Abstract
Background
Premna integrifolia Linn. is a medicinal plant of an Ayurvedic importance and proved to have an anti-inflammatory, anti-diabetic, anti-microbial and hypo-lipidemic activity. Glutathione (GSH) redox status is an important parameter to assess the antioxidant activity of any neutraceuticals.
Objective
In order to assess the anti-oxidant potential of hydro alcoholic extract (HAE) of P. integrifolia, this study was aimed to evaluate the GSH redox status in high fat diet induced experimental atherosclerosis.
Materials and methods
The present study comprises sixty Wistar rats and they were divided into six groups: the first group served as control, the second group was fed with high fat diet and the third, fourth and fifth groups were fed with high fat diet along with various concentrations of HAE of 200, 400 and 500 g/kg.b.wt respectively and the sixth group was administered high fat diet along with 10 mg/kg b.wt of atorvastatin for 30 days. GSH-dependent enzymes like GSH-peroxidase (GPx), GSH-reductase (GR) and glucose 6-phosphate dehydrogenase (G6PD) were estimated in hemolysate, kidney, heart and liver of experimental rats.
Results
Analysis of GSH levels showed a significant decrease in hemolysate, heart and kidney (p < 0.05) and liver (p < 0.01) in high fat-fed rats when compared to control. Activities of GPx, GR and G6PD in hemolysate and heart (p < 0.001), liver and kidney (p < 0.05) in high fat-fed rats when compared to control. Dose-dependent increase was observed in rats treated with various concentrations of HAE.
Conclusion
The HAE of root bark of P. integrifolia is proved to have a protective role on antioxidant defense in high fat diet induced atherosclerosis model. As a whole P. integrifolia increases the GSH content in a dose-dependent manner and in turn altered the redox cycle.
Keywords: Premna integrifolia, GSH-dependant enzymes, High fat diet, Atherosclerosis, Antioxidants
1. Introduction
Atherosclerosis is characterized by an accumulation of cholesterol deposits in macrophages in large- and medium-sized arteries, the cholesterol deposition leads to the proliferation of certain cell types within the arterial wall that gradually impinge on the vessel lumen and impede blood flow. The World Health Organization predicts that global economic prosperity could lead to an epidemic of atherosclerosis as developing countries acquire Western habits [1]. Familial hypercholesterolemia is an autosomal dominant disorder that affects approximately 1 in 500 persons of the general population [2]. An excessive oxidative stress is characterized by lipid and protein oxidation in the vascular wall leads to atherosclerosis. Oxidation of low density lipoprotein (LDL) is an earlier event in the process of atherogenesis and it has potentially pro-atherogenic activity [3]. The production of reactive oxygen and nitrogen species by vascular cells, as well as oxidative changes contributing to important clinical manifestations of coronary artery disease such as endothelial dysfunction and plaque disruption. A pathophysiologically important cause for atherosclerosis is oxidative modification which includes poor performance of antioxidant strategies limiting to atherosclerosis. Oxidative stress is considered to be a secondary process in the development of atherosclerosis [4].
GSH-redox cycle play an important role in alleviating the free radical generation during oxidative stress in pathological condition. The red blood corpuscle (RBCs) are intrinsically prone to oxidative stress because they are exposed to high oxygen tension, and have a characteristic structural composition with poly unsaturated fatty acids in the membrane and antioxidant metal iron in hemoglobin [5]. However, membrane and cytoplasmic compartments of RBC have an efficient antioxidant mechanism that maintains their integrity. GSH play a key role in protecting cells against free radicals might be due to the nucleophilicity of thiols (-SH) with free radicals [6]. GSH levels are maintained enzymatically via GR, G6PD, GPx, glutathione S-transferase (GST) and gamma glutamyl transferase (γ-GT). GSH is converted into oxidized GSH (GSSG) by trans-hydrogenation and reduction of GSSG is mediated by GSSG reductase which uses a co-factor, NADPH which is recycled by G6PD via Pentose phosphate pathway. GPx is responsible for most of lipid peroxide decomposition in cells and this enzyme protects the cells from peroxidative damage. Lipooxygenase-induced lipid peroxidation causes suicidal inactivation and further produces oxidation products which may initiate free radical-mediated secondary reactions [7]. Under oxidative stress, the microsomal GST in the cell can be activated through direct hydroperoxide-mediated S-thiolation with GSH and thiol exchange-mediated dethiolation are imposed by the intracellular GSH redox state [8]. Free radicals are involved in normal biochemical processes like oxidative reduction and cellular metabolism; however, they also mediate disease processes [9]. Hydrogen peroxide (H2O2) is metabolized by catalase (CAT) and cellular peroxidases [10]. The contribution of mitochondrial oxidants and DNA damage to the progression of atherosclerotic lesions in human arterial wall and in atherosclerosis-prone mice [11]. The maintenance of G6PD, phosphoglucose isomerase, and 6-phosphogluconate dehydrogenase which are generated from ribose-5-phosphate and the reduction of NADP+ via hexose monophosphate shunt [12]. A plant, P. integrifolia belongs to Verbinaceae family are rich in flavonoids and bioflavonoids are known for their antioxidant activities. Methanolic extract of this plant roots showed in-vitro antioxidant activities such as anti-radical, superoxide scavenging, anti lipid peroxidation, hydroxyl radical and nitric oxide scavenging properties. P. integrifolia is a potential source of natural antioxidant and its aqueous extract showed a significant antioxidant activity [13]. Chloroform: methanol (1:1) extract of P. integrifolia roots showed its beneficial effect on human leucocytes and erythrocytes against H2O2 induced oxidative damage [14]. Hence the present study was aimed to focus on anti-oxidant mediated activity of HAE in high fat diet-induced atherosclerosis via GSH redox system.
