Abstract
People with metastatic breast cancer face many challenges and disparities in obtaining optimal cancer care. These challenges are accentuated in underserved patient populations across Europe, who are less likely to receive quality healthcare for reasons including socioeconomic inequalities, educational or cultural status, or geographic location. While there are many local and national initiatives targeted to address these challenges, there remains a need to reduce disparities and improve access to healthcare to improve outcomes, with a focus on multidisciplinary stakeholder engagement.
In October 2019, a range of experts in metastatic breast cancer, including healthcare professionals, patient representatives, policymakers and politicians, met to discuss and prioritize the critical needs of underserved patient populations with metastatic breast cancer in Europe. Six key challenges faced by these communities were identified: the need for amplification of the metastatic breast cancer patient voice, better and wider implementation of high-quality guidelines for metastatic breast cancer, more collaboration between stakeholders, tailored support for patients from different cultural and ethnic backgrounds, improved data sharing, and work-related issues. The Expert Panel then conceived and discussed potential actionable goals to address each key challenge. Their conclusions present a set of interrelated approaches to address the different challenges and could serve as the basis for concerted improvement of the lives of patients with metastatic breast cancer in Europe.
Keywords: Metastatic breast cancer, Underserved patient population, Europe, Cancer care disparities, Challenges, Oncology
Highlights
-
•
A pan-European approach is needed to tackle disparities in metastatic breast cancer treatment.
-
•
Multi-stakeholder collaboration may overcome challenges faced by underserved patients.
-
•
New interventions integrated with existing initiatives could improve patient care.
1. Introduction
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer-related death in women across Europe [1]. Although early BC is treatable and potentially curable, up to 10% of patients are diagnosed at an advanced stage in developed countries, and 20–30% of patients experience progression to metastatic BC (mBC), even if diagnosed early and appropriately managed [2,3]. Despite advancements in our understanding of the disease, mBC survival rates have remained stable [2,[4], [5], [6], [7]], although available data pre-date the advent of treatments such as cyclin-dependent kinase 4/6 and phosphoinositide 3-kinase inhibitors [8], newer human epidermal growth factor receptor 2 (HER2) targeted agents [9], poly-ADP ribose polymerase inhibitors [10] and a PD-L1 inhibitor [11], which will hopefully help improve outcomes in future. Nevertheless, mBC remains a treatable but incurable disease.
The main goals of mBC treatment are prolongation of life and symptom palliation [[12], [13], [14]]. Following diagnosis, median survival is approximately 2–3 years [2,6], and the relative 5-year survival rate for distant metastatic disease is only 28% compared with 99% and 86% for localized and regional disease, respectively [7]. In addition, the quality of life of people with mBC has only minimally improved in recent years [2,15]. Therefore, mBC represents an area of high unmet need and is a significant global health burden.
Although several new treatments for mBC have been recently introduced [[12], [13], [14],16], positive outcomes depend not only on the response to treatment, which varies according to disease and patient characteristics, but also on access to those treatments, which is affected by a host of factors. The wide variation in these factors leads to disparities in cancer care between and within countries, including high-income countries, and not all patients benefit equally from the healthcare services available [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. For example, registry data from New Zealand found that the median survival after mBC diagnosis was 18.8 months (2010–2015), around half that of other developed countries [27]. Similarly, there are significant differences in both BC incidence and mortality between European countries [1,28], and regionally within each country.
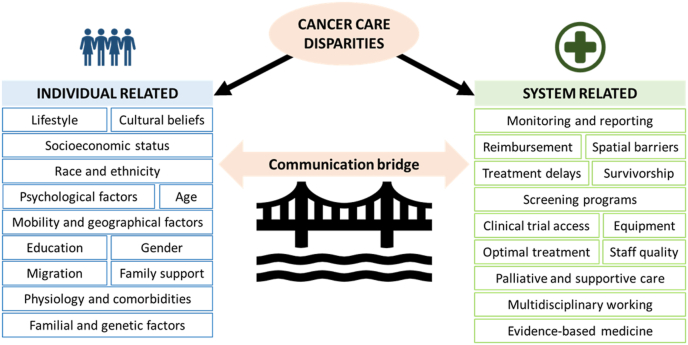
Disparities in cancer care can be classified as individual-related (e.g. age, socioeconomic status, etc.) or system-related (e.g. reimbursement, policy, etc.) (Fig. 1).
