Abstract
Background
Phyllanthusfraternus is a pantropical weed of family phyllanthaceae, mainly found in northeast India. It has been used in the folklore medicine of Manipur tribe for treating type 2 diabetes.
Objective
The present study was commenced to evaluate the anti-diabetic and renoprotective potential of P.fraternus (aerial parts) in alloxan-induced diabetes in rats.
Materials and methods
Alloxan (130 mg/kg, ip) was used for the induction of diabetes in adult male wistar rats. Animals with blood glucose level greater than 280 mg/dL were treated once daily for 14 days with various test extracts. The biochemical parameters were measured from serum on the 15th day post-treatment. Necropsy samples harvested from pancreas and kidneys were examined for histopathological changes in these organs.
Results
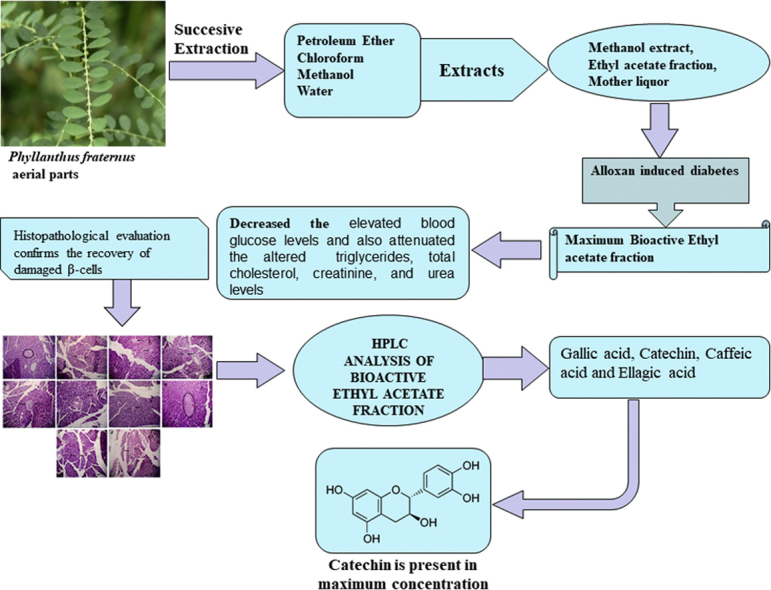
Alloxan-induced diabetes not only caused significant increases in blood glucose, triglycerides, total cholesterol, creatinine and urea levels, but also provoked high oxidative stress in pancreas and kidneys. Profound morphological injuries were observed in islets of Langerhans and kidneys of diabetic animals. Administration of methanol extract (200 and 400 mg/kg) and mother liquor (200 and 400 mg/kg) ameliorate the elevated levels of blood glucose, triglycerides, total cholesterol as well as other biochemical parameters, but highest reduction in blood glucose concentration was observed with the largest dose of ethyl acetate fraction (400 mg/kg) of P.fraternus. Histopathological examination of pancreas and kidneys also exhibited greater protection by treatment with acetate fraction (400 mg/kg). The HPLC analysis showed the presence of four polyphenols such as catechin, gallic acid, caffeic acid and ellagic acid in ethyl acetate fraction of P. fraternus during HPLC analysis.
Conclusion
The results suggest that polyphenols present in P.fraternus may be responsible for the anti-diabetic and renoprotective activity in rats. Such protective effects of could be mediated through flavonol-induced anti-oxidant and anti-inflammatory activities in the pancreas and kidneys.
Keywords: Phyllanthusfraternus, Antidiabetic, Antioxidant, Polyphenols
Graphical abstract
1. Introduction
Diabetes is a immedicable metabolic disorder that occurs when there is not enough insulin secretion form pancreas and/or when the body is not able to efficiently utilize insulin and resistance to this hormone is developed by the patients. Unbridled release of glucose from the liver and poor uptake by skeletal muscles leads to hyperglycemia, glycosuria, and oxidative stress. The consequences of uncontrolled diabetes consist of a host of micro- and macro-vascular complications affecting several vital organs, particularly the cardiovascular system, kidneys, eyes, and peripheral nerve damage [1,2].
The upward trend of type 2 diabetes or Diabetes mellitus constitutes a serious global public health problem and puts a heavy burden on health care costs in both developed and developing countries. The global prevalence of diabetes mellitus affects around 250 million individuals, and it is predicted that by the year 2025, there will be an increase in 72% of diabetic patients globally [3]. Several epidemiologic studies have established diabetes link with sedentary life-style, urbanization and increased economic prosperity, unhealthy dietary habits, intake of sugar-loaded drinks, obesity and stressful conditions. Some people are genetically prone to type 2 diabetes caused by pancreatic beta-cells dysfunction [4]. Also, there is an autoimmune disorder involved in causing pancreatic malfunction among diabetic patients.
Several synthetic drugs (insulin, oral antidiabetic agents) are available for the treatment of diabetes mellitus, but the prolonged usage of synthetic drugs not only produces drug resistance, but also causes adverse drug reactions. Due to the high cost of synthetic drugs, many poor diabetic patients cannot afford expensive drugs and are desperately looking for safe alternative therapies. Besides the enormous cost, there is also poor availability of modern therapies in the rural areas, especially developing countries like India [5]. With the increasing incidence of diabetes mellitus in urban and rural populations throughout the world, there is a urgent need to develop safe and effective anti-diabetic indigenous and cost-effective botanical remedies. Different alternative strategies for the treatment of diabetes mellitus are urgently needed to manage harmful pathological conditions, morbidity and mortality associated with this disorder.