2. Materials and methods
2.1. Preparation of plant extract from root bark
The root bark of P. integrifolia was procured from Indian Medical Practitioners Co-operative Pharmacy and Stores (IMPCOPS), Chennai, Tamil Nadu, India and authenticated by Shri. C. Arunachalam, Research Officer (Botany), Captain Srinivasa Murthy Regional Ayurveda Drug Development Institute, Chennai and the voucher specimen (00641/2014) was deposited in the Botany Department. The coarse powder of root bark of 500 g was soaked in 5L of hydro alcohol (60:40, v/v; water: ethanol) for 72 h with intermittent shaking at room temperature. The extract was filtered through Whatmann No. 1 filter paper; the filtrate was evaporated to dryness and stored in an air-tight container. The percentage yield was higher (7.6%) at 60:40 (v/v) than that at 50:50 (v/v) and 70:30 (v/v). Therefore, the ratio of 60:40 was chosen for the following study.
2.2. Animals
Wistar rats of 6–8 weeks of age were purchased from King's Institute of Preventive Medicine, Guindy, Chennai-600032, India. Experiment was conducted in 60 rats (30 males + 30 females), 10 rats (5M + 5F) in each group. The rats were maintained in institutional animal house facility with 12 h light/dark cycles. Temperature was maintained at 25 ± 3 °C and feeding schedule consisted of rat pellet diet and water ad libitum. HAE, atorvastatin and vehicle (0.5% SCMC) were administered orally for 30 days using gavage and SCMC was served as a solvent. The proposal was duly approved by the Institutional Animal Ethical Committee as per CPCSEA guidelines (Registration No. IAEC/CSMDRIAS/10/2014) prior to initiation of experiments.
Group I- Control group (Rats fed with 0.5% SCMC).
Group II- High fat-fed rats.
Group III- High fat-fed rats treated with 200 mg HAE/kg b.wt.
Group IV- High fat-fed rats treated with 400 mg HAE/kg b.wt.
GroupV- High fat-fed rats treated with 500 mg HAE/kg b.wt.
Group VI- High fat-fed rats treated with 10 mg of Atorvastatin/kg b.wt.
2.3. Atherosclerosis model
Rats were fed with atherogenic diet consisting of 2.0 g of cholesterol, 8.0 g of saturated fat and 0.1 g of calcium were mixed thoroughly with 90 g of powdered standard commercial pellet diet. The rats were fed with high fat diet along with weekly challenge of oral vitamin D (3,00,000 IU) through per oral route [15]. Feed was prepared daily and intake was recorded. Acute toxicity study was performed based on the OECD guideline 423. No toxicity was found at the maximum dose level and the therapeutic dose was calculated.
2.4. Biochemical analysis
At the end of experimental period (i.e.31st day) 4 mL of blood sample was collected in an EDTA-K2 tube from retro-orbital plexus under mild ether anesthesia. Plasma was separated using cooling centrifuge (Remi C-24) at 3000 rpm for 10 min at 4 °C, the bottom portion of red blood cells were washed with ice cold phosphate buffered saline (0.1 M; pH 7.4) in a high speed cooling centrifuge and this procedure was repeated till the red cell pellet changed to pale red color. The pellet obtained was suitably diluted with phosphate buffered saline and used for the estimations. Tissues such as liver, heart and kidney were removed and homogenized using Teflon homogenizer samples were kept on crushed ice until analysis. Biochemical parameters like GSH [16], GPx [17], GR [18], G6PD [19] and total protein [20] were estimated in the liver, heart and kidney by standard procedures using UV-Visible Spectrophotometer (Perkin–Elmer Lambda EZ-201).
