Abstract
Background
Arsenic is an environmental contaminant of global concern. Consumption of ground water contaminated with inorganic arsenic (iAs) continues to be the major source of its exposure. The developing nervous system is especially vulnerable to environmental insults due to its higher rate of oxygen consumption and provision of weaker antioxidant (AOX) machinery.
Objective
Since oxidative stress has been reported as one of the major factors underlying iAs induced toxicity, the aim of the present study is to study the effect of two AOXs i.e., Alpha Lipoic Acid (ALA) and Curcumin (Cur) in developing cerebellum of rats exposed to arsenic during postnatal period.
Materials and Methods
The study was carried out on mother reared neonatal rat pups grouped as normal (Ia) and sham (vehicle) controls (Ib,c,d), while the experimental groups IIa/ IIb received sodium arsenite (NaAsO2) [(1.5/2.5 mg/kg body weight (bw)] alone or along with ALA (70 mg/kg bw)- IIIa/ IIIb or along with Cur (150 mg/kg bw)- IVa/ IVb. Behavioural, biochemical and immunohistochemical procedures were carried out to understand the underlying mechanisms.
Results
The observations indicated deficits in locomotor function, accumulation of iAs, increased levels of oxidative stress markers along with downregulation of the expression of proteins closely associated with synaptic functioning (Synaptophysin and Postsynaptic density protein95) in the cerebellum of iAs treated animals. Substantial recovery in all these parameters was observed in AOX co-treated groups.
Conclusion
Our results support the potential of ALA and Cur in amelioration of iAs induced developmental neurotoxicity. ALA and Cur can be proposed as dietary adjuvants amongst populations inhabiting areas with high iAs contamination as a safe and cost effective antidotes.
Keywords: curcumin, alpha lipoic acid, synaptophysin, PSD95
1. Introduction
The developmental processes in the nervous system are highly susceptible to disruption by various environmental contaminants even at doses that may not be toxic to mature systems [1]. Accordingly, the pre-natal and early postnatal periods have been identified as the critical periods in nervous system development. In humans, extensive development of brain occurs during the period of brain growth spurt (third trimester of pregnancy to early infancy) and the developing nervous system is highly vulnerable to environmental influences during this period. In laboratory animals, such as the rat, the brain growth spurt occurs primarily during the first 2–3 weeks after birth, with a peak around postnatal day (PND) 6–8 [2].
Cerebellum, an integral component of hindbrain, is involved in the control of posture, balance and fine coordination of motor movements. It is one of the initial regions of the brain to undergo differentiation and one of the last to attain maturity. This protracted developmental period makes it highly vulnerable to exogenous and endogenous insults, thereby targeting rapidly ongoing complex processes such as neuronal proliferation, their migration, myelination and synaptogenesis [3]. Thus, exposure to toxic substances during this vulnerable period could have an adverse effect on cytogenetics, morphogenesis and synaptic connectivity [4]. Also, cerebellum is an easy target for harmful environmental agents owing to its propensity towards oxidative stress, based on provision of relatively poor antioxidant (AOX) machinery and redox–active transition metal ions [5].
Arsenic (As) is a metalloid and ranks first in the USEPA (United States Environmental Protection Agency) list of prioritized pollutants [6]. Inorganic arsenic (iAs) induced toxicity has been described on a vast scale, with approximately 140 million people suffering from it in 70 countries across the globe [7]. Consumption of iAs contaminated groundwater continues to be the chief source of iAs exposure. Exposure to iAs via consumption of poultry, sea-food, rice and rice products (the later being major dietary supplements amongst infants) has been described [8]. Also, the pregnant women are often exposed to high levels of As due to its omnipresence in the environment. Thus, early postnatal exposure to iAs could influence various neurodevelopmental processes and subsequently result in functional deficits in later life [9]. The paucity of data pertaining to various effects induced by iAs exposure during the vulnerable periods of development raises special concern, though iAs induced neurotoxic effects have largely been reported in adult models [10], [11], [12].
Oxidative stress has been identified as one of the major mechanisms underlying iAs induced toxicity [13] and few studies have suggested the role of various AOXs in combating iAs induced adverse effects [11], [12]. Among the phytochemicals, the availability of exogenously administered alpha lipoic acid (ALA) and Curcumin (Cur) is ensured by their ability to cross blood brain barrier (BBB) [14], [15]. In the present study, the role of ALA and Cur was evaluated on neurobehavioral, biochemical and synaptic alterations in cerebellum of rats exposed to sodium arsenite (NaAsO2) from PND 1–21 (critical window period of cerebellar development).
2. Material and methods
2.1. Ethical approval
The ethical clearance for the study was obtained from the Institute Ethical Animal Committee (IEAC 594/11). Pregnant Wistar rats (gestation day 18–19), obtained from the Experimental Animal Facility (EAF) were housed in temperature (20°C–24 °C) and humidity (50–60%) controlled rooms with 12 h light/dark cycle and fed on standard rodent diet (Ashirwad Industries, India) with ad libitum access to drinking water. The study was carried out in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
2.2. Experimental design and treatments
The animals were checked for delivery status daily at 10 AM and 4 PM and the day of delivery of pups was considered as PND 0. Animals from each litter were assigned to different groups so that no two animals from the same litter belonged to the same group and average litter size was 6 animals/lactating mother. The protocol for evaluation of ameliorative role of test substance in toxicity model was adopted from previously reported literature [16], [17]. The mother reared pups were divided as normal controls receiving no treatment (Ia) and the sham controls (Ib, Ic, Id) receiving sterile water, ethanol (Merck 1009830511) and Di methylsulfoxide (DMSO) (Sigma Aldrich D5879) (vehicles for NaAsO2, ALA and Cur) respectively. The pups belonging to experimental groups IIa/IIb received only NaAsO2 (Sigma Aldrich 71287) (1.5/2.5 mg/kg body weight (bw) whereas pups in groups IIIa/IIIb and IVa/IVb received 1.5/2.5 mg/kg bw NaAsO2 along with ALA (Sigma Aldrich 62320) (70 mg/kg bw) and Cur (Sigma Aldrich C1386) (150 mg/kg bw) respectively (Fig. 1). As the study design involved intervention from PND 1, the animals were randomly assigned to different groups irrespective of sex with each group having mixed population (sex determination based on anogenital distance though reported, continues to be illusive at the time of birth) [18].
