Abstract
Background
Amoora rohituka is described in Ayurveda, an Indian traditional system of medicine for management of disorders of blood, diseases of eye, helminthiasis disease, ulcer, liver disorders and splenomegaly. However, the leaves were not reported to have anticancer properties till date.
Objective
This study was carried out to evaluate the cytotoxic potential of leaf extracts of Amoora rohituka.
Materials and methods
The leaves powder was macerated in petroleum ether, ethyl acetate and methanol and evaluated their anticancer activities in vitro. The phytochemical constituents of the active (ethyl acetate) extract were screened by FTIR analysis and phytochemical screening methods.
Results
The ethyl acetate extract (RLEA) showed the presence of alkaloids, flavonoids, steroids, tannins, saponins and terpenoids. The RLEA exhibited high cytotoxic effect against human breast cancer cells, MCF-7 (IC50 = 9.81 μg/mL) and induced apoptosis by altering nuclear morphology and DNA laddering. Wound healing assays explained the potency of extract to decrease the cell migration.
Conclusion
The extract of Amoora rohituka leaves exhibited anticancer activity with less toxicity and it could be used for development of alternative drugs in the treatment of human breast cancer.
Keywords: Traditional medicine, Breast cancer, FTIR, HPTLC, Apoptosis, Cell migration
Graphical abstract
1. Introduction
The commonest cancer among global women population is breast cancer with a lifetime risk estimated to be one in eight in industrialized countries. In the year 2012, 1.7 million new cases and about 522,000 deaths were reported over the world and alarmed the such burden of breast cancer will increase to almost double of the present cases by 2030 [1], [2]. The conventional procedure for cancer treatments includes surgical removal of the malignant tissue, chemotherapy and ionizing radiation etc., which causes severe side effect as well as destroying the adjacent normal cells. Next alternative medicine has been discovered for improving its efficacy against cancer with reduced side effects of chemotherapy and boost up the immune system to fight against metastatic cells, however some tumors developed resistance to conventional drugs [3]. About 70% of the anticancerous compounds are isolated from medicinal plants or their derivatives and play as a lead molecules for the drug development with unique novel pharmacophores [4], [5]. The reverse pharmacology approaches could offer an efficient development platforms for herbal drugs and reduce the time and economize investments with better safety [1]. The phytochemicals having the potency to induce apoptosis in cancer cells are promising to play significant role in the managing and treating the cancer. It has been reported that several isolates from medicinal plants showed their anticancer activity.
Amoora rohituka (Roxb.) Wight & Arn. is belonging to Meliaceae family and used in several medicinal formulations [6]. A. rohituka is well known one of important Indian medicinal plant which is commonly used in disorders of blood, diseases of eye, helminthiasis disease, ulcer or wound, liver disorders, splenomegaly, internal tumours, leucorrhoea and urinary disorders [7]. A. rohituka has different kinds of metabolites such as alkaloids, flavonoids, glycosides, terpenoids, and anthraquinones, which are medicinally importance [8]. Most of them isolated from the bark of stem have anticancer properties [9], [10], [11], [12], [13], [14]. Although the study on medicinal properties of leaves compounds from A. rohituka are still limited.
Leaves are the renewable source of the natural product without harming the plant, also an economical and environment friendly [8]. Therefore, present study has emphasized to investigate the anticancer activity of A. rohituka leaves extracts against human breast adenocarcinoma (MCF-7) cells.
2. Material and methods
2.1. Plant material and preparation of extracts
A. rohituka leaves were collected from the medicinal plant garden, Banaras Hindu University, India and its leaves were dried at room temperature for two weeks and ground into a coarse powder using the electrical domestic grinder. The 100 g of leaves powder was macerated for 24 h with continuous stirred in 500 mL each of petroleum ether, ethyl acetate and methanol respectively thrice in each. Then, the supernatant was recovered by filtration through Whatman filter paper of 11 μm pore size after maceration using a suction apparatus. Further, each filtrate was completely dried by rotary vacuum evaporator.
2.2. Phytochemical screening and fingerprinting
Chemical Method: Phytochemical screening was performed using standard procedures as reported in the previous study [15]. Briefly, Dragendroff's test, Mayer's test and Wagner's test for alkaloids, ferric chloride test and Shinoda test for flavonoids, Salkowski reaction and Liebermann–Burchard reaction for steroids, ferric chloride and lead acetate test for tannins and phenolic compounds, Kellar Killani test for glycosides, foam test saponins, etc.
Fourier Transformed Infrared (FTIR) Spectroscopy: Fourier Transformed Infrared (FTIR) Spectrometer (Perkin Elmer spectrum 2) was also used to study the functional group of Ethyl acetate fraction of the leaves for confirmation of phytochemical constituents as it was active fraction against cancer cells [16]. The samples were analyzed in the range of wave number 4000–400 cm–1 to study the chemical nature of the extract. The sample was added into KBr crystal and powdered to make KBr pellet. 100 interferograms were averaged with a spectral resolution of ±4 cm–1 to improve the signal to noise ratio for each spectrum. In the case of identical condition, the background spectra were subtracted from the sample spectra to directly relate the intensities of the percentage transmittance of functional group to the concentration of the related functional groups.
