Abstract
Background
Hyperlipidemia triggers atherosclerosis by involving immune cells, such as T-cells. T-cells plays a role in worsening conditions during a high-fat diet (HFD).
Objective
The research aimed to analyze the role of single garlic oil (SGO) on T-cells activation and its proinflammatory cytokine expression in HFD mice.
Methods
Mice were divided into six groups: ND (normal diet); HFD (high-fat diet without treatment); HFD + Simv (HFD + simvastatin 2.6 mg/kg body weight); and HFD + SGO 1–3 (high-fat diet + single garlic oil in a dose of 12.5, 25, and 50 mg/kg body weight), respectively. Treatments were orally given every day for 45 days. At the end of treatments, lymphocytes were isolated from mice spleen. The relative number of T-cells and proinflammatory cytokines were measured using flow-cytometry and analyzed using one-way ANOVA (p < 0.05).
Result
Our result indicated that HFD mice had lower naive T cells (CD4+CD62L+) than normal mice (p < 0.05). SGO treatment in HFD mice increased the relative number of naïve T cells. HFD treatment increased the expression of TNF-α and IFN-γ through NF-κB expression. Furthermore, SGO treatment improved the expression of TNF-α and IFN-γ.
Conclusion
Our study suggests that SGO could act as a promising prospect for therapy to improve chronic inflammation in a HFD.
Keywords: High-fat diet, Single garlic, T-cells
1. Introduction
Hyperlipidemia is closely related to the increase of plasma lipids, especially cholesterol. A high-fat diet is realized as the primary factor causing an imbalance between cholesterol serum of low-density lipoproteins (LDL) and high-density lipoproteins (HDL) [1]. An increasing LDL level in serum is followed by a decrease in HDL level, which is the critical characteristic in atherosclerosis development [2]. Atherosclerosis is a complex disease that gradually develops in arterial blood vessels and the leading cause of cardiovascular disease [3].
Atherosclerosis is marked with the change in arterial size due to the formation of atherosclerosis plaques consisted of necrotic cells, calcified area, modified lipid accumulation, smooth muscle cells, endothelial cells, leukocyte, and foam cells [4]. Atherosclerosis is the leading risk factor of HFD and causing hyperlipidemia. The innate and adaptive immunity cells play a significant role in atherosclerosis progression [5,6].
T-lymphocyte is part of adaptive immunity that existed in the tunica adventitia of normal artery [7]. Dynamic change in blood lipid levels could trigger naïve CD4+ T-cells to differentiate into effector cells and produce cytokine during atherogenesis [8]. T-helper 1 cell (Th1) is the subset of T lymphocyte mostly found in atherosclerotic lesions based on cytokine it produced. Th1 cells secrete IFN-γ and TNF-α proinflammatory cytokines to enhance immune response through macrophage activation, smooth muscle cells, and endothelial cells during atherogenesis [9].
Hyperlipidemia increase T-cells recruitment to the aorta in the initial and advanced stages [7]. Active T-cells will initiate proinflammatory mediator, thus cause an increase in immune response and worsen atherosclerosis development [10]. NFκB is the main transcription factor which plays a significant role in proinflammatory cytokines synthesis [11]. One of the therapeutic strategies to prevent and inhibit atherosclerosis lesion development is by using natural compounds to suppress T-cells activation and its proinflammatory cytokines production through NFκB.
Garlic (Allium sativum) is a cooking spice used globally. Also, it has a positive effect on health; thus, it commonly used as an ingredient for traditional medicine [12,13]. Organosulfur compounds in garlic, such as S-allyl-l-cysteine, diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, and allicin are the essential components [14,15]. The beneficial effects of garlic are among others, to protect from cardiovascular diseases [16] and as anti-inflammation [17].
Single garlic or in Indonesian called Bawang lanang is garlic with one bulb due to growth in an extreme environmental condition. Indonesian communities often use it as traditional medicine or supplement. However, the scientific information about single garlic, especially on atherosclerosis, is still limited. The present study focused on analyzing the effect of single garlic on T-cells activation along with its pro-inflammatory cytokines. The phytochemicals effect of single garlic and its derivate for health is expected to be one of the alternatives to prevent the formation of new or formed plaques in atherosclerosis through suppression of T-cells activation.