Fig. 1.
Grouping of animals based on the exposure pattern.
LD50 of NaAsO2 in adult Wistar rats when administered intraperitoneally (i.p.) has been reported as 15.86 mg/kg bw [19]. In the present study, the dose of NaAsO2 (1.5 and 2.5 mg/kg bw) approximately equivalent to 9.5 and 16% of its LD50 was used. Similar doses for evaluation of iAs induced toxicity have been used in previous studies [19], [20], [21], [22], [23], [24]. The doses of ALA (70 mg/kg bw) and Cur (150 mg/kg bw) were adopted from reported literature [22], [25]. To the best of our knowledge, there are no reported toxic effects for these AOXs at these dose levels which make them suitable candidates for trial use in toxicological studies [11], [12]. The AOXs (ALA and cur) were administered half an hour later to NaAsO2 [26]. The i.p. route was chosen to ensure rapidity in uptake and uniformity in exposure to the test substance(s) that were administered on daily basis (once a day from PND 1–21).
2.3. Rota-rod test
To investigate the status of motor coordination, postural balance and cerebellar dependent learning in control and experimental animals, automated rota-rod test (TSE Systems, Germany) was carried out (n = 6/group). To familiarize the animals to the procedure, four practice sessions, with the rod moving at a fixed speed of 5 rotations per minute (rpm) for 5 min each were carried out on PND 19. For test trials, rats were placed on the rod and the initial rpm adjusted at 5 was steadily increased to 25 rpm over a period of 300 s. The animals were tested for 3 trials per day for three consecutive days (PND 20, 21, 22) representing day 1, 2 and 3 respectively, with 1 h inter-trial interval to avoid fatigue. The time spent by the animal in balancing itself on the rotating rod before falling off from the rod (latency to fall) was recorded as detected by the photo-beam sensor [27].
2.4. Tissue collection
The animals were either perfusion fixed (4% paraformaldehyde) or sacrificed by cervical dislocation on PND 22. The cerebella obtained from perfusion fixed animals (n = 6/group) were processed for immunohistochemical localization of synaptic proteins. The freshly (unfixed) obtained cerebella (n = 6/group/technique) were either snap frozen (−80 °C) and processed for estimation of oxidative stress marker levels, synaptic protein levels (Western Blotting) or digested for analysis of tissue iAs levels (Atomic absorption spectrophotometer- AAS).
2.5. Tissue arsenic level
The tissue samples were weighed and subjected to acid (15 mL of 50% HNO3) digestion (Aurora Biomed Microwave Digester). The final volume of the digested sample was made upto 50 mL (Mili Q) and analyzed by Atomic Absorption Spectrophotometer (AA 7000, Lab India) with working range between 0.1 and 0.8 absorbance for estimation of iAs levels. The values were expressed in μg/gm of wet tissue weight [28].
2.6. Reduced glutathione (GSH) and malondialdehyde (MDA) levels
GSH level was estimated by the method of Ellman [29]. In brief, 150 μL of the tissue homogenate (10% w/v in 0.3 M Na2HPO4) and 150 μL of 5% TCA (trichloro-acetic acid) were centrifuged (5000 rpm for 10 min at 4 °C) and 100 μL of the supernatant was added to the cuvette with 4 mL of 0.3 M Na2HPO4; 400 μL of 5% TCA and 500 μL of Ellman's reagent (DTNB -5, 5′-dithiobis 2-nitrobenzoic acid). Immediately after addition of DTNB, the absorbance was read at 412 nm (Biomate 3S Spectrophotometer, Thermo Scientific) against a reagent blank. A standard curve was plotted using known concentrations of GSH and the tissue GSH level was expressed as μg/gm of tissue [30].
MDA, a by-product of LPO and Thiobarbituric acid reactive substance (TBARS) was estimated by the method of Ohkawa et al. [31]. Briefly, a mixture of 500 μL of tissue homogenate; 50 μL of 8.1% SDS (sodium dodycyl sulfate); 1500 μL of 20% acetic acid in aqueous solution (v/v) pH 3.5; 1500 μL of 0.8% thiobarbituric acid; and 700 μL of distilled water was vortexed, the reaction being carried out in a hot water bath (80–90 °C) for 1 h. After cooling and further centrifugation at 4000 rpm for 10 min, the pink staining in the supernatant was read at 532 nm (Biomate 3S Spectrophotometer, Thermo Scientific). A calibration curve was generated using 1,1,3,3-tetramethoxypropane as a standard and the calculated MDA level was expressed as nMol/gm of tissue.
2.7. Immunohistochemistry
Cryocut (HS 525, Microm GmbH, Germany) sagittal sections (30 μm) of the cerebellum were processed for standardized free floating imunohistochemical technique [24]. Specific monoclonal antibodies: Synaptophysin (Syp: SantaCruz Biotechnology. sc9116) and Post Synaptic Density marker (PSD 95: Pierce Biotechnology, Inc. MA1045) in dilutions of 1:200 were used for primary incubation whereas Ultravision Plus Detection system kit (Thermo Scientific TP-060-HLX) was used as secondary antibody. 3′,3’ diaminobenzidine (DAB) was used as chromogen for visualizing immunoreactivity. Negative and positive controls were processed simultaneously by carrying out incubation with specific normal serum instead of primary antibody for the former and staining of hippocampus for the later.
2.8. Western Blotting
The standardized protocol [24] was adopted for Western Blotting. Specific primary antibodies- Syp (Santa Cruz Biotechnology. sc9116); PSD95 (Pierce Biotechnology, Inc. MA1045) and loading control (GAPDH & β actin; Bioss, USA) in dilutions of 1:1000 were used for primary incubation followed by overnight incubation with secondary antibody (Goat anti mouse sc 2005 or Goat anti rabbit sc 2004, SantaCruz Biotechnology; dilution- 1:2000). Bands were visualized using DAB. The blots were scanned for densitometric analysis using Quantity 1 software of Gel Documentation System (Bio-Rad, USA). The result was expressed as change in terms of control as follows: where, ODPE, LE, PC, LC referred to OD of experimental group, loading control in experimental group; control group; loading control in control group respectively.
2.9. Statistical analysis
One Way ANOVA followed by Newman Keuls posthoc test was applied for statistical analysis using Graphpad prism 6. p value < 0.05 was considered significant. As the data among the normal controls and the sham controls did not show significant difference, the values of corresponding sham controls were considered for comparison.