Fig. 1.
Cancer care disparities classified according to patient-related or system-related factors (Adapted from Ref. [29], with permission from the author).
Individual-related disparities, such as race [30], socioeconomic status [31,32], and social and psychological factors [33] are well known to adversely affect patient outcomes.
System-related disparities include significant gaps in mBC-specific reporting and data collection, cancer care policies, including access to supportive care services, and healthcare provider education [15,18,24,26,[34], [35], [36]], as well as barriers in access to treatments, such as systemic therapies and radiation therapy [18,37,38]. Intra-country disparities also exist, with geographical location playing a major part in access to appropriate healthcare services, such as women in the UK experiencing “postcode prescribing”, resulting in variable access to medication in different parts of the country [39]. There also remains a need for a greater understanding of how mBC affects the family and professional life of both patients and their caregivers, and the best ways to support them [15,35].
Underserved patient populations (UPP) with mBC may be defined as less likely to receive quality healthcare owing to the patient- or system-related factors outlined above. Successful strategies aimed at reducing disparities and increasing access to optimal treatment and care must be developed for these UPPs.
To better understand the challenges facing UPPs with mBC, it is important to get the perspective and input of all stakeholders, including healthcare professionals (HCPs), patient advocacy group (PAG) representatives, policy makers, politicians and others. The challenges not only include access to the right treatment and care, but also improvements in HCP education, policy-related issues, support for patients, caregivers and employers, investment in medical innovations and efficient use of healthcare resources. A multidisciplinary team approach has been shown to improve patient care and health outcomes in BC [40], including survival [41]. Therefore, multidisciplinary collaboration can build on existing initiatives, identify remaining gaps, and develop new actions to drive access to the appropriate care for patients with unmet needs.
In October 2019, a multidisciplinary group of European experts met in Brussels, Belgium to discuss the access barriers to healthcare facing UPPs with mBC. Their goal was to conceive and prioritize actionable solutions that countries and healthcare systems can implement with multifunctional stakeholder groups, according to local needs. In this review, we discuss the outcomes and implications of this debate.
2. Materials and methods
Prior to the meeting, a literature search was conducted to identify the main risk factors associated with the UPPs in mBC and define categories of unmet needs. The electronic database, PubMed, was used to identify relevant English language articles, with filters employed to limit studies to those in humans and published within the last five years. Search terms included “breast cancer” AND (“underserved” OR “disparity” OR “barrier” OR “unmet need” OR “under resourced” OR “inequality”). Additional studies were identified through searching key cancer and think-tank institution websites. All types of publications, including reviews, clinical trials, white papers and consensus statements were considered. In order to provide a more accurate picture of the total volume of evidence pertaining to UPP with BC, the literature search was not limited to mBC or to Europe.
The findings of the literature search were supported by insights, gathered through a premeeting survey of attendees, about how UPPs with mBC are defined in different countries, the priority areas to address and existing best practices.
The meeting in Brussels was attended by principal European stakeholders in mBC (“the Expert Panel”), including 10 clinicians (oncologists), 4 PAG representatives, 2 (ex)payers, a nurse, a psychologist, a politician, a policy affairs manager, and the Managing Coordinator of the ABC Global Alliance. A series of multidisciplinary-group workshops were conducted to identify the key challenges faced by UPPs and develop tangible solutions, which were explored in more detail before being subjected to wider group discussion to refine the ideas developed.