HPTLC fingerprinting: For phytochemical fingerprinting, CAMAG Linomat 5 (Switzerland) HPTLC was used with slightly modification of reported method [17]. Briefly, Aluminum TLC Silica gel 60 F254 plates (5 cm × 10 cm; 175–225 μm thickness, Merck Limited, Mumbai, India) was used as stationary phase and Toluene: Acetone: Ethyl acetate (8:2:0.5 v/v) as mobile phase.
The extract band (sample concentration 2 mg/mL; application volume 10 μL, width 6 mm, the distance between tracks 10 mm) was applied on the plate with CAMAG Linomat 5 sample applicator syringe. Chamber was kept to saturation for 45 min at room temperature. The length of chromatogram run was 80 mm. Then, densitometric analysis was done with CAMAG TLC scanner of the system using radiation source D2 & W lamp and the procedure was operated by win CATS software integrated with the system. Chromatogram recorded on two modes, first before at 254 nm and 366 nm, and then after spraying 2% sulphuric acid solution.
2.3. Cell culture
Human breast adenocarcinoma (MCF-7) cells, human triple negative breast carcinoma (MDA-MB-231) cells, mice undifferentiated carcinoma (Ehrlich-Lettre ascites carcinoma, EAC) cells and mice fibroblast (NCTC clone 929, L929) cells were procured from National Centre for Cell Sciences (NCCS), Pune, India. The cells were cultured in DMEM with high-glucose, sodium bicarbonate, l-glutamine and sodium pyruvate which supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics-antimycotic (10,000 unit/mL of penicillin G, 10 mg/mL of streptomycin sulfate and 25 μg/mL amphotericin B in 0.9% normal saline) solution at 37 °C in a humidified environment with 5% CO2 gas. About 70–80% confluent cells at log phase were harvested by trypsinization (1X Trypsin-EDTA Solution, Himedia). MCF-7 cells were used throughout the study while EAC and L929 cells were used in cytotoxicity assay [18].
2.4. Cytotoxicity assay
MTT assay was applied for evaluation of the cytotoxicity of the extracts in which tetrazolium rings of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide is cleaved by mitochondrial enzyme succinate dehydrogenase of live cells. The yellowish MTT solution is turned into water-insoluble violet colored formazan crystals depends on the number of viable cells. This assay is also sensitive, reliable, quantitative and colorimetric method to measure cell viability. The 100 μL of cell solution in fresh media at a cell density of 1 × 104 cells/mL was seeded in each well of 96-well plate and incubated overnight. Then, the old culture media was replaced with 200 μL fresh media and treated with the extracts with serial dilution which was further incubated for 48 h. On the completion of treatment duration, the culture media with treatment was replaced with 100 μL of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) solution at a concentration of 0.5 mg/mL in incomplete culture media, i.e., the culture media without fetal bovine serum, and kept for 4 h at 37 °C. The plate with cells were centrifuged at 750 rpm for 5 min (Remi R8C) and the supernatant was replaced with 100 μL DMSO to solubilize water-insoluble purple colored-formazan crystal and absorbance was measured at 570 nm using microtitre plate reader (Biorad, India) [19]. The percentage of cell viability was calculated as: Percentage cell viability = (OD of the cells treated with extract/OD of the un treated control cells) × 100, then IC50 was calculated [20], [21].
2.5. Cell morphology analysis
Human breast cancer (MCF-7) cells were seeded into 96 well plate at a density of 1 × 103 cells in each well and incubated for 48 h in fresh media. Then, the culture medium was changed with fresh culture medium and the ethyl acetate extract was administered at the concentration of its IC50 value which was further incubated for 24 h in the same environmental condition. After incubation, the culture medium was removed and cells were washed 10 mM PBS (pH 7.4) and observed under the inverted light microscope (Dewinter, India) at 400× magnification.
2.6. Apoptosis assay
Acridine orange (AO) and Ethidium bromide (EtBr) double staining were performed for detection of the mode of cell death. Acridine orange is a cell permeable dye which binds with the nucleic acid to emit green fluorescence while ethidium bromide can enter the cell after the destruction of the membrane and binds to the nucleic acid to emit red fluorescence. Hence, these two dye facilitate the detection of mode of cell death [19]. The MCF-7 cells were seeded into 96 well plate at the concentration of 1 × 104 cell per well and incubated for 24 h. Then, the culture medium was changed with fresh culture medium and 200 μL volume was maintained. The ethyl acetate extract was administered at the concentration of its IC50 value as test drug, Paclitaxel was added in control positive group while 1X PBS was taken in control negative group. It was further incubated for 24 h at 37 °C in a humidified environment with 5% CO2 gas. After completion of incubation, the culture medium was removed and cells were washed with 10 mM PBS (pH 7.4) and cells were incubated for further 30 min in the solution of 100 μg/mL AO and 100 μg/mL EtBr in dark. Again, it was washed 10 mM PBS (pH 7.4) and cells were detected by the blue filter of fluorescence inverted microscope (Dewinter, India) at 400× magnification [18].
