Bolded entries refer to complications that are statistically significant.
Discussion
This present study demonstrated that patients undergoing TKA via robot-assisted surgery had lower revision rates at 1-year after discharge, as well as lower rates of MUA at both 90 days and 1 year after discharge. In addition, there was a lower risk for systemic complications for patients in the robotic TKA cohort both during the in-patient hospital stay and at 90 days after discharge. These complications included DVT, AMS, PE, anemia, ARF, cerebrovascular event, pneumonia, RF, and UTI. Finally, patients in the robotic cohort were prescribed significantly lower average cumulative MME at 90 days after discharge, 6 months after discharge, and 1 year after discharge relative to patients in the nonrobotic cohort.
Since the advent of the ROBODOC into orthopaedic operating rooms, technological advances have resulted in the production of more robot-assisted surgical platforms. This greater access to robotic technologies has led to increases in its utilization for TKA performed in the United States [34]. In a study using the Nationwide Inpatient Sample database, Antonios et al identified 6,060,901 patients from 2005 to 2014 who had undergone TKA via conventional means, computer navigation, and robot assistance. It was found that in that period, despite only representing 0.4% of all TKAs performed, robot-assisted TKA demonstrated a steady increase in usage [34]. Much similar to the data from the study by Antonios et al, the present study highlights the increasing occurrence in TKA performed robotically in the United States, with the total number of robot-assisted TKAs representing 0.65% of all primary TKAs identified within this study.
There are several limitations inherent to utilization of a database system. A potential limitation to this study was during the time period of data collection, the only Unites States Food and Drug Administration (FDA)–approved robotic platform during this collection for TKA was the Stryker Mako Robotic-arm Assist (Stryker Corporation, Kalamazoo, MI). There were potentially test sites of other robotic platforms who were performing TKA captured in this data set as pilot studies as Rosa (Zimmer Biomet, Warsaw, IN) received FDA approval on January 25, 2019, Think Surgical (THINK surgical, Fremont, CA) received FDA approval on October 10, 2019, and Navio (Smith and Nephew, London, United Kingdom) received FDA clearance in April of 2018. However, with the timing of collection, it would be more likely than not that the overwhelming majority of these cases were performed with the Stryker Mako robotic system. In regard to the implant type, each of these robotic platforms is based on FDA-approved implants also available for conventional use, so there should be no differences detected that are attributed to the implant choice. A possible confounder with the MME data is the lack of amount of available opioid data for patients in both cohorts at the individual time periods analyzed. However, this reduction in available opioid data is likely due to selection bias, given we excluded patients who were on opioids preoperatively and we excluded opioid use because of other procedures that could have occurred in the year after the index procedure. In addition, this reduction can also likely be due to patients not being started on opioids postoperatively, as well as many patients finishing their opioid tapers well before the measured MME time period. Given that the longevity on TKA prosthetics is multiple years, by measuring complications up to 1 year after discharge, potential further complications could have occurred. In addition, by measuring complication measurements at 1 year, this study is limited to short-term outcomes. Similarly, examination of systemic complications was limited to a 90-day evaluation. However, this decision was made to maximize the chance of finding a correlation between the systemic complications and the performed procedure. Furthermore, there exists a possibility of coding bias with the manual entry of diagnosisand procedural codes used for this study. In addition, codes between ICD-9 and ICD-10 do not exactly match. To address possible coding bias and the lack of continuity between ICD-9 and ICD-10 codes, a code translator was used to match corresponding codes. Despite the use of multivariate logistic regression to diminish the effect of confounders, there still remains the chance of other confounders influencing the data. Although this study could have incorporated more elements into our adjustment to control for other confounders, the decision to control for age, BMI, gender, CCI, tobacco use, and diabetes mellitus was only because these represented ‘high-impact’ confounders. Finally, another limitation with the use of the PearlDiver database is that patients in both cohorts could not be identified by the type of anesthesia received (general vs spinal orepidural). With the Current Procedural Terminology (CPT) coding, there is no stratification between general or regional anesthesia, as anesthesia only codes for time units.
At both 90 days after discharge and 1 year after discharge, patients in the nonrobotic TKA cohort had significantly higher occurrences of MUA (90 days: OR: 2.50, 95% CI: 1.96-3.28; 1 year: OR: 2.18, 95% CI: 1.78-2.71) than patients in the robotic TKA cohort. These findings match the findings by Malkan et al [35], who found a 4.5-fold decrease in the rates of MUA for patients undergoing robot-assisted TKA in comparison with conventional MUA (1.06% vs 4.79%, P = .032). Although this study’s results are similar to findings by Malkan et al, it contains a much greater sample size (conventional TKA sample size: 750,122 vs 188; robotic TKA sample size: 5228 vs 188), thus allowing for more generalizability. These findings regarding MUA are particularly interesting as they have implications on the long-term outcomes from a TKA. In a recent study by Crawford et al that examined 2193 patients who underwent a primary TKA between the years 2003 and 2007 with a 2-year minimum follow-up, patients who underwent MUA after primary TKA were at risk for higher revision rates, worse long-term clinical scores, range of motion, and prosthetic survivorship [36]. Although continued research is needed to investigate long-term outcomes in patients undergoing MUA after a robot-assisted TKA, the present data in this study demonstrated that robot-assisted TKA results in lower rates of MUA, which could potentially translate into positive long-term results.
