Abstract
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a facultative intracellular pathogen that causes salmonellosis and mortality worldwide. S. Typhimurium infects macrophages and survives within phagosomes by avoiding the phagosome-lysosome fusion system. Phagosomes sequentially acquire different Rab GTPases during maturation and eventually fuse with acidic lysosomes. Lysophosphatidylcholine (LPC) is a bioactive lipid that is associated with the generation of chemoattractants and reactive oxygen species (ROS). In our previous study, LPC controlled the intracellular growth of Mycobacterium tuberculosis by promoting phagosome maturation. In this study, to verify whether LPC enhances phagosome maturation and regulates the intracellular growth of S. Typhimurium, macrophages were infected with S. Typhimurium. LPC decreased the intracellular bacterial burden, but it did not induce cytotoxicity in S. Typhimurium-infected cells. In addition, combined administration of LPC and antibiotic significantly reduced the bacterial burden in the spleen and the liver. The ratios of the colocalization of intracellular S. Typhimurium with phagosome maturation markers, such as early endosome antigen 1 (EEA1) and lysosome-associated membrane protein 1 (LAMP-1), were significantly increased in LPC-treated cells. The expression level of cleaved cathepsin D was rapidly increased in LPC-treated cells during S. Typhimurium infection. Treatment with LPC enhanced ROS production, but it did not affect nitric oxide production in S. Typhimurium-infected cells. LPC also rapidly triggered the phosphorylation of IκBα during S. Typhimurium infection. These results suggest that LPC can improve phagosome maturation via ROS-induced activation of NF-κB pathway and thus may be developed as a therapeutic agent to control S. Typhimurium growth.
Keywords: bactericidal activity, lysophosphatidylcholine, macrophage, phagosome maturation, reactive oxygen species, Salmonella Typhimurium
INTRODUCTION
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a gram-negative facultative intracellular pathogen that causes salmonellosis and septic shock (Johnson et al., 2018). Salmonella can infect and proliferate within phagocytic and nonphagocytic host cells. Following infection, S. Typhimurium is recognized by pattern-recognition receptors (PRRs) that activate the NF-κB or MAPK signaling pathways to induce the expression of inflammatory cytokines, chemokines, and inducible nitric oxide synthase (NOS2), which encodes the enzyme that is responsible for producing the potent antibacterial molecule nitric oxide (NO) (Broz et al., 2012; Dougan et al., 2011). Furthermore, macrophage recognition of S. Typhimurium leads to the activation of NADPH oxidase in the phagosomal membrane and the production of reactive oxygen species (ROS), which are critical for innate defense (Bae et al., 2011; Cho et al., 2020; Rhen, 2019; Wong et al., 2009). This activation results in the control of bacterial growth.
S. Typhimurium is also transferred from microfold cells (M cells) to macrophages and eliminated by phagocytosis (Monack, 2013; van Asten et al., 2005). However, recent studies have shown that S. Typhimurium enters macrophages via phagocytosis and forms a special membrane structure to avoid fusion with lysosomes and survive in the host cell. This unique membrane structure is called the Salmonella-containing vacuole (SCV) (Bakowski et al., 2008; Garcia-del Portillo et al., 2008). SCVs protect S. Typhimurium from host immunity and alter virulence to effectively survive within host cells. Because of the formation of these structures, S. Typhimurium has various mechanisms to avoid the host immune system, including lysosomal degradation and the inhibition of phagosome maturation (Wick, 2011). The biogenesis of SCVs occurs through sequential interactions with the endocytic pathway (Steele-Mortimer, 2008).
The phagocytic process involves interactions with endosomes and lysosomes and changes the distribution of proteins that interact with various Rab proteins belonging to the Ras superfamily of small GTPases (Hutagalung and Novick, 2011; Prashar et al., 2017). During phagosome maturation, the early phagosome is formed through the interactions of PtdIns3P (phosphatidylinositol-3-phosphate), EEA1 (early endosome antigen 1) and SNARE with Rab5 (Levin et al., 2016). Early phagosomes also become slightly acidic and recruit Rab7 to the phagosomal membrane (Vieira et al., 2003). The late phagosome expresses Rab7 and LAMP-1 (lysosome-associated membrane protein 1) and becomes more acidic via v-ATPase activity, promoting fusion with the lysosome (Huynh et al., 2007). Phagolysosomes eliminate microorganisms by using lysosomal degradative enzymes, including various cathepsins, proteases and hydrolases. Although phagocytosis removes intracellular bacteria, some intracellular bacteria have diverse strategies to survive in host cells, including the regulation of phagosome maturation or the host environment (Uribe-Querol and Rosales, 2017).
Lysophosphatidylcholine (LPC) is an oxidized low-density lipoprotein that can stimulate monocytes, macrophages, T lymphocytes, smooth muscle cells, and neutrophils (Hong and Song, 2008). It has been suggested that LPC plays a role in various biological processes, including T lymphocyte and macrophage chemotaxis, antibody formation by B lymphocytes and the bactericidal activity of neutrophils (Kabarowski, 2009). LPC protects mice from cecal ligation and puncture (CLP)-induced sepsis and enhances bacterial clearance by neutrophils (Yan et al., 2004). In addition, a recent study showed that LPC controls intracellular M. tuberculosis growth by modulating phagosome maturation in mouse macrophages (Lee et al., 2018). However, it has not been established whether LPC has bactericidal activity to inhibit bacterial growth during S. Typhimurium infection. In this study, we investigated whether LPC controls intracellular bacterial growth by regulating phagosome maturation in mouse macrophages during S. Typhimurium infection, similar to M. tuberculosis infection. Our study demonstrated that LPC suppressed intracellular bacterial growth by accelerating phagosome maturation in S. Typhimurium-infected cells, suggesting that LPC may be a potential candidate for controlling S. Typhimurium.
MATERIALS AND METHODS
Reagents
LPC (1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine [18:0]) was purchased from Avanti Polar Lipids, Inc. (USA). The other reagents used were 2′,7′-dichlorofluorescein diacetate (DCF-DA; Calbiochem, USA), apocynin (Sigma-Aldrich, USA), bay11-7082 (Cayman Chemical, USA), streptomycin (Sigma-Aldrich), and cephalexin (Sigma-Aldrich).
Cell culture
The mouse macrophage cell line Raw264.7 was purchased from American Type Culture Collection (ATCC, USA). Raw264.7 cells were maintained in RPMI 1640 (Cellgro, USA) culture medium containing 10% fetal bovine serum (FBS; Lonza, USA) and penicillin/streptomycin (Gibco-BRL, USA) at 37°C with 5% CO2.
Bacterial strain and culture conditions
For this study, we used Salmonella enterica serovar Typhimurium strain ATCC 14028 or SL1344. S. Typhimurium was inoculated from frozen stocks onto LB agar (Difco™ LB Broth, Miller; BD Bioscience, USA) and incubated for 24 h at 37°C. A total of 1-3 colonies were inoculated in 5 ml of LB broth containing 10 g/L tryptone (BD Bioscience), 5 g/L yeast extract (USB, USA), and 5 g/L sodium chloride (Bio Basic Inc., Canada) and incubated at 37°C with vigorous shaking until an optical density at 600 nm (OD600) of 1 was reached. After culture, S. Typhimurium was used for further analysis.