2.7. DAPI staining
Nuclear Morphology was studied by DAPI (4′-6-diamidino-2-phenylindole) stain (Genetix, India) which forms fluorescent complexes with natural double-stranded DNA [22]. The 1 × 104 cell per well and incubated for 24 h. Then, the treatments were given as per previous experiment followed by washing of cells with PBS and stained with DAPI solution. After 30 min incubation, the cells were again washed with 10 mM PBS (pH 7.4) and cells were studied by fluorescence inverted microscope using the blue filter [23].
2.8. Detection of DNA fragmentation
MCF-7 cells were seeded into 6 well plate at the concentration of 1 × 105 cell per well and incubated for 24 h. Then, the culture medium was changed with fresh culture medium and the ethyl acetate extract was administered at the concentration of its IC50 value as test drug, Paclitaxel was added in control positive group while 1X PBS was taken in control negative group. It was further incubated for 24 h at 37 °C in a humidified environment with 5% CO2 gas. After completion of incubation, the cells were harvested and DNA isolated as per manufacturer instruction using DNASure tissue mini kit (Genetix, India). The isolated DNA was dissolved in DNA loading buffer separately and electrophoresis was done on 1.5% agarose gel [24].
2.9. Wound scratch healing assay
The MCF-7 cells were grown to 80% confluent and collected by trypsinization for seeding into 6-well plate at a density of 1 × 105 cells/ml. It was incubated at 37 °C for 24 h in DMEM complete culture medium. Then the medium was replaced with medium without fetal bovine serum and incubated further 24 h, then scratch wound was made passing through the center of the well containing monolayer of cells by using a sterile 20 μL pipette tip and washed thrice with 10 mM PBS. 1 mL of complete medium was given to the wound containing cells and extract was administrated in test group while medium in control group and followed by incubation for 24 h, 48 h and 72 h. Images of the migrated cells were taken using an inverted microscope (Dewinter, India) at 100× magnification [25].
2.10. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 for Windows. The analysis of variance was performed on three independent cytotoxicity results by one way ANOVA analysis using tukey as post test. The results were considered significant at p value < 0.05. The data were expressed as mean ± SD.
3. Results
3.1. Phytochemical screening and fingerprinting of different extracts
The yields of the extracts were found to be petroleum ether extract 5.20 g; ethyl acetate extract 7.70 g and methanol extract 6.47 g respectively which were evaluated for their phytochemical constituents. The results of the phytochemical screening were presented in Table 1 which revealed the variation in phytochemicals of different extracts such as the petroleum extract contained steroids, tannins and glycosides, ethyl acetate extract contained alkaloids and steroids while methanolic extract showed flavonoids, terpenoids and glycosides. These results were supported by Mahajan et al. [8] reported that the alkaloids, flavonoids, steroids, phenols, terpenoids, glycosides and saponins were main components of the leaves and seed extract from the same plant.
Table 1.
Presenting the phytochemicals constituents of different extracts of A. rohituka leaves.
| Chemical methods | |||
|---|---|---|---|
| Phytochemicals | Petroleum Ether Extract | Ethyl acetate Extract | Methanolic Extract |
| Alkaloids | – | +++ | – |
| Flavonoids | – | + | +++ |
| Steroids | + | +++ | – |
| Terpenoids | – | + | ++ |
| Glycosides | ++ | – | +++ |
| Tannins | ++ | – | _ |
| Saponins | – | – | + |
| Phenols | – | + | – |
“+” Present; “–” Absent.
Spectroscopic and chemical fingerprinting analysis Fig. 1B show the FTIR spectra of phytochemical and confirmed the various chemical constituents in ethyl acetate fraction of A. rohituka leaves. Broad stretching band of hydroxyl at 3398 cm–1 indicated the presence of O—H stretching in intermolecular bond of alcohols. The peaks obtained at 2927 cm–1 had strong stretching of N—H in amine group. The peaks at 2891 indicated medium stretching in alkane with C—H group. The strong peaks generated at 1729 cm–1 C O stretching for ester compounds and other strong peak observed at 1731.8 cm–1 for C O in conjugated anhydride. The medium-weak peak at 1645 cm–1 indicated C—H bending for aromatic compounds. Another strong peak at 1457 showed C—H bending for methyl, at 1377 for O—H stretching for hydroxyl in phenol compounds and at 1240 cm–1 stretching of C—N in amine functions group. Along with above peaks some medium sharp peaks also observed at 1171, 1070 and 901 for stretching of C—N in amine functions group, C—O in primary alcohol and bending of C C in alkenes [26]. The peak at 600 cm–1 represents for C—N—C and detailed information given in Table 2. The obtained peak values was also similar to earlier reported work of Burman et al. and Saleem et al. [27], [28].
Fig. 1.
Chemical Fingerprinting of A. rohituka leaf extracts in different organic solvents. Solvent System: (Toluene: Acetone: Ethyl acetate: 7.5: 2.0: 0.5); Track 1 = Petroleum Ether Extract, Track 2 = Ethyl acetate and Track 3 = Methanolic Extract; and Infrared Spectroscopy.
Table 2.