3. Results
3.1. Rota-rod testing
A significant (p < 0.05) impairment in the motor coordination, evident by quicker fall of the animals from the rotating rod, was observed in iAs alone treated groups, the performance being significantly impaired on day one and two of the trial in group IIb as compared to the controls. An overall improvement in the performance was noted across the AOX (ALA, Cur) supplemented groups on day two and three of the trial. The motor co-ordination of the AOX (ALA, Cur) co-treated groups increased significantly (p < 0.05) from day 1 to day 3 as compared to the iAs alone treated groups, the increase being 71.44% and 99.18% in the IIIa and IIIb and 67.87% and 94.31% in IVa and IVb as compared to IIa and IIb respectively (Fig. 2).
Fig. 2.
Time spent on the rotating rod (rota-rod apparatus) by the control (I) and the experimental (IIa, b; IIIa, b; IVa, b) animals on day 1 (PND 20), 2 (PND 21) & 3 (PND 22) of trial. Significant at p < 0.05 values × compared to I, @ compared to IIa and # compared to IIb respectively. Note: Improved performance was evidenced by increased time spent by the animals receiving antioxidants (ALA or Cur) with NaAsO2 on the rotating rod.
3.2. Cerebellar arsenic levels
A significant (p < 0.05) dose dependent accumulation of iAs (μg/g cerebellar tissue) was observed in animals belonging to group IIa and IIb. The cerebellar iAs levels in the animals receiving ALA with low (IIIa) and high (IIIb) dose of iAs decreased significantly by 39.26% and 29.49% respectively. The animals co-treated with Cur and iAs (IVa, IVb) presented a significant (p < 0.05) decrease by 38.64% and 39.60% in cerebellar iAs levels as compared to iAs alone treated groups (IIa, IIb). The cerebellar iAs levels were comparable among the animal groups receiving either of the two AOXs with lower dose of iAs (IIIa, IVa), whereas, in animal groups receiving either of the AOXs with higher dose of iAs (IIIb, IVb), the tissue iAs levels were comparatively (p < 0.05) lower in group co-treated with Cur as against group receiving ALA (Fig. 3).
Fig. 3.
Cerebellar iAs levels (μg/g tissue) in the control (I) and the experimental (IIa, b; IIIa, b; IVa, b) animals. Values are Mean ± SD. Significant at p < 0.05 levels × compared to I, @ compared to IIa, # compared to IIb & ˆ compared to IIIb respectively. Note: Significant decrease in iAs levels of antioxidant co-treated groups (IIIa, b; IVa, b) as compared to iAs alone (IIa, b) treated animals.
3.3. Biochemical parameters (GSH and MDA)
The cerebellar GSH levels revealed a significant (p < 0.05) dose dependent decrease (40.30%, 46.93%) in the animals exposed to iAs alone (IIa, IIb) as compared to the controls (Fig. 4A). Co-administration of ALA/Cur with iAs led to significant increase (p < 0.05) in cerebellar GSH by 17.09% and 20.51% in ALA co-treated groups (IIIa, IIIb) and 24.4% and 28.8% in Cur co-treated groups (IVa, IVb) as compared to iAs alone treated groups (IIa, IIb). A significant (p < 0.05) and dose dependent increase (36.79%, 47.19%) in cerebellar MDA levels was observed in iAs alone treated groups as compared to the controls (Fig. 4B). Simultaneous administration of ALA with iAs decreased MDA levels by 16.05% and 20.42% (IIIa, IIIb) whereas Cur co-treatment with iAs led to 15.55% and 17.91% decrease in MDA level as compared to iAs alone treated animals (IIa, IIb).
Fig. 4.
Levels of GSH (A) and MDA (B) in cerebellum of control (I) and experimental (IIa, b; IIIa, b; IVa, b) groups. Values are Mean ± SD. Significant at p < 0.05 levels × compared to I, @ & # compared to IIa & IIb respectively. Note: Significant recovery in GSH & MDA levels in the antioxidant co-treated groups.
3.4. Syp and PSD95 expression
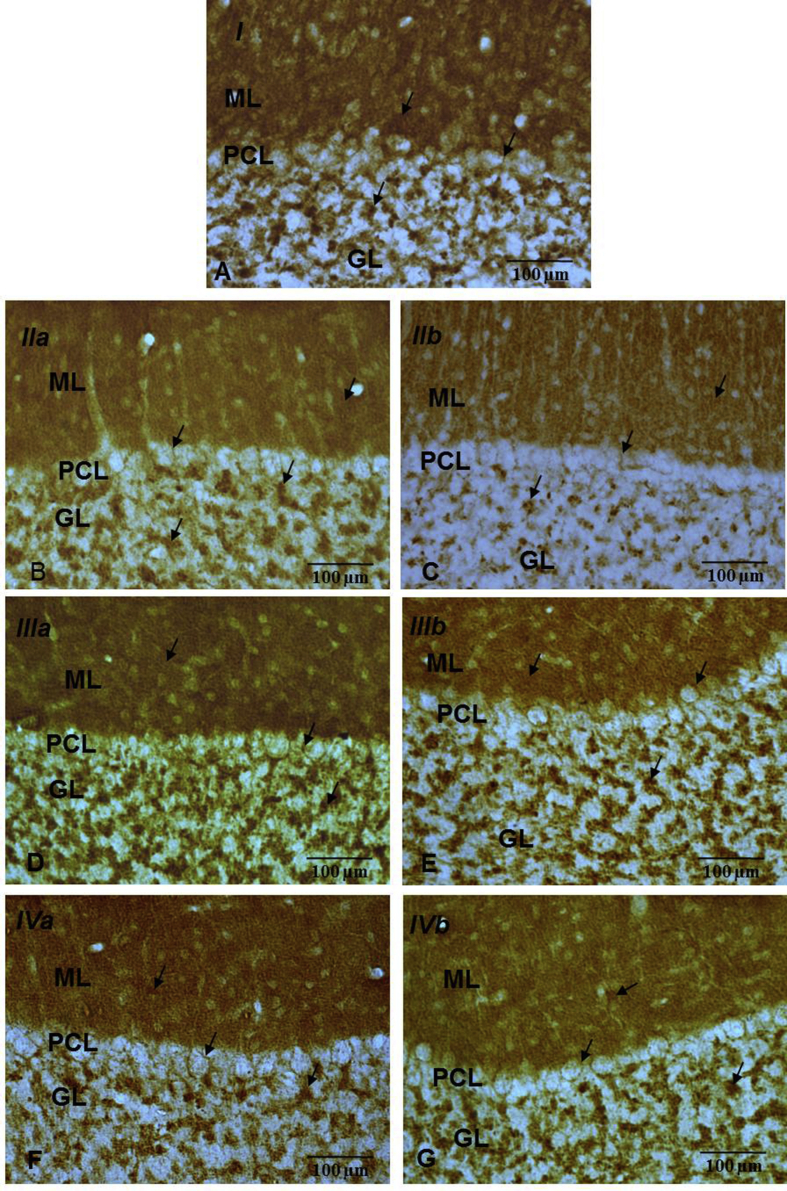
The pattern of Syp immuno expression was somewhat uniform across all the groups with intense immuno-reactivity in the molecular layer (ML) [possibly depicting synaptic zones between fibres (parallel and climbing) with Purkinje cell dendrites and interneurons and substantial immunoreactivity around the Purkinje cells (PCL) and the glomeruli in the Granule cell layer (GL). The Syp immunoexpression was less intense in iAs alone treated animals as compared to the controls as well as AOX co-treated animals (Fig. 5). These observations were substantiated by Western blot data analysis, which showed a significant (p < 0.05) dose dependent decrease (28% and 37%) in the Syp level of iAs alone treated groups (IIa, IIb). Cotreatrment of iAs and AOXs (ALA/Cur) increased the Syp levels by 23.40% and 30.76% (IIIa, IIIb) and 23.07% and 32.40% (IVa, IVb) respectively (Fig. 6).