2.5. Statistical analysis
Statistical analysis was carried out using graph pad prism software, version 5. All the values were expressed as mean ± SD (n = 10). Analysis of variance (ANOVA) was used for multiple comparison of treatment groups with the vehicle control and disease control followed by Dunnett's test. p < 0.05 was considered to be statistically significant.
3. Results
3.1. P. integrifoliaextract increases the GSH levels
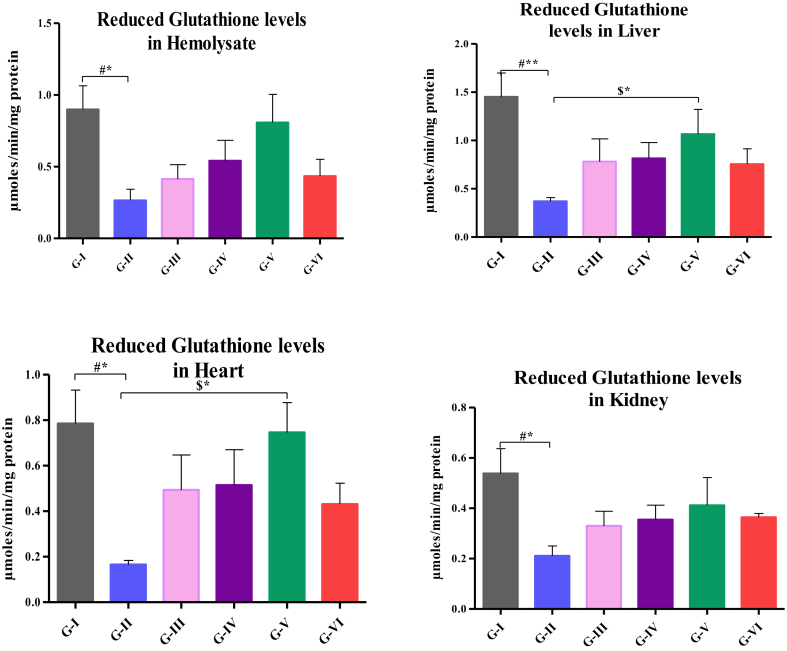
Levels of GSH in hemolysate, liver, heart and kidney are depicted in Fig. 1. The level of GSH in hemolysate was significantly decreased in high fat-fed rats (p < 0.05) when compared to control. The levels of GSH was significantly reduced in liver (p < 0.01), heart and kidney (p < 0.05) of high fat-fed rats than that of control and significant increase was noticed in high fat-fed rats treated with 500 mg/kg.b.wt (p < 0.05) of heart and liver than that of high fat-fed rats.
Fig. 1.
Analysis of GSH levels in hemolysate, liver, heart and kidney. Values are compared with group I & group II; and expressed as mean ± SD (n=10);*p<0.05;**p<0.01 G-I: Control; G-II: Atherosclerosis induced rats; G-III: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-IV: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-V: Atherosclerosis induced ratstreated with 200mg HAE/kg b.wt; G-VI: Atherosclerosis induced rats treated with 10mg of Atorvastatin/kg b.wt.