Phyllanthus is the largest genus of flowering plants that belongs to family Phyllanthaceae having about 750–1250 species. Researchers are showing greater interest on the plants belonging to the genus Phyllanthus, owing to their therapeutic roles in non-communicable diseases [6]. The tribal communities located in the Asian countries have been using the Phyllanthus species for different herbal remedies since time immemorial [7]. Phyllanthus fraternus is a pantropical weed which is mostly found in northeast India. This plant is locally known as Hajarmani and Kanocha (Hindi language), and as herbal formulation named “Bhumyamlaki” [8,9]. This plant was known to be highly effective for treating different diseases such as hepatitis, cold, flu, tuberculosis, viral infections, anemia, biliary and urinary disorders and other bacterial and fungal infections [8,10]. This plant has the ability to act as antioxidant, antinociceptive, hepatoprotective, and antifibromyalgic [[11], [12], [13]]. Literature survey revealed the presence of various phytoconstituents in P. fraternus such as phyllanthin, hypophyllanthin, nirphyllin, phyllnirurin, niranthin, nirtetralin, niruriside, securinine, limonene, 4-methoxy-securinine, 4-methoxy-norsecurinine, niruretin, phyllanthol, phyllanthenol, phyllanthenone, lintetralin, astragalin, cymene, niruodine and phyllanthimide [14].
Ethnic communities in the Manipur state of India have been traditionally using P. fraternus for treating Diabetes mellitus [15]. Moreover, various species of Phyllanthus such as Phyllanthus reticulatus, Phyllanthus niruri and Phyllanthus amarus has been evaluated in alloxan induced diabetes [[16], [17], [18]]. The antihyperglycemic effect of P. amarus pertains to the presence of various secondary metabolites such as flavonoids, tannins and alkaloids [18]. Similarly, the antidiabetic effect of P. niruri may be due to the presence of flavonoids, alkaloids, glycosides and tannins in the plant [17]. The phytochemical screening of P. fraternus also showed the presence of similar phytoconstituents as other species contain. Garg et al., 2008 and Kushwah et al., 2010 had evaluated the aqueous and ethanolic extracts of P. fraternus in fructose induced insulin resistance and alloxan induced diabetes in rats [19, 20]. Therefore, on the basis of traditional use and previous published reports, the present study was undertaken to further investigate the anti-diabetic potential of methanol extract and bioactive fractions of P. fraternus in alloxan-induced diabetes in rats and various biochemical and histopathological studies were carried out. Diabetic nephropathy and oxidative stress are the most common complications in patients suffering from diabetes. Therefore, the current study was also intended to determine the renoprotective and antioxidant potential of P. fraternus. The overall objective of these studies was to provide science-based rationale for the usage of this herbal remedy for treating diabetes and associated kidney problems in humans.
2. Materials and methods
2.1. Plant material
The aerial parts (stem: leaves: 1:1) of the plant P. fraternus were obtained, dried and identified from the Department of Botany, Sri Venkateswara University, Tripura. The dried plant was deposited as voucher specimen number 1023 in the herbarium of department of same university. The aerial parts of the plant were air dried under shade and then coarsely powdered. Different extracts of the powdered plant material were prepared using Soxhlet extraction and different solvents based on their polarity viz. petroleum ether, chloroform, and methanol. The remaining marc was soaked in water for 24 h for obtaining the respective aqueous extract. The extracts were then concentrated using rotary evaporator (IKA, Works INC., North America) and stored at −20 °C until further use.
The further fractionation procedure involved the suspension of 50 g methanol extract in 100 mL water and rapidly shaken in the separating funnel following the addition of hexane (200 mL). The separating funnel was allowed to stand till the separation of the two layers. The aqueous layer from the separation funnel was collected and was further subjected to using ethyl acetate (3 × 150 mL) and the remaining mother liquor was stored. The yield of all the obtained fraction was calculated by weighing the amount of extract in contrast to the amount of dried raw material. The concentrated fractions were packed and stored to avoid microbial contamination.
2.2. Drugs and chemicals
Alloxan (LobaChemie, Mumbai, India), 1,1,3,3 tetra methoxy propane (Sigma Aldrich, Bangalore, India), reduced glutathione (GSH) (LobaChemie, Mumbai, India), thio-barbituric acid (Loba Chem, Mumbai, India), tris buffer (Merck specialities, Mumbai), glacial acetic acid, sodium di-hydrogen phosphate and di-sodium hydrogen phosphate (Thermo Fisher Scientific, Mumbai) were used in the present study.
2.3. Preliminary phytochemical screening of extracts
The prepared extracts of the powdered plant material were subjected to preliminary phytochemical screening using standard procedures [21].
2.4. Experimental animals
Adult male Wistar rats weighing 200–250 g were procured from Indian Institute of Integrated Medicine (IIIM), Jammu, India. Animals were kept under standard animal husbandry conditions with 12 h cycle of light and darkness and had free access to tap water and laboratory pellet chow diet at all times. The study procedure was duly approved by the Institutional Animal Ethics Committee (IAEC) no. 226/CPCSEA-2015-33 (Guru Nanak Dev University). Animals were housed and handled according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
2.5. Induction of diabetes
A standardized dose of alloxan monohydrate in normal saline (130 mg/kg body weight i. p.) was used for the induction of diabetes. After 72 h of alloxan administration, blood was withdrawn under anesthesia (chloral hydrate 300 mg/kg) by retro orbital vein puncture and Omron HGM-112 blood glucose monitoring system (DEL Bio Inc., Taiwan) was used for measuring blood glucose level of all animals. Further experiments were done on animals having blood glucose level more than 280 mg/dL. Diabetic rats were treated once daily for 14 consecutive days with various test extracts at different doses.