3. Results
The literature searches yielded 364 articles that met the search criteria. After examination of study abstracts, 45 were deemed not relevant for various reasons (not specific to BC, duplicates, non patient-centric, theoretical modeling tools, conceptual frameworks). The remaining 319 articles were categorized according to the main risk factor(s) reported (Table 1).
Table 1.
Key risk factors associated with UPPs in mBC, identified by literature search. ∗Some articles reported on more than one risk factor.
| Risk factor | Number of articles∗ |
|---|---|
| Ethnicity/culture | 115 |
| Geographical location | 44 |
| Socioeconomic status | 43 |
| Social and psychological factors | 35 |
| Barriers to healthcare services (e.g. screening, care support etc.) | 33 |
| Physiological and lifestyle factors, including comorbidities | 21 |
| Age | 21 |
| Access to treatment or disease awareness information | 21 |
| Familial and genetic factors | 14 |
| Education | 9 |
| Requirement for HCP education or training | 8 |
| Gender | 4 |
| Workplace issues | 2 |
| Healthcare resource utilization | 2 |
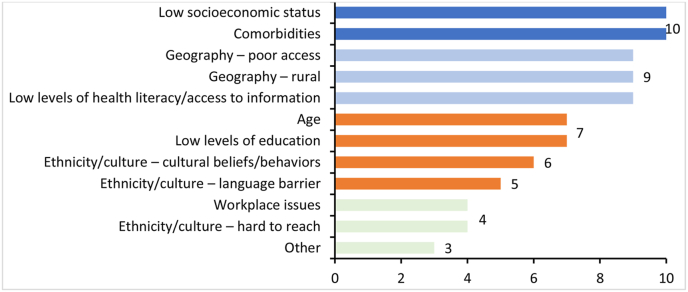
The literature searches were supported by the insights gathered through the survey (Appendix A), which was completed by 15 respondents who identified the key characteristics of UPPs in mBC (Fig. 2.).
Fig. 2.
Most frequently identified UPP characteristics for patients with mBC according to premeeting survey. “Other” characteristics comprised the following: attitude/culture of healthcare staff; health system characteristics; quality control; poor information; living on an island; diagnosis in young women with related issues, such as fertility, childcare and work.
Based on the outputs of the literature search and survey, challenges identified were grouped according to the following categories: socioeconomic and workplace issues, education and awareness needs, healthcare availability and access, and ethnicity and cultural issues. During workshop discussions, different groups of experts identified and prioritized multiple challenges associated with UPPs with mBC and classified them according to the categories above (Appendix B). The six challenges identified as the most feasible to address were as follows:
-
1.
Need for improved awareness and amplification of the mBC voice
-
2.
Better and wider implementation of high-quality guidelines for mBC
-
3.
Need for improved mBC understanding in non-oncologists and better communication between primary care physicians (PCPs), oncologists and patients
-
4.
Need for improved awareness of and tailored approaches to support patients with mBC in multicultural communities
-
5.
Need for improved mBC data gathering and clinical trials
-
6.
Issues within the workplace
Each of these challenges was debated, and potential solutions involving all stakeholders were proposed and developed during the meeting. The proposals are discussed below and summarized in Table 2.
Table 2.
Summary of the potential actionable goals and their main requirements to address the six main challenges in access to care for the UPPs with mBC. ∗This initiative would not work in Belgium since specialists play the role of GPs for these patients owing to resource constraints and the way the patient pathway is structured. BC, breast cancer; BCN, Breast Cancer Now; EU, European Union; GP, general practitioner; HCP, healthcare professional; HRU, healthcare resource utilization; mBC, metastatic breast cancer; PAG, Patient Advocacy Group; QoL, quality of life; UPP, underserved patient population.