S. Typhimurium infection in vitro
Raw264.7 cells were seeded at 1 × 104 cells/well on 6-well cell culture plates (SPL Lifesciences, Korea) and incubated for 2 days at 37°C with 5% CO2. The cells were washed twice with phosphate-buffered saline (PBS; Gibco Life Technologies, USA) and mixed with S. Typhimurium at an multiplicity of infection (MOI) of 1 or 10. The plates were centrifuged at 500g for 5 min and incubated for 30 min at 37°C with 5% CO2. After 30 min, the cells were washed twice with PBS and treated with 30 μM or 60 μM LPC in the presence of 60 μg/ml gentamicin, which removes extracellular bacteria.
S. Typhimurium infection in vivo
Six-week-old male BALB/c mice were used in this study and handled according to a protocol approved by the Institutional Animal Care and Use Committee of Kangwon National University (KIACUC) (No. KW-130613-1). The mice were inoculated orally with PBS or S. Typhimurium strain SL1344 (1 × 108 CFU/mouse). After 2 h, the mice were injected subcutaneously with LPC (20 mg/kg) or PBS in the presence or absence of the antibiotics cephalexin (1 mg/kg, oral injection) every 12 h for 3 days. Then, mouse survival was monitored daily for 10 days. For bacterial enumeration, tissues (liver and spleen) were collected from infected mice at 5 days post-infection (dpi). Tissues were homogenized, diluted and plated onto LB agar plates supplemented with 100 μg/ml streptomycin. LB agar plates were incubated overnight at 37°C. Colonies were counted and normalized to the weight of the tissue to obtain CFU/g.
Colony-forming unit (CFU) assay
A total of 1 × 104 cells were infected with S. Typhimurium at an MOI of 1 or 10 for 30 min and treated with LPC for the indicated times. The cells were washed twice with PBS and lysed with 200 μl of PBS containing 0.1% Triton X-100, and the number of intracellular bacteria was determined by calculating the CFU.
Cell viability assay
Raw264.7 cells were seeded at 1 × 104 cells/well on a 6-well cell culture plate and cultured for 24 h at 37°C with 5% CO2. The cells were washed twice with PBS and infected with S. Typhimurium at an MOI of 10 for 30 min. Then, the cells were washed with PBS and treated with LPC for the indicated times in the presence of gentamicin. The number of viable cells was counted using the trypan blue dye exclusion method.
Total RNA isolation and reverse transcription polymerase chain reaction (RT-PCR)
Raw264.7 cells were infected with S. Typhimurium at an MOI of 10 and then treated with LPC for 1 h. Then, the cells were washed with PBS and harvested. Total RNA extraction was performed as previously described (Lee et al., 2015). The oligonucleotide primers used were 5′-TTCTGTCTACTGAACTTCGGGGTAATCGGTCC-3′ (sense) and 5′-GTATGAGATAGCAATCGGCTGACGGTGTGGG-3′ (antisense) for tumor necrosis factor alpha (TNF-α), 5′-GACCCAGATGCAGGAAAGGAA-3′ (sense) and 5′-TCATGGTGCACAGCAAAGTGAT-3′ (antisense) for NOX2 (NADPH oxidase), and 5′-AGGCTGTGCTGTCCCTGTATGC-3′ (sense) and 5′- ACCCAAGAAGGAAGGCTGGAAA-3′ (antisense) for actin. The amplified PCR products were analyzed with a 1.5% agarose gel and visualized under UV light after ethidium bromide staining.
Western blot analysis
SDS-PAGE and western blot analysis were performed as previously described (Woo et al., 2018). Briefly, Raw264.7 cells were infected with S. Typhimurium and treated with LPC for the indicated times. The cells were then harvested and lysed with RIPA buffer in the presence of a protease inhibitor cocktail. Each sample was incubated for 40 min on ice and collected by centrifugation at 14,000 rpm for 40 min at 4°C. Equivalent amounts of protein were resolved by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, USA). For the western blot analysis, anti-MEK1/2, anti-phospho-MEK1/2, anti-phospho-ERK, anti-ERK, anti-phospho-SAPK/JNK, anti-SAPK/JNK, anti-Rab5, anti-Rab7, and anti-Cathepsin D were purchased from Cell Signaling Technologies (USA). Anti-phospho-IκBα and α-tubulin were purchased from Santa Cruz Biotechnology (USA). The target proteins were detected by chemiluminescence using an enhanced ECL detection system (Advansta, USA). α-Tubulin was used to verify that the proteins were equally loaded.
Immunofluorescence analysis
Immunofluorescence assays were performed as previously described (Lee et al., 2016a). Briefly, to detect markers associated with phagosome maturation, the cells were seeded on coverslips in 12-well plates and infected with FITC-labeled S. Typhimurium at an MOI of 10 for 30 min. Then, extracellular bacteria were washed away with PBS, and the cells were cultured in the presence or absence of LPC without antibiotics for the indicated times. The cells were washed with PBS and fixed with PBS containing 4% paraformaldehyde for 15 min at room temperature. Then, the cells were permeabilized with PBS containing 1% Triton X-100 for 10 min and incubated with anti-EEA1-Alexa Fluor® 647 (MBL, USA) or Alexa Fluor® 647 anti-mouse CD107a LAMP-1 (BioLegend, USA) for 2 h at room temperature. The coverslips were mounted onto slides with Fluoromount-GTM mounting medium (Southern Biotech, USA) and observed by using confocal microscopy (FV1000 SPD; Olympus, Japan). To quantify phagosome maturation, a total of 3-5 fields containing more than 50 cells were randomly selected. The percentage of phagosome markers that colocalized with S. Typhimurium was normalized to the total number of cells.
To quantify colocalization, the number of total cells, FITC-labeled S. Typhimurium puncta (green puncta) and FITC-labeled S. Typhimurium+ + EEA1+ double-positive puncta (yellow puncta) was counted in images. The number of FITC-labeled S. Typhimurium+ + EEA1+ double positive puncta (yellow puncta) was divided by the number of FITC-labeled S. Typhimurium puncta (green puncta). The number of FITC-labeled S. Typhimurium-positive puncta was divided by the number of total cells. The percentage of colocalization was calculated as (the number of FITC-labeled S. Typhimurium+ + EEA1+ double positive puncta/the number of total cells)/(the number of FITC-labeled S. Typhimurium-positive puncta/the number total cells). To quantify the accumulation of EEA1 or LAMP-1, the number of EEA1- or LAMP-1-positive puncta and total cells counted, and then these values were normalized to the number of total cells. The ratio of EEA1 or LAMP-1 accumulation obtained from uninfected cells was set as 1. All experiments were based on three independent samples and repeated three times.
Measurement of intracellular ROS production
Intracellular ROS production was assessed using 2′,7′-dichlorofluorescein diacetate (DCF-DA). Raw264.7 cells were infected with S. Typhimurium in the presence or absence of LPC for the indicated times. Then, the cells were treated with DCF-DA for 20 min at 37°C with 5% CO2, washed with PBS and fixed with PBS containing 4% paraformaldehyde. The coverslips were mounted onto slides with Fluoromount-GTM mounting medium and observed by using confocal microscopy. To quantify intracellular ROS production, images of cells expressing DCF fluorescence were randomly selected in 3-5 fields and analyzed using ImageJ software (National Institutes of Health, USA). The level of DCF fluorescence was normalized to the DCF fluorescence intensity in control cells. All experiments were based on three independent samples and were repeated three times.