2. Materials & methods
2.1. Chemicals
FITC-conjugated anti-mouse CD4 (clone number: GK1.5), PE/Cy5-conjugated anti-mouse CD62L (clone number: MEL-14), PE-conjugated rat anti-mouse TNFα (clone number: MP6-XT22), and PerCP/Cy5.5-conjugated rat anti-mouse IFNγ (clone number: XMG1.2) antibodies were purchased from BioLegend (San Diego, CA). PE/Cy5-conjugated rabbit anti-mouse NFκB (Polyclonal) was purchased from Bioss Inc (Massachusetts, USA). Simvastatin (10 mg) was purchased from Dexa Medica, Tangerang, Indonesia.
2.2. SGO preparation
Single garlic was obtained from Ngadas Village, Malang Regency and Sarangan Village, Magetan Regency. Single garlic was identified at UPT Materia Medica, Batu with no. specimen T0-T0975. The extraction process was conducted at UPT Materia Medika Batu, Indonesia. 250 g single garlic were dried at 50 °C. The garlic was then grounded into powder. The single garlic powder was extracted by soxhletation method with 1.5 L N-hexane. The extraction result was evaporated using a rotary evaporator and kept in a freezer at –4 °C until further use.
2.3. Experimental animals
Twenty-four male (Mus musculus) Balb/C mice, 10 ± 2 weeks old with 38 ± 5 g of body weight were used in this study. The mice obtained from The Integrated Research and Testing Laboratory-Unit IV, Gadjah Mada University, Yogyakarta. The mice acclimatized for one-week and given free access of food and ad libitum water.
2.4. Experimental design
After one-week acclimatized, the mice were divided into two groups, normal diet (ND) and HFD groups for 45 days. HFD contained with 37.33% carbohydrate, 35.85% fat, 11.17% protein, and mineral and fiber as remain. After 45 days, HFD mice groups were re-divided into five groups randomly : mice with high fat diet without treatment (HFD) + simv (HFD + simvastatin in a dose of 2.6 mg/kg body weight); HFD + SGO1 (12.5 mg/kg body weight); HFD + SGO2 (25 mg/kg body weight); and HFD + SGO3 (50 mg/kg body weight). Treatments were given orally every day for 4 weeks. The animal welfare and experimental procedures were approved by the Research Ethics Committee, Brawijaya University, Indonesia (Approval no. 880-KEP-UB).
2.5. Lymphocyte cells isolation, immunostaining, and FACS analysis
At the end of treatment, mice were fasted overnight and then dissected by using euthanasia. Spleen was collected and washed three times using PBS. The spleen then mashed in clockwise on a Petri dish containing 1 mL PBS. The suspension obtained was centrifuged at 2500 rpm for 5 min at 4 °C. Homogenate added with 1 mL PBS and re-centrifuged at 2500 rpm at a temperature of 4 °C for 5 min. Pellets were stained using FITC-conjugated anti-mouse CD4 (clone number: GK1.5) and PE/Cy5-conjugated anti-mouse CD62L (clone number: MEL-14) antibodies for extracellular staining.
Intracellular staining was performed by adding 100 μl Cytofix/Cytoperm (BD-Biosciences Pharmingen) cytofix-cytoperm into cells stained with FITC-conjugated anti-mouse CD4 antibody and incubated at 4 °C for 20 min. After incubation, 500 μL washsperm was added into cells suspension and then centrifuged at 2500 rpm, 4 °C for 5 min. The obtained supernatant was discarded, and pellets were stained using PE/Cy5-conjugated rat anti-mouse NFκB (Polyclonal), PE-conjugated rat anti-mouse TNFα (clone number: MP6-XT22), and PerCP/Cy5.5-conjugated rat anti-mouse IFNγ (clone number: XMG1.2) antibodies according to staining combination used. Stained pellets were incubated at 4 °C for 15 min in a dark room and analyzed using flow-cytometry (BD Biosciences FACS Calibur™). The relative number of each parameter were analyzed using BD CellQuest™ Pro sofware and then performed the statistical analysis.