At the 1-year after discharge period, patients in the nonrobotic TKA cohort also had a significantly higher risk of prosthetic revision than patients in the robotic TKA cohort (OR: 1.21, 95% CI: 1.03-1.43). Although limited research has shown lower revision rates for patients undergoing robot-assisted knee arthroplasty [37], this research has been limited to unicompartmental knee arthroplasty. Moreover, Kim et al followed patients who received TKA via conventional means or robot-assisted surgery over a 10-year period in a prospective, randomized controlled trial and reported no differences in the 2 groups in terms of survivorship (98%). With survivorship end point being defined as having a revision TKA, his study suggests that both groups had comparable revision rates at 10 years since initial TKA [38]. Given the paucity of research explicitly examining prosthetic revision rates for patients undergoing robot-assisted TKA, the impact of robot-assisted TKA on prosthetic revision rates in the short-, intermediate-, and long-term postoperative period remains unclear. Despite this, the results of this study in regard to prosthetic revision rates can possibly be explained by the improved radiographic alignment, accuracy, and component position achieved through the use of robotics [27,29]. Whether these factors only have implications for the short-intermediate postoperative period remains unclear, and future research should continue to investigate the differences in revision rates for robot-assisted TKA as compared with conventional TKA.
Patients in the nonrobotic TKA cohort were generally older (age: 65-79: 57.71% vs 50.34%, P < .001), had higher levels of morbidly obese classifications (BMI: ≥40: 53.40% vs 32.34%, P < .001), and had a higher burden of medical comorbidities (CCI: 1.38 vs 1.06, P < .001). The presence of these characteristics represents increased risks for perioperative and postoperative complications in patients undergoing a TKA. However, this study used multivariate logistic regression to diminish the confounding effects of these characteristics; thus, the differences in systemic complications for this study were not attributed to the incongruous populations (age, BMI, comorbidities). Despite adjusting for these factors, patients in the nonrobotic TKA cohort during the inpatient hospital stay were more likely to experience DVT (OR: 2.40, 95% CI: 1.50-4.18), AMS (OR: 2.40, 95% CI: 1.29-5.26), PE (OR: 3.76, 95% CI: 1.94-8.76), anemia (OR: 2.26, 95% CI: 2.08-2.46), ARF (OR: 2.56, 95% CI: 1.89-3.60), cerebrovascular event (OR: 1.80, 95% CI: 1.17-2.98), pneumonia (OR: 3.64, 95% CI: 1.88-8.49), RF (OR: 2.70, 95% CI: 1.69-4.70), and UTI (OR: 1.47, 95% CI: 1.25-1.75). In addition, at the 90 days after discharge, the same cohort of patients were more likely to experience DVT (OR: 1.55, 95% CI: 1.28-1.90), AMS (OR: 1.44, 95% CI: 1.01-2.16), PE (OR: 2.16, 95% CI: 1.52-3.21), anemia (OR: 2.50, 95% CI: 2.12-2.98), ARF (OR: 1.71, 95% CI: 1.29-2.32), RF (OR: 1.89, 95% CI: 1.24-3.07), and UTI (OR: 1.47, 95% CI: 1.25-1.75). These results are interesting considering patients in this cohort had a shorter LOS than those in the robotic cohort (3.00 vs 4.38, P < .001), and longer hospital durations increase the risk for hospital-acquired infections. The use of robotics for total joint replacement has been linked to lower rates of PE and DVT; however, studies on this are limited to robotics for total hip arthroplasty [39,40]. Although the results of this study suggest that robotic TKA carries a lower risk of systemic complications, further research should aim to expand on this before definitive conclusions can be made.
Aside from regaining joint functionality, one of the primary goals of orthopaedic surgeons is to successfully control postoperative pain after performing a TJA [41]. One method of attaining this is via opioid prescriptions. Owing to the current opioid epidemic in the United States and the risk it carries of translating into long-term opioid use and overdose, proper opioid prescription management for patients undergoing TKA is of utmost importance [[41], [42], [43]]. Given the heightened risk of opioid consumption after TKA, findings from this present study would indicate that the use of robotics for TKA is associated with lower postoperative opioid consumption. At all time periods analyzed, patients in the robotic TKA cohort had significantly lower levels of MME consumption than those in the nonrobotic TKA cohort (90 days: 989 vs 1299, P < .001; 6 months: 2934 vs 3420, P < .001; 1 year: 3578 vs 6203, P < .001). These findings match those in a recent study by Kayani et al [44], in which patients undergoing robotic TKA had lower levels of opioid consumption and pain in the days after TKA. However, in their study, opioid consumption and pain were only examined in the immediate 3 days postoperatively after TKA. The present study largely expanded on their opioid findings by showing significantly lower opioid levels for the robotic TKA group up to 1 year.
With the outcomes from robot-assisted TKA showing promising results, it is worthwhile to discuss the differences between results of this study and prior studies that have sought to examine outcome results in computer-assisted TKA vs conventional TKA. Although computer-assisted surgery (CAS) allows for similar procedural techniques as robot-assisted surgery, such as improved component alignment and implant positioning, there exists minimal evidence to show for better clinical outcomes and improved implant survivorship in the short and intermediate postoperative term [45]. In a similar study using the New Zealand Joint Registry for 19,221 TKAs performed from 2006 to 2018, Roberts et al analyzed revision rates and functional data at 6 months, 5 years, and 10 years, between those that had undergone CAS vs conventional surgery [46]. It was found that there was no difference between the 2 cohorts in terms of revision rates and implant survival, suggesting that CAS and conventional surgery achieved safe and comparable results [46]. Since the implementation of robot-assisted TKA, several studies have shown an improvement in alignment and precision with robotics; however, this was not shown to have a measurable effect in the short-term period despite no outliers in alignment in the robotic group and a range of 19%-24% of outliers in the conventional group [30,32,33]. There are advances with robotic arm–assisted surgery that have demonstrated less soft-tissue damage with saw precision [47], and balancing sensors being available on these platforms may allow for more surgeon feedback. There is also a potential confounding factor that low-volume total knee surgeons may not have the skill with conventional instrumentation as a high-volume fellowship-trained surgeon, such that previous studies performed by high-volume fellowship-trained surgeons comparing short-term results may not reflect the entire population of surgeons as well as a large database may capture.