Describing the peak value with respective functional group of phytocontituents of FTIR analysis of A. rohituka leaves extract in ethyl acetate.
| Sl. No | Peak value | Functional group | FTIR Vibrations |
|---|---|---|---|
| 1. | 3398 | OH | Strong broad stretch (in alcohol or phenol) |
| 2. | 2924 | N—H | Strong stretch of amine group |
| 3. | 2891 | C—H | medium stretching in alkane |
| 4. | 1729 | C—O | Strong Stretch in ester |
| 5. | 1645 | C=C | Medium bending in aromatic compound |
| 6. | 1457 | C—H | Strong bending in methyl |
| 7. | 1377 | O—H | strong stretch in phenols |
| 8. | 1240 | C—N | stretch vibration in amide |
| 9. | 1171 | C—N | stretch vibration in tert-alcohol |
| 10. | 1070 | C—O | stretch in primary alcohol |
| 11. | 901 | C=C | Bending in alkene |
| 12. | 600 | C—N—C | Bending in structure |
Again phytochemical standardization was carried out through chemical fingerprinting using HPTLC chromatogram for all extracts. The number of bands 6, 7 and 8 were observed for petroleum ether, ethyl acetate, and methanol extracts respectively. There were four chemical bands, were common in either of two extracts with Rf 0.04, 0.58, 0.69 and 1.00; and only one band was common in petroleum ether extract and ethyl acetate with Rf 0.69 whereas two chemical bands were common in ethyl acetate and methanolic extracts with Rf 0.04 and 0.58 (Fig. 1).
3.2. Cytotoxicity effect of leaves extract
The cytotoxicity of leaves extract against cancer cells are summarized in Fig. 2. It was observed that the ethyl acetate extract showed promising anticancer activity toward the cell lines. Human breast adenocarcinoma (MCF-7), triple-negative human breast cancer (MDA-MB-231) cells, mice undifferentiated carcinoma (EAC) and mice fibroblast (L929) cell lines were used to evaluate the cytotoxicity of the leaf extracts of three different solvents and L929 cells were taken as normal cells. A significant decrease in cell viability was recorded with increasing concentration of the extracts by MTT assay. The L929 cells showed the least sensitivity to the extracts while MCF-7 cells had highest sensitivity followed by MDA-MB-231 cells and EAC cells (Fig. 2). The IC50 values of these cells revealed that the ethyl acetate extract had highest cytotoxicity effect for MCF-7 cells (Fig. 2). The ethyl acetate extract was selected for further study against MCF-7 cell line on the basis of cell toxicity.
Fig. 2.
Describing the cytotoxicity of different extracts of A. rohituka leaves with their respective IC50 values (PE: Petroleum ether extract; EA: Ethyl acetate extract; M: Methanol extract).
3.3. The ethyl acetate extract altered cell morphology
Cell morphology of treated and untreated cells was studied under light microscopy to examine the cellular alteration. It revealed the induction of cytoplasmic vacuolization in treated cells with the extract, which was absent in untreated cells (Fig. 3A–C) and indicated the induction of apoptosis-like cell death.
Fig. 3.
Microscopy of the cells showing alteration in their cellular and nuclear morphology in the treated group (A: treated with packlitaxel; B: treated with Amoora rohituka leave extract, RLEA; C: without any treatment, control negative) and also DNA laddering in the treated group (A: treated with packlitaxel; B: treated with RLEA; C: without any treatment, control negative) in agarose gel which supporting the induction of apoptosis by the extract.
3.4. Analysis of apoptosis by ethyl acetate extract
After 24 h of the MCF-7 cells treatment with extract, the cells were stained with acridine orange and ethidium bromide for examination under a fluorescence microscope. The apoptosis-like cell death was not detected in the untreated negative control and all cells were emitting green color fluorescence with intact nuclear morphology and cell membrane. The treated cells with the extract at the concentration of its IC50 value showed yellowish to orange-red color fluorescence with nuclear fragmentation, nuclear contraction and cytoplasmic membrane blebbing. The similar phenomenon observed in the treated cells with the conventional drug (Paclitaxel) which showed orange and red-colored cells (Fig. 3D–F).
3.5. Alteration in nuclear morphology
The DAPI staining was used for the treated MCF-7 and untreated cells and followed by microscopic examination, which revealed a significance alteration in nuclear morphology in the treated cells, where nuclear fragmentation, chromatin condensation and shrunken nuclear morphology were observed. The untreated control group of cells showed intact nuclear morphology no indicating for apoptosis (Fig. 3G–I).
3.6. Assessment of DNA fragmentation
The fragmentation of genomic DNA was confirmed by DNA laddering assay to support the apoptosis induction by the extract in the cancer cells. It was observed that the treated cells showed DNA laddering pattern and a single genomic DNA band was in untreated cells. The cells treated with paclitaxel as control positive had also DNA laddering pattern (Fig. 3J).