Fig. 5.
Immuno-histochemical localization of Syp (→) in cerebellar cortical layers (ML, PCL & GL) of control (A) & experimental (B, C, D, E, F, G) groups (40X). Note: Decreased Syp immunoreactivity in B (IIa) & C (IIb) as compared to group A (I), D (IIIa), E (IIIb), F (IVa) & G (IVb).
Fig. 6.
(A) Immunoblots of Syp (cerebellum) of control (I) & the experimental (IIa, b; IIIa, b; IVa, b) animals along with loading control (β actin). (B) Bar diagram showing fold change in Syp expression in the experimental (IIa, b; IIIa, b; IVa, b) vs control group. Values are Mean ± SD. Significant at p < 0.05 levels × compared to I, @ & # compared to IIa & IIb respectively.
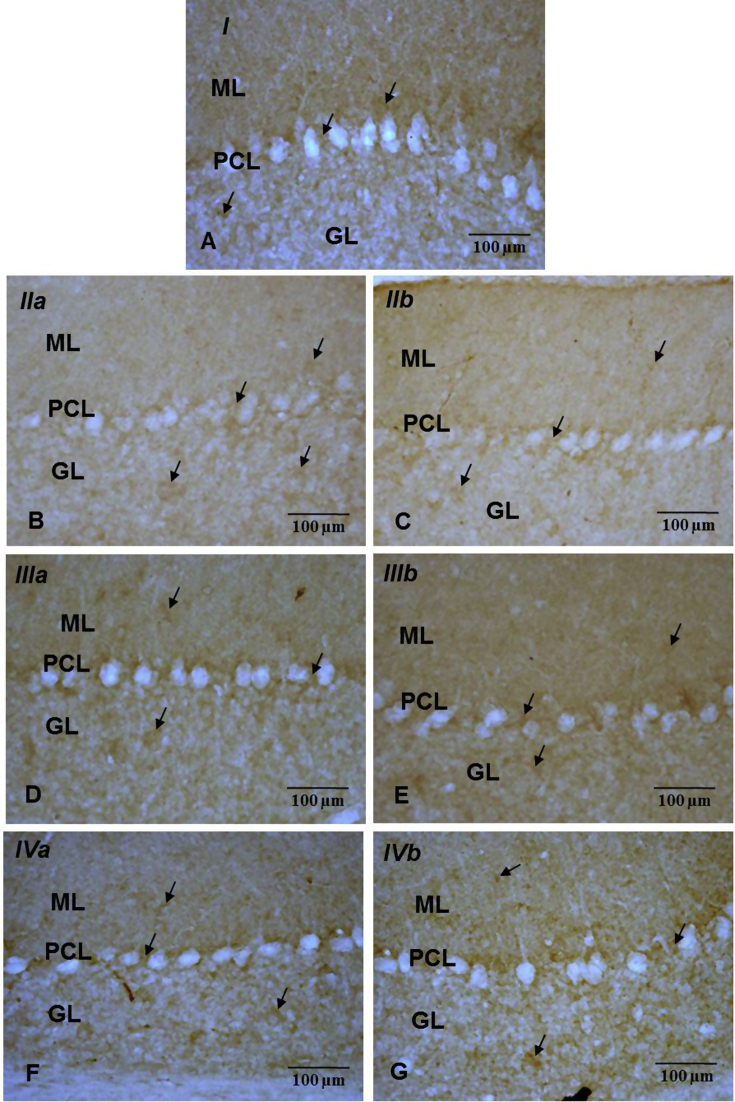
On the whole, PSD95 immunoreactivity presented a punctate appearance in the ML (possibly denoting the synapses of Purkinje cell dendrites with parallel and climbing fibres) and GCL (possibly denoting glomeruli) whereas substantial immunoreactivity was noted around the Purkinje cells. PSD95 immunoexpression was less intense in the cerebella of iAs alone treated animals as compared to the controls and animals co-treated with iAs and AOX (ALA/Cur) (Fig. 7). These results were validated by Western Blot analysis which revealed significant (p < 0.05) down-regulation (24% and 22%) in PSD95 level in iAs alone treated animals (IIa, IIb) and significant (p < 0.05) up-regulation in ALA (20% and 10.34%) and Cur (16.4% and 14.28%) co-treated groups (Fig. 8).
Fig. 7.
Immuno-histochemical localization of PSD95 (→) in ML, GCL & areas intervening between PCs of cerebellar cortex from control (A) & experimental (B, C, D, E, F, G) groups (40X). Note: Decrease in the cerebellar PSD95 immunoreactivity in B (IIa) & C (IIb) as compared to group A (I), D (IIIa), E (IIIb), F (IVa) & G (IVb).
Fig. 8.