This study is unique in that it is the first of its kind to examine the effect robot- vs non–robot-assisted TKA can have on multiple systemic complication risks. In addition, this study is also the first to explicitly examine prosthetic revision rates after robotic TKA in the short-intermediate period after initial TKA and quantifying pain medication usage up to 1 year postoperatively. Finally, this study allows for confidence in extrapolating the data to the general population with its use of leveraging a large national patient database.
Conclusion
The use of robotics in performing TKA has been increasing over the past few decades, and with more robot arm–assisted platforms being introduced into orthopaedic operating rooms, it is reasonable to expect this trend to continue. This present study demonstrated that the use of robot-assisted surgical equipment for a TKA resulted in lower 1-year revision rates, decreased occurrences of MUA, lower risk of systemic complications, and lower opiate consumption for postoperative pain management. Continued research and expansion on long-term data for robotics in knee arthroplasty procedures will help establish the future role of robotics in orthopaedic operating rooms.
Conflict of Interest
The authors declare there are no conflicts of interest.
Appendix A. Supplementary data
Appendix
Appendix Table A1.
Codes used to define initial cohorts.
| Primary TKA codes | |||
|---|---|---|---|
| ICD-9-P-8154 | ICD-10-P-0SRC0LZ | ICD-10-P-0SRT0J9 | ICD-10-P-0SRV0J9 |
| ICD-10-P-0SRC07Z | ICD-10-P-0SRD0J9 | ICD-10-P-0SRT0JA | ICD-10-P-0SRV0JA |
| ICD-10-P-0SRC0J9 | ICD-10-P-0SRD0JA | ICD-10-P-0SRT0JZ | ICD-10-P-0SRV0JZ |
| ICD-10-P-0SRC0JA | ICD-10-P-0SRD0JZ | ICD-10-P-0SRU0J9 | ICD-10-P-0SRW0J9 |
| ICD-10-P-0SRC0JZ | ICD-10-P-0SRD0KZ | ICD-10-P-0SRU0JA | ICD-10-P-0SRW0JA |
| ICD-10-P0SRC0KZ | ICD-10-P-0SRD0L9 | ICD-10-P-0SRU0JZ | ICD-10-P-0SRW0JZ |
| ICD-10-P-0SRC0L9 | ICD-10-P-0SRD0LZ | ICD-10-P-0SRU0KZ | ICD-10-P-0SRW0KZ |
| Robotic surgery of lower extremity codes | ICD-10-P-8E0YXCZ | ||
| ICD-9-P-1741 | ICD-9-P-1744 | ICD-10-P-8E0Y0CZ | |
| ICD-9-P-1742 | ICD-9-P-1745 | ICD-10-P-8E0Y3CZ | |
| ICD-9-P-1743 | ICD-9-P-1749 | ICD-10-P-8E0Y4CZ | |
| Exclusion codes for knee | |||
| ICD-9-D-73315 | ICD-9-D-82382 | ICD-10-D-S72456A | ICD-10-D-S82401A |
| ICD-9-D-73397 | ICD-9-D-82390 | ICD-10-D-S72499A | ICD-10-D-S82202A |
| ICD-9-D-82100 | ICD-9-D-82392 | ICD-10-D-S72409B | ICD-10-D-S82402A |
| ICD-9-D-82110 | ICD-9-P-0080 | ICD-10-D-S72453B | ICD-10-D-S82201B |
| ICD-9-D-82120 | ICD-9-P-0081 | ICD-10-D-M84469A | ICD-10-D-S82201C |
| ICD-9-D-82123 | ICD-9-P-0082 | ICD-10-D-M84369A | ICD-10-D-S82401B |
| ICD-9-D-82129 | ICD-9-P-0083 | ICD-10-D-S82109A | ICD-10-D-S82202B |
| ICD-9-D-82130 | ICD-9-P-0084 | ICD-10-D-S82101A | ICD-10-D-S82402B |
| ICD-9-D-82132 | ICD-9-P-8155 | ICD-10-D-S82831A | ICD-10-P-0SPC0JZ |
| ICD-9-D-82133 | ICD-9-P-8006 | ICD-10-D-S82102A | ICD-10-P-0SPD0JZ |
| ICD-9-D-82139 | ICD-10-D-M84453A | ICD-10-D-S82832A | |
| ICD-9-D-73316 | ICD-10-D-M84750A | ICD-10-D-S82109B | |
| ICD-9-D-73393 | ICD-10-D-M84353A | ICD-10-D-S82109C | |
| ICD-9-D-82300 | ICD-10-D-S7290XA | ICD-10-D-S82101B | |
| ICD-9-D-82302 | ICD-10-D-S7290XB | ICD-10-D-S82831B | |
| ICD-9-D-82310 | ICD-10-D-S7290XC | ICD-10-D-S82102B | |
| ICD-9-D-82312 | ICD-10-D-S72409A | ICD-10-D-S82832B | |
| ICD-9-D-82380 | ICD-10-D-S72453A | ICD-10-D-S82201A |
Appendix Table A2.