3.2. P. integrifolia extract increases the GPx activity
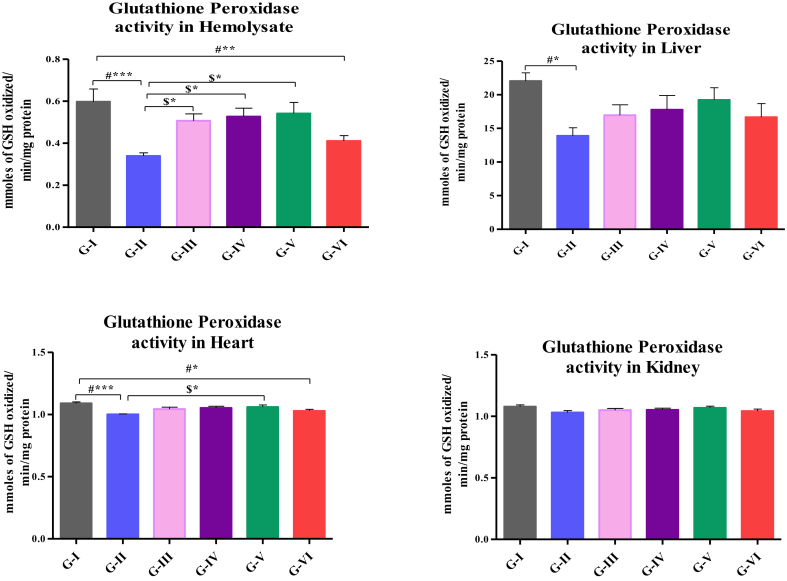
Activity of GPx in hemolysate, liver, heart and kidney are shown in Fig. 2. The activity of GPx in hemolysate was significantly decreased in high fat-fed rats (p < 0.001), high fat-fed rats treated with atorvastatin (p < 0.01) than that of control and significantly increased (p < 0.05) in high fat-fed rats treated with 200, 400 and 500 mg/kg b.wt of HAE than that of rats fed with high fat-diet alone. The activity was significantly decreased in liver (p < 0.05) and heart of high fat-fed rats (p < 0.001) and heart of high fat-fed rats treated with atorvastatin (p < 0.05) than that of control. The enzyme activity was significantly increased (p < 0.05) in high fat-fed rats treated with 200 mg, 400 and 500 mg/kg.b.wt of HAE of hemolysate and 500 mg/kg.b.wt of HAE in heart than that of rats fed with high fat-fed diet alone.
Fig. 2.
Assay of GPx in hemolysate, liver, heart and kidney. Values are compared with group I & group II; and expressed as mean ± SD (n=10);∗p<0.05∗∗p<0.01;∗∗∗p<0.001; G-I: Control; G-II: Atherosclerosis induced rats; G-III: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-IV: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-V: Atherosclerosis induced ratstreated with 200mg HAE/kg b.wt; G-VI: Atherosclerosis induced rats treated with 10mg of Atorvastatin/kg b.wt.
3.3. P. integrifolia extract increases the GR activity
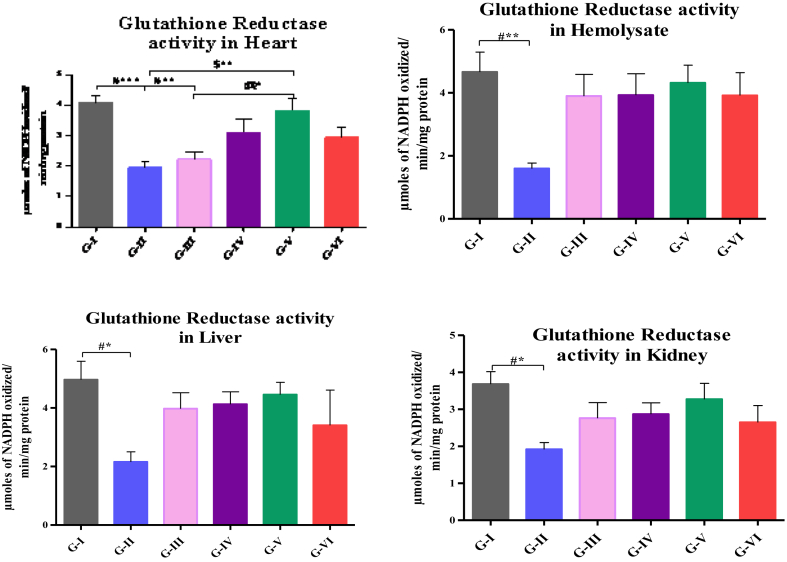
Activity of GR in hemolysate, liver, heart and kidney are depicted in Fig. 3. The activity of GR in hemolysate (p < 0.001), heart (p < 0.01), liver and kidney (p < 0.05) was significantly reduced in high fat-fed rats and high fat-fed rats treated with 200 mg/kg.b.wt HAE (p < 0.01) than that of control. The activity of GR was significantly increased in high fat-fed rats treated with 500 mg (p < 0.01) of hemolysate than that of high fat-fed rats. Dose-dependent increase was observed in group high fat-fed rats treated with 500 mg/kg.b.wt (p < 0.05) than that of high fat-fed rats treated with 200 mg/kg.b.wt HAE.
Fig. 3.