NO detection assay
The NO detection assay was previously described (Lee et al., 2019) and performed using an NO detection kit according to the manufacturer’s instructions (iNtRON Biotechnology, Korea). In brief, culture supernatants were mixed with sulfanilamide in reaction buffer (N1 buffer) for 10 min, and then naphthylethylenediamine in stabilizer buffer (N2 buffer) was added to the mixture and incubated for 10 min. The absorbance value at 540 nm was measured using a microplate reader. NO production was calculated using a standard curve with a nitrite standard solution.
Statistical analysis
All experiments were based on three independent samples and were repeated three times. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, USA). The significance of differences between groups was tested with Student’s t-test and one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. A P value < 0.05 (*P < 0.05; **P < 0.01; ***P < 0.001; and ns, not significant [P > 0.05]) was considered a significant difference.
RESULTS
Suppression of intracellular bacterial growth by LPC treatment of S. Typhimurium-infected mouse macrophages
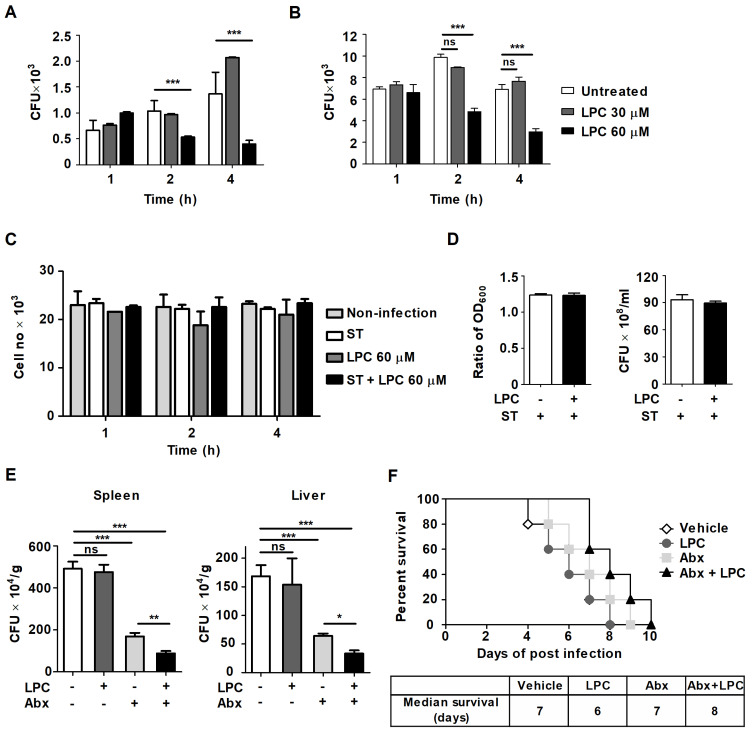
Our previous report showed that LPC enhances phagosome maturation and suppresses proinflammatory cytokine production, suggesting that LPC plays a role in controlling intracellular M. tuberculosis growth without excessive inflammation (Lee et al., 2018). In addition, a recent study showed that LPC increases Escherichia coli-killing activity in neutrophils via the enhanced fusion of azurophil granule-phagosomes (Hong et al., 2010). Therefore, we first examined the effect of LPC on intracellular S. Typhimurium growth in mouse macrophages. LPC treatment significantly decreased intracellular bacterial growth in S. Typhimurium-infected Raw264.7 cells at an MOI of 1 or 10 compared with that in untreated S. Typhimurium-infected cells (Figs. 1A and 1B). As LPC exhibits cytotoxicity to macrophages by enhancing ROS production (Park et al., 2009), we determined whether LPC directly affected the growth of bacteria or host macrophages and that it did not show toxicity to S. Typhimurium-infected mouse macrophages or S. Typhimurium (Figs. 1C and 1D). To demonstrate the possibility as a therapeutic adjuvant for Salmonella treatment, mice were inoculated orally with PBS or S. Typhimurium (1 × 108 CFU/mouse) and then injected subcutaneously (s.c.) with LPC or PBS in the presence or absence of antibiotics cephalexin (1 mg/kg) which is a 1st generation cephalosporin antibiotics and is effective against β-lactamase enzyme producing strains, every 12 h for 3 days. Combined administration of LPC and the antibiotic, cephalexin, significantly improved the bacterial clearance in spleen and liver compared with treatment of cephalexin alone (Fig. 1E). Combined administration of LPC and cephalexin slightly increased mouse survival compared with LPC-injected or cephalexin-injected mice (Fig. 1F), indicating that combined administration of LPC and antibiotics could improve the therapeutic effect than single antibiotics treatment. Taken together, these results suggest that LPC controls the intracellular growth of S. Typhimurium in mouse macrophages without host cell damage and has the potential to be developed as a therapeutic adjuvant for S. Typhimurium treatment.
Fig. 1. LPC controls the intracellular growth of S. Typhimurium in mouse macrophages.
Raw264.7 cells were infected with S. Typhimurium (ST) at an MOI of 1 (A) or 10 (B) and then treated with LPC for the indicated times. (A and B) Intracellular bacterial growth was determined by CFU assays. (C) The cytotoxic effect of LPC on Raw264.7 cells was assessed using a trypan blue exclusion assay. (D) The cultures were grown in LB broth in the presence or absence of LPC for 4 h. Left panel: bacterial growth was assessed by the OD600. Right panel: CFUs in LB agar plates were determined. (E and F) Mice were inoculated orally with PBS or S. Typhimurium (SL1344 strain, 1 × 108 CFU/mouse) and then injected s.c. with LPC (20 mg/kg) or PBS in the presence or absence of the antibiotic cephalexin (Abx, 1 mg/kg) every 12 h for 3 days. (E) Bacterial burdens carried at 5 dpi in liver and spleen (3 mice per group). Data are expressed as the mean ± SD (n = 3). (F) Mouse survival was monitored daily for 10 days (n = 3 in uninfected groups, n = 5 in SL1344-infected groups). Median survival time was determined using Prism5 software. The data in (A-E) were analyzed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons; *P < 0.05, **P < 0.01, ***P < 0.001 and not significant (ns) (P > 0.05).