2.6. Statistical analysis
The relative number of CD4+CD62L+, CD4+NFκB+, CD4+IFN-γ+, and CD4+TNF-α+ T-cells was analyzed using one-way ANOVA. If significance (p < 0.05), Duncan Multiple Range Test (DMRT) was performed as a post hoc test. Data presented as the mean ± standard deviation (SD).
3. Result
3.1. SGO suppress CD4 T-cells activation in high fat diet mice
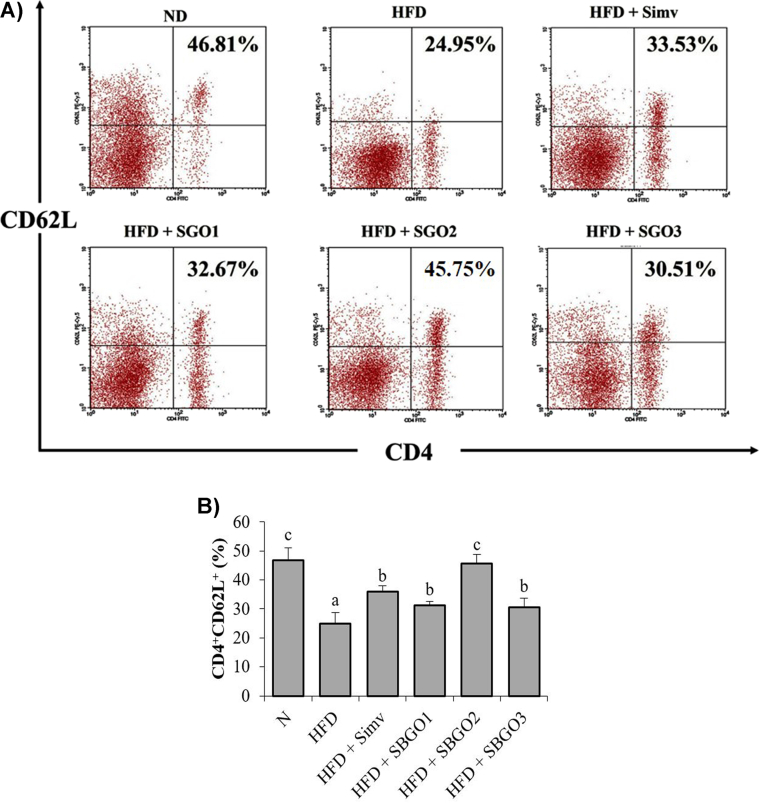
Our result indicated that the CD4+CD62L+ has significantly difference between the normal group and the HFD group (p < 0.05) (Fig. 1A and B). HFD mice showed an increase in T-cells activation indicated by the decrease in the relative number of CD4+CD62L+ T-cells. SGO treatment restores the relative number of CD4+CD62L+ cells in HFD (Fig. 1A). Interestingly, simvastatin treatment did not significantly differ in the relative number of CD4+CD62L+ T-cells compared to SGO 12.5 mg/kg and 50 mg/kg (Fig. 1B). Furthermore, SGO 25 mg/kg did not have significantly differ in the relative number of CD4+CD62L+ T-cells compared to the normal group (Fig. 1B).
Fig. 1.
SGO treatment increases CD4+CD62L+ T-cells in HFD mice. A) the relative number of CD4+CD62L+ T-cells resulted from flow-cytometry B) Bart chart of the relative number of CD4+CD62L+ T-cells represented in mean ± SD. Different letters indicate a significant difference (p < 0.05) based on the DMRT test. SGO = Single Garlic Oil; ND = Normal Diet; HFD = High-Fat Diet; HFD + Simv = HFD + Simvastatin 2.6 mg/kg; HFD + SGO1 = HFD + SGO 12.5 mg/kg BW; HFD + SGO2 = HFD + SGO 25 mg/kg BW; HFD + SGO3 = HFD + SGO 50 mg/kg BW.