2.6. Experimental protocol
Sixty rats were randomly divided into ten groups, each comprising of six animals per group. Standard antidiabetic drug (Gliclazide) and test agents (methanol extract, ethyl acetate fractions and mother liquor) were administered orally once daily for 14 consecutive days. Suspension of various test doses was prepared using 0.5% aqueous solution of carboxymethyl cellulose (CMC) as a vehicle.
2.6.1. Group I (normal control)
No treatment was given to this group of six rats.
2.6.2. Group II (diabetic control)
Single intraperitonial injection of alloxan monohydrate 130 mg/kg was used to induce diabetes. No test substance was administered to this group.
2.6.3. Group III (positive control)
Comparator antidiabetic drug, Gliclazide (10 mg/kg, po), was given for 14 consecutive days to diabetic rats.
2.6.4. Group IV & V
Diabetic animals were treated with methanol extract of P. fraternus (200 and 400 mg/kg, po) for 14 consecutive days.
2.6.5. Group VI & VIII
Diabetic animals were administered with ethyl acetate fraction of P. fraternus (200 and 400 mg/kg, p. o.) for 14 consecutive days.
2.6.6. Group IX & X
Diabetic animals were treated with mother liquor of P. fraternus (200 or 400 mg/kg, po) for 14 consecutive days.
2.7. Biochemical measurements
After 15 days of treatment all the animals were fasted overnight, and blood was withdrawn under anesthesia using chloral hydrate 300 mg/kg [22] by retro orbital vein puncture. The collected blood was allowed to clot and centrifuged for 10 min at 10,000 rpm to separate serum for various estimations. Animals were sacrificed as per the guidelines of CPCSEA following the protocol of cervical dislocation. The two important organs viz. Pancreas and kidneys of the rats were removed aseptically for analyzing the different biochemical and histopathological studies.
Biochemical parameters like total protein content, total cholesterol, triglycerides, urea and creatinine were estimated using commercially available kits (ErbaLachema) and concentrations were expressed in mg/dl.
2.8. Evaluation of diabetes-induced oxidative stress in pancreas and renal tissues
Thio-barbituric acid reactive substances (TBARS) were quantitatively measured according to the method of Okhava [23] and the results were expressed in the units nanomoles per mg of protein. GSH level in tissue was also determined using standardized method [24], and the results were expressed in the units of microgram of reduced glutathione per mg of protein. The SAG (superoxide anion generation) in tissue was assayed by measuring reduced nitroblue tetrazolium (NBT) in renal tissue [25]. The results were expressed as reduced NBT pmoles per minute per mg of tissue.
2.9. Histopathological studies
Samples harvested from the pancreas and kidneys were preserved in 10% buffered formaldehyde in a stoppered container. The samples fixed in buffered formaldehyde were dehydrated in graded concentrations of ethanol, immersed in xylene, and then embedded in paraffin. The 5-μm thick sections were stained with hematoxylin and eosin (H&E). The slides were examined under binocular microscope for gross histopathological changes. Photomicrographs were taken with attached camera on the microscope.
2.10. High performance liquid chromatography (HPLC) of bioactive ethyl acetate fraction of P.fraternus
The bioactive ethyl acetate fraction was analyzed on Shimadzu UHPLC Nexera system (Shimadzu, MA, USA), provided with a photodiode array (PDA) detector using C18 (150 mm × 4.6 mm, i. d. 5 μm) column. The gradient mobile phase consisting of 0.1% acetic acid aqueous as solution A and Methanol as solution B was used. The gradient elution is: 0–1 min, 30% B; 1–10 min, 65% B; 10–14 min, 80% B; 14–16 min, 80% A, 16–17 min: 40% B, 17–20 min: 35% B and 20–21 min: 30% B. The flow rate was set as 1 ml/min and the injection volume was 5 mL. Quantification of peaks was also done using software provided with Shimadzu UHPLC Nexera system.
2.11. Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) and the post hoc analysis was done using Tukey's test by Sigma Stat Version- 3.5. All results were expressed in the form of mean ± standard error of the mean (S.E.M). The p value less than 0.05 was considered to be statistically significant.
3. Results
3.1. Preliminary phytochemical screening
The percentage yield of each extract was as follows: petroleum ether (4.41%), chloroform (3.42%), methanol (13.4%) and aqueous (20.05%). It was observed from the preliminary phytochemical screening that the petroleum ether extract was rich in lipids, methanol extract was rich in alkaloids, glycosides and flavonoids and the chloroform extract did not contain any phytoconstituent. Carbohydrates and proteins were found in aqueous extract.
3.2. Effects of various P.fraternus extracts on elevated blood glucose level
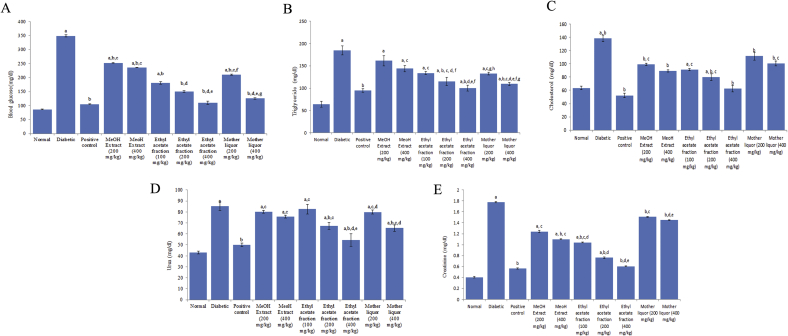
Administration of methanol extract (200, 400 mg/kg), ethyl acetate fraction (100, 200, 400 mg/kg) and mother liquor (200, 400 mg/kg) for 14 days reduced the blood glucose levels in diabetic rats. However, the elevated blood glucose were lowered to the normal level by the largest dose of ethyl acetate fraction (400 mg/kg)in diabetic animals as compared to untreated or normal controls (Fig. 1A).