LPC promotes phagosome maturation in S. Typhimurium-infected mouse macrophages
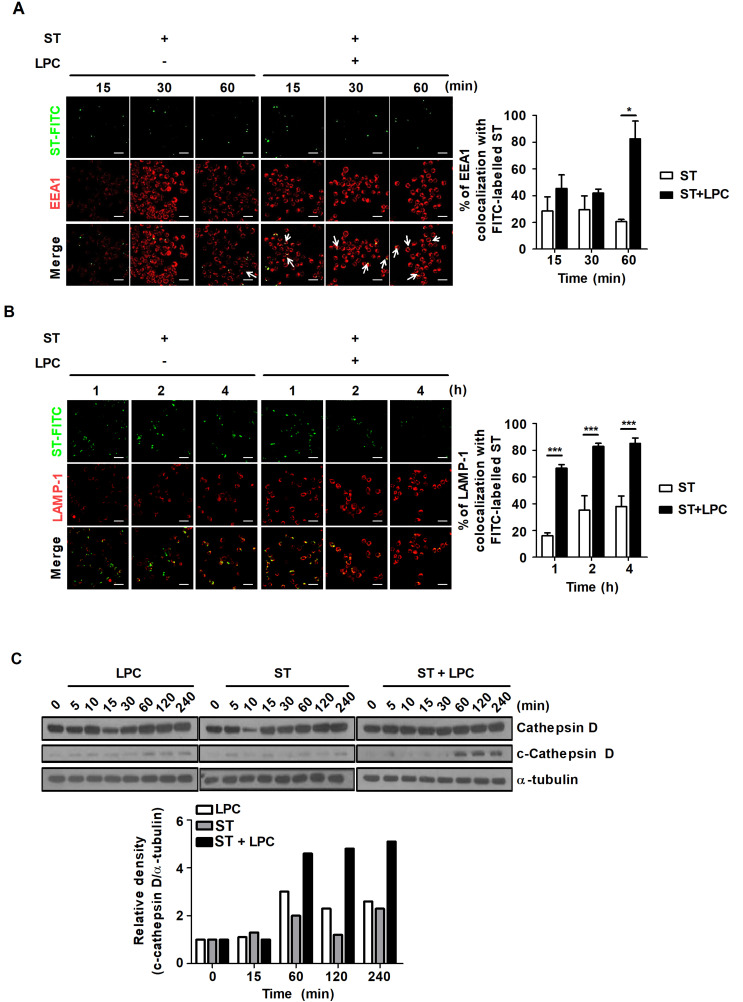
During maturation, phagosomes sequentially acquire or lose new proteins according to the ‘kiss and run’ hypothesis (Desjardins, 1995). Briefly, the formation of early phagosomes involves Rab5, which cooperates with EEA1, which functions as tethering proteins. Rab5 and EEA1 are rapidly converted to Rab7 and LAMP-1, which leads to the formation of the late phagosome and phagolysosome progression (Pauwels et al., 2017). S. Typhimurium can generate a replicate niche known as the SCV to avoid fusion with lysosomes in host cells. Because of the various strategies to avoid host immune responses, it is important to discover a novel drug that effectively targets the SCV (Brumell et al., 2002; Kamaruzzaman et al., 2017). It is also necessary to identify the mechanisms by which SCV-targeting drugs control bacterial growth. Thus, we sought to determine how LPC regulates intracellular bacterial growth in S. Typhimurium-infected mouse macrophages. The percentage of EEA1 that colocalized with FITC-labeled S. Typhimurium increased approximately 4-fold in S. Typhimurium-infected cells treated with LPC compared to that of cells infected with S. Typhimurium without LPC treatment (Fig. 2A). In addition, LPC treatment contributed to the increased percentage of FITC-labeled S. Typhimurium that colocalized with the late phagosome marker LAMP-1 compared to that of untreated S. Typhimurium-infected cells (Fig. 2B).
Fig. 2. LPC augments phagosome maturation in S. Typhimurium-infected cells.
Raw264.7 cells were infected with FITC-labeled S. Typhimurium (ST) at an MOI of 10 and then treated with LPC for the indicated times. Cells were fixed and stained with anti-EEA1-Alexa647 (A) or anti-LAMP-1-Alexa647 (B). Colocalization of FITC-labeled S. Typhimurium and each marker was observed under confocal microscopy. Bar graph represents the percentage of EEA1+ (A) or LAMP-1+ (B) phagosomes containing FITC-labeled S. Typhimurium. Scale bars = 100 μm. (C) Western blot analysis was performed on total cell lysates using antibodies for the indicated proteins. Bar graph represents the densitometric quantification of bands corresponding to cleaved cathepsin D normalized to total cathepsin D protein. Data are presented as the mean ± SD (n = 3) and were analyzed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons; *P < 0.05, ***P < 0.001 and not significant (ns) (P > 0.05).
Cathepsin D is a lysosomal aspartyl protease that functions in the endocytic pathway and contributes to killing intracellular pathogens (Pires et al., 2016). During phagosome maturation, cathepsin D is trafficked to late phagosomes and proteolytically activated at an acidic pH (pH 4.5-5.0) (Benes et al., 2008). Thus, cleaved cathepsin D can be used as an indirect marker of phagosome maturation. To further demonstrate that LPC promotes phagosome maturation during S. Typhimurium infection in macrophages, we performed a western blot assay to detect the level of cleaved cathespin D. The level of cleaved cathepsin D clearly increased at 1 h after LPC treatment in S. Typhimurium-infected cells (Fig. 2C). These results suggest that LPC promotes phagosome maturation during S. Typhimurium infection in macrophages.
LPC rapidly induces the phosphorylation of IκBα and enhances the level of intracellular ROS in S. Typhimurium-infected mouse macrophages
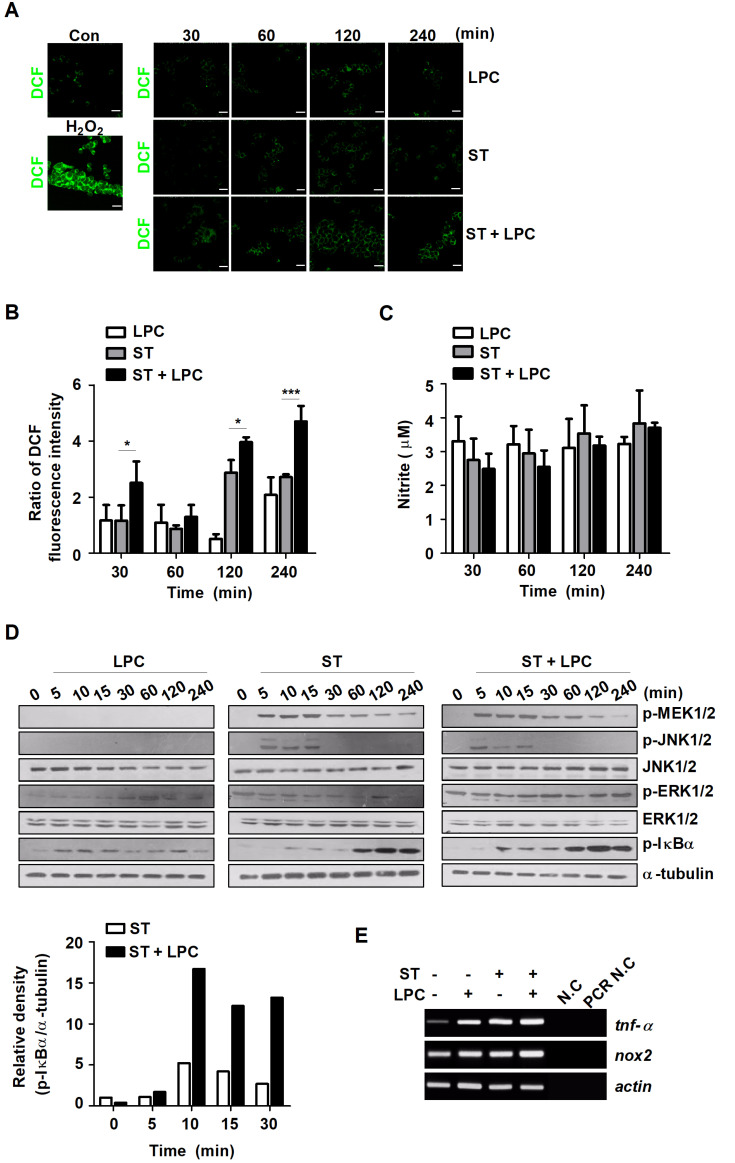
Our previous study showed that LPC controls intracellular M. tuberculosis by increasing intracellular ROS levels that enhance NO production and phagosome maturation in mouse macrophages (Lee et al., 2018). Consistent with this, we investigated whether LPC enhances the production of NO and ROS during S. Typhimurium infection in mouse macrophages. Treatment with LPC increased intracellular ROS levels from early (30 min) to late (4 h) time points after S. Typhimurium infection (Figs. 3A and 3B). However, NO production did not change in S. Typhimurium-infected cells in the presence or absence of LPC (Fig. 3C). Next, we sought to explore the intracellular pathway involved in LPC-mediated control of S. Typhimurium. The level of phosphorylated IκBα was significantly increased at 10 min after LPC treatment in S. Typhimurium-infected cells (Fig. 3D). The expression levels of TNF and NOX2 (NADPH oxidase 2) also slightly enhanced in LPC-treated cells during S. Typhimurium infection (Fig. 3E). These results suggest that LPC accelerates intracellular ROS production and the activation of the NF-κB signaling pathway in S. Typhimurium-infected macrophages.