3.7. The cellular migration and delayed wound healing by ethyl acetate extract
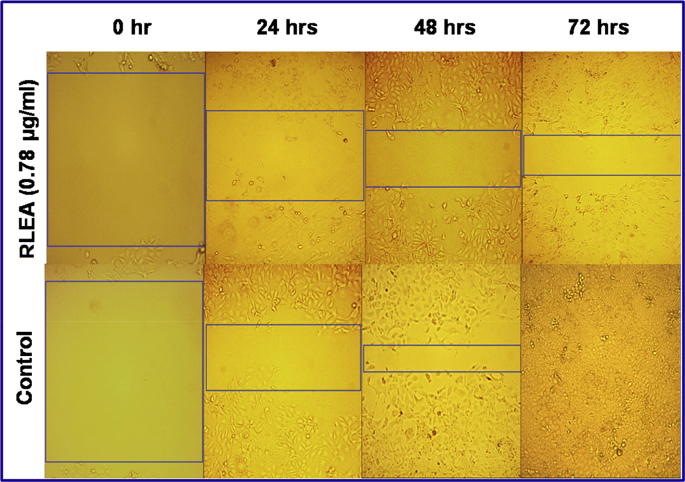
Cellular migration is an important characteristics feature of cancer cells which helps them in spreading and metastasis. It was commonly evaluated by the wound scratch healing assay. In this study, the effect of ethyl acetate extract from leaves was evaluated for cellular migration and showed a significant decrease in cellular migration and delayed in wound healing (Fig. 4).
Fig. 4.
Wound healing showing inhibition of cellular migration in treated group of MCF-7 cells at the concentration of 0.78 μg/mL ethyl acetate extract of A. rohituka leaves (RLEA).
4. Discussion
Medicinal plants are used as prime source for development of therapeutic agents against several diseases. The phytochemicals have molecular target for inducing the apoptosis in different cancer cells [29], [30], [31], [32], [33]. The traditional system for diseases treatment based on medicinal plants in different regions of the world provides information for the urgent development of drugs. Breast cancer is a challenging problem for oncologist to treat effectively without severe side effects. Although, the development of chemoresistance in cancer cells is the major obstacle for the treatment of cancer by pharmacological approach. The alternative drugs require for overcoming such challenging problems. The traditional knowledge of medicinal plants offers the alternative approach to develop the leading molecules or drugs with better compatibility, cost-effective and less toxicity.
The bark of A. rohituka described in preparation of many polyherbal formulations in Ayurveda, Homeopathy, etc. for the treatment of several diseases [11], [34]. The active extract from the leaves of A. rohituka showed rich in alkaloids and steroids content are pharmaceutically important and many drugs from these phytochemicals are in pre-clinical trial, such as rohitukine (chromane alkaloid) from the stem bark of A. rohituka exhibits anticancer, immuno-modulatory, anti-inflammatory, anti-implantation and anti-fertility properties [13], [35], The isolated Amooranin from the stem bark is a triterpenoid inducing cell growth arrest, apoptosis and also overcomes the drug resistance developed in cancer cells [12], [34], A triterpenoid: Aphanin is also extracted from stem bark, which downregulates K-Ras and STAT3 gene expression in pancreatic carcinoma cells [6]. The fingerprinting is an important tool for the identification and quality control of herbal drugs and formulations by means of qualitative and quantitative analysis of phytochemicals [17], [36]. The FTIR study also shows the different functional group in crude extract of selected plant [26]. These compounds are alkane, alkenes, amines, amides, alcohols, methyl, phenols, aromatics, anhydride and esters. These findings support the primary phytochemical screening results, i.e. presence of alkaloids, flavonoids, steroids, terpenoids and phenols [16], [37], [38], [39], [40]. This analysis helps in understanding the phytochemistry of active compounds and the active constituents may be present at Rf 0.10, 0.39, 0.66 and 0.76 in both extracts (Fig. 1). The cytotoxicity of all three leaf extracts on MCF-7, EAC and mice fibroblast L929 cells showed inhibitory activity against cancer, indicating that the extracts of A. rohituka could be alternatives of conventional drugs against cancer. The extracts also showed the potency as an anticancer activity by inducing cell toxicity in the cancer cell lines as described in Fig. 2. The IC50 values of the leaves extracts showed the potential activity with IC50 value of 17.65 μg/mL, 9.81 μg/mL and 31.23 μg/mL for petroleum ether, ethyl acetate and methanol extracts respectively against human breast carcinoma (MCF-7) cells and petroleum ether extract of stem bark had IC50 value 41 μg/mL whereas ethyl acetate and methanol had no activity [6], [12], [41], [42], [43]. The several cytoplasmic vacuoles were also seen in the treated cells. The two types of cytoplasmic vacuolization in mammalian cells have been reported, namely transient and irreversible vacuolization. Transient vacuolization is an important symbol which is observed during an exposure of cells to an inducer or cytotoxic materials, which progressively affects the cell cycle and migration of the cells reversibly [44]. Irreversible vacuolization is hallmarks of cytopathological conditions that lead to cell death in cytotoxic stimulus where irreversible vacuolization combines with acidic organelles and affects the endoplasmic reticulum and Golgi apparatus leads to apoptosis like cell death. Both natural as well as synthetic cytotoxic chemical entities were reported to induce cytoplasmic vacuolization, which either affects on cell cycle and cell migration or induce apoptosis-like cell death [45]. The primary goal of anticancer chemotherapeutic drugs is the destroying cancer cells by inducing apoptosis in affected cells. Apoptosis is a highly systematic and programmed cell death, wherein the cell debris is phagocytosed by the adjacent cells. The drugs having apoptosis inducing property in cancer cells are considered as the potential anticancer chemotherapeutic agent could also reduce the tumor volume. Acridine orange and ethidium bromide double fluorescent staining are able to detect morphological changes occurred in apoptotic cells and differentiates from normal cells, early apoptotic cells, late apoptotic cells and necrotic cells. Acridine orange could penetrate the intact plasma membrane and bind to DNA that exhibit green fluorescence after staining the normal and early apoptotic cells. Ethidium bromide enters into the damaged membranes of cells, which emit orange-red fluorescence on the staining of late apoptotic and dead/necrotic cells. The double staining of acridine orange and ethidium bromide enables to distinguish normal, early apoptotic, late apoptotic and necrotic cells along with the nuclear morphology [46]. DAPI staining is the most commonly used assay to examine the apoptosis at nuclear morphology and DNA level [22]. In this study, the extract of ethyl acetate induced apoptosis in human breast cancer (MCF-7) cells and altered morphology of the cells and their nuclei were observed in DAPI staining. Genomic DNA fragmentation is an important characteristic of apoptosis [24], [47]. DNA fragmentation in MCF-7 cells was induced by the ethyl acetate extract of A. rohituka showing a typical ladder pattern of apoptosis mode of cell death that indicated to apoptosis-induced cell death. The cancer cell migration i.e., metastasis of tumor cells into the normal organ tissues limits the effective surgery for tumor removal. Several researches have shown the cancer cell migration inhibitory effect of phyto-constituents [25]. It has been reported that the stem bark of A. rohituka has potency to inhibit cancer cell migration [48]. This study also explored the cell migration inhibitory effect of the ethyl acetate extract from A. rohituka leaves on the migration of MCF-7 cells.