Fig. 1.
(A) Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the blood glucose levels. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. normal control; pb < 0.05 Vs. diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH (200 mg/kg); pe < 0.05 Vs. MeOH (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (400 mg/kg); pg < 0.05 Vs. mother liquor (200 mg/kg) (B): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the blood triglyceride levels. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. normal control; pb < 0.05 Vs diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH extract (200 mg/kg); pe < 0.05 Vs. MeOH extract (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (100 mg/kg): pg < ethyl acetate fraction (200 mg/kg) (C): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the blood cholesterol levels. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. normal control; pb < 0.05 Vs. diabetic; pc < 0.05 Vs. positive control (D): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the blood urea levels. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. normal control; pb < 0.05 Vs. diabetic; pc.<0.05 Vs. positive control; pd < 0.05 Vs. MeOH extract (200 mg/kg); pe < 0.05 Vs. MeOH extract (400 mg/kg) (E): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the creatinine levels. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. normal control; pb < 0.05 Vs. diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH extract (200 mg/kg); pe < 0.05 Vs. MeOH extract (400 mg/kg).
3.3. Effects of various P.fraternus extracts on elevated blood triglycerides and total cholesterol level
Noticeable alleviation in total cholesterol and triglycerides were observed in alloxan-induced diabetic animals. Treatment with methanol extract (200,400 mg/kg), ethyl acetate fraction (100, 200, 400 mg/kg) and mother liquor (200, 400 mg/kg) reduce the varied triglycerides and cholesterol content, but a significant reduction occurred in ethyl acetate fraction (400 mg/kg) as compared to the control group (Fig. 1B and C).
3.4. Effects of various P.fraternus extracts on elevated urea and creatinine level
Significant increases in serum urea and creatinine levels were found in diabetic animals. Administration of methanol extract (200, 400 mg/kg), ethyl acetate fraction (100, 200, 400 mg/kg) and mother liquor (200, 400 mg/kg) prevent the increase of urea and creatinine. The group treated with ethyl acetate fraction at a dose of 400 mg/kg showed significant reductions in urea and creatinine levels (Fig. 1D and E).
3.5. Effect of various P.fraternus extracts on oxidative stress parameters
Diabetes-induced oxidative stress caused marked increase in lipid peroxides such as thiobarbituric acid reactive substances (TBARS), superoxide anion generation (SAG) and reduction in reduced glutathione (GSH) concentration in the pancreas and kidney tissues. Treatment with methanol extract (200, 400 mg/kg), ethyl acetate fraction (100, 200, 400 mg/kg) and mother liquor (200, 400 mg/kg) caused reduced levels of lipid peroxides and increase in GSH level. A significant effect in these biochemical parameters was observed with ethyl acetate fraction at a dose of 400 mg/kg (Fig. 2).
Fig. 2.
(A) Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the TBARS levels in pancreatic tissue. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. normal control; pb < 0.05 Vs diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH (200 mg/kg); pe < 0.05 Vs. MeOH (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (100 mg/kg); pg < ethyl acetate fraction (200 mg/kg); ph < 0.05 Vs. ethyl acetate fraction (400 mg/kg) (B): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the TBARS levels in kidney tissues. Values are expressed as mean ± S.E.M (N = 6). Pa<0.05 Vs normal; pb < 0.05 Vs diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs MeOH (200 mg/kg); pe < 0.05 Vs. MeOH (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (100 mg/kg); pg < ethyl acetate fraction (200 mg/kg) (C): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the GSH levels in pancreatic tissue. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs normal control; pb < 0.05 Vs diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH (200 mg/kg); pe < 0.05 Vs. MeOH (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (100 mg/kg) (D): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on the GSH levels in kidney tissue. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs. diabetic; pb < 0.05 Vs. diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH (200 mg/kg); pe < 0.05 Vs. MeOH (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (100 mg/kg) (E): Showing effects of methanol extract, ethyl acetate fraction and mother liquor on SAG levels. Values are expressed as mean ± S.E.M (N = 6). Pa < 0.05 Vs normal; pb < 0.05 Vs diabetic; pc < 0.05 Vs. positive control; pd < 0.05 Vs. MeOH (200 mg/kg); pe < 0.05 Vs MeOH (400 mg/kg); pf < 0.05 Vs. ethyl acetate fraction (100 mg/kg); pg < ethyl acetate fraction (200 mg/kg); ph < 0.05 Vs ethyl acetate fraction (400 mg/kg); pi < 0.05 Vs. mother liquor (200 mg/kg).