3.2. SGO suppress NFkB expression in high fat diet mice
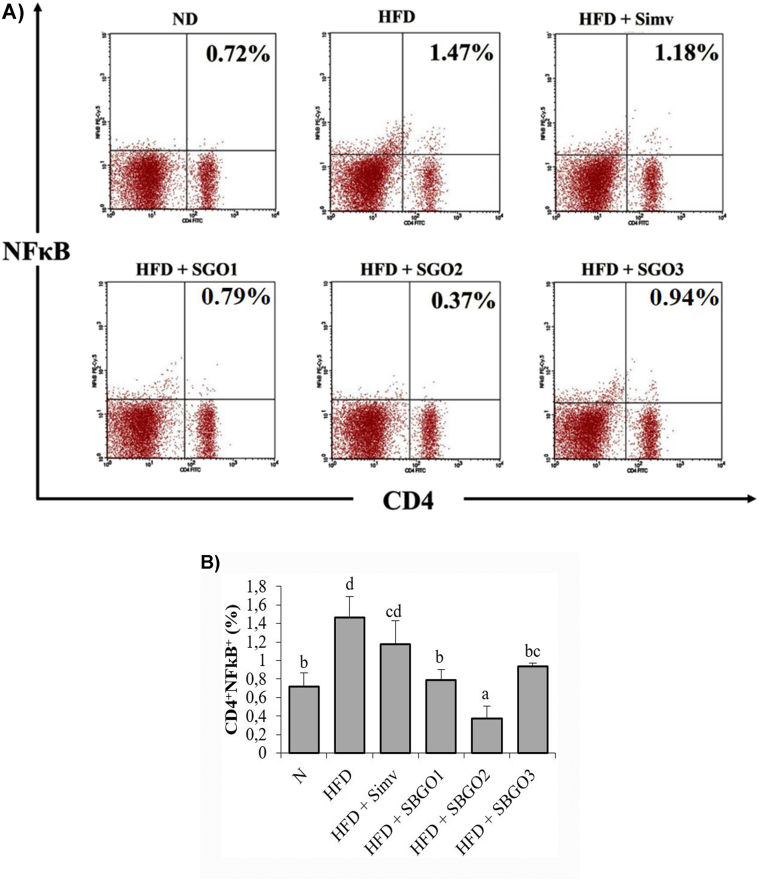
Our result indicated that there was a significant difference in the CD4+NFκB+ in the HFD group compared to the normal group (p < 0.05) (Fig. 2A and B). HFD mice showed an increase in NFκB activity in CD4 T-cells. SGO treatment decrease NFκB expression in T-cells in the HFD condition (Fig. 2A). Interestingly, SGO treatment had a better effect in suppressing NFκB activity on T-cells compared to simvastatin as drug control (Fig. 2B). In addition, the result indicates that SGO dose 25 mg/kg had lower NFκB activity in T-cells and even lower than the normal group (Fig. 2B).
Fig. 2.
SGO treatment suppresses CD4+NFκB+ T-cells expression in HFD mice. A) The relative number of CD4+NFκB+ T-cells resulted from flow-cytometry B) Bar chart of the relative number of CD4+NFκB+ T-cells represented in mean ± SD. Different letters indicate a significant difference (p < 0.05) based on the DMRT test. SGO = Single Garlic Oil; ND = Normal Diet; HFD = High-Fat Diet; HFD + Simv = HFD + Simvastatin 2.6 mg/kg; HFD + SGO1 = HFD + SGO 12.5 mg/kg BW; HFD + SGO2 = HFD + SGO 25 mg/kg BW; HFD + SGO3 = HFD + SGO 50 mg/kg BW.