Assay of GR in hemolysate, liver, heart and kidney. Values are compared with group I & group II; and expressed as mean ± SD (n=10);∗p<0.05∗∗p<0.01; ∗∗∗p<0.001; G-I: Control; G-II: Atherosclerosis induced rats; G-III: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-IV: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-V: Atherosclerosis induced ratstreated with 200mg HAE/kg b.wt; G-VI: Atherosclerosis induced rats treated with 10mg of Atorvastatin/kg b.wt.
3.4. P. integrifolia extract increases theG6PD activity
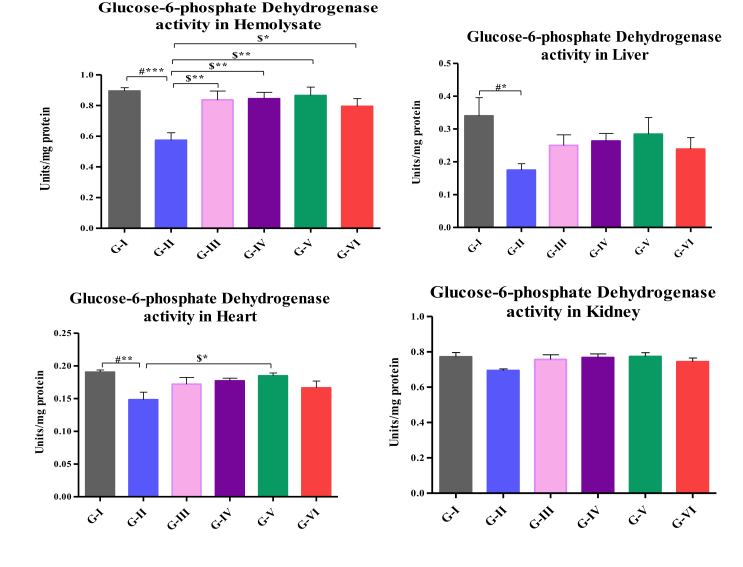
Activities of G6PD in hemolysate, liver, heart and kidney are shown in Fig. 4. Activities of G6PD was decreased in hemolysate (p < 0.001), liver (p < 0.05), heart (p < 0.01) of high fat-fed rats when compared to control. The activity was significantly increased in high fat-fed rats treated with 200, 400 and 500 mg/k.b.wt (p < 0.01) and high fat-fed rats treated with atorvastatin (p < 0.05) in hemolysate and heart of high fat-fed rats treated with 500 mg/kg.b.wt (p < 0.05) when compared rats fed with high fat diet alone.
Fig. 4.
Assay of G6PD in hemolysate, liver, heart and kidney. Values are compared with group I & group II; and expressed as mean ± SD (n=10); G-I: Control; G-II: Atherosclerosis induced rats; G-III: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-IV:Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-V: Atherosclerosis induced rats treated with 200mg HAE/kg b.wt; G-VI: Atherosclerosis induced rats treated with 10mg of Atorvastatin/kg b.wt.
4. Discussion
Reactive oxygen species (ROS) are key mediators of signaling pathways that underlie vascular inflammation in atherogenesis, starting from the initiation of fatty streak development, through lesion progression, to ultimate plaque rupture. The pathophysiological consequences of GSH depletion promote generation of reactive oxygen species and oxidative stress with the subsequent cascade effects affecting the functional and structural integrity of cell [21]. In the present study, GSH levels in hemolysate, liver, heart and kidney were decreased in high fat-fed rats. GSH acts as a substrate for GPx, catalase, GST and GR. Catalase and GPx catalyze the conversion of H2O2 to water [22]. During the reduction of H2O2, GSH is converted into GSSG, thus reverted back by GR [23]. H2O2 produced may further metabolized by catalase mediated mechanism [24]. GR is also essential for the maintenance of GSH level in vivo [25]. An association between oxidative stress and the development of a pro-inflammatory state may demand the utilization of intracellular GSH [26], [27]. GSH-dependent antioxidant system is impaired in atherosclerotic vessels were reported [28]. The respiratory epithelium regulates enzymes involved in maintaining redox equilibrium [29]. Lipid peroxidation and atherogenesis is preceded by the depletion of GSH in the atheroma-prone aortic arch, further supporting a critical role of GSH-dependent antioxidant system in protecting the vasculature against atherosclerosis [30]. GSH is required to counteract the deleterious effects of oxidative stress induced during atherogenesis. Supportive evidence showed that an important role of free radicals in ischemic injuries and tissue injury and hence the present study is in agreement with other findings.