(A) Immunoblots of PSD95 (cerebellum) of control (I) & the experimental (IIa, b; IIIa, b; IVa, b) animals along with loading control (GAPDH). (B) Bar diagram showing fold change in PSD95 expression in the experimental (IIa, b; IIIa, b; IVa, b) vs control group. Values are Mean ± SD. Significant at p < 0.05 levels × compared to I, @ & # compared to IIa & IIb respectively.
4. Discussion
The preliminary observations of the present study have demonstrated iAs induced adverse effects on behavioral, biochemical and immunohistochemical parameters associated with developing rat cerebellum. Also, evaluation of the role of exogenously administered AOXs (ALA, Cur) revealed their ameliorating effect on iAs induced neurotoxicity.
Our observations of dose dependent decrease in rota-rod performance of iAs alone exposed animals (IIa, IIb) could be suggestive of deranged motor coordination and the same is in agreement with previous reports [12], [32], [33], [34]. Although there are conflicting reports in literature of increased as well as decreased motor activity following iAs exposure in rats and mice [35], yet, the role of various factors such as dose of iAs as well as duration of exposure have been suggested as important determinants of altered response in locomotor activity. Association has been drawn between various plausible mechanisms being involved in iAs induced effects on locomotor activity. Optimal Calbindin (a vital calcium binding protein) level with in the Purkinje cells is considered an essential determinant of normal motor coordination and sensory integration [36]. iAs induced decreased expression of Calbindin could be associated with deficits in the precision of motor coordination. A number of investigators have also linked behavioral alterations with iAs induced disturbances in various neurotransmitter systems such as inhibition of cholinesterase [37] and glutamate decarboxylase [38]. Besides, iAs induced alterations in the AOX status and dopaminergic elements of the nervous system have been suggested as underlying factors in behavioral alterations [33]. In the present study, the animals co-treated with ALA/Cur along with iAs spent significantly more time on the rotating rod. Although, improvement in the motor function has been previously reported [12] following co-exposure to iAs (20, 100 mg/kg bw NaAsO2) and Cur (orally for 28 days) in adult rats, yet the present study demonstrated enhanced motor coordination of juvenile rats following exposure to iAs and Cur postnatally. The observations of the earlier reports as well as of the present study suggest the scope for recovery in iAs induced altered motor activity, provided AOXs are supplemented during the period of exposure.
Our observations of dose dependent accumulation of iAs at tissue level are in coherence with the earlier studies whereby increased tissue levels of iAs have been reported in various animal models following exposure to iAs [11], [12], [32], [33], [34], [35]. However, the variation in the tissue iAs levels, as observed in the present study and the previously reported studies, could be due to adoption of varied routes for iAs administration (i.p. v/s oral) and different durations of exposure (acute vs chronic), the difference in the age groups of the animal models (pups v/s adults) as well as due to differential affinity for iAs at tissue level.
Accumulation of iAs at tissue level (cerebellum) could be traced to iAs induced structural and functional alterations of the defensive barriers (blood brain and blood Cerebrospinal fluid barrier) of the central nervous system. Furthermore, accumulation of iAs at tissue level could be reinforced by its binding to molecules containing thiol groups such as GSH, Cysteine etc., though variation in thiol content of the tissues could be the determining factor for its differential accumulation [39]. Our data of significant dose dependent decrease in cerebellar iAs levels of animals co-treated with AOXs is in coherence with earlier reports [11], [12], [40]. AOX induced decrease in cerebellar iAs levels could be correlated with the metal chelating properties of these two AOXs. The chelating ability of ALA could be linked to its dithiol groups and the resultant decrease in the metallic load on the biological system [41]. On the other hand, the role of Cur in chelation is linked to its unique conjugated structure of two methoxylated phenols and an enol form of beta diketone whereby it is able to trap as well as donate H atoms from methylene and phenolic groups [42]. From the observations of the present study, it could be proposed that these AOXs possess the potential of decreasing the tissue iAs levels, thereby protecting the tissues from its toxic effects. However, there is the need to explore whether it is the chelating activity of AOXs alone or any other action in combination with chelation that enables these to lower tissue iAs levels.
Oxidative stress resulting from deranged balance of pro-oxidant/AOX system has been reported as the major mechanism underlying iAs induced neurotoxicity [13]. In the present study, we focused on the levels of GSH as this AOX is closely associated with the metabolism of iAs, while MDA levels were estimated to evaluate iAs induced damage to the cell membranes. We noted decrease in the levels of GSH, while MDA levels were found to be elevated in the cerebellar tissue of iAs alone treated animals, thereby indicating iAs induced depletion of AOX machinery as well as induction of lipid peroxidation (LPO) respectively. Neuronal tissue is highly vulnerable to oxidative stress with direct impact on synaptic plasticity, dendritic morphology and neurogenesis, thereby highlighting the importance of optimal levels of AOXs in the nervous system. Accumulation of iAs and its metabolites directly or indirectly promote ROS induction which in turn induces COX-2 expression through activation of the mitogen-activated protein kinase (MAPK) pathway [43]. Furthermore, compromised levels of GSH could adversely affect processes like cellular detoxification capacity, methylation of iAs and stress protein gene expression, besides rendering the cells more sensitive to cell death by Fas ligand and 12-lipooxygenase mediated apoptotic pathways [44], [45]. Also, iAs induced LPO results in loss of transmembrane potential, alteration in membrane fluidity and permeability, increase in intracellular calcium levels and inhibition of various cell membrane proteins (Na+/K-ATPases and glutamate transporters) [46]. iAs induced activation of the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) has been implicated in regulation of expression of several AOX enzymes such as NADPH, quinine oxido-reductase-1, Heme oxygenase-1 and glutathione S-transferase (GST) [47].