Codes used to evaluate for knee joint complications.
| Joint infection | |||
|---|---|---|---|
| ICD-9-D-99666 | ICD-10-D-T8453XA | ICD-10-D-T8453XS | ICD-10-D-T8454XD |
| ICD-9-D-99667 | ICD-10-D-T8453XD | ICD-10-D-T8454XA | ICD-10-T8454XS |
| Periprosthetic fracture | ICD-10-D-T84043S | ||
| ICD-9-D-99644 | ICD-10-D-M9712XA | ICD-10-D-T84042D | |
| ICD-10-D-M9711XA | ICD-10-D-M9712XD | ICD-10-D-T84042S | |
| ICD-10-D-M9711XD | ICD-10-D-M9712XS | ICD-10-D-T84043A | |
| ICD-10-D-M9711XS | ICD-10-D-T84042A | ICD-10-D-T84043D | |
| Aseptic loosening | ICD-10-D-T84033S | ||
| ICD-9-D-99641 | ICD-10-D-T84032D | ICD-10-D-T84033A | |
| ICD-10-D-T84032A | ICD-10-D-T84032S | ICD-10-D-T84033D | |
| Prosthetic dislocation | |||
| ICD-9-P-7976 | ICD-10-P-OSSC0ZZ | ICD-10-P-0SSCXZZ | ICD-10-P-OSSDX5Z |
| ICD-9-P-7986 | ICD-10-P-OSSC3ZZ | ICD-10-P-0SSD04Z | ICD-10-P-0SSDXZZ |
| ICD-10-P-OSSC04Z | ICD-10-P-0SSC4ZZ | ICD-10-P-OSSD0ZZ | |
| Prosthetic revision | |||
| ICD-9-P-0080 | ICD-10-P-0QPF0JZ | ICD-10-P-0SPD08Z | ICD-10-P-0SRC06A |
| ICD-9-P-0081 | ICD-10-P-0QRF3JZ | ICD-10-P-0SRU0JA | ICD-10-P-0SRC06Z |
| ICD-9-P-0082 | ICD-10-P-0SUD09C | ICD-10-P-0SRU0JZ | ICD-10-P-0SRC0J9 |
| ICD-9-P-0083 | ICD-10-P-0QPF3JZ | ICD-10-P-0SPD48Z | ICD-10-P-0SRC0JA |
| ICD-9-P-0084 | ICD-10-P-0QRF4JZ | ICD-10-P-0SPD4JZ | ICD-10-P-0SRC0JZ |
| ICD-10-P-0SPC09Z | ICD-10-P-0QUF0JZ | ICD-10-P-0SPW0JZ | ICD-10-P-0SPC4JZ |
| ICD-10-P-0SUV09Z | ICD-10-P-0QUF4JZ | ICD-10-P-0SRV0J9 | ICD-10-P-0SPC0JZ |
| ICD-10-P-0SUW09Z | ICD-10-P-0SRT0J9 | ICD-10-P-0SRV0JA | ICD-10-P-0SRD069 |
| ICD-10-P-0SPD09Z | ICD-10-P-0SPC08Z | ICD-10-P-0SRV0JZ | ICD-10-P-0SRD0JA |
| ICD-10-P-0QRD0JZ | ICD-10-P-0SRT0JA | ICD-10-P-0SPT0JZ | ICD-10-P-0SRD0JZ |
| ICD-10-P-0QPD0JZ | ICD-10-P-0SRT0JZ | ICD-10-P-0SRW0J9 | ICD-10-P-0SRD0J9 |
| ICD-10-P-0QRD3JZ | ICD-10-P-0SPC48Z | ICD-10-P-0SRW0JA | ICD-10-P-0SRD06A |
| ICD-10-P-0QUD0JZ | ICD-10-P-0SPC4JZ | ICD-10-P-0SRW0JZ | ICD-10-P-0SRD06Z |
| ICD-10-P-0SUC09C | ICD-10-P-0SPV0JZ | ICD-10-P-0SPU0JZ | ICD-10-P-0SPD0JZ |
| ICD-10-P-0QRF0JZ | ICD-10-P-0SRU0J9 | ICD-10-P-0SRC069 | |
| Manipulation under anesthesia | |||
| CPT-27570 |
Appendix Table A3.
Codes used to evaluate for systemic complications.