The decomposition of lipid peroxides causes deleterious effects in cells and GPx may protect the cell from those effects. It was observed that a significant decrease in the level of GPx in atherosclerosis induced rats of the present study may be due to the deficiency of the GSH-dependent GPx does not appear to increase the severity of atherosclerosis [31]. The disruption of antioxidant system in atherosclerotic vasculature may occur at an initial stage in GSH homeostasis or the GSH redox state. Selenium-dependent GPx and GR activity was dramatically reduced in human atherosclerotic plaques than in the normal arteries [32]. Oxygen free radicals have been implicated in the development of hyperlipedimic atherosclerosis. The present study did not show any significant change in quenching of free radicals by atorvastatin during the ingestion of high fat diet fed rats. The activity of GPx of our present study coincides well with the above findings.
High fat diet is accompanied by an increase oxidative stress in tissues which is characterized by a reduction in antioxidant enzyme activities and GSH levels, correlate with the increased MDA levels in most tissues [33]. Same observations were noticed in the present study (un published data). In addition, NADPH-dependent GR has the main role in repairing the cell from oxidative stress induced damage [34]. An imbalance between oxidants and antioxidants within the cell are due to some pathological conditions of the body [35]. The dysregulation of the GSH redox system is likely to impact any cell functions that rely on thiol redox-sensitive signaling pathways [36]. The formation of atherosclerotic lesions is associated with increased production of reactive oxygen species (ROS) and the accumulation of lipid and protein oxidation products-processes suggesting that oxidative stress plays a central role in atherogenesis [37]. The role of the GSH-dependent antioxidant system and GSH/GSSG redox ratio as a causative factor in the development of atherosclerotic lesions. This fundamental mechanism includes GSH synthesis and export, and the regeneration of GSH by GR [38], [39]. Present study showed a significant reduction in the GR activity in tissues and blood indicates GR also play an emerging role in alleviating the oxidative stress induced during atherosclerosis.
G6PD activity is rapidly upregulated in response to the initial stage of oxidative stress, presumably to maintain GSH in its reduced form [40], [41], [42]. Acute oxidative stress is often accompanied by depletion of GSH and induction of G6PD activity. In such cases inhibiting G6PD may limit GR activity which regenerates GSH via mechanisms by which GSH levels are maintained [43]. GSH synthesis is induced by oxidized-LDL in macrophages and known to be upregulated by oxidative stress [29] hence increased GSH levels are compensated by increased de novo synthesis. G6PD deficiency contributes to high blood pressure and lower serum high density lipoprotein cholesterol which leads to cardiovascular mortality associated with atherosclerosis [44]. Emerging evidence suggests that the GSH redox state of the vasculature plays a crucial role in the development of atherosclerosis. These data suggest that improving GSH homeostasis and strengthening the GSH redox state protects against cardiovascular disease.
Treatment with HAE of P. integrifolia increases the GSH content, which in turn protects the cellular proteins against oxidation through GSH redox cycle and also directly detoxifies the reactive oxygen species generation on high fat diet. Simultaneous treatment with HAE brought these levels to near normalcy. GR activity is directly proportional to the concentration of the drug was clearly noticed and the higher doses of drug treatment showed a better prognosis in atherosclerotic conditions. P. integrifolia roots are the potential source of natural antioxidants which would be helpful in treating and preventing many free radical mediated diseases [45]. GSH is required to counteract the deleterious effects of oxidative stress. This may be the reason for the non-significant reduction of GSH levels at low dose (200 mg/kg) of HAE treatment. It is clearly depicted that the HAE treated rats showed increased activities of GSH-dependent enzymes when compared to high fat diet induced rats. Hence HAE of P. integrifolia protects liver from the free radical damage at the higher dose level (400, 500 mg/kg). This could be due to P. integrifolia inhibited the free radicals mediated lipid peroxidation and preserved the antioxidant enzyme status. This ameliorative effect of drug on tissue lipid peroxidation may be used as a therapeutic tool in the management of various pathological complications in which induction of oxidative stress is the major contributing mechanism [46]. The HAE of root bark of P. integrefolia individually maintains the lipids, lipoproteins through metabolic and cardioprotective key enzymes [47], [48]. LDL oxidation may be prevented by HAE of this plant inturn protects the GSH-dependent enzymes via GSH redox cycle.
5. Conclusion
The present study showed a significant decrease in the GSH-dependent enzyme activities in high fat fed rats and the activities were improved on the treatment of HAE of P. integrifolia L. A dose dependent increase in the activities of GSH-dependant enzymes were noticed in rats treated with HAE. Further study on activity-guided fractionation of P. integrifolia L. will be helpful in finding the exact component involved in the maintenance of GSH-redox status in atherosclerosis induced rats.