3.3. SGO decrease proinflammatory cytokines production in high fat diet mice
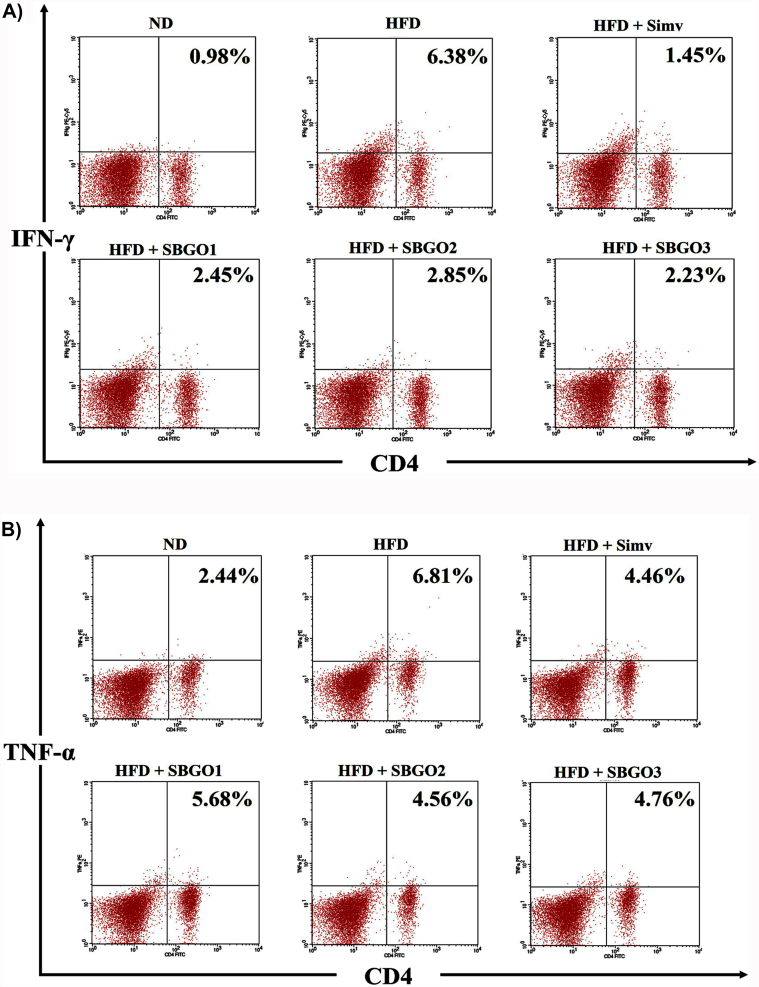
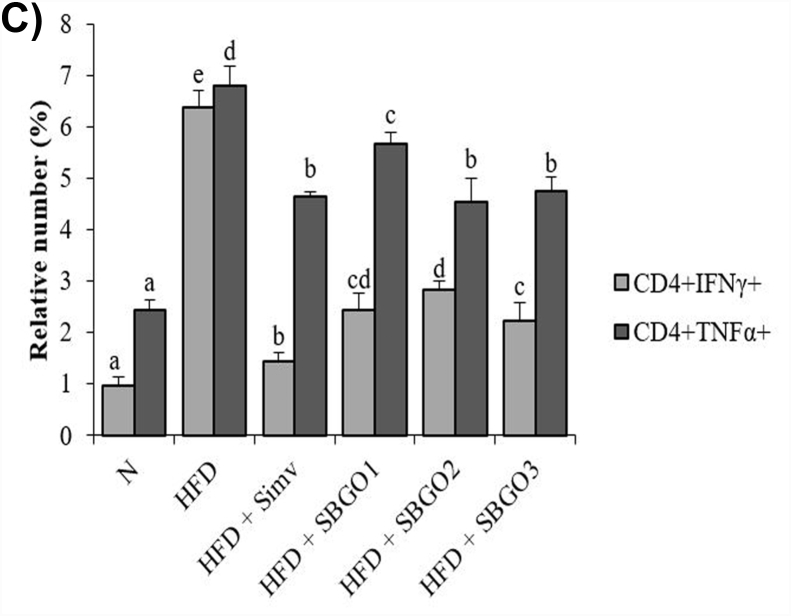
Our result indicated that there was a significant difference CD4+IFN-γ+ and CD4+TNF-α+ cells in the HFD group compared to the normal group (p < 0.05) (Fig. 3A–C). HFD mice have an increase in IFN-γ and TNF-α synthesis in CD4 T-cells. SGO treatment decreases the IFN-γ and TNF-α synthesis in CD4 T-cells in the HFD condition (Fig. 3A and B). Interestingly, simvastatin had a better effect in suppressing IFN-γ but had the same effect as SGO 25 mg/kg and 50 mg/kg in suppressing TNF-α synthesis (Fig. 3C).