Fig. 3. LPC rapidly increases intracellular ROS levels and NF-κB activation in S. Typhimurium-infected cells.
Raw264.7 cells were infected with S. Typhimurium (ST) at an MOI of 10 and then treated with LPC for the indicated times. (A) Intracellular ROS levels were measured using dichlorofluorescein (DCF) under confocal microscopy. Scale bars = 100 μm. (B) Bar graph represents the relative levels of intracellular ROS calculated from the fluorescence intensity. (C) NO production was determined in the culture supernatants at the indicated time points. Data are represented as the mean ± SD (n = 3), and the experiments were performed in triplicate. Statistical significance was analyzed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons (*P < 0.05 and ***P < 0.001). (D) Western blot analysis was performed on total cell lysates using antibodies for the indicated proteins. Bar graph represents the densitometric quantification of bands corresponding to phospho- IκBα normalized to α-tubulin. (E) The mRNA levels of TNF-α, NOX2 and actin were determined by RT-PCR. N.C, negative control; PCR N.C, PCR negative control.
LPC activates the NF-κB signaling pathway by inducing intracellular ROS production in S. Typhimurium-infected macrophages
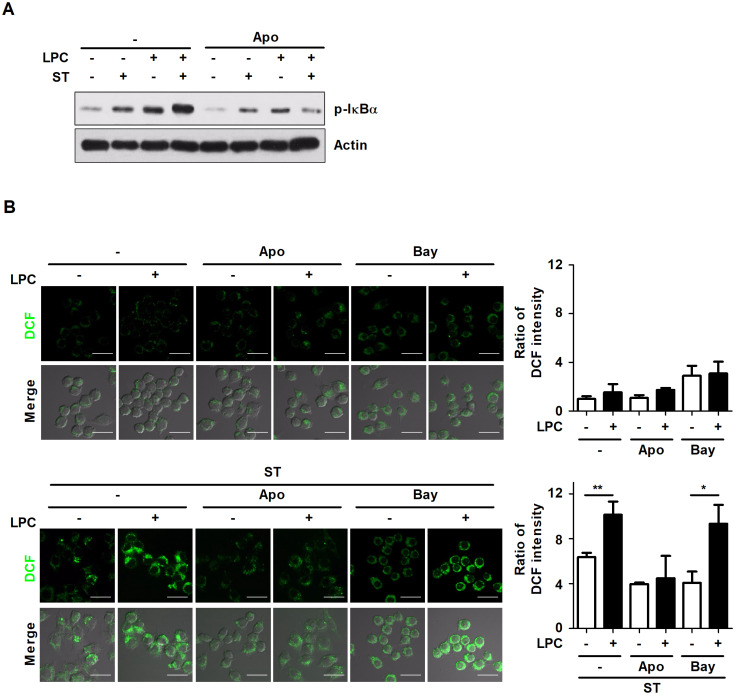
A recent study showed that the activation of NF-κB enhances phagocytic activity and cytokine production (Jeon et al., 2013). Based on our results that LPC induces an increase in intracellular ROS production and NF-κB activation, we determined whether LPC induces intracellular ROS production via NF-κB activation or NF-κB activation via intracellular ROS production during S. Typhimurium infection in macrophages. To investigate whether intracellular ROS production induces NF-κB activation, Raw264.7 cells were pretreated with apocynin, an NADPH oxidase inhibitor. Inhibition of ROS production using apocynin reduced the phosphorylation of IκBα, which was increased in LPC-treated cells during S. Typhimurium infection (Fig. 4A). Next, to investigate whether the NF-κB pathway affects intracellular ROS production in LPC-treated cells during S. Typhimurium infection, Raw264.7 cells were pretreated with bay11-7082, an inhibitor of NF-κB. Treatment with LPC enhanced the level of intracellular ROS in S. Typhimurium-infected cells; however, blockade of NF-κB using bay11-7082 failed to suppress intracellular ROS production in LPC-treated cells during S. Typhimurium infection (Fig. 4B). Therefore, these results suggest that LPC enhances NF-κB activation by inducing intracellular ROS production during S. Typhimurium infection.
Fig. 4. LPC activates the NF-κB signaling pathway by increasing intracellular ROS production in S. Typhimurium-infected cells.
(A) Raw264.7 cells were pretreated with apocynin (Apo) for 1 h and infected with S. Typhimurium (ST) at an MOI of 10 in the presence or absence of LPC for 30 min. Western blot assays were performed using whole cell lysates to analyze the indicated proteins. (B) Raw264.7 cells were pretreated with apocynin (Apo) or bay11-7082 (Bay) for 1 h and infected with S. Typhimurium (ST) at an MOI of 10 in the presence or absence of LPC for 30 min. Intracellular ROS levels were measured using dichlorofluorescein (DCF) under confocal microscopy. Scale bars = 20 μm. Bar graph represents the relative levels of intracellular ROS calculated from fluorescence intensity. Data are represented as the mean ± SD (n = 3), and the experiments were performed in triplicate. Statistical significance was analyzed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons (*P < 0.05 and **P < 0.01).