The significant recovery in oxidative stress of animals co-treated with ALA or Cur is in coherence with the reports put forth by earlier investigators [11], [12], [48]. The decrease in oxidative stress could either be due to direct ROS scavenging property of these AOXs or by chelating action. The neuroprotective role of ALA and Cur could be attributed to their potential of increasing the de-novo synthesis of GSH [49], [50]. The up-regulatory effects of Cur on Nrf2 and phase II detoxifying enzymes, also plays a major role in decreasing oxidative stress [51]. Furthermore, it was interesting to note substantial restoration of redox status in the groups receiving AOXs (ALA and Cur) along with higher dose of iAs as evidenced by 20.51% and 28.8% increase in GSH and 47.19% and 20.42% decrease in the MDA levels. In the groups receiving low dose of iAs along with ALA and Cur, the alteration in redox status was less substantial such as 17.09% and 24.4% increase in GSH and 36.79% and 16.05% decrease in MDA levels. This response could be linked to rigorous activation of cellular defense machinery following exposure to higher dose of iAs. It could thus be hypothesized that iAs induced decrease in GSH beyond a threshold level might initiate the feed-back activation for its further formation. Various investigators have reported iAs induced increased uptake of cystine by the cells as a secondary stress response to compensate the loss of GSH [52]. Thus, the cumulative effect of the cell's intrinsic protective mechanisms and the additive effect of exogenously administered AOX could have boosted the cellular ability to combat iAs induced oxidative stress.
Synapses, the highly specialized sites for quick signaling amongst neurons, are rapidly formed during the postnatal period. Since, Syp and PSD95 are amongst the important proteins associated with structural and functional aspects of pre and post synaptic membranes, we studied their expression in the developing rat cerebellum following exposure to iAs alone or in combination with AOXs (ALA, Cur) during the peak period of synaptogenesis.
The pattern of Syp and PSD95 immuno-expression observed in our study is similar to that reported by earlier investigators [53]. The decreased expression of Syp in iAs alone treated animals is indicative of iAs induced derangement in the neurotransmission and neuronal plasticity along with poor nerve terminal differentiation and synaptogenesis [54]. Fujimura and coworkers [55] noted decreased levels of Syp in the cerebellum of rats exposed perinatally to methyl mercury (5 ppm, orally) and associated it with motor dysfunction (Rota-rod testing), thereby reiterating the vital role of optimal Syp expression in maintenance of neurotransmission at chemical synapses. Syp is crucial in synaptic vesicle (SV) exocytosis [54] and modulation of synaptic plasticity [56]. Besides, it plays an important role in release of neurotransmitters by binding to calcium [57]. Changes in the expression of Syp have also been associated with external stimuli such as constraint stress and internal factors like deficits of neurotrophic factors [58].
Our observation of iAs induced decrease in expression of PSD95 is in congruence with the recent study of Luo and coworkers [59], who, reported decreased expression of PSD95 in hippocampus of adult rats exposed to NaAsO2 (2.72, 13.6,68 mg/l orally) for 3 months. These investigators hypothesized, iAs induced alterations in the expression of various proteins such as PSD95, NR2A, CaMKII, synaptic Ras GTPase-activating protein (SynGAP) and nuclear activated extracellular signal regulated kinase (ERK1/2) as the molecular basis of iAs induced neurotoxicity. Decreased level of PSD95 in iAs alone treated animals is of immense relevance in the present study as this protein is implicated to play a crucial role in the formation and maturation of excitatory synapses during synaptogenesis, the period corresponding to the experimental period of the present study [60]. Furthermore, PSD95 (a PDZ domain scaffold protein) being a prominent component of post synaptic structure is involved in synaptic development, neurotransmission, signal transduction and synaptic plasticity [61]. By virtue of its location and interaction with a number of other proteins including NMDA and AMPA receptors, it plays a central role in post synaptic transmission [60]. Thus, the neurochemical basis of iAs induced neurotoxicity could be linked to down-regulated expression of two vital synaptic proteins, Syp and PSD95. However, further studies focusing on the up- and down-stream molecules required for the expression of these vital proteins of synaptogenesis would be helpful to decipher various mechanisms underlying iAs induced neurotoxicity.
5. Conclusion
The major observations of our study suggest that exposure of animals to iAs during the critical window period of cerebellar development results in neurobehavioral alterations which could be associated with iAs induced decrease in levels of Synaptic proteins (Syp and PSD95) and increase in oxidative stress. The neuroprotective activity of the supplemented AOXs (ALA/Cur) could be linked to their role in up-regulation of synaptic proteins, augmentation of AOX defense system and chelation of iAs at tissue level. Hence, these AOXs can be proposed as dietary adjuvants among the populations inhabiting areas with high iAs contamination as safe and cost effective antidotes.
Source(s) of funding
This study was partially funded by Department of Science and Technology (DST INSPIRE Fellowship IF10053 to Ms. Parul Kaushal) and Department of Anatomy, AIIMS.
Conflict of interest
None
Acknowledgement
Technical support of Mr. Kirpal Singh is acknowledged.
Footnotes
Peer review under responsibility of Trans disciplinary University, Bangalore.