5. Conclusion
In the present work, we have explored the therapeutic effect of A. rohituka leaf extracts in three different organic solvents; petroleum ether, ethyl acetate and methanol. The ethyl acetate extract has shown more effective and less toxic in comparison to others. The phytochemical screening also reveals the alkaloids and terpenoids which may be similar to previously isolated phytochemicals from the stem bark of the same plant. The most potent ethyl acetate extract exhibits anticancer activity by inducing cell toxicity and apoptosis (morphology change, nuclear fragmentation, and DNA fragmentation) in MCF-7 cells and EAC cells, and safer for L929 cells. The ethyl acetate extract also inhibits MCF-7 cell migration. Therefore, our data confirm the potential of A. rohituka leaf extract as a source for the development of future chemotherapeutic agents.
Source(s) of funding
The author Rajesh Kumar Singh is grateful to BHU and UGC for financial supports as fellowships (No. R/Dev/Scholarship (SRF-JRF)/Ayurveda/57032.
Conflict of interest
None
Acknowledgement
We are thankful to the Centre of Experimental Medicine & Surgery (CEMS), Institute of Medical Sciences, Banaras Hindu University, Varanasi, India for instrument facilities.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Arya R.K., Singh A., Yadav N.K., Cheruvu S.H., Hossain Z., Meena S., et al. Anti-breast tumor activity of Eclipta extract in-vitro and in-vivo: novel evidence of endoplasmic reticulum specific localization of Hsp60 during apoptosis. Sci Rep. 2015;5:18457. doi: 10.1038/srep18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer: GLOBOCAN: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Cancer fact sheets. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 3.Patwardhan B., Vaidya A.D.B., Chorghade M. Ayurveda and natural products drug discovery. Curr Sci. 2004;86:789–799. [Google Scholar]
- 4.Cragg G.M., Grothaus P.G., Newman D.J. Impact of natural products on developing new Anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S.B., Gupta R. Drug development from natural Resource: a systematic approach. Mini Rev Med Chem. 2015;15:52–57. doi: 10.2174/138955751501150224160518. [DOI] [PubMed] [Google Scholar]
- 6.Rabi T., Catapano C.V. Aphanin, a triterpenoid from Amoora rohituka inhibits K-Ras mutant activity and STAT3 in pancreatic carcinoma cells. Tumour Biol. 2016;37:12455–12464. doi: 10.1007/s13277-016-5102-2. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous . Government of India; New Delhi: 2008. The ayurvedic pharmacopoeia of India, part I, vol. VI, first edi., department of AYUSH, ministry of health & family welfare; p. 135. [Google Scholar]
- 8.Mahajan V., Sharma N., Kumar S., Bhardwaj V., Ali A., Khajuria R.K., et al. Production of rohitukine in leaves and seeds of Dysoxylum binectariferum: an alternate renewable resource. Pharm Biol. 2015;53:446–450. doi: 10.3109/13880209.2014.923006. [DOI] [PubMed] [Google Scholar]
- 9.Sahu N., Meena S., Shukla V., Chaturvedi P., Kumar B., Datta D. Extraction , fractionation and re-fractionation of Artemisia nilagirica for anticancer activity and HPLC-ESI-QTOF-MS/MS determination. J Ethnopharmacol. 2018;213:72–80. doi: 10.1016/j.jep.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Mishra R., Sharma S., Sharma R.S., Singh S., Sardesai M.M., Sharma S., et al. Author's accepted manuscript. J Ethnopharmacol. 2018 doi: 10.1016/j.jep.2018.03.005. [DOI] [Google Scholar]
- 11.Chowdhury R., Hasan C.M., Rashid M.A. Guaiane sesquiterpenes from Amoora rohituka. Phytochemistry. 2003;62:1213–1216. doi: 10.1016/s0031-9422(02)00698-2. [DOI] [PubMed] [Google Scholar]
- 12.Rabi T., Wang L., Banerjee S. Novel triterpenoid 25-hydroxy-3-oxoolean-12-en-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-006-9275-z. [DOI] [PubMed] [Google Scholar]