LPC controls the intracellular growth of S. Typhimurium by promoting phagosome maturation via ROS-induced NF-κB activation
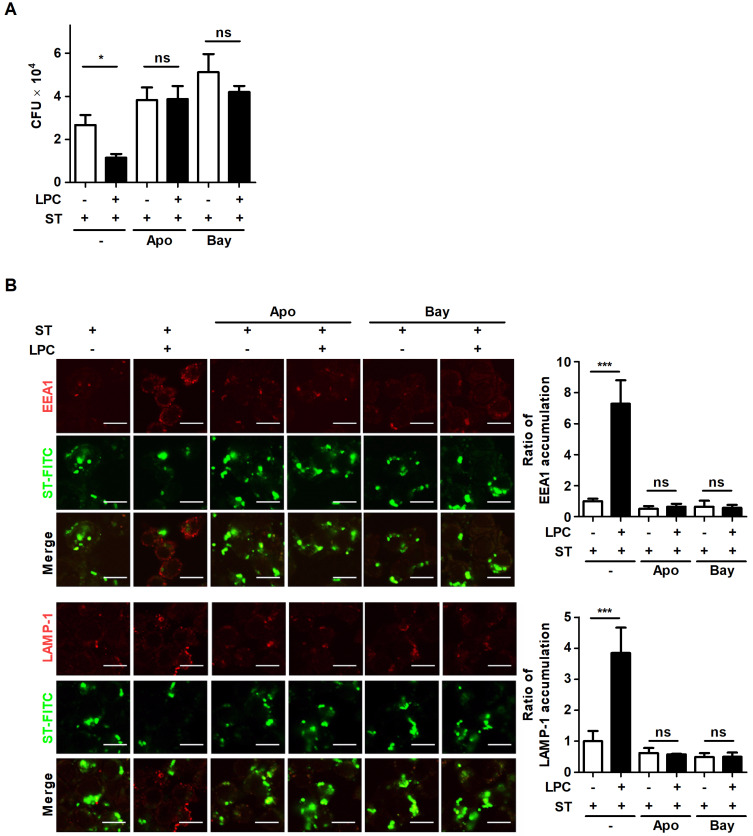
According to the results that LPC activates the NF-κB pathway through the increased level of intracellular ROS in macrophages, the intracellular growth of S. Typhimurium was measured in cells that were pretreated with inhibitors of NF-κB or NADPH oxidase. Treatment with LPC caused an approximately 2-fold decrease in the intracellular growth of S. Typhimurium (Fig. 5A). However, inhibition of ROS production or the NF-κB pathway restored intracellular S. Typhimurium growth, even though the cells were treated with LPC (Fig. 5A). Treatment with LPC rapidly facilitated the accumulation of EEA1 or LAMP-1, while this accumulation was significantly diminished in cells that were pretreated with apocynin or bay11-7082 (Fig. 5B). Consistent with the decreased intracellular growth of S. Typhimurium shown in Fig. 5A, the intensity of FITC-labeled S. Typhimurium declined in LPC-treated cells compared with that in untreated S. Typhimurium-infected cells (Fig. 5B). These data reveal that LPC accelerates phagosome maturation through activation of the NF-κB pathway by upregulating intracellular ROS production, and thus LPC controls intracellular S. Typhimurium growth in mouse macrophages.
Fig. 5. LPC controls the intracellular growth of S. Typhimurium through the enhanced activation of the NF-κB signaling pathway by upregulating intracellular ROS production.
(A) Raw264.7 cells were pretreated with apocynin (Apo) or bay11-7082 (Bay) for 1 h and infected with S. Typhimurium (ST) at an MOI of 10 in the presence or absence of LPC. Intracellular bacterial growth was determined by CFU assay at 2 h after LPC treatment. (B) Raw264.7 cells were pretreated with apocynin (Apo) or bay11-7082 (Bay) for 1 h and infected with FITC-labeled S. Typhimurium at an MOI of 10 in the presence or absence of LPC. Cells were fixed and stained with anti-EEA1-Alexa647 (top panel) or anti-LAMP-1-Alexa647 (bottom panel). Accumulation of each marker was observed under confocal microscopy. Scale bars = 10 μm. Bar graph represents the ratio of EEA1+ or LAMP-1+ accumulation. Data are represented as the mean ± SD (n = 3), and the experiments were performed in triplicate. Statistical significance was analyzed with one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons; *P < 0.05, ***P < 0.001 and not significant (ns) (P > 0.05).
DISCUSSION
S. Typhimurium is an intracellular pathogen that causes typhoid-like disease in mice (Johnson et al., 2018). This pathogen can survive and replicate within host cells, including macrophages, dendritic cells and M cells, which contributes to the systemic spread this organism (Haraga et al., 2008). When S. Typhimurium passes through the intestinal epithelium and reaches macrophages, the macrophages internalize S. Typhimurium via phagocytosis and control the intracellular growth of S. Typhimurium through phagosome maturation. Phagosome maturation is a homeostatic mechanism that removes intracellular bacteria and apoptotic cells (Lee et al., 2020). Phagosomes contain target substances that enter host cells through phagocytosis and undergo a series of maturation processes. Phagosomes acquire membrane molecules involved in maturation by interacting with the compartments of the endocytic pathway. The early-to-late phagosome transition is mediated by the conversion of two small GTPases, Rab5 and Rab7, and various molecules (Bohdanowicz and Grinstein, 2010). Rab5-expressing early phagosomes induce the recruitment of EEA1, which acts as a functional link between Rab proteins and SNARE to trigger membrane fusion (Simonsen et al., 1999). During phagosome maturation, Rab5 is converted to Rab7, which induces the recruitment of RILP, a Rab7 effector protein (Harrison et al., 2003). Rab7-expressing late phagosomes are ultimately converted to phagolysosomes by fusion with lysosomes expressing LAMP-1 and LAMP-2 (Huynh et al., 2007). Phagolysosomes trigger phagosome acidification through the activity of v-ATPase, resulting in the inactivation or degradation of target molecules by lysosomal enzymes, such as cathepsin D and various hydrolases (Flannagan et al., 2012). However, S. Typhimurium has various immune evasion strategies, including the recognition or activation of PRRs (Wong et al., 2009), regulation of host cell death (Wemyss and Pearson, 2019) and inhibition of phagolysosome formation, which is the final step of phagosome maturation (Bernal-Bayard and Ramos-Morales, 2018; Buchmeier and Heffron, 1991). In particular, to hamper the fusion of S. Typhimurium-containing phagosomes and lysosomes, S. Typhimurium constantly recruits the early phagosome markers Rab5 and EEA1 to S. Typhimurium-containing vacuoles (SCVs) to prevent the accumulation of Rab7, LAMP-1 and v-ATPase, which characterizes late phagosomes (Smith et al., 2007). Brumell et al. (2002) showed that intracellular replication of S. Typhimurium was increased in cells that expressed a constitutively active mutant of Rab5 or a dominant-negative mutant of Rab7, suggesting that SCVs maintain the characteristics of early phagosomes to allow S. Typhimurium to survive and replicate within macrophages. We found that LPC enhanced the accumulation of the early and late phagosome markers EEA1 and LAMP-1, respectively, in S. Typhimurium-containing phagosomes. In addition, we confirmed that the protein level of activated cathepsin D (cleaved cathepsin D) was markedly enhanced in LPC-treated macrophages during S. Typhimurium infection. Previous studies have shown that the conversion of Rab5 to Rab7 or the expression of these proteins regulates phagosome maturation (Meresse et al., 1999; Rink et al., 2005). Therefore, we confirmed that the expression of Rab5 and Rab7 was affected by LPC treatment at the transcriptional and translational levels; however, there was no notable difference in the expression of these proteins in the presence or absence of LPC during S. Typhimurium infection (data not shown). Our results suggest that the expression of Rab proteins is not promoted or inhibited by LPC treatment, indicating that Rab proteins are already expressed in macrophages and act as bridges between other proteins during phagosome maturation.