| Acute renal failure | |||
|---|---|---|---|
| ICD-9-D-5845 | ICD-9-D-58081 | ICD-10-D-N179 | ICD-10-D-N004 |
| ICD-9-D-5846 | ICD-9-D-58089 | ICD-10-D-N19 | ICD-10-D-N005 |
| ICD-9-D-5847 | ICD-9-D-5809 | ICD-10-D-N990 | ICD-10-D-N006 |
| ICD-9-D-5848 | ICD-10-D-N170 | ICD-10-D-N000 | ICD-10-D-N007 |
| ICD-9-D-5849 | ICD-10-D-N171 | ICD-10-D-N001 | ICD-10-D-N008 |
| ICD-9-D-5800 | ICD-10-D-N172 | ICD-10-D-N002 | ICD-10-D-N009 |
| ICD-9-D-5804 | ICD-10-D-N178 | ICD-10-D-N003 | |
| Anemia | |||
| ICD-9-D-2851 | ICD-9-D-2800 | ICD-10-D-D500 | ICD-10-D-D62 |
| Altered mental status | |||
| ICD-9-D-78097 | ICD-10-D-R4182 | ||
| Cerebrovascular event | |||
| ICD-9-D-430 | ICD-10-D-I610 | ICD-10-D-I6320 | ICD-10-D-I63442 |
| ICD-9-D-431 | ICD-10-D-I611 | ICD-10-D-I6329 | ICD-10-D-I63443 |
| ICD-9-D-4320 | ICD-10-D-I612 | ICD-10-D-I658 | ICD-10-D-I63449 |
| ICD-9-D-4321 | ICD-10-D-I613 | ICD-10-D-I659 | ICD-10-D-I6349 |
| ICD-9-D-4329 | ICD-10-D-I614 | ICD-10-D-I6501 | ICD-10-D-I6350 |
| ICD-9-D-4359 | ICD-10-D-I615 | ICD-10-D-I6502 | ICD-10-D-I63511 |
| ICD-9-D-4358 | ICD-10-D-I616 | ICD-10-D-I6503 | ICD-10-D-I63512 |
| ICD-9-D-43300 | ICD-10-D-I618 | ICD-10-D-I6509 | ICD-10-D-I63513 |
| ICD-9-D-43301 | ICD-10-D-I619 | ICD-10-D-I6521 | ICD-10-D-I63519 |
| ICD-9-D-43310 | ICD-10-D-I6200 | ICD-10-D-I6522 | ICD-10-D-I63521 |
| ICD-9-D-43311 | ICD-10-D-I6201 | ICD-10-D-I6523 | ICD-10-D-I63522 |
| ICD-9-D-43320 | ICD-10-D-I6202 | ICD-10-D-I6529 | ICD-10-D-I63523 |
| ICD-9-D-43321 | ICD-10-D-I6203 | ICD-10-D-G458 | ICD-10-D-I63529 |
| ICD-9-D-43330 | ICD-10-D-I629 | ICD-10-D-G459 | ICD-10-D-I63531 |
| ICD-9-D-43331 | ICD-10-D-I6302 | ICD-10-D-I6330 | ICD-10-D-I63532 |
| ICD-9-D-43380 | ICD-10-D-I6312 | ICD-10-D-I63311 | ICD-10-D-I63533 |
| ICD-9-D-43381 | ICD-10-D-I6322 | ICD-10-D-I63312 | ICD-10-D-I63539 |
| ICD-9-D-43390 | ICD-10-D-I651 | ICD-10-D-I63313 | ICD-10-D-I63541 |
| ICD-9-D-43391 | ICD-10-D-I63031 | ICD-10-D-I63319 | ICD-10-D-I63542 |
| ICD-9-D-43400 | ICD-10-D-I63032 | ICD-10-D-I63321 | ICD-10-D-I63543 |
| ICD-9-D-43401 | ICD-10-D-I63033 | ICD-10-D-I63322 | ICD-10-D-I63549 |
| ICD-9-D-43410 | ICD-10-D-I63039 | ICD-10-D-I63323 | ICD-10-D-I6359 |
| ICD-9-D-43411 | ICD-10-D-I63131 | ICD-10-D-I63329 | ICD-10-D-I636 |
| ICD-9-D-43490 | ICD-10-D-I63132 | ICD-10-D-I63331 | ICD-10-D-I638 |
| ICD-9-D-43491 | ICD-10-D-I63133 | ICD-10-D-I63332 | ICD-10-D-I639 |
| ICD-10-D-I6000 | ICD-10-D-I63139 | ICD-10-D-I63333 | ICD-10-D-I6601 |
| ICD-10-D-I6001 | ICD-10-D-I63231 | ICD-10-D-I63339 | ICD-10-D-I6602 |
| ICD-10-D-I6002 | ICD-10-D-I63232 | ICD-10-D-I63341 | ICD-10-D-I6603 |
| ICD-10-D-I6010 | ICD-10-D-I63233 | ICD-10-D-I63342 | ICD-10-D-I6609 |
| ICD-10-D-I6011 | ICD-10-D-I63239 | ICD-10-D-I63343 | ICD-10-D-I6611 |
| ICD-10-D-I6012 | ICD-10-D-I63011 | ICD-10-D-I63349 | ICD-10-D-I6612 |
| ICD-10-D-I602 | ICD-10-D-I63012 | ICD-10-D-I6339 | ICD-10-D-I6613 |
| ICD-10-D-I6020 | ICD-10-D-I63013 | ICD-10-D-I6340 | ICD-10-D-I6619 |
| ICD-10-D-I6021 | ICD-10-D-I63019 | ICD-10-D-I63411 | ICD-10-D-I6621 |
| ICD-10-D-I6022 | ICD-10-D-I63111 | ICD-10-D-I63412 | ICD-10-D-I6622 |
| ICD-10-D-I6030 | ICD-10-D-I63112 | ICD-10-D-I63413 | ICD-10-D-I6623 |
| ICD-10-D-I6031 | ICD-10-D-I63113 | ICD-10-D-I63419 | ICD-10-D-I6629 |
| ICD-10-D-I6032 | ICD-10-D-I63119 | ICD-10-D-I63421 | ICD-10-D-I668 |
| ICD-10-D-I604 | ICD-10-D-I63211 | ICD-10-D-I63422 | ICD-10-D-I669 |
| ICD-10-D-I6050 | ICD-10-D-I63212 | ICD-10-D-I63423 | |
| ICD-10-D-I6051 | ICD-10-D-I63213 | ICD-10-D-I63429 | |
| ICD-10-D-I6052 | ICD-10-D-I63219 | ICD-10-D-I63431 | |
| ICD-10-D-I606 | ICD-10-D-I6300 | ICD-10-D-I63432 | |
| ICD-10-D-I607 | ICD-10-D-I6309 | ICD-10-D-I63433 | |
| ICD-10-D-I608 | ICD-10-D-I6310 | ICD-10-D-I63439 | |
| ICD-10-D-I609 | ICD-10-D-I6319 | ICD-10-D-I63441 | |
| Deep vein thrombosis | |||
| ICD-9-D-45340 | ICD-10-D-I82403 | ICD-10-D-I824Z9 | ICD-10-D-I825Z1 |
| ICD-9-D-45341 | ICD-10-D-I82409 | ICD-10-D-I82501 | ICD-10-D-I825Z2 |
| ICD-9-D-45342 | ICD-10-D-I82491 | ICD-10-D-I82502 | ICD-10-D-I825Z3 |
| ICD-9-D-45111 | ICD-10-D-I82492 | ICD-10-D-I82503 | ICD-10-D-I825Z9 |
| ICD-9-D-45119 | ICD-10-D-I82493 | ICD-10-D-I82509 | |
| ICD-9-D-45389 | ICD-10-D-I82499 | ICD-10-D-I82591 | |
| ICD-9-D-4539 | ICD-10-D-I824Y1 | ICD-10-D-I82592 | |