References
- 1.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobbing J., Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 3.Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145(4):399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- 4.Bouet V., Dijk F., Ijkema-Paassen J., Wubbels R.J., van der Want J.J., Gramsbergen A. Early hypergravity exposure effects calbindin-D28k and inositol-3-phosphate expression in Purkinje cells. Neurosci Lett. 2005;382(1, suppl 2):10–15. doi: 10.1016/j.neulet.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Kalia M. Brain development: anatomy, connectivity, adaptive plasticity, and toxicity. Metabolism Suppl. 2008;57(2):S2–S5. doi: 10.1016/j.metabol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Ng J.C., Wang J., Shraim A. A global health problem caused by arsenic from natural sources. Chemosphere. 2003;52(9):1353–1359. doi: 10.1016/S0045-6535(03)00470-3. [DOI] [PubMed] [Google Scholar]
- 7.Freeman K. Nutrient protection against arsenic toxicity: folate, cysteine support methylation in children. Environ Health Perspect. 2009;117:A10–A211. doi: 10.1289/ehp.117-a211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis M.A., Mackenzie T.A., Cottingham K.L., Gilbert-Diamond D., Punshon T., Karagas M.R. Rice consumption and urinary arsenic concentrations in U.S. Children. Environ Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vahter M. Health effects of early life exposure to arsenic. Basic Clin Pharmacol Toxicol. 2007;102:204–211. doi: 10.1111/j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 10.Flora S.J.S. Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin Exp Pharmacol Physiol. 1999;26(11):865–869. doi: 10.1046/j.1440-1681.1999.03157.x. [DOI] [PubMed] [Google Scholar]
- 11.Shila S., Kokilavani V., Subathra M., Panneerselvam C. Brain regional responses in antioxidant system to alpha-lipoic acid in arsenic intoxicated rat. Toxicology. 2005;210(1):25–36. doi: 10.1016/j.tox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Yadav R.S., Sankhwar M.L., Shukla R.K., Chandra R., Pant A.B., Islam F. Attenuation of arsenic neurotoxicity by Curcumin in rats. Toxicol Appl Pharmacol. 2009;240(3):367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka K., Hayashi H., Tachikawa M., Kato K., Hasegawa A., Oku N. Metabolic methylation is a possible genotoxicity-enhancing process of inorganic arsenics. Mutat Res. 1997;394(1–3):95–101. doi: 10.1016/s1383-5718(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 14.Seaton T.A., Jenner P., Marsden C.D. The isomers of thioctic acid alter C-deoxyglucose incorporation in rat basal ganglia. Biochem Pharmacol. 1996;51(7):983–986. doi: 10.1016/0006-2952(95)02250-3. [DOI] [PubMed] [Google Scholar]
- 15.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 16.Mittal M., Flora S.J.S. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem Toxicol. 2007;30(3):263–281. doi: 10.1080/01480540701380075. [DOI] [PubMed] [Google Scholar]
- 17.Saha S.S., Ghosh M. Comparative study of antioxidant activity of alpha-eleostearic acid and punicic acid against oxidative stress generated by sodium arsenite. Drug Chem Toxicol. 2009;47(10):2551–2556. doi: 10.1016/j.fct.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Faber K.A., Hughes C.L. Anogenital distance at birth as a predictor of volume of the sexually dimorphic nucleus of the preoptic area of the hypothalamus and pituitary responsiveness in castrated adult rats. Biol Reprod. 1992;46(1):101–104. doi: 10.1095/biolreprod46.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Maiti S., Chatterjee A.K. Effects on levels of glutathione and some related enzymes in tissues after an acute arsenic exposure in rats and their relationship to dietary protein deficiency. Arch Toxicol. 2001;75(9):531–537. doi: 10.1007/s002040100240. [DOI] [PubMed] [Google Scholar]
- 20.Dhar P., Mohari N., Mehra R.D. Preliminary morphological and morphometric study of rat cerebellum following sodium arsenite exposure during rapid brain growth (RBG) period. Toxicology. 2007;234(1–2):10–20. doi: 10.1016/j.tox.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Dhar P., Somesh M.S., Kaushal P., Sehgal R., Mehra R.D. Effects of arsenic exposure during early postnatal period on leydig cells of rat testis. Toxicol Environ Chem. 2010;92(6):1157–1168. [Google Scholar]
- 22.Dixit S., Dhar P., Mehra R.D. Protective role of exogenous α-lipoic acid (ALA) on hippocampal antioxidant status and memory function in rat pups exposed to sodium arsenite during the early post-natal period. Toxicol Mech Methods. 2011;21(3):216–224. doi: 10.3109/15376516.2010.538751. [DOI] [PubMed] [Google Scholar]
- 23.Kaushal P., Dhar P., Shivaprasad S.M., Mehra R.D. Postnatal exposure to sodium arsenite (NaAsO2) induces long lasting effects in rat testes. Toxicol Int. 2012;19(2):215–222. doi: 10.4103/0971-6580.97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushal P., Mehra R.D., Dhar P. Curcumin induced up-regulation of Myelin basic protein (MBP) ameliorates sodium arsenite induced neurotoxicity in developing rat cerebellum. J Anat Soc India. 2014;63(1):3–11. [Google Scholar]
- 25.Gupta Y.K., Briyal S., Sharma M. Protective effect of curcumin against kainic acid induced seizures and oxidative stress in rats. Indian J Physiol Pharmacol. 2009;53(1):39–46. [PubMed] [Google Scholar]
- 26.Neves R.N.P., Carvalho F., Carvalho M., Fernandes E., Soares E., de Bastos M.L. Protective activity of hesperidin and lipoic acid against sodium arsenite acute toxicity in mice. Toxicol Pathol. 2004;32(5):527–535. doi: 10.1080/01926230490502566. [DOI] [PubMed] [Google Scholar]
- 27.Catre C.D., Lopes M.F., Cabrita A.S. Lasting developmental effects of neonatal fentanyl exposure in preweanling rats. Anesthesiol Res Pract. 2012;2012:10. doi: 10.1155/2012/180124. Article ID 180124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaiswal A.K., Kaushal P., Dhar P., Millo T., Murty O.P. Microwave digestor and its forensic application - a review. J Forensic Med Toxicol. 2011;28(1):65–74. [Google Scholar]
- 29.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 30.Pari L., Latha M. Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipid peroxidation in STZ diabetic male wistar rats. BMC Complement Altern Med. 2004;2:4–16. doi: 10.1186/1472-6882-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez V.M., Carrizales L., Jiménez-Capdeville M.E., Dufour L., Giordano M. The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull. 2001;55(2):301–308. doi: 10.1016/s0361-9230(01)00477-4. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez V.M., Limón-Pacheco J.H., Carrizales L., Mendoza-Trejo M.S., Giordano M. Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol Teratol. 2010;32(6):640–647. doi: 10.1016/j.ntt.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Markowski V.P., Reeve E.A., Onos K., Assadollahzadeh M., McKay N. Effects of prenatal exposure to sodium arsenite on motor and food-motivated behaviors from birth to adulthood in C57BL6/J mice. Neurotoxicol Teratol. 2012;34(2):221–231. doi: 10.1016/j.ntt.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh T., Zhang Y.F., Murai S., Saito H., Nagahama H., Miyate H. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol Lett. 1990;54(2–3):345–353. doi: 10.1016/0378-4274(90)90202-w. [DOI] [PubMed] [Google Scholar]