| Challenge | 1. Need for improved awareness and amplification of the mBC voice | 2. Better and wider implementation of high-quality guidelines for mBC | 3. Need for improved mBC understanding in non-oncologists and better communication between PCPs, oncologists and patients | 4. Need for improved awareness of and tailored approaches to support patients with mBC in multicultural communities | 5. Need for improved mBC data gathering and clinical trials | 6. Issues within the workplace | |
|---|---|---|---|---|---|---|---|
| Objective | To improve knowledge and awareness of the unmet needs in mBC among non-specialist audiences | Increase implementation of guidelines | Drive increased free time for oncologists to treat more patients with mBC | Increase awareness of mBC with culturally specific campaigns | Accurate capture of mBC prevalence across the EU (Create a ‘Metastatic Center’) | Create a supportive work environment for patients with mBC | |
| Potential solution(s) | Evidence package for politicians (and, subsequently, other stakeholders) | Erasmus programs for HCPs through extension of existing Erasmus programs | Improve patient education and empowerment through patient advocacy groups | Develop a series of small interventions that support management of non-oncology-related healthcare | Develop a targeted education program for specific subpopulations, partnering with local community organizations and HCPs | Leverage existing registries to improve what already exists and implement cancer registration capacity | An accreditation program that encourages the establishment of a cancer-friendly work environment |
| Target audience | Primarily national politicians; could also be adapted for other audiences | All cancer healthcare professionals | Oncologists and patients | PCPs, oncologists | To be determined, based on country | Healthcare payers, government, hospitals | Employers, policy makers, payers |
| Implementation | Funding and creation of evidence-based benefit package for healthcare (data gathering, analysis, and reporting) | Funding and support from the European Union | Multidisciplinary engagement and collaboration | GPs, BC centers, and patients willing to be part of the pilot scheme | Leverage local in-country knowledge to support with resource ideas | Work with EUROCARE (for cancer registry initiative) | Agree a ‘Charter’/checklist for company and qualifier ‘seal’ |
| Requirements | Engage countries where data collection on ethnicities is permitted | ||||||
| Harmonized action plan (timelines, data-gathering directives) | Education to raise awareness of and improve implementation of mBC guidelines | Rapid review of available initiatives and apps that could be leveraged as part of the intervention stage | Decide on whether scheme is targeted to cancer or expanded to other disease areas | ||||
| Guideline endorsement by payers, to avoid any financial shortcomings | Identification of a way to capture baseline and postintervention metrics | Involvement of a government department to endorse and/or help create | |||||
| Metrics for success | Levels of awareness among the political class | Number of HCPs participating | Change in patient-reported outcomes after intervention (guideline awareness, patient empowerment and actions) | Change in pre- and post-non-mBC related HRU | Levels of awareness among patients and the general public (hard to measure) | Accurate data | Number of accredited employers |
| (hard to measure) | |||||||
| Changes in reported outcomes in HCP practice | Physician-reported outcomes after intervention (anonymized prescription behavior) | Measure of reported patient satisfaction | Number of communities that adopt the program | Hospital participation rate | Number of patients who take time off work without vs with the new charter | ||
| Patient outcomes and satisfaction | Market research data | Measure of reported physician satisfaction | Disaggregation of the outcomes for gender, age, setting of care, education, income level, social status | Awards for early adopters | |||
| Feedback on recruitment, staff retention, staff wellbeing | |||||||
| Role of clinician | To provide support through active data gathering | To participate in and follow structured programs | To treat patients according to guidelines and inform patients accordingly | To support shaping the evaluation metrics and providing content for the ‘red flags’ guide | To measure outcomes in treated patients from different cultural groups | To play an active role in developing registries; to be an advocate within the clinical community | To articulate the benefit of program/build argument |
| To support identification of locally relevant populations | |||||||
| To engage with GPs to co-create the program | |||||||
| Role of policy maker | To be open to engagement and discussion and facilitate data gathering in understanding the magnitude of the disparities issue in Europe | To implement and regulate the programs across Europe | To support provision of a cancer budget sufficient to implement guidelines | To increase the priority of the initiative (need to see measurable results) | To identify a clearer picture of the local issues (via metrics) | To engage with DG Sante to coordinate/lead the data collection | To facilitate funding and tax incentives |