| ICD-9-D-4512 | ICD-10-D-I824Y2 | ICD-10-D-I82593 | |
| ICD-9-D-45350 | ICD-10-D-I824Y3 | ICD-10-D-I82599 | |
| ICD-9-D-45351 | ICD-10-D-I824Y9 | ICD-10-D-I825Y1 | |
| ICD-9-D-45352 | ICD-10-D-I824Z1 | ICD-10-D-I825Y2 | |
| ICD-10-D-I82401 | ICD-10-D-I824Z2 | ICD-10-D-I825Y3 | |
| ICD-10-D-I82402 | ICD-10-D-I824Z3 | ICD-10-D-I825Y9 | |
| Myocardial infarction | |||
| ICD-9-D-41000 | ICD-9-D-41041 | ICD-9-D-41072 | ICD-10-D-I2121 |
| ICD-9-D-41001 | ICD-9-D-41042 | ICD-9-D-41060 | ICD-10-D-I229 |
| ICD-9-D-41002 | ICD-9-D-41050 | ICD-9-D-41061 | ICD-10-D-I2101 |
| ICD-9-D-41010 | ICD-9-D-41051 | ICD-9-D-41062 | ICD-10-D-I221 |
| ICD-9-D-41011 | ICD-9-D-41052 | ICD-10-D-I214 | ICD-10-D-I220 |
| ICD-9-D-41012 | ICD-9-D-41080 | ICD-10-D-I213 | ICD-10-D-I228 |
| ICD-9-D-41020 | ICD-9-D-41081 | ICD-10-D-I2119 | |
| ICD-9-D-41021 | ICD-9-D-41082 | ICD-10-D-I2109 | |
| ICD-9-D-41022 | ICD-9-D-41090 | ICD-10-D-I2129 | |
| ICD-9-D-41030 | ICD-9-D-41091 | ICD-10-D-I240 | |
| ICD-9-D-41031 | ICD-9-D-41092 | ICD-10-D-I2111 | |
| ICD-9-D-41032 | ICD-9-D-41070 | ICD-10-D-I2102 | |
| ICD-9-D-41040 | ICD-9-D-41071 | ICD-10-D-I222 | |
| Pneumonia | |||
| ICD-9-D-413 | ICD-9-D-48232 | ICD-9-D-4831 | ICD-10-D-J150 |
| ICD-9-D-4800 | ICD-9-D-48239 | ICD-9-D-4838 | ICD-10-D-J1289 |
| ICD-9-D-4801 | ICD-9-D-48240 | ICD-9-D-4841 | ICD-10-D-J09X1 |
| ICD-9-D-4802 | ICD-9-D-48241 | ICD-9-D-485 | ICD-10-D-J851 |
| ICD-9-D-4803 | ICD-9-D-48242 | ICD-9-D-486 | ICD-10-D-J1001 |
| ICD-9-D-4808 | ICD-9-D-48249 | ICD-9-D-4870 | ICD-10-D-J1108 |
| ICD-9-D-4809 | ICD-9-D-48281 | ICD-9-D-99731 | ICD-10-D-J153 |
| ICD-9-D-481 | ICD-9-D-48282 | ICD-9-D-99732 | ICD-10-D-J122 |
| ICD-9-D-4820 | ICD-9-D-48283 | ICD-10-D-J189 | ICD-10-D-J1281 |
| ICD-9-D-4821 | ICD-9-D-48284 | ICD-10-D-J188 | |
| ICD-9-D-4822 | ICD-9-D-48289 | ICD-10-D-J180 | |
| ICD-9-D-48230 | ICD-9-D-4829 | ICD-10-D-J151 | |
| ICD-9-D-48231 | ICD-9-D-4830 | ICD-10-D-J157 | |
| Pulmonary embolism | |||
| ICD-9-D-41511 | ICD-9-D-41519 | ICD-10-D-I2609 | ICD-10-D-I2782 |
| ICD-9-D-41519 | ICD-9-D-4162 | ICD-10-D-I2699 | |
| Respiratory failure | |||
| ICD-9-D-51853 | ICD-9-D-51882 | ICD-10-D-J9611 | ICD-10-D-J9612 |
| ICD-9-D-51851 | ICD-10-D-J9601 | ICD-10-D-J9602 | ICD-10-D-J9692 |
| ICD-9-D-51883 | ICD-10-D-J9600 | ICD-10-D-J9620 | ICD-10-D-J95822 |
| ICD-9-D-51884 | ICD-10-D-J9690 | ICD-10-D-J9622 | ICD-10-D-J952 |
| ICD-9-D-51881 | ICD-10-D-J9621 | ICD-10-D-J9691 | ICD-10-D-J953 |
| ICD-9-D-51852 | ICD-10-D-J9610 | ICD-10-D-J95821 | |
| Urinary tract infection | |||
| ICD-9-D-5990 | ICD-10-D-N390 |
References
- 1.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz A.M., Farley K.X., Guild G.N. Projections and Epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. 2020;35:S79. doi: 10.1016/j.arth.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vessely M.B., Whaley A.L., Harmsen W.S. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop Relat Res. 2006;452:28. doi: 10.1097/01.blo.0000229356.81749.11. [DOI] [PubMed] [Google Scholar]
- 4.Bouras T., Bitas V., Fennema P. Good long-term results following cementless TKA with a titanium plasma coating. Knee Surg Sports Traumatol Arthrosc. 2017;25:2801. doi: 10.1007/s00167-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 5.Jauregui J.J., Cherian J.J., Pierce T.P. Long-term survivorship and clinical outcomes following total knee arthroplasty. J Arthroplasty. 2015;30:2164. doi: 10.1016/j.arth.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.H., Park J.W., Kim J.S. Long-term clinical outcomes and survivorship of press-fit condylar sigma fixed-bearing and mobile-bearing total knee prostheses in the same patients. J Bone Joint Surg Am. 2014;96:e168. doi: 10.2106/JBJS.M.01130. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y.J., Ra H.J. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi: 10.5792/ksrr.2016.28.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourne R.B., Chesworth B.M., Davis A.M. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468:57. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson J.G., Wixson R.L., Tsai D. Functional outcome and patient satisfaction in total knee patients over the age of 75. J Arthroplasty. 1996;11:831. doi: 10.1016/s0883-5403(96)80183-5. [DOI] [PubMed] [Google Scholar]