Source(s) of funding
The authors gratefully acknowledge the Director General, Central Council for Research in Ayurvedic Sciences, M/o AYUSH, Govt. of India for the financial support.
Conflict of interest
None
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Berrios X., Koponen T., Huiguang T., Khaltaev N., Puska P., Nissinen A. Distribution and prevalence of major risk factors of non-communicable diseases in selected countries: the WHO inter-health programme. Bull World Health Organ. 1997;75:99–108. [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein J.L., Brown M.S. Molecular medicine. The cholesterol quartet. Science. 2001;292(5520):1310–1312. doi: 10.1126/science.1061815. [DOI] [PubMed] [Google Scholar]
- 3.Berliner J.A., Heinecke J.W. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 4.Stocker R., Keaney J.F. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira A.L.A., Machado P.E.A., Matsubara L.S. Lipid peroxidation, antioxidant enzymes and GSH levels in human erythrocytes exposed to colloidal iron hydroxide in vitro. Braz J Med Biol Res. 1999;32:689–694. doi: 10.1590/s0100-879x1999000600004. [DOI] [PubMed] [Google Scholar]
- 6.Jones D.P., Go Y.M., Anderson C.L., Ziegler T.R., Kinkade J.M., Jr., Kirlin W.G. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita H., Nakamura A., Noguchi N., Niki E., Kuhn H. Oxidation of low density lipoprotein and plasma by 15-lipoxygenase and free radicals. FEBS Lett. 1999;445:287–290. doi: 10.1016/s0014-5793(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 8.Dafre A.L., Sies H., Akerboom T. Protein S-thiolation and regulation of microsomal glutathione transferase activity by the glutathione redox couple. Arch Biochem Biophys. 1996;332:288–294. doi: 10.1006/abbi.1996.0344. [DOI] [PubMed] [Google Scholar]
- 9.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold R.S., Shi J., Murad E., Whalen A.M., Sun C.Q., Polavarapu R. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci Unit States Am. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballinger S.W., Patterson C., Knight-Lozano C.A., Burow D.L., Conklin C.A., Hu Z. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 12.Baquer N.J., Lean M.C.P. Evidences for the existence and functional activity of pentose phosphate pathway enzymes in the large particles fraction isolate from rat tissues. Biochem Biophys Res Commun. 1972;46:169–174. doi: 10.1016/0006-291x(72)90646-8. [DOI] [PubMed] [Google Scholar]
- 13.Gokani R.H., Lahiri S.K., Santani D.D., Shah M.B. Evaluation of in-vitro anti-oxidant activity of Premna integrifolia Linn. root. Res J Pharmacogn Phytochem. 2010;2:196–199. [Google Scholar]
- 14.Mali P.Y. Beneficial effect of extracts of Premna integrifolia root on human leucocytes and erythrocytes against hydrogen peroxide induced oxidative damage. Chronicles Young Sci. 2014;5:53–58. [Google Scholar]
- 15.Altman R.F. A simple method for rapid production of atherosclerosis in rats. Experentia. 1973;29:256. doi: 10.1007/BF01945508. [DOI] [PubMed] [Google Scholar]
- 16.Moron Μ.S., Depierre J.W., Mannervik Β. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 17.Rotruck J.T., Pope A.L., Gantler H.E., Swanson A., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;79:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 18.Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;50:5475–5480. [PubMed] [Google Scholar]
- 19.Baquer N.J., Lean M.C.P. Evidences for the existence and functional activity of pentose phosphate pathways enzymes in the large particles fraction isolate from rat tissues. Biochem Biophys Res Commun. 1972;46:169–174. doi: 10.1016/0006-291x(72)90646-8. [DOI] [PubMed] [Google Scholar]
- 20.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Feuvray D., James F., De Leiris J. Protective effect of endogenous catecholamine depletion against hypoxic and reoxygenation damage in isolated rat heart: an ultrastructural study. J de Physiologie. 1980;76(7):717–722. [PubMed] [Google Scholar]