- 13.Kumara P.M., Soujanya K.N., Ravikanth G., Vasudeva R., Ganeshaiah K.N., Shaanker R.U. Rohitukine, a chromone alkaloid and a precursor of flavopiridol, is produced by endophytic fungi isolated from Dysoxylum binectariferum Hook.f and Amoora rohituka (Roxb).Wight & Arn. Phytomedicine. 2014;21:541–546. doi: 10.1016/j.phymed.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Rabi T., Banerjee S. Novel semisynthetic triterpenoid AMR-Me inhibits telomerase activity in human leukemic CEM cells and exhibits in vivo antitumor activity against Dalton's lymphoma ascites tumor. Cancer Lett. 2009;278:156–163. doi: 10.1016/j.canlet.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Mohan S., Gupta D. Phytochemical analysis and differential in vitro cytotoxicity assessment of root extracts of Inula racemosa. Biomed Pharmacother. 2017;89:781–795. doi: 10.1016/j.biopha.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Heneczkowski M., Kopacz M., Nowak D., Kuzniar A. Infrared spectrum analysis of some flavonoids. Acta Pol Pharm. 2001;58:415–420. [PubMed] [Google Scholar]
- 17.Mallick N., Singh M., Parveen R., Khan W., Ahmad S., Najm M.Z., et al. HPTLC analysis of bioactivity guided anticancer enriched fraction of hydroalcoholic extract of picrorhiza kurroa. BioMed Res Int. 2015 doi: 10.1155/2015/513875. article id: 513875. 18 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh M., Singh R.K., Singh S.K., Mahto S.K., Misra N. In vitro biocompatibility analysis of functionalized poly(vinyl chloride)/layered double hydroxide nanocomposites. RSC Adv. 2018;8:40611–40620. doi: 10.1039/C8RA06175K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel D.K., Singh R.K., Singh S.K., Aswal V.K., Rana D., Ray B., et al. Graphene as a chain extender of polyurethanes for biomedical applications. RSC Adv. 2016;6:58628–58640. doi: 10.1039/C6RA12792D. [DOI] [Google Scholar]
- 20.Ghosh S., Singh R.K., Kumar Dubey V., Rangan L. Antileishmanial activity of labdane diterpenes isolated from alpinia nigra seeds. Lett Drug Des Discov. 2017;14:119–124. doi: 10.2174/1570180813666160725100300. [DOI] [Google Scholar]
- 21.Patere S.N., Pathak P.O., Kumar Shukla A., Singh R.K., Kumar Dubey V., Mehta M.J., et al. Surface-modified liposomal formulation of amphotericin B: in vitro evaluation of potential against visceral leishmaniasis. AAPS PharmSciTech. 2017;18:710–720. doi: 10.1208/s12249-016-0553-8. [DOI] [PubMed] [Google Scholar]
- 22.Alam F., Najum Q., Waheed A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Compl Altern Med. 2017:1–9. doi: 10.1186/s12906-017-1882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza M.B., Elkady A.I., Al-Attar A.M., Syed F.Q., Mohammed F.A., Hakeem K.R. Induction of apoptosis and cell cycle arrest by ethyl acetate fraction of Phoenix dactylifera L. (Ajwa dates) in prostate cancer cells. J Ethnopharmacol. 2018;218:35–44. doi: 10.1016/j.jep.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S., Prasad G.V.R.K., Mukhopadhaya A. Vibrio cholerae porin OmpU induces caspase-independent programmed cell death upon translocation to the host. Cell. 2015;290:31051–31068. doi: 10.1074/jbc.M115.670182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikseresht M., Kamali A., Rahimi H., Delaviz H., Toori M., Kashani I., et al. The hydroalcoholic extract of Matricaria chamomilla suppresses migration and invasion of human breast cancer MDA-MB-468 and MCF-7 cell lines. Pharmacognosy Res. 2017;9:87. doi: 10.4103/0974-8490.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar V., Roy B.K. Population authentication of the traditional medicinal plant Cassia tora L. based on ISSR markers and FTIR analysis. Sci Rep. 2018;8:10714. doi: 10.1038/s41598-018-29114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burman S., Bhattacharya K., Mukherjee D., Chandra G. Antibacterial efficacy of leaf extracts of Combretum album Pers. against some pathogenic bacteria. BMC Compl Altern Med. 2018;18:213. doi: 10.1186/s12906-018-2271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleem U., Hussain K., Ahmad M., Bukhari N.I., Malik A., Ahmad B. Report: physicochemical and phytochemical analysis of Euphorbia helioscopia (L.) Pak J Pharm Sci. 2014;27:577–585. [PubMed] [Google Scholar]
- 29.Pacifico S., Gallicchio M., Lorenz P., Potenza N., Galasso S., Marciano S., et al. Apolar Laurus nobilis leaf extracts induce cytotoxicity and apoptosis towards three nervous system cell lines. Food Chem Toxicol. 2013;62:628–637. doi: 10.1016/j.fct.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 30.Raina K., Tyagi A., Kumar D., Agarwal R., Agarwal C. Role of oxidative stress in cytotoxicity of grape seed extract in human bladder cancer cells. Food Chem Toxicol. 2013;61:187–195. doi: 10.1016/j.fct.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajkumar V., Guha G., Ashok Kumar R. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem Toxicol. 