3.6. Histopathological findings
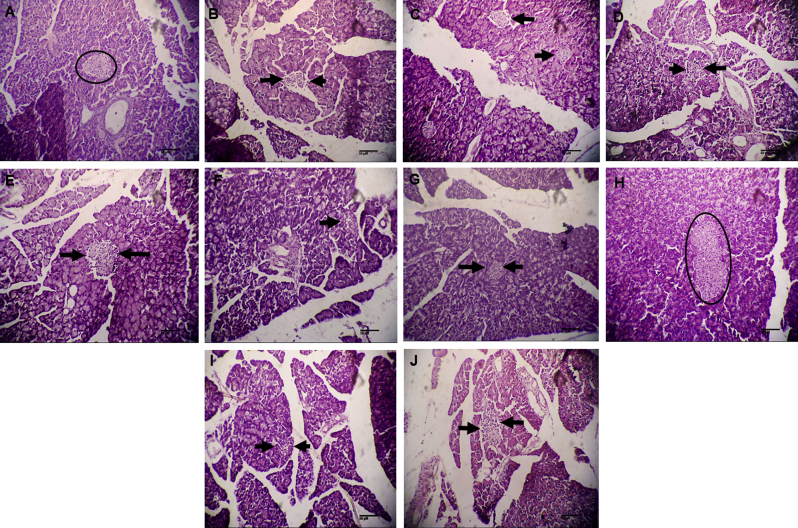
As was expected, the histopathology examination revealed that alloxan destroyed islets of Langerhans, which were replaced by fibrotic cells in the pancreas (Fig. 3B). There was no marked change in pancreas when diabetic animals were treated with ethyl acetate fraction (100 mg/kg), methanol extract and mother liquor at the doses of 200 mg/kg, respectively (Fig. 3D,F, and I). The islets of Langerhans of rats treated with ethyl acetate fraction showed mild regeneration (200 mg/kg) (Fig. 3G), while a higher dose of ethyl acetate fraction caused (400 mg/kg) a significant regeneration and expansion of islet cells (Fig. 3H and J).
Fig. 3.
(A) Pancreatic tissue section is unremarkable and shows normal islets of Langerhans in normal control group (H&E; 100X) (B) Photomicrograph shows degenerated islets of Langerhans replaced by fibrosis in diabetic control group (H&E; 100X) (C) Photomicrograph shows normal islets of Langerhans and features like binucleation and eosinophilia in positive control (H&E; 100X) (D) Section showing fibrotic islets of Langerhans in MeOH extract (200 mg/kg) treated group (E) Section showing mildly preserved islets of Langerhans in MeOH extract (400 mg/kg) treated group (H&E; 100X) (F and G) Photomicrographs showing degeneration of islets of Langerhans and lymphocytic infiltration in ethyl acetate fraction (100 and 200 mg/kg) treated groups (H&E; 100X) (H) Section showing preserved integrity of islets of Langerhans and hyperplasia of islet cells, exhibiting protective response to large dose of ethyl acetate fraction (400 mg/kg) (H&E; 100X) (I and J) Photomicrographs showing degenerated islets of Langerhans in mother liquor (200 and 400 mg/kg) treated groups (H&E; 100X).
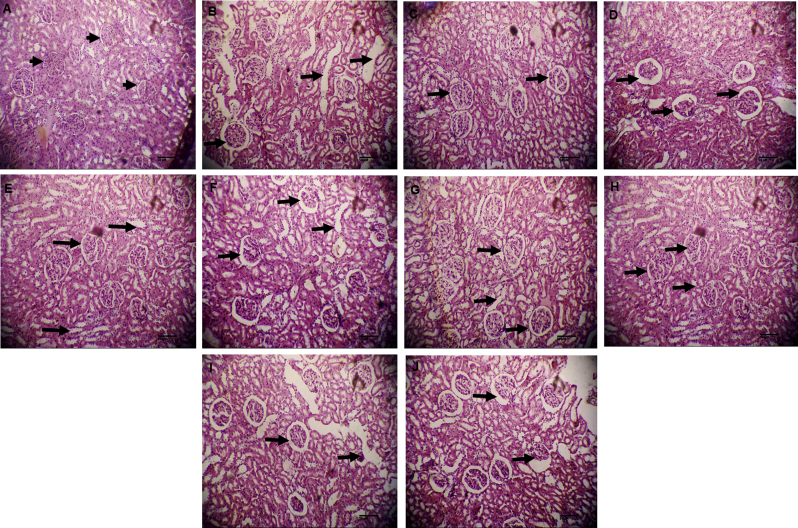
Histopathological examination of kidney sections revealed the thickening of glomerular membrane, mesangial expansion and inflammation of interstitial cells in diabetic animals (Fig. 4B). When diabetic animals were treated with 10 mg/kg gliclazide no tubular changes were observed in glomerular membrane and it was normal looking (Fig. 4D). There was no reduction in diabetes-induced kidney injury with the administration of ethyl acetate fraction at the dose of 100 mg/kg, and methanol extract and mother liquor at the dose of 200 mg/kg, respectively (Fig. 4E,G and J). There was marked reduction of inflammation and lipoid nephrosis in diabetic rats with the administration of large dose of ethyl acetate fraction (400 mg/kg) (Fig. 4I).
Fig. 4.
(A) Photomicrograph of kidney section showing normal glomeruli, tubules and vessels in normal control group (H&E; 100X) (B) Kidney section showing tubular changes, thickening of glomerular membrane and mesangial hypercellularity in diabetic control group (H&E; 100X) (C) Kidney section showing normal glomeruli in positive control group (H&E; 100X) (D) Kidney section showing mild inflammation in MeOH extract (200 mg/kg) treated group (H&E; 100X) (E) Kidney section showing interstitial inflammation and fibrosis in MeOH extract (400 mg/kg) treated group (H&E; 100X) (F and G) Kidney sections showing mild inflammation in ethyl acetate fraction (100 and 200 mg/kg) treated groups (H&E; 100X) (H) Photomicrograph showing kidney protection (i.e. normal glomerulus, tubules and blood vessels). in diabetic rats treated with ethyl acetate fraction (400 mg/kg) of Phyllanthusfraternus (H&E; 100X) (I and J) Kidney sections showing mild inflammatory changes in mother liquor (200 and 400 mg/kg) treated groups (H&E; 100X).