| To advise if the focus should be women’s health generally or BC specifically | |||||||
| To negotiate with budget holders and provide solutions to increase or stretch the budget | To conduct a spend-to-save analysis to demonstrate broader impact and efficient use of resources | To advise on possible legislative impact | |||||
| To demonstrate the impact on underserved patients | To lobby the relevant governmental department to adopt the program | ||||||
| Role of PAG | Highlight the needs of the UPP, lobby for change, and disseminate publicly available data | To support and raise awareness of the programs | To represent the UPPs with mBC to implement the solutions | To promote the initiative to patients, highlighting the benefits | To ensure content is appropriate and drive uptake | To be an advocate for a nationwide campaign for registries | To promote/campaign to policymakers |
| To raise awareness of the impact on patients’ QoL | |||||||
| To use campaigning know-how, e.g. BCN in the UK | To create the seal | ||||||
| To take a seat on the board of accreditation | |||||||
| Breadth of implementation | National | International (Europe-wide) | National and international (Europe-wide) | National∗ | Local | National and international (Europe-wide) | National |
3.1. Need for improved awareness and amplification of the mBC voice
One of the greatest needs associated with mBC is raising awareness of the disease among all stakeholders (patients; patient advocates; payers; HCPs, including students; policy makers; politicians; cancer charities; research funding bodies such as the European Union [EU] and the public). Improved awareness leads to better understanding and communication across stakeholder groups and facilitates best-practice sharing. A number of existing initiatives already work to raise awareness of mBC, including international organizations, such as ABC Global Alliance, Union for International Cancer Control, the Metastatic Breast Cancer Alliance and several others, as well as national schemes (e.g. the UK Unsurvivors campaign from UK charity Breast Cancer Now, Collectif 1310 in France, and the German Cancer 10-year plan, to name a few).
The Expert Panel discussed additional ways in which to raise awareness and felt that two main channels of communication would be most effective: an HCP exchange program and an evidence package.
The European Community Action Scheme for the Mobility of University Students (ERASMUS), is a program established in 1987 by the EU that allows students to study at universities in the EU member states, EU candidate countries and European Free Trade Association/European Economic Area members for set periods of time [42,43]. Since then it has given 6 million students the opportunity to spend part of their university career in another country while achieving credit for their degree at home [[42], [43]]. ERASMUS PLUS is a branch of ERASMUS started in 2014 which targets a broader scope of individuals, including specialized staff, teachers, trainees, volunteers, and more. ERASMUS PLUS is an invaluable learning and training experience, and therefore promoting it among HCPs treating mBC as well as easing the access and securing adequate grants for this group would give individuals an opportunity to experience different healthcare systems and allow them to learn and bring home ideas that could improve their own practice, similarly to how ERASMUS helps medical students broaden their intellectual horizons [[42], [43]]. This move would promote mBC awareness in two ways. Firstly, it should be expected that a HCP who has had the opportunity to treat patients and engage in other healthcare systems will be able to educate their colleagues back home, as sessions could be held during which HCPs who attended the program gather to discuss their observations with other HCPs. Secondly, the HCP will have a better understanding of patients from different backgrounds, will be more culturally sensitive, and will know better how to approach them. This could help support continuous HCP education and facilitate information and best practice sharing across European countries, in line with wider-reaching initiatives such as comparative data sharing (see also “3.5 Need for improved mBC data gathering” below). This initiative would be targeted towards nurses, physicians, physiotherapists, psycho-oncologists, palliative care professionals, social workers and other allied HCPs across Europe, with political level support, such as funding from the EU.
The Expert Panel also proposed an “evidence package” of standardized data to identify and validate challenges and unmet needs faced by patients with mBC in a particular country. This would be targeted towards national politicians, providing consistent information to support governments in raising awareness of mBC and addressing challenges and offering a starting point from which to identify the greatest priorities for healthcare resources, in line with pan-European initiatives such as the “Europe Beating Cancer” Plan, which aims to reduce the burden of cancer and address cancer-related inequalities between countries [44], and National Cancer Control Program (NCCPs) that are designed to improve all cancer care in individual countries through evidence-based strategies [45].