- 10.Dunbar M.J., Robertsson O., Ryd L. Appropriate questionnaires for knee arthroplasty. Results of a survey of 3600 patients from the Swedish Knee Arthroplasty Registry. J Bone Joint Surg Br. 2001;83:339. doi: 10.1302/0301-620x.83b3.11134. [DOI] [PubMed] [Google Scholar]
- 11.Noble P.C., Conditt M.A., Cook K.F. The John Insall Award: patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35. doi: 10.1097/01.blo.0000238825.63648.1e. [DOI] [PubMed] [Google Scholar]
- 12.Kayani B., Konan S., Ayuob A. Robotic technology in total knee arthroplasty: a systematic review. EFORT Open Rev. 2019;4:611. doi: 10.1302/2058-5241.4.190022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandher D.S., Oh K.J., Boaparai R.S. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2007;454:281. doi: 10.1097/01.blo.0000246562.50467.3d. [DOI] [PubMed] [Google Scholar]
- 14.Chin P.L., Yang K.Y., Yeo S.J. Randomized control trial comparing radiographic total knee arthroplasty implant placement using computer navigation versus conventional technique. J Arthroplasty. 2005;20:618. doi: 10.1016/j.arth.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Hetaimish B.M., Khan M.M., Simunovic N. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27:1177. doi: 10.1016/j.arth.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Matziolis G., Krocker D., Weiss U. A prospective, randomized study of computer-assisted and conventional total knee arthroplasty. Three-dimensional evaluation of implant alignment and rotation. J Bone Joint Surg Am. 2007;89:236. doi: 10.2106/JBJS.F.00386. [DOI] [PubMed] [Google Scholar]
- 17.Leung T., Vyas D. Robotic surgery: Applications. Am J Robot Surg. 2014;1:1. [PMC free article] [PubMed] [Google Scholar]
- 18.Honl M., Dierk O., Gauck C. Comparison of robotic-assisted and manual implantation of a primary total hip replacement. A prospective study. J Bone Joint Surg Am. 2003;85:1470. doi: 10.2106/00004623-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara S., Sugano N., Nishii T. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21:957. doi: 10.1016/j.arth.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Bargar W.L., Parise C.A., Hankins A. Fourteen year follow-up of randomized clinical trials of active robotic-assisted total hip arthroplasty. J Arthroplasty. 2018;33:810. doi: 10.1016/j.arth.2017.09.066. [DOI] [PubMed] [Google Scholar]
- 21.Siebel T., Käfer W. [Clinical outcome following robotic assisted versus conventional total hip arthroplasty: a controlled and prospective study of seventy-one patients] Z Orthop Ihre Grenzgeb. 2005;143:391. doi: 10.1055/s-2005-836776. [DOI] [PubMed] [Google Scholar]
- 22.Barrett A.R., Davies B.L., Gomes M.P. Computer-assisted hip resurfacing surgery using the acrobot navigation system. Proc Inst Mech Eng H. 2007;221:773. doi: 10.1243/09544119JEIM283. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian P., Wainwright T.W., Bahadori S. A review of the evolution of robotic-assisted total hip arthroplasty. Hip Int. 2019;29:232. doi: 10.1177/1120700019828286. [DOI] [PubMed] [Google Scholar]
- 24.Jacofsky D.J., Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31:2353. doi: 10.1016/j.arth.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Park S.E., Lee C.T. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22:1054. doi: 10.1016/j.arth.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Bellemans J., Vandenneucker H., Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111. doi: 10.1097/BLO.0b013e318126c0c0. [DOI] [PubMed] [Google Scholar]
- 27.Hampp E.L., Chughtai M., Scholl L.Y. Robotic-arm assisted total knee arthroplasty demonstrated greater accuracy and precision to plan compared with manual techniques. J Knee Surg. 2019;32:239. doi: 10.1055/s-0038-1641729. [DOI] [PubMed] [Google Scholar]
- 28.Moon Y.W., Ha C.W., Do K.H. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17:86. doi: 10.3109/10929088.2012.654408. [DOI] [PubMed] [Google Scholar]
- 29.Sultan A.A., Samuel L.T., Khlopas A. Robotic-arm assisted total knee arthroplasty more accurately restored the posterior condylar offset ratio and the insall-salvati index compared to the manual technique; A cohort-matched study. Surg Technol Int. 2019;34:409. [PubMed] [Google Scholar]