3.7. HPLC analysis of the bioactive ethyl acetate fraction of P.fraternus
Presence of four polyphenolic compounds: namely gallic acid, catechin, caffeic acid and ellagic acid was found in the HPLC analysis of ethyl acetate fraction of P. fraternus. Among all polyphenols, ellagic acid (70.1 mg/L) and catechin (464.6 mg/L) was present in highest concentration. The HPLC results are summarized in Table 1.
Table 1.
Polyphenolic compounds detected by HPLC in ethyl acetate fraction of Phyllanthusfraternus.
| Peak | Retention time | AUC | Peak height | Concentration (mg/L) | Compound |
|---|---|---|---|---|---|
| 1 | 2.247 | 176,073 | 8455 | 12.348 | Gallic acid |
| 2 | 4.020 | 110,8757 | 40,126 | 464.551 | Catechin |
| 3 | 6.936 | 200,106 | 8904 | 14.380 | Caffeic acid |
| 4 | 6.900 | 318,72 | 6670 | 70.063 | Ellagic acid |
| Total | 1,803,508 | 64,156 |
4. Discussion
It is well known that the toxic glucose analogue alloxan selectively destroys insulin hormone producing pancreatic beta-cells in rodents, thereby causing insulin-dependent diabetes in these animals, which has similar characteristics as observed in humans [26]. In the present study, a single intraperitoneal injection of alloxan monohydrate (130 mg/kg)led to significant pancreatic damage as was judged by pertinent biochemical and histopathological changes. Fourteen days continuous treatment with methanol extract (200 and 400 mg/kg), ethyl acetate fraction (100 and 200 mg/kg), and mother liquor (200 and 400 mg/kg) moderately alleviated the elevated levels of blood glucose, triglycerides, total cholesterol as well as other biochemical parameters, but the highest reduction in blood glucose concentration, triglycerides, total cholesterol, creatinine, and urea occurred with the largest dose of ethyl acetate fraction (400 mg/kg) of P. fraternus (Fig. 1 – see histograms A-E). Histopathological examination of pancreas (Fig. 3, see photomicrographs A-J), and kidneys (Fig. 4, see photomicrographs A-J) also revealed highest protection with the P. fraternus ethyl acetate fraction (400 mg/kg). To the best of our knowledge, this is the first study to report the antidiabetic effects of P. fraternus extracts in alloxan-induced diabetes in the rat model.
Alloxan, in the presence of intracellular thiols, generates reactive oxygen species (ROS) through redox reaction. It appears that alloxan-induced toxic injury in the pancreatic β-cells is initiated by the free radicals formed via redox reaction [27]. Oxidative stress resulting from metabolic syndrome can damage the cell membranes and components, including lipids, proteins, glutathione, metabolic enzymes and DNA. In our study, alloxan-induced diabetes resulted in significant oxidative stress related changes in the pancreas and kidneys. There was a marked increase in lipid peroxides (TBARS, SAG) and reduction in GSH concentration in both pancreas and kidney tissues. There was significant reduction in lipid peroxides accompanied by marked increase in GSH level by treatment with methanol extract (200, 400 mg/kg), ethyl acetate fraction (100, 200, 400 mg/kg) and mother liquor (200, 400 mg/kg). The greatest attenuation of these biochemical parameters was observed with ethyl acetate fraction of P. fraternus (400 mg/kg) (Fig. 2).
Histopathological studies of pancreas and renal tissues support noticeable destruction of islets of Langerhans which were then replaced by fibrotic cells in pancreatic tissue (Fig. 3-p), and thickening of glomerular membrane, mesangial expansion and inflammation of interstitial cells in renal tissues (Fig. 4). These histopathological observations lend additional support and are consistent with the previous alloxan-induced diabetic studies [[28], [29], [30]]. Fourteen days continuous treatment with P. fraternus extracts not only decreased inflammation and lipoid nephrosis in renal tissues in comparison to alloxan diabetic control group, but also significantly reduced morphological abnormalities in kidneys and pancreas, accompanied by regeneration and expanded islet cells in pancreas.
Phytochemical screening of ethyl acetate fraction of P. fraternus extracts showed the presence of various polyphenols. The HPLC analysis of ethyl acetate fraction of P. fraternus revealed the presence of four polyphenol ingredients: gallic acid, catechin, caffeic acid and ellagic acid (Table 1). Among the four polyphenolic compounds, the largest amounts were of ellagic acid (70.1 mg/L) and catechin (464.6 mg/L). Polyphenols are the most widely spread compounds present in higher plants, and are known to be involved in the healing of non-communicable diseases, including diabetes, through their ability to scavenge free radicals [31,32].