The evidence package could also be used by other stakeholders, such as physicians, nurses and other HCPs, to lobby for change. The first step would be to gather accurate and up-to-date statistics (see also “3.5 Need for improved mBC data gathering” below), beginning with national cancer registries and other sources of objective information. To implement such an initiative would require funding for gathering evidence (data collecting, analysis, and reporting) and creating action plans. The evidence would require evaluation by multiple stakeholders, such as HCPs and policymakers.
3.2. Better and wider implementation of high-quality guidelines for mBC
There are international evidence-based guidelines specific to the treatment of mBC [12,13,16] and specialist nurse support [46] as well as many individual country-specific guidelines, but these are not always faithfully implemented in practice [[47], [48], [49]]. Since cancer plans are not universal throughout Europe, the European Commission has decided to develop an inclusive ‘‘Europe Beating Cancer’’ plan for all EU members, which would provide standards for therapy planning and management [44].
The Expert Panel felt that the empowerment and education of patients, through the support of PAGs, including bodies like the European Cancer Patient Coalition, could compel physicians to treat according to high-quality guidelines, thereby increasing their widespread implementation. The more educated patients are about their treatment options, the more likely they are to discuss guidelines and recommended treatments with their HCPs, primarily oncologists, encouraging them to treat according to the guidelines. It is thought that many patients are afraid to challenge HCPs and ask about guidelines, so their empowerment should initially come from PAGs (provided they are without any financial conflicts of interest), before translating to the individual patient. Patients should also be entitled to rely on the expertise of an HCP who is fully aware and compliant with certified and validated national guidelines, something that should be included in HCP training initiatives. This could be actively supported by European cancer organizations, such as the European School of Oncology and the European Society for Medical Oncology (ESMO), in addition to national professional organizations that provide national guidelines adapted to each country’s reality. This may ultimately facilitate national access to specific treatments and care modalities. Guideline implementation could be monitored by national cancer registries through regular surveys of the stakeholders, documenting treatments that are guideline-compliant, although anonymity of individuals would need to be guaranteed in order to get an accurate picture, since there are often instances where guidelines are not followed due to external factors, such as financial reasons. Alternatively, information could be sourced from existing databases, real-world data projects and/or market research, which would involve considerable effort, but would yield important intelligence. Utilizing data to enable individual HCPs to see how they would compare with the national average could also be useful to promote good practice. This proposal would also align with the ECC’s European Cancer Patient’s Bill of Rights, which advocates a patient-centric approach to cancer care, including every patient’s right to receive the most accurate information and to be proactively involved in their own care [50, 51].
3.3. Need for improved mBC understanding in non-oncologists and better communication between PCPs, oncologists and patients
A holistic approach to treating mBC involves a variety of HCPs. PCPs have an important role in the care of patients with BC, since they can be the initial point of contact for the first presentation of BC symptoms, symptoms of relapse, for the management of long-term side effects after cancer treatment or comorbidities, and for screening psychosocial needs for appropriate referral [34,[52], [53], [54]]. Skilled PCPs could provide support throughout therapy, particularly in areas or centers that may not have extensive multidisciplinary support teams to manage specific comorbidities. Many patients with cancer have concerns about seeing their PCP for cancer-related follow-up care [54,55]. Therefore, clear guidance is needed on the respective roles of the PCP, the home nurse, and the pharmacist, with a need to drive coordination and communication between the oncologist and PCPs. Some such initiatives are currently in existence. For example, in the UK, Macmillan has produced toolkits, guidance documents, and online training to support PCPs in connecting with patients with cancer. There is also an e-learning community to provide cancer-specific educational opportunities for registered nurses.