- 30.Song E.K., Seon J.K., Yim J.H. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471:118. doi: 10.1007/s11999-012-2407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sousa P.L., Sculco P.K., Mayman D.J. Robots in the operating room during hip and knee arthroplasty. Curr Rev Musculoskelet Med. 2020;13:309. doi: 10.1007/s12178-020-09625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liow M.H., Xia Z., Wong M.K. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29:2373. doi: 10.1016/j.arth.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Song E.K., Seon J.K., Park S.J. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19:1069. doi: 10.1007/s00167-011-1400-9. [DOI] [PubMed] [Google Scholar]
- 34.Antonios J.K., Korber S., Sivasundaram L. Trends in computer navigation and robotic assistance for total knee arthroplasty in the United States: an analysis of patient and hospital factors. Arthroplast Today. 2019;5:88. doi: 10.1016/j.artd.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malkan A.L., Roche M.W., Kolisek F.R. Manipulation under anesthesia rates in technology-assisted versus conventional-instrumentation total knee arthroplasty. Surg Technol Int. 2020;36:336. [PubMed] [Google Scholar]
- 36.Crawford D.A., Adams J.B., Morris M.J. Manipulation under anesthesia after knee arthroplasty is associated with worse long-term clinical outcomes and survivorship. J Knee Surg [Epub ahead of print]. PMID: 31645072. 2019 doi: 10.1055/s-0039-1700569. [DOI] [PubMed] [Google Scholar]
- 37.Batailler C., White N., Ranaldi F.M. Improved implant position and lower revision rate with robotic-assisted unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27:1232. doi: 10.1007/s00167-018-5081-5. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y.H., Yoon S.H., Park J.W. Does robotic-assisted TKA result in better outcome scores or long-term survivorship than conventional TKA? A randomized, controlled trial. Clin Orthop Relat Res. 2020;478:266. doi: 10.1097/CORR.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagio K., Sugano N., Takashina M. Effectiveness of the ROBODOC system in preventing intraoperative pulmonary embolism. Acta Orthop Scand. 2003;74:264. doi: 10.1080/00016470310014175. [DOI] [PubMed] [Google Scholar]
- 40.Perets I., Walsh J.P., Close M.R. Robot-assisted total hip arthroplasty: clinical outcomes and complication rate. Int J Med Robot. 2018;14:e1912. doi: 10.1002/rcs.1912. [DOI] [PubMed] [Google Scholar]
- 41.Lespasio M.J., Guarino A.J., Sodhi N. Pain management associated with total joint arthroplasty: a primer. Perm J. 2019;23:18. doi: 10.7812/TPP/18-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam A., Juurlink D.N. The prescription opioid epidemic: an overview for anesthesiologists. Can J Anaesth. 2016;63:61. doi: 10.1007/s12630-015-0520-y. [DOI] [PubMed] [Google Scholar]
- 43.Dwyer M.K., Tumpowsky C.M., Hiltz N.L. Characterization of post-operative opioid use following total joint arthroplasty. J Arthroplasty. 2018;33:668. doi: 10.1016/j.arth.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Kayani B., Konan S., Tahmassebi J. Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty: a prospective cohort study. Bone Joint J. 2018;100-b:930. doi: 10.1302/0301-620X.100B7.BJJ-2017-1449.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnett R.S., Barrack R.L. Computer-assisted total knee arthroplasty is currently of no proven clinical benefit: a systematic review. Clin Orthop Relat Res. 2013;471:264. doi: 10.1007/s11999-012-2528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts T.D., Frampton C.M., Young S.W. Outcomes of computer-assisted surgery compared with conventional instrumentation in 19,221 total knee arthroplasties: results after a mean of 4.5 years of follow-up. J Bone Joint Surg Am. 2020;102:550. doi: 10.2106/JBJS.19.00852. [DOI] [PubMed] [Google Scholar]
- 47.Hampp E.L., Sodhi N., Scholl L. Less iatrogenic soft-tissue damage utilizing robotic-assisted total knee arthroplasty when compared with a manual approach: a blinded assessment. Bone Joint Res. 2019;8:495. doi: 10.1302/2046-3758.810.BJR-2019-0129.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.