Cardiovascular protective effects are shown by catechins through multiple actions that include: anti-oxidant [33], anti-inflammatory [34], anti-thrombogenic, anti-proliferative and anti-hypertensive potentials [35,36]. Antioxidant effects by catechins are produced by capturing free radicals and inducing antioxidant enzymes [37,38]. Increased insulin sensitivity in diabetic patients by catechin, a flavan-3-ol, has been reported [39]. They cause the influx of Ca2++ and lead to the release of insulin act by activating cAMP/PKA signaling. According to Qin et al., 2003, Cinnamomum verum and C. aromaticum are rich in polyphenolic catechins. Insulin-induced glucose utilization is increased by enhancing the insulin-signaling pathway in the skeletal muscle of rats by oral administration of cinnamon extracts (30 and 300 mg/kg) for 3-weeks [40]. Keeping in view these observations, we hypothesize that polyphenolic catechin present in P. fraternus may be primarily responsible or it may act synergistically with other constituents for the anti-diabetic and renoprotective activity in rats. Such protective effects of catechin could be mediated through its flavonol-induced anti-oxidant and anti-inflammatory activity in the kidneys and enhanced release of insulin from regenerated pancreas [41,42]. Further studies are needed to ascertain the underlying antidiabetic mechanism of P. fraternus extracts containing catechin and other ingredients present in this medicinal plant.
5. Conclusion
Oral administration of P. fraternus extracts to rats significantly improved alloxan-induced biochemical alterations. There was also a significant reduction in lipid peroxidation and also significantly reduced the morphological changes in pancreas and kidneys supporting the biochemical improvements.
The findings of this investigation suggest that polyphenol catechin present in P. fraternus may be primarily responsible for the anti-diabetic and renoprotective activity in rats which could be mediated through its flavonol-induced anti-oxidant and anti-inflammatory activities in the pancreas and kidneys. Further studies are warranted to ascertain the underlying antidiabetic mechanism of P. fraternus extracts containing catechin and other ingredients found in this medicinal plant.
Source(s) of funding
None declared.
Conflict of interest
Harpal Singh Buttar is the member of the Editorial Board of J-AIM. However, he was not involved in the peer review process of this manuscript.
Acknowledgements
The authors duly acknowledge the Department of Pharmaceutical Sciences of Guru Nanak Dev University for supporting this study and providing technical facilities for this work.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Paula H. John Wiley and Sons; UK: 2009. Diabetes in hospital: a practical approach for healthcare professionals. [Google Scholar]
- 2.Rang H.P., Dale M.M., Ritter J.M., Flower R.J., Henderson G. 7th ed. Churchill Livingstone; 2012. Rang and Dale's pharmacology; p. 377. [Google Scholar]
- 3.Koschinsky M.L., Marcoina S.M. The relationship between lipoprotein alpha and the complications of diabetes mellitus. Acta Diabetol. 2003;40:65–76. doi: 10.1007/s005920300007. [DOI] [PubMed] [Google Scholar]
- 4.Cuschieri S. The genetic side of type 2 diabetes - a review. Diabetes Metab Syndr. 2019;13(4):2503–2506. doi: 10.1016/j.dsx.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Pareek A., Suthar M. Antidiabetic activity of extract of Berberis aristate root in streptozotocin induced diabetic rats. Pharmacol online. 2010;2:179–185. [Google Scholar]
- 6.Calixto J.B., Santos A.R., Cechinel V., Yunes R.A. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology and therapeutic potential. Med Res Rev. 1998;18:225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Sarin B., Verma N., Martin J., Mohanty A. An overview of important ethnomedicinal herbs of Phyllanthus species: present status and future prospects. Sci World J. 2014;214:200–212. doi: 10.1155/2014/839172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavit M., Patel B.N., Jain B.K. Phytochemical analysis of leaf extract of Phyllanthus fraternus. Res J Recent Sci. 2013;2:12–15. [Google Scholar]
- 9.Sharma S.K., Sheela M.A. Pharmacognostic evaluation of leaves of certain Phyllanthus species used as a botanical source of Bhumyamalaki in Ayurveda. Ayu. 2011;32(2):250. doi: 10.4103/0974-8520.92552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta K., Patel B.N., Jain B. Antibacterial and antifungal potentiality of leaf extract of Phyllanthus fraternus Webster: an Ethnomedicinal plant. Am J Microbiol Res. 2014;2:74–79. [Google Scholar]
- 11.Chopade A.R., Sayyad F.J. Antifibromyalgic activity of standardized extracts of Phyllanthusamarus and Phyllanthusfraternus in acidic saline induced chronic muscle pain. Biomed Aging Pathol. 2014;4:123–130. [Google Scholar]
- 12.Chopade A.R., Sayyad F.J. Antinociceptive effect of Phyllanthusfraternus extract in complete Freund’s adjuvant induced chronic pain in mice. Biomed Aging Pathol. 2013;3:235–240. [Google Scholar]
- 13.Lata S., Singh S., Tiwari K.N., Upadhyay R. Evaluation of the antioxidant and hepatoprotective effect of Phyllanthusfraternus against a chemotherapeutic drug cyclophosphamide. Appl Biochem Biotechnol. 2014;173:2163–2173. doi: 10.1007/s12010-014-1018-8. [DOI] [PubMed] [Google Scholar]