Fig. 3.
SGO treatment decrease IFN-γ and TNF-α proinflammatory cytokines production by T-cells in HFD mice. A) The relative number of IFN-γ+ synthesis CD4+ T-cells resulted from flow-cytometry B) The relative number of CD4+TNF-α+T-cells, (C) Bar chart of the relative number of CD4+IFN-γ+ and CD4+TNF-α+ T-cells represented in mean ± SD. Different letters indicate a significant difference (p < 0.05) based on the DMRT test. SGO = Single Garlic Oil; ND = Normal Diet; HFD = High-Fat Diet; HFD + Simv = HFD + Simvastatin 2.6 mg/kg; HFD + SGO1 = HFD + SGO 12.5 mg/kg BW; HFD + SGO2 = HFD + SGO 25 mg/kg BW; HFD + SGO3 = HFD + SGO 50 mg/kg BW.
4. Discussion
The role of nutrition as an agent for the prevention of degenerative disease receives rapid attention in the last few years. Phytochemicals diet can be used as a protective agent for the cardiovascular disease since it has beneficial effects, among others, as an antioxidant, and for fat homeostatic repair [13]. Rich fibre diet and antioxidant can be used as an option for cardiovascular disease management [18]. Garlic has known in the whole world for its medicinal and culinary values. Besides that, our previous study suggested that SGO were safe to consumes continuously [19]. Garlic contains sulfur compounds presumed to play a role in most of its biological activities [20,21].
Naïve T-cells are mature T-cells but not exposed to antigen and not yet differentiated [22]. The naïve T-cells express CD62L molecules functioned as an attachment and rolling in endothelium from blood to tissues. The number of CD4+ T-cells effector/memory was significantly increased in HFD mice, whereas the number of naïve CD4+ T-cells in HFD mice experienced a decrease compared to healthy mice [23]. A HFD triggers an increase in T-cells activation and imbalance between erythrocyte and lymphocyte [24] thus initiate inflammation [25].
Our result indicates that the HFD increased the activation of T-cells number, whereas SGO treatment suppress T-cells activation. These result also in line with our previous study that lymphocyte concentration were decline in HFD mice after SGO treatment [26]. Allicin, one of the active compounds in garlic inhibits T-cell adhesion in endothelial cells by inhibiting ICAM-1 and VCAM-1 expression [27]. Other research results suggest that compound content in garlic could decrease cholesterol and LDL, prevent LDL oxidation, and decrease atherosclerosis progress [28,29]. Oxidized LDL could act as damage-associated molecular patterns (DAMP) in HFD conditions thus trigger the initiation of inflammation through TLR4 [30]. Allicin compound in garlic plays a vital role in preventing excessed LDL uptake into cells [24] thus it is assumed to participate in decreasing the number of oxidized LDL followed by the decrease in activated T-cell number.
NFκB is an inflammation mediator center that regulates more than 200 genes including those genes that involved in inflammation and innate immunity through proinflammatory cytokines production [11]. HFD triggers the activity of NFκB [31], which in turn promotes the excessive proinflammatory cytokine synthesis [32]. Our result indicated that SGO treatment decrease NFκB in CD4 T-cells significantly. Allicin compound plays a role in inhibiting NFκB activity. Thus it also inhibits pro-inflammatory cytokines production like TNF-α and IL-1β [27]. Organosulfur compounds in garlic can prevent IκB degradation. Thus NFκB activities reduced. Also, the sulfur content in garlic can inhibit p38 phosphorylation and ERK pathway [17].
Our next result indicated that a decrease in NFκB activity could decrease TNF-α and IFN-γ cytokines production. TNF-α has a strong proinflammatory activity, whereas IFN-γ induce local chemokine to recruit other immune cells, including increasing T-cells infiltration to tissues [33]. In line with our previous study, SGO treatment also reduce TNF- α concentration in serum [34]. Furthermore, SGO restores the regulatory T-cells (Tregs) and its anti-inflammatory cytokine IL-10 and TGF-β [35]. Thus resulting in the delay of T-cell activation and decline the proinflammatory cytokine. Our result indicates that phytochemical compounds in garlic oil had a robust anti-inflammation ability and can be used as a therapy in HFD caused inflammation.