Recently, Shivcharan et al. (2018) demonstrated that LPC promotes the invasive ability of Salmonella by enhancing the Salmonella invasion-promoting molecules spiA and spiC. In addition, LPC continuously maintains the expression of hilA, one of the major transcription factors of Sips. Although LPC enhanced the invasive ability of epithelial cells, Miyazaki et al. (2017) found that LPC induced bacterial membrane permeability and suppressed the survival of various types of bacteria, including gram-positive methicillin-resistant Staphylococcus epidermidis (MRSE) and gram-negative E. coli and Pseudomonas aeruginosa. Our previous study showed that LPC promoted intracellular bacterial killing in M. tuberculosis-infected macrophages (Lee et al., 2018). In line with our previous study, we also demonstrated that treatment with 18:0 LPC enhanced intracellular bacterial killing in S. Typhimurium SL1344-infected mouse macrophages.
Because antibiotic resistance is one of the global health threat, it is an important to develop candidates which overcome antibiotic resistance to protect the host against antibiotic resistant bacterial infection. Orsi et al. (2006) showed that propolis had an antibacterial activity on S. Typhi. In addition, combined treatment of propolis and antibiotics, including ampicillin, amoxicillin and cephalexin, synergistically enhanced antibacterial susceptibility. Hossain et al. (2020) also demonstrated that combined treatment of gallic acid and antibiotic ceftiofur decreased significantly bacterial biofilm viability and the motility of S. Typhimurium than ceftiofur single treatment. In line with these studies, we found that combined administration of LPC and cephalexin significantly decreased bacterial burden in liver and spleen and moderately protected mouse survival against S. Typhimurium infection. However, since the effective concentration and frequency for the combination therapy could not be determined, further study will be designed to investigate the effective concentration and the administration frequency of LPC as an adjunctive therapy with antibiotic treatment.
During S. Typhimurium infection, macrophages also activate intracellular NF-κB or MAPK signaling pathways by recognizing S. Typhimurium through various PRRs and generate cytokines, chemokines, ROS and reactive nitrogen species, which have microbicidal activities (Behnsen et al., 2015; Lee et al., 2016b; Yang et al., 2009). Our study demonstrated that treatment with LPC increased intracellular ROS but not NO production in S. Typhimurium-infected macrophages. Our previous study showed that LPC promoted phagosome maturation by augmenting intracellular ROS production through cAMP-induced activation of PKA in M. tuberculosis-infected macrophages (Lee et al., 2018). Consistent with this finding, we also demonstrated that LPC-induced intracellular ROS production promoted phagosome maturation via NF-κB pathway in S. Typhimurium-infected macrophages. Blander and Medzhitov (2004) demonstrated that TLR2/4-/- and MyD88-/- macrophages internalized S. Typhimurium and fused with lysosomes, suggesting that TLR2/4-MyD88 signaling contributes to the internalization of S. Typhimurium and phagosome maturation in macrophages. Consistent with the findings of study, we also demonstrated that LPC enhanced phagosome maturation by rapidly activating the NF-κB pathway in S. Typhimurium-infected cells.
Taken together, our results demonstrate that LPC facilitates bactericidal activities by enhancing phagosome maturation via NF-κB activation through LPC-induced intracellular ROS production in S. Typhimurium-infected macrophages. Therefore, these findings suggest that LPC could be developed as a potential therapeutic agent to improve host antibacterial activities.
ACKNOWLEDGMENTS
This research was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (2017R1A6A3A11032251, 2018R1D1A1B07049097, and 2020R1I1A1A01066916), and a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2020R1A6C101A195). Funding was also provided by a 2017 Research Grant from Kangwon National University (No. 520170491).
Footnotes
AUTHOR CONTRIBUTIONS
H.J.L. and Y.J.J. designed the study. H.J.L., W.G.H., Y.W., and J.H.A. performed the experiments. H.J.K., H.K., S.M., T.W.H., and D.K.S. contributed materials and analysis tools. H.J.L., Y.M.J., and Y.J.J. provided funding. H.J.L. and Y.J.J. wrote the paper with input from the other authors.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski M.A., Braun V., Brumell J.H. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 2008;9:2022–2031. doi: 10.1111/j.1600-0854.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- Behnsen J., Perez-Lopez A., Nuccio S.P., Raffatellu M. Exploiting host immunity: the Salmonella paradigm. Trends Immunol. 2015;36:112–120. doi: 10.1016/j.it.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes P., Vetvicka V., Fusek M. Cathepsin D--many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Bayard J., Ramos-Morales F. Molecular mechanisms used by Salmonella to evade the immune system. Curr. Issues Mol. Biol. 2018;25:133–167. doi: 10.21775/cimb.025.133. [DOI] [PubMed] [Google Scholar]
- Blander J.M., Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Bohdanowicz M., Grinstein S. Vesicular traffic: a Rab SANDwich. Curr. Biol. 2010;20:R311–R314. doi: 10.1016/j.cub.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Broz P., Ohlson M.B., Monack D.M. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes. 2012;3:62–70. doi: 10.4161/gmic.19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell J.H., Tang P., Zaharik M.L., Finlay B.B. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar typhimurium in the cytosol of epithelial cells. Infect. Immun. 2002;70:3264–3270. doi: 10.1128/iai.70.6.3264-3270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N.A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 1991;59:2232–2238. doi: 10.1128/IAI.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D.H., Kim J.K., Jo E.K. Mitophagy and innate immunity in infection. Mol. Cells. 2020;43:10–22. doi: 10.14348/molcells.2020.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M. Biogenesis of phagolysosomes: the 'kiss and run' hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- Dougan G., John V., Palmer S., Mastroeni P. Immunity to salmonellosis. Immunol. Rev. 2011;240:196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- Flannagan R.S., Jaumouille V., Grinstein S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- Garcia-del Portillo F., Nunez-Hernandez C., Eisman B., Ramos-Vivas J. Growth control in the Salmonella-containing vacuole. Curr. Opin. Microbiol. 2008;11:46–52. doi: 10.1016/j.mib.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Haraga A., Ohlson M.B., Miller S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- Harrison R.E., Bucci C., Vieira O.V., Schroer T.A., Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 2003;23:6494–6506. doi: 10.1128/mcb.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C.W., Kim T.K., Ham H.Y., Nam J.S., Kim Y.H., Zheng H., Pang B., Min T.K., Jung J.S., Lee S.N., et al. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J. Immunol. 2010;184:4401–4413. doi: 10.4049/jimmunol.0902814. [DOI] [PubMed] [Google Scholar]
- Hong C.W., Song D.K. Immunomodulatory actions of lysophosphatidylcholine. Biomol. Ther. 2008;16:69–76. [Google Scholar]