2011;49:363–369. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Abu Bakar M.F., Mohamad M., Rahmat A., Burr S.A., Fry J.R. Cytotoxicity, cell cycle arrest, and apoptosis in breast cancer cell lines exposed to an extract of the seed kernel of Mangifera pajang (bambangan) Food Chem Toxicol. 2010;48:1688–1697. doi: 10.1016/j.fct.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Ren M.R., Hur J.S., Kim J.Y., Park K.W., Park S.C., Seong C.N., et al. Anti-proliferative effects of Lethariella zahlbruckneri extracts in human HT-29 human colon cancer cells. Food Chem Toxicol. 2009;47:2157–2162. doi: 10.1016/j.fct.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran C., Nair P.K.R., Alamo A., Cochrane C.B., Escalon E., Melnick S.J. Anticancer effects of amooranin in human colon carcinoma cell line in vitro and in nude mice xenografts. Int J Cancer. 2006;119:2443–2454. doi: 10.1002/ijc.22174. [DOI] [PubMed] [Google Scholar]
- 35.Mohanakumara P., Sreejayan N., Priti V., Ramesha B.T., Ravikanth G., Ganeshaiah K.N., et al. Dysoxylum binectariferum Hook.f (Meliaceae), a rich source of rohitukine. Fitoterapia. 2010;81:145–148. doi: 10.1016/j.fitote.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Nile S.H., Park S.W. HPTLC analysis. Antioxidant Antigout Activ Indian Plants. 2014;13:531–539. [PMC free article] [PubMed] [Google Scholar]
- 37.Andleeb S., Naseer A., Ali S., Mustafa R.G., Zafar A., Shafique I., et al. Biological activities and secondary metabolite screening of rumex hastatus extract through fourier transform infrared and Raman spectroscopy. Infect Disord Drug Targets. 2018;18:164–176. doi: 10.2174/1871526517666170728130836. [DOI] [PubMed] [Google Scholar]
- 38.Deepika S., Harishkumar R., Dinesh M., Abarna R., Anbalagan M., Roopan S.M., et al. Photocatalytic degradation of synthetic food dye, sunset yellow FCF (FD&C yellow no. 6) by Ailanthus excelsa Roxb. possessing antioxidant and cytotoxic activity. J Photochem Photobiol B. 2017;177:44–55. doi: 10.1016/j.jphotobiol.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Tarpley W., Vitiello C. Infrared spectra of steroids. Anal Chem. 1952;24(2):315–318. doi: 10.1021/ac60062a017. [DOI] [Google Scholar]
- 40.Marion B.L., Ramsay D.A., Jones R.N., Marion L., Ramsay D.A. The infrared absorption spectra of Alkaloids1. J Am Chem Soc. 1951;73(1):305–308. doi: 10.1021/ja01145a100. [DOI] [Google Scholar]
- 41.Chan L.L., George S., Ahmad I., Gosangari S.L., Abbasi A., Cunningham B.T., et al. Cytotoxicity effects of Amoora rohituka and chittagonga on breast and pancreatic cancer cells. Evid Based Compl Alternat Med. 2011:860605. doi: 10.1155/2011/860605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramachandran C., Rabi T., Fonseca H.B., Melnick S.J., Escalon E.A. Novel plant triterpenoid drug amooranin overcomes multidrug resistance in human leukemia and colon carcinoma cell lines. Int J Cancer. 2003;105:784–789. doi: 10.1002/ijc.11180. [DOI] [PubMed] [Google Scholar]
- 43.Rabi T., Ramachandran C., Fonseca H.B., Nair R.P.K., Alamo A., Melnick S.J., et al. Novel drug amooranin induces apoptosis through caspase activity in human breast carcinoma cell lines. Breast Cancer Res Treat. 2003;80:321–330. doi: 10.1023/A:1024911925623. [DOI] [PubMed] [Google Scholar]
- 44.Ohoka N., Nagai K., Shibata N., Hattori T., Nara H., Cho N., et al. 2017. And sensitizes cancer cells to Bortezomib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shubin A.V., Demidyuk I.V., Komissarov A.A., Rafieva L.M., Kostrov S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget. 2016 Aug 23;7(34):55863–55889. doi: 10.18632/oncotarget.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar N., Biswas S., Mathew A.E., Varghese S., Mathew J.E., Nandakumar K., et al. Pro-apoptotic and cytotoxic effects of enriched fraction of Elytranthe parasitica ( L .) Danser against HepG2 Hepatocellular carcinoma. BMC Compl Altern Med. 2016:1–11. doi: 10.1186/s12906-016-1395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S., Sharma V.K., Yadav S., Dey S. Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem Cent J. 2017:1–10. doi: 10.1186/s13065-017-0281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha D., Dutta K., Ganguly K.K., Biswas J., Bishayee A., Metastasis T., et al. HHS Public Access. 2016;54:654–667. doi: 10.1002/mc.22136.A. [DOI] [Google Scholar]