Successful HCP-patient (and caregiver) communication in the advanced cancer setting helps patients come to terms with their disease and its treatment, cope with the impact on their lives, and may help to improve clinical outcomes [56,57]. Understanding and addressing patients’ concerns, needs and preferences requires effective communication skills, which has been recognized as an area for improvement in medical education and clinical care. Recommendations for HCP communication skills training and clinical practice guidelines for its implementation have been made [58,59].
Another potential improvement is the greater use of patient-reported outcomes (PROs). PROs can enhance physician and nurse understanding of patient symptoms and the impact they have on quality of life, thereby facilitating a more tailored approach to clinical decision making and reducing service utilization for uncontrolled symptoms [60].
The Expert Panel identified a lack of knowledge of the statistics of PCP involvement in mBC as a factor affecting HCP resource management and proposed a pilot scheme to evaluate healthcare resource utilization before and after a series of small interventions. These interventions would aim to increase PCP awareness and understanding of mBC and facilitate communication between patients, PCPs, and oncologists (Table 2). Enhancing non-oncologist awareness of the needs of patients with mBC would increase the time available to oncologists, thereby potentially enabling them to devote more time to UPPs. The pilot scheme would require baseline evaluation of healthcare resource utilization related to non-mBC follow-up, i.e. management of comorbidities, infections, psychosocial needs, etc., that could be delivered by non-oncology staff. It would require the development of a series of small interventions that support management of oncology-related healthcare. One intervention could be devising a “red flags for mBC” guide – a handy reference guide of top tips for busy PCPs, which could provide helpful advice on how to manage patients with mBC and when to seek specialist care, with a focus on “red flag situations” in follow-up after early BC and in management of mBC. Another intervention could be promoting patient education on how to effectively communicate with non-oncologists (“What do I need to tell my PCP about my cancer?“); available commercial apps like the multilingual, Cankado, could be utilized. Finally, a third intervention would aim to connect oncologists and PCPs who care for the same patient, to improve communication channels between them. The effect of all the interventions on healthcare resource utilization in the pilot center would ideally be measured one year after implementation and, if successful, the scheme could be rolled out nationally.
3.4. Need for improved awareness of and tailored approaches to support patients with mBC in multicultural communities
Racial disparities in BC care are well documented [61,62]. For example, black women are more likely to die of BC than white women [62]. Black women are also significantly less likely to receive cancer-directed surgery, radiation therapy, and hormonal therapy, are underrepresented in clinical trials, and have poorer access to trial-based innovations in cancer care compared with white women [61,62]. Other cultural and socioeconomic differences also exist. For example, immigrant and low-income women living in Switzerland were less likely to take up BC-preventative methods, such as mammography and breast self-examination, compared with Swiss nationals [63]. Another study showed the general trend of lower breast screening uptake in immigrant women in Italy compared with Italian women between 2005 and 2013. However, it also showed that the prevalence of breast screening is higher among immigrant women in Northern Italy than among Italian women in Southern Italy [64]. In general, migrant women are more likely to utilize emergency health services than non-migrants [65], which is likely related to low rates of acculturation [66]. Lack of awareness and misrepresentation may also affect the lesbian, gay, bisexual and transgender community and there is a need for improved appropriate social support for sexual and gender minority BC survivors [67].
Every European patient should have the right to optimal and timely access to appropriate specialized care [50,51]. Evaluation of subpopulations in each region is required to understand where best to focus efforts and identify those patient groups that need support (e.g. cultures, ethnicities, ages, sexual orientations, migrants and traveling communities), although this may be limited by data protection laws in some countries. There is also the need to approach any intervention in a culturally sensitive and appropriate manner.