- 22.Rocha K.K.R., Souza G.A., Ebaid G.X., Seiva F.R.F., Cataneo A.C., Novelli E.L.B. Resveratrol toxicity: effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem Toxicol. 2009;7:1362–1367. doi: 10.1016/j.fct.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Masella R., Di Benedetto R., Vari R., Filesi C., Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 24.George P. The effect of the peroxide concentration and other factors on the decomposition of hydrogen peroxide by catalase. Biochem J. 1949;44:197–205. doi: 10.1042/bj0440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zwieten R., Verhoeven A.J., Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic Biol Med. 2014;67:377–386. doi: 10.1016/j.freeradbiomed.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Hassan M.Q., Hadi R.A., Al-Rawi Z.S., Pardon V.A., Stohs S.J. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol. 2001;21:69–73. doi: 10.1002/jat.736. [DOI] [PubMed] [Google Scholar]
- 27.Mates J.M., Peres-Gomez C., Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 28.Diaz M.N., Frei B., Vita J.A., Keaney J.F. Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Qiao M., Mieyal J.J., Asmis L.M., Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized LDL-induced cell injury in human macrophages: role of glutathione reductase and glutaredoxin. Free Radic Biol Med. 2006;41:775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Biswas S.K., Newby D.E., Rahman I., Megson I.L. Depressed glutathione synthesis precedes oxidative stress and atherogenesis in Apo-E (-/-) mice. Biochem Biophys Res Commun. 2005;338:1368–1373. doi: 10.1016/j.bbrc.2005.10.098. [DOI] [PubMed] [Google Scholar]
- 31.Judy B.D.H., Paul K.W., Nada S., Josefa P., Michael D., Ismail K. Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet. J Lipid Res. 2006;47:1157–1167. doi: 10.1194/jlr.M500377-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Lapenna D., de Gioia S., Ciofani G., Mezzetti A., Ucchino S., Calafiore A.M. Glutathione- related antioxidant defenses in human atherosclerotic plaques. Circ. 1998;97:1930–1934. doi: 10.1161/01.cir.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 33.Noeman S.A., Hamooda H.E., Baalash A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndrome. 2011;3:3–17. doi: 10.1186/1758-5996-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultatos L.G. Effects of acute ethanol administration on the hepatic xanthine Dehydrogenase/xanthine oxidase system in the rat. J Pharmacol Exp Therapeut. 1988;246:946–949. [PubMed] [Google Scholar]
- 35.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res Int. 2014:1–19. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chisolm G.M., Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 38.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 39.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 40.Jain M., Brenner D.A., Cui L., Lim C.C., Wang B., Pimentel D.R. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res. 2003;93:9–16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- 41.Salvemini F., Franze A., Iervolino A., Filosa S., Salzano S., Ursini M.V. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J Biol Chem. 1999;274:2750–2757. doi: 10.1074/jbc.274.5.2750. [DOI] [PubMed] [Google Scholar]
- 42.Matsui R., Xu S., Maitland K.A., Mastroianni R., Leopold J.A., Handy D.E. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E (-/-) mice. Arterioscler Thromb Vasc Biol. 2006;26:910–916. doi: 10.1161/01.ATV.0000205850.49390.3b. [DOI] [PubMed] [Google Scholar]
- 43.Tsai K.J., Hung I.J., Chow C.K., Stern A., Chao S.S., Chiu D.T.Y. Impaired production of nitric oxide, superoxide, and hydrogen peroxide in glucose 6-phosphate-dehydrogenase-deficient granulocytes. FEBS Lett. 1998;436:411–414. doi: 10.1016/s0014-5793(98)01174-0. [DOI] [PubMed] [Google Scholar]
- 44.Peter A.H., Jane A.L., Sachin A.G., Fabio A.R., William C.S. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H491–H500. doi: 10.1152/ajpheart.00721.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajendran R., Saleem Basha N., Ruby S. Evaluation of in vitro antioxidant activity of stem-bark and wood of Premna serratifolia Linn. (Verbenaceae) Phytochemistry. 2009;1:11–14. [Google Scholar]
- 46.Jones D.P. Redefining oxidative stress. Antioxidants Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 47.Chitra S., Arivukkodi R., Arunadevi R., Sudesh G., Ilavarasan R., Dhiman VdK.S. Anti-atherosclerotic activity of root bark of Premna integrifolia Linn. in high fat diet induced atherosclerosis model rats. J Pharmaceut Anal. 2016;7:123–128. doi: 10.1016/j.jpha.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chitra S., Arivukkodi R., Gaidhani S., Ilavarasan R., Vd Dhiman K.S. Premna integrifolia L. on enzymatic biomarkers in atherosclerosis. Int J Biochem Res Rev. 2017;17(3):1–9. [Google Scholar]