5. Conclusion
SGO suppressed the CD4 T-cells activation through CD4+CD62L+ T-cells. The SGO decrease inflammation through the decrease in NFκB activities and its proinflammatory cytokines, such as TNF-α and IFN-γ. The result suggests that SGO played a role as an athero-protective agent in the HFD condition. Phytochemicals content in garlic could be a promising therapeutic agent in the future for treatment of inflammatory-related diseases.
Source(s) of funding
The research was funded by Ministry of Research, Technology and Higher Education of the Republic of Indonesia no. grant 3.4.8/UN.32.14 LT/2018.
Conflict of interest
None
Acknowledgement
We would like to thank to Siti Nur Arifah and Alif Rofiqotun Nurul Alimah to assist this research.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Figueiredo P.S., Inada A.C., Marcelino G., Cardozo C.M., Freitas K., Guimarães R. de C.A. Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients. 2017;9(10):1–32. doi: 10.3390/nu9101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamashita T., Sasaki N., Kasahara K., Hirata Kichi. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol. 2015;66(1):1–8. doi: 10.1016/j.jjcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Cardiovascular diseases (CVDs) 2017. http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Accessed.
- 4.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., Lichtman A.H., Hansson G.K. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilhan F. Atherosclerosis and the role of immune cells. World J Clin Cases. 2015;3(4):345. doi: 10.12998/wjcc.v3.i4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galkina E., Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620.Immune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabas I., Lichtman A.H. Monocyte-Macrophages and T Cells in atherosclerosis. Immunity. 2017;47(4):621–634. doi: 10.1016/j.immuni.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu M.Y., Li C.J., Hou M.F., Chu P.Y. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. 2017;18(10) doi: 10.3390/ijms18102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramji D.P., Davies T.S. Cytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26(6):673–685. doi: 10.1016/j.cytogfr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welty F.K., Alfaddagh A., Elajami T.K. Targeting inflammation in metabolic syndrome. Transl Res. 2016;167(1):257–280. doi: 10.1016/j.trsl.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh P., Singh J., Singh S. B Singh Medicinal values of garlic (Allium sativum L.) in human life: an overview. Greener J Agric Sci. 2014;4(6):265–280. doi: 10.15580/GJAS.2014.6.031914151. [DOI] [Google Scholar]
- 13.Ourouadi S., Moumene H., Zaki N. A Boulli Garlic (Allium sativum): a source of Multiple nutraceutical and functional components. J Chem Biol Phys Sci. 2016;7(1):9–21. [Google Scholar]
- 14.Amagase Significance of garlic and its constituents in cancer and cardiovascular disease garlic and cardiovascular disease: a critical review 1, 2. J Nutr. 2006;136(2):736–740. 136/3/810S [pii] [Google Scholar]
- 15.Molina-Calle M., de Medina V.S., Priego-Capote F., de Castro M.D.L. Establishing compositional differences between fresh and black garlic by a metabolomics approach based on LC–QTOF MS/MS analysis. J Food Compos Anal. 2017;62(May):155–163. doi: 10.1016/j.jfca.2017.05.004. [DOI] [Google Scholar]
- 16.Santo S.E., Keiss H.P., Meyer K., Nuytenhek R., Roos Th, Dirsch V. Garlic and cardiovascular disease. Med Aromat Plant Sci Biotechnol. 2007;1(1):31–36. [Google Scholar]
- 17.Lee D.Y., Li H., Lim H.J., Lee H.J., Jeon R., Ryu J.-H. Anti-inflammatory activity of sulfur-containing compounds from garlic. J Med Food. 2012;15(11):992–999. doi: 10.1089/jmf.2012.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukthamba P., Srinivasan K. Hypolipidemic and antioxidant effects of dietary fenugreek (Trigonella foenum-graecum) seeds and garlic (Allium sativum) in high-fat fed rats. Food Biosci. 2016;14:1–9. doi: 10.1016/j.fbio.2016.01.002. [DOI] [Google Scholar]