- 14.Sittie A.A., Lemmich E., Olsen C.E., Hviid L., Christensen S. Alkamides from Phyllanthusfraternus. Planta Med. 1998;64:192–193. doi: 10.1055/s-2006-957405. [DOI] [PubMed] [Google Scholar]
- 15.Khan M.H., Yadava P. Antidiabetic plants used in Thoubal district of Manipur, northeast India. Ind J Trad Knowl. 2009;9(3):510–514. [Google Scholar]
- 16.Kumar S., Kumar D., Deshmukh R.R., Lokhande P.D., More S.N., Rangari V.D. Antidiabetic potential of Phyllanthusreticulatus in alloxan-induced diabetic mice. Fitoterapia. 2008;79:21–23. doi: 10.1016/j.fitote.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Okoli C.O., Ibiam A.F., Ezike A.C., Akah P.A., Okoye T.C. Evaluation of antidiabetic potentials of Phyllanthus niruri in alloxan diabetic rats. Afr J Biotechnol. 2010;9(2) [Google Scholar]
- 18.Adeneye A.A. The leaf and seed aqueous extract of Phyllanthus amarus improves insulin resistance diabetes in experimental animal studies. J Ethnopharmacol. 2012;144:705–711. doi: 10.1016/j.jep.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Kushwah A.S., Patil B.M., Thippeswamy B.S. Effect of Phyllanthusfraternus on fructose induced insulin resistance in rats. IJP-International J Pharmacol. 2010;6(5):624–630. [Google Scholar]
- 20.Garg M., Dhar V., Kalia A. Antidiabetic and antioxidant potential of Phyllanthusfraternus in alloxan induced diabetic animals. Pharmacogn Mag. 2008;4(14):138. [Google Scholar]
- 21.Khandelwal K.R. Nirali Parkashan; Pune: 2004. Preliminary phytochemical screening in practical pharmacognosy; p. 1449. 1156. [Google Scholar]
- 22.Chen S., Ye Z.Q., Li Z.W., Zhao C.X., Chen G.J., Zhou J.Z. WenyangHuoxueJiedu formula inhibits thin-cap fibroatheroma plaque formation via the VEGF/VEGFR signaling pathway. J Ethnopharmacol. 2018;219:213–221. doi: 10.1016/j.jep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Okhawa H., Ohishi N., Yagi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E., Durgun O., Kelly B.M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;51:882–888. [PubMed] [Google Scholar]
- 25.Wang H.D., Pagano P.J., Du Y., Cayatte A.J., Quinn M.T., Brecher P. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 26.Dunn J.S., Sheehan H.L., Mclethie N.G.B. Necrosis of islets of Langerhans produced experimentally. The Lancet. 1943;1:484–487. [Google Scholar]
- 27.Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- 28.Hamden K., Elfeki A., Ayadi F., Jamoussi K., Masmoudi H. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors. 2008;33:165–175. doi: 10.1002/biof.5520330302. [DOI] [PubMed] [Google Scholar]
- 29.Al-Noory A.S., Amreen A.N., Hymoor S. Antihyperlipidemic effects of ginger extracts in alloxan-induced diabetes and propylthiouracil-induced hypothyroidism in rats. Pharmacogn Res. 2013;5:157–161. doi: 10.4103/0974-8490.112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed M.F., Kazim S.M., Ghori S.S., Mehjabeen S.S., Ahmed S.R. Antidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic rats. Internet J Endocrinol. 2010;2010:1–6. doi: 10.1155/2010/841090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czinner E., Hagymasi K., Blazovies A., Kery A., Szoke E., Lemverkovics E. In vitro antioxidant properties of Helichrysum arenarium (L) Moench. J Ethnopharmacol. 2000;73:437–443. doi: 10.1016/s0378-8741(00)00304-4. [DOI] [PubMed] [Google Scholar]
- 32.Carini M., Adlini G., Furlanetto S., Stefani R., Facino R.M. LC coupled to ion- trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas. J Pharm Biomed Sci. 2001;24:517–526. doi: 10.1016/s0731-7085(00)00431-3. [DOI] [PubMed] [Google Scholar]
- 33.Rice E.C. Implications of the mechanisms of action of tea polyphenols as antioxidants in vitro for chemoprevention in humans. Proc Soc Exp Biol Med. 1999;220:262–266. doi: 10.1046/j.1525-1373.1999.d01-45.x. [DOI] [PubMed] [Google Scholar]
- 34.Sang S., Lambert J.D., Ho C.T., Yang C.S. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Islam M.A. Cardiovascular effects of green tea catechins: progress and promise. Recent Pat Cardiovasc Drug Discov. 2012;7:88–99. doi: 10.2174/157489012801227292. [DOI] [PubMed] [Google Scholar]
- 36.Babu P.V., Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higdon J.V., Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 38.Choi J.H., Cha B.K., Rhee S.J. Effects of green tea catechin on hepatic microsomal phospholipase A2 activities and changes of hepatic phospholipid species in streptozotocin induced diabetic rats. J Nutr Sci Vitaminol. 1998;44:673–683. doi: 10.3177/jnsv.44.673. [DOI] [PubMed] [Google Scholar]
- 39.Chemler J.A., Lock L.T., Koffas M.A., Tzanakakis E.S. Standardized biosynthesis of flavon-3-ols with effect on pancreatic beta-cell insulin secretion. Appl Microbiol Biotechnol. 2007;77:797–807. doi: 10.1007/s00253-007-1227-y. [DOI] [PubMed] [Google Scholar]
- 40.Qin B., Nagasaki M., Ren M., Bajotto G., Oshida Y., Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 41.Jangir R.N., Jain G.C. Evaluation of antidiabetic activity of hydroalcoholic extract of Cassia fistula Linn. pod in streptozotocin-induced diabetic rats. Pharmacogn J. 2017;9(5) [Google Scholar]
- 42.Meng J.M., Cao S.Y., Wei X.L., Gan R.Y., Wang Y.F., Cai S.X. Effects and mechanisms of tea for the prevention and management of diabetes mellitus and diabetic complications: an updated review. Antioxidants. 2019;8(6):170. doi: 10.3390/antiox8060170. [DOI] [PMC free article] [PubMed] [Google Scholar]