- Hossain M.A., Park H.C., Lee K.J., Park S.W., Park S.C., Kang J. In vitro synergistic potentials of novel antibacterial combination therapies against Salmonella enterica serovar Typhimurium. BMC Microbiol. 2020;20:118. doi: 10.1186/s12866-020-01810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung A.H., Novick P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh K.K., Eskelinen E.L., Scott C.C., Malevanets A., Saftig P., Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J.W., Park B.C., Jung J.G., Jang Y.S., Shin E.C., Park Y.W. The soluble form of the cellular prion protein enhances phagocytic activity and cytokine production by human monocytes via activation of ERK and NF-kappaB. Immune Netw. 2013;13:148–156. doi: 10.4110/in.2013.13.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Mylona E., Frankel G. Typhoidal Salmonella: distinctive virulence factors and pathogenesis. Cell. Microbiol. 2018;20:e12939. doi: 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- Kabarowski J.H. G2A and LPC: regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 2009;89:73–81. doi: 10.1016/j.prostaglandins.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaruzzaman N.F., Kendall S., Good L. Targeting the hard to reach: challenges and novel strategies in the treatment of intracellular bacterial infections. Br. J. Pharmacol. 2017;174:2225–2236. doi: 10.1111/bph.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Kim K.C., Han J.A., Choi S.S., Jung Y.J. The early induction of suppressor of cytokine signaling 1 and the downregulation of toll-like receptors 7 and 9 induce tolerance in costimulated macrophages. Mol. Cells. 2015;38:26–32. doi: 10.14348/molcells.2015.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Ko H.J., Jung Y.J. Insufficient generation of mycobactericidal mediators and inadequate level of phagosomal maturation are related with susceptibility to virulent Mycobacterium tuberculosis infection in mouse macrophages. Front. Microbiol. 2016a;7:541. doi: 10.3389/fmicb.2016.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Ko H.J., Kim S.H., Jung Y.J. Pasakbumin A controls the growth of Mycobacterium tuberculosis by enhancing the autophagy and production of antibacterial mediators in mouse macrophages. PLoS One. 2019;14:e0199799. doi: 10.1371/journal.pone.0199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Ko H.J., Song D.K., Jung Y.J. Lysophosphatidylcholine promotes phagosome maturation and regulates inflammatory mediator production through the protein kinase A-phosphatidylinositol 3 kinase-p38 mitogen-activated protein kinase signaling pathway during Mycobacterium tuberculosis infection in mouse macrophages. Front. Immunol. 2018;9:920. doi: 10.3389/fimmu.2018.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Woo Y., Hahn T.W., Jung Y.M., Jung Y.J. Formation and maturation of the phagosome: a key mechanism in innate immunity against intracellular bacterial infection. Microorganisms. 2020;8:1298. doi: 10.3390/microorganisms8091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Wi S.M., Min Y., Lee K.Y. Peroxiredoxin-3 is involved in bactericidal activity through the regulation of mitochondrial reactive oxygen species. Immune Netw. 2016b;16:373–380. doi: 10.4110/in.2016.16.6.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R., Grinstein S., Canton J. The life cycle of phagosomes: formation, maturation, and resolution. Immunol. Rev. 2016;273:156–179. doi: 10.1111/imr.12439. [DOI] [PubMed] [Google Scholar]
- Meresse S., Steele-Mortimer O., Finlay B.B., Gorvel J.P. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H., Midorikawa N., Fujimoto S., Miyoshi N., Yoshida H., Matsumoto T. Antimicrobial effects of lysophosphatidylcholine on methicillin-resistant Staphylococcus aureus . Ther. Adv. Infect. Dis. 2017;4:89–94. doi: 10.1177/2049936117714920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D.M. Helicobacter and Salmonella persistent infection strategies. Cold Spring Harb. Perspect. Med. 2013;3:a010348. doi: 10.1101/cshperspect.a010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi R.D., Sforcin J.M., Funari S.R.C., Fernandes A., Bankova V. Synergistic effect of propolis and antibiotics on the Salmonella typhi. Braz. J. Microbiol. 2006;37:108–112. [Google Scholar]
- Park C.H., Kim M.R., Han J.M., Jeong T.S., Sok D.E. Lysophosphatidylcholine exhibits selective cytotoxicity, accompanied by ROS formation, in RAW 264.7 macrophages. Lipids. 2009;44:425–435. doi: 10.1007/s11745-009-3286-6. [DOI] [PubMed] [Google Scholar]
- Pauwels A.M., Trost M., Beyaert R., Hoffmann E. Patterns, receptors, and signals: regulation of phagosome maturation. Trends Immunol. 2017;38:407–422. doi: 10.1016/j.it.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires D., Marques J., Pombo J.P., Carmo N., Bettencourt P., Neyrolles O., Lugo-Villarino G., Anes E. Role of cathepsins in Mycobacterium tuberculosis survival in human macrophages. Sci. Rep. 2016;6:32247. doi: 10.1038/srep32247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashar A., Schnettger L., Bernard E.M., Gutierrez M.G. Rab GTPases in immunity and inflammation. Front. Cell. Infect. Microbiol. 2017;7:435. doi: 10.3389/fcimb.2017.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M. Salmonella and reactive oxygen species: a love-hate relationship. J. Innate Immun. 2019;11:216–226. doi: 10.1159/000496370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J., Ghigo E., Kalaidzidis Y., Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Shivcharan S., Yadav J., Qadri A. Host lipid sensing promotes invasion of cells with pathogenic Salmonella . Sci. Rep. 2018;8:15501. doi: 10.1038/s41598-018-33319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Gaullier J.M., D'Arrigo A., Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Smith A.C., Do Heo W., Braun V., Jiang X.J., Macrae C., Casanova J.E., Scidmore M.A., Grinstein S., Meyer T., Brumell J.H. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J. Cell Biol. 2007;176:263–268. doi: 10.1083/jcb.200611056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer O. The Salmonella-containing vacuole: moving with the times. Curr. Opin. Microbiol. 2008;11:38–45. doi: 10.1016/j.mib.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Querol E., Rosales C. Control of phagocytosis by microbial pathogens. Front. Immunol. 2017;8:1368. doi: 10.3389/fimmu.2017.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asten A.J., Koninkx J.F., van Dijk J.E. Salmonella entry: M cells versus absorptive enterocytes. Vet. Microbiol. 2005;108:149–152. doi: 10.1016/j.vetmic.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Vieira O.V., Bucci C., Harrison R.E., Trimble W.S., Lanzetti L., Gruenberg J., Schreiber A.D., Stahl P.D., Grinstein S. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol. 2003;23:2501–2514. doi: 10.1128/mcb.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemyss M.A., Pearson J.S. Host cell death responses to non-typhoidal Salmonella infection. Front. Immunol. 2019;10:1758. doi: 10.3389/fimmu.2019.01758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M.J. Innate immune control of Salmonella enterica serovar Typhimurium: mechanisms contributing to combating systemic Salmonella infection. J. Innate Immun. 2011;3:543–549. doi: 10.1159/000330771. [DOI] [PubMed] [Google Scholar]
- Wong C.E., Sad S., Coombes B.K. Salmonella enterica serovar typhimurium exploits Toll-like receptor signaling during the host-pathogen interaction. Infect. Immun. 2009;77:4750–4760. doi: 10.1128/IAI.00545-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Y., Kim H., Kim K.C., Han J.A., Jung Y.J. Tumor-secreted factors induce IL-1beta maturation via the glucose-mediated synergistic axis of mTOR and NF-kappaB pathways in mouse macrophages. PLoS One. 2018;13:e0209653. doi: 10.1371/journal.pone.0209653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J.J., Jung J.S., Lee J.E., Lee J., Huh S.O., Kim H.S., Jung K.C., Cho J.Y., Nam J.S., Suh H.W., et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Yuk J.M., Jo E.K. The role of nitric oxide in mycobacterial infections. Immune Netw. 2009;9:46–52. doi: 10.4110/in.2009.9.2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]