- 19.Lestari S.R., Rifai M. The effect of single-bulb garlic oil extract toward the hematology and histopathology of the liver and kidney in mice. Braz J Pharm Sci. 2019;55 doi: 10.1590/s2175-97902019000218027. [DOI] [Google Scholar]
- 20.Arreola R., Quintero-Fabián S., López-Roa R., Flores-Gutiérrez E., Reyes-Grajeda J., Carrera-Quintanar L. Immunomodulation and anti-inflammatory effects of garlic compounds: discovery service for endeavour college of natural health library. J Immunol Res. 2015;2015:1–13. doi: 10.1186/1471-244X-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaafar M.R. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J Med. 2012;48(1):59–66. doi: 10.1016/j.ajme.2011.12.003. [DOI] [Google Scholar]
- 22.Rifa’i M., Widodo N. Significance of propolis administration for homeostasis of CD4+CD25+immunoregulatory T cells controlling hyperglycemia. J Kor Phys Soc. 2014;3(1):1–8. doi: 10.1186/2193-1801-3-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H., Youm Y.-H., Vandanmasgar B., Ravussin A., Gimble J.M., Greenway F. Obesity increases the production of pro-inflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safitri Y.D., Atho’illah M.F., Nur’aini F.D., Widyarti S., Rifa’i M. The Effects of Elicited Soybean (Glycine max) Extract on Hematopoietic Cells of High Fat-Fructose Diet Balb/C Mice Model. JJBS. 2018;11(6):241–246. [Google Scholar]
- 25.Wu H., Ghosh S., Perrard X.D., Feng L., Garcia G.E., Perrard J.L. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115(8):1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 26.Ilmawati R.R., Gofur A., Lestari S.R. Single bulb garlic oil improves interleukin-6 via decreased reactive oxygen species (ROS) in high-fat diet male mice. Universa Medicina. 2019;38:100–107. doi: 10.18051/UnivMed.2019.v38.100-107. [DOI] [Google Scholar]
- 27.Bruck R., Aeed H., Brazovsky E., Noor T., Hershkoviz R. Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005;25(3):613–621. doi: 10.1111/j.1478-3231.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y., He Z., Shen X., Xu X., Fan J., Wu S. Cholesterol-lowering effect of allicin on hypercholesterolemic ICR mice. Oxid Med Cell Longev. 2012;2012 doi: 10.1155/2012/489690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalal I., Sengupta M., Paul S., Mishra A. Comparative study of the effect of atorvastatin and garlic extract in experimentally induced hypercholesterolemia in rabbits. Int J Basic Clin Pharmacol. 2013;2(4):397. doi: 10.5455/2319-2003.ijbcp20130810. [DOI] [Google Scholar]
- 30.Atho’illah M.F., Safitri Y.D., Nur’aini F.D., Widyarti S., Hideo T., Rifa’i M. Soybean extract suppresses B cell activation through TLR3/TLR4 in high fat-high fructose diet mice. Turkish J Immunol. 2018;6(3):95–103. doi: 10.25002/tji.2018.866. [DOI] [Google Scholar]
- 31.Nur’aini F.D., Rahayu S., Rifa’i M. NFКB activity decreased in BALB/c mice with high fat diet and fructose. AIP Conference Proceeding. 2017 doi: 10.1063/1.4983416. [DOI] [Google Scholar]
- 32.Jialal I., Kaur H., Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99(1):39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 33.Sologuren I., Rodriguez-Gallego C., Lara P.C. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Cancer Res. 2014;3(1):18–31. doi: 10.3978/j.issn.2218-676X.2014.02.05. [DOI] [Google Scholar]
- 34.Arifah S.N., Atho’illah M.F., Lukiati B., Lestari S.R. Herbal medicine from single clove garlic oil extract ameliorates hepatic steatosis and oxidative status in high fat diet mice. Malays J Med Sci. 2020;27:46–56. doi: 10.21315/mjms2020.27.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lestari S.R., Rifa M. Regulatory T cells and anti-inflammatory cytokine profile of mice fed a high-fat diet after single-bulb garlic (Allium sativum L.) oil treatment. Trop J Pharmac Res. 2018;17(November):2157–2162. [Google Scholar]