- 36.Barski J.J., Hartmann J., Rose C.R., Hoebeek F., Mörl K., Noll-Hussong M. Calbindin in cerebellar Purkinje cells is a critical determinant of the precision of motor coordination. J Neurosci. 2003;23(8):469–477. doi: 10.1523/JNEUROSCI.23-08-03469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patlolla A.K., Tchounwou P.B. Cytogenetic evaluation of arsenic trioxide toxicity in Sprague-Dawley rats. Mutat Res. 2005;587(1–2):126–133. doi: 10.1016/j.mrgentox.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Xi S., Guo L., Qi R., Sun W., Jin Y., Sun G. Prenatal and early life arsenic exposure induced oxidative damage and altered activities and mRNA expressions of neurotransmitter metabolic enzymes in offspring rat brain. J Biochem Mol Biol. 2010;24(6):368–378. doi: 10.1002/jbt.20349. [DOI] [PubMed] [Google Scholar]
- 39.Aposhian H.V., Aposhian M.M. Newer developments in arsenic toxicity. Int J Toxicol. 1989;8:1297–1305. [Google Scholar]
- 40.Dwivedi N., Flora G., Kushwaha P., Flora S.J.S. Alpha-lipoic acid protects oxidative stress, changes in cholinergic system and tissue histopathology during co-exposure to arsenic-dichlorvos in rats. Environ Toxicol Pharmacol. 2014;37(1):7–23. doi: 10.1016/j.etap.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Rochette L., Ghibu S., Richard C., Zeller M., Cottin Y., Vergely C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol Nutr Food Res. 2013;57(1):114–125. doi: 10.1002/mnfr.201200608. [DOI] [PubMed] [Google Scholar]
- 42.Manikandan P., Sumitra M., Aishwarya S., Manohar B.M., Lokanadam B., Puvanakrishnan R. Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int J Biochem Cell Biol. 2004;36(10):1967–1980. doi: 10.1016/j.biocel.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Xi S., Xu Y., Wang F., Zheng Y., Li B. Sodium arsenite induces cyclooxygenase-2 expression in human uroepithelial cells through mapk pathway activation and reactive oxygen species induction. Toxicol In Vitro. 2013;27(3):1043–1048. doi: 10.1016/j.tiv.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Anderson C.P., Tsai J.M., Meek W.E., Liu R.M., Tang Y., Forman H.J. Depletion of glutathione by buthionine sulfoxine is cytotoxic for human neuroblastoma cell lines via apoptosis. Exp Cell Res. 1999;246(1):183–192. doi: 10.1006/excr.1998.4303. [DOI] [PubMed] [Google Scholar]
- 45.Higuchi Y., Yoshimoto T. Arachidonic acid converts the glutathione depletion-induced apoptosis to necrosis by promoting lipid peroxidation and reducing caspase-3 activity in rat glioma cells. Arch Biochem Biophy. 2002;400(1):133–140. doi: 10.1006/abbi.2002.2784. [DOI] [PubMed] [Google Scholar]
- 46.Mattson M.P. Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci. 1998;21(2):53–57. doi: 10.1016/s0166-2236(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 47.Li B., Li X., Zhu B., Zhang X., Wang Y., Xu Y. Sodium arsenite induced reactive oxygen species generation, nuclear factor (erythroid-2 related) factor 2 activation, heme oxygenase-1 expression, and glutathione elevation in Chang human hepatocytes. Environ Toxicol. 2013;28(7):401–410. doi: 10.1002/tox.20731. [DOI] [PubMed] [Google Scholar]
- 48.El Demerdash F.M., Yousef M.I., Radwan F.M.E. Ameliorating effect of Curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009;47(1):249–254. doi: 10.1016/j.fct.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Han D., Sen C.K., Roy S., Kobayashi M.S., Tritschler H.J., Packer L. Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am J Physiol. 1997;273(5 Pt 2):R1771–R1778. doi: 10.1152/ajpregu.1997.273.5.R1771. [DOI] [PubMed] [Google Scholar]
- 50.Biswas S.K., McClure D., Jimenez L.A., Megson I.L., Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7(1–2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 51.Gao S., Duan X., Wang X., Dong D., Liu D., Li X. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol. 2013;59:739–747. doi: 10.1016/j.fct.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 52.Deneke S.M. Induction of cystine transport in bovine pulmonary artery endothelial cells by sodium arsenite. Biochim Biophys Acta. 1992;1109(2):127–131. doi: 10.1016/0005-2736(92)90075-w. [DOI] [PubMed] [Google Scholar]
- 53.Castejón O.J., Fuller L., Dailey M.E. Localization of synapsin-I and PSD-95 in developing postnatal rat cerebellar cortex. Brain Res Dev Brain Res. 2004;151(1–2):25–32. doi: 10.1016/j.devbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Bergmann M., Schuster T., Grabs D., Marquèze-Pouey B., Betz H., Traurig H. Synaptophysin and synaptoporin expression in the developing rat olfactory system. Brain Res Dev Brain Res. 1993;74(2):235–244. doi: 10.1016/0165-3806(93)90009-y. [DOI] [PubMed] [Google Scholar]
- 55.Fujimura M., Cheng J., Zhao W. Perinatal exposure to low-dose methylmercury induces dysfunction of motor coordination with decreases in synaptophysin expression in the cerebellar granule cells of rats. Brain Res. 2012;29(1464):1–7. doi: 10.1016/j.brainres.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Sanyal T., Kumar V., Nag T.C., Jain S., Sreenivas V., Wadhwa S. Prenatal loud music and noise: differential impact on physiological arousal, hippocampal synaptogenesis and spatial behavior in one day-old chicks. PLoS One. 2013;8(7):e67347. doi: 10.1371/journal.pone.0067347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehm H., Wiedenmann B., Betz H. Molecular characterization of synaptophysin, a major calcium-binding protein of the synaptic vesicle membrane. EMBO. 1986;5(3):535–541. doi: 10.1002/j.1460-2075.1986.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hisatsune C., Kuroda Y., Akagi T., Torashima T., Hirai H., Hashikawa T. Inositol 1,4,5-trisphosphate receptor type 1 in granule cells, not in Purkinje cells, regulates the dendritic morphology of Purkinje cells through brain-derived neurotrophic factor production. J Neurosci. 2006;26(42):10916–10924. doi: 10.1523/JNEUROSCI.3269-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo J.H., Qiu Z.Q., Zhang L., Shu W.Q. Arsenite exposure altered the expression of NMDA receptor and postsynaptic signaling proteins in rat hippocampus. Toxicol Lett. 2012;211(1):39–44. doi: 10.1016/j.toxlet.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Losi G., Prybylowski K., Fu Z., Luo J., Wenthold R.J., Vicini S. PSD-95 regulates NMDA receptors in developing cerebellar granule neurons of the rat. J Physiol. 2003;548(Pt 1):21–29. doi: 10.1113/jphysiol.2002.034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El-Husseini A.E., Schnell E., Chetkovich D.M., Nicoll R.A., Bredt D.S. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;17(290,5495):1364–1368. [PubMed] [Google Scholar]