The Expert Panel suggested targeting community-based peer groups and HCPs embedded in communities to collect data and create tailored education on mBC. This would include information on how to appropriately refer to the disease and patients’ needs within the medical communities that serve these subpopulations, help with language/socioeconomic issues, and to create and verify information to support local UPPs (Table 2). Local PAGs, cultural organizations, and community-based HCPs should identify existing local communities and projects at a grass roots level, to advise on the content of new initiatives aiming to tackle health-related problems. Many cultural community projects already exist, for example the Breast Cancer Project by Race Equality Foundation or the MOPA (Muslim and Palliative Care Antwerp) and similar initiatives by Kom op tegen Kanker in Belgium, which provides culturally-tailored guidance to Muslim cancer patients requiring palliative care and their families, and engages with Muslim clerics to help disseminate appropriate messages to the community through the mosques. Initiatives such as these can help provide the appropriate knowledge and insight needed to address the disparities in local underserved mBC populations. The proposed intervention for mBC should encompass minorities with cultural taboos and/or language barriers and include both men and women. It would operate at a locoregional level to identify the relevant subpopulations and their needs and then develop a targeted education program for specific subpopulations. It would be adapted to different communities, with different content styles to allow for language barriers, i.e. visuals rather than words, translation of content into different languages, etc. This initiative would be driven by PAGs, who would provide the insight and knowledge needed to inform the program, as well as implement local rollout and encourage the targeted subpopulation(s) to get involved. Further considerations would include involvement of local educational establishments and social security departments for further information gathering, and support in rolling out local initiatives.
3.5. Need for improved mBC data gathering and clinical trials
There are evident data gaps in the recording of mBC statistics. There are very few accurate records of the number of people who are living with mBC, how many patients with early-stage BC have recurrences, and how the incidence and outcome of mBC have changed over time for the common subtypes of BC [2, [4], [5], [6]]. Furthermore, cancer registration in Europe is challenged by significant disparities in the quality and coverage of cancer registries [68], while some countries do not have a cancer registry at all. Although several groups in different countries are working to improve data collection, further work is clearly needed. Primary data-generation needs of mBC management identified by the Expert Panel include the creation of registries where they do not yet exist; active sharing of resources and real-world data between BC clinics; the introduction of biorepositories for studies to identify biomarkers for treatment response. There is also a need to reconsider the approach to conducting mBC clinical trials, making them more sustainable and more efficient in terms of data generation [69]. Considering the aforementioned disparities between countries affecting the quality and consistency of the reported data, it seems plausible from the perspective of the local health authority that making investments in trial innovation, such as increasing the expenditures going into mBC research and strengthening ties with global academia and industry, and promoting transparency in collection and reporting of trial data could improve both sustainability and efficacy of the trials. It would also heighten the country’s reputation for research capacity and promote research globalization and quality.
The preferred solution discussed by the Expert Panel was to create a platform where accurate capturing of mBC data across Europe would be the main goal (Table 2). The scheme would leverage existing registries to improve what already exists by examining current examples of good practice and working with pan-European and international organizations such as EUROCARE, AROME, or IARC in Lyon, France. Other ideas discussed included introducing an outcome measure by the ESMO, and an annual mBC census where the government compels centers to collect up-to-date data on metastatic disease.
Clinical trials have resulted in significant advances in many aspects of BC care, for example as a way of offering emerging new therapies to patients [24]. Furthermore, there is some evidence to suggest that access to clinical trials is associated with improved survival for mBC patients [70]. Clinical trial information is, rightly, aimed primarily at oncologists, but should also be publicly accessible through national/regional databases (e.g. as it is via cancertrials.be in Belgium). However, although the Expert Panel acknowledged the need for greater awareness of and improved access to clinical trials for mBC patients, potential measures to address this were not discussed at the meeting.
3.6. Issues within the workplace
Many patients with cancer want to return to work in order to attain a sense of normality, to maintain a positive outlook and for financial reasons. A survey by Macmillan in the UK showed that 87% of employed people diagnosed with cancer said that it was important to them to continue working [71]. However, the same survey also showed that patients need support to return to work following a cancer diagnosis, with one in five facing discrimination at work. In some countries, legal measures protect the employment rights of cancer patients. For example, in the UK, cancer is considered as a disability under Equality Act 2010, meaning that an employee cannot lose their job or be treated less favorably for having cancer, yet many employers remain unaware of this [72]. In addition, many employers need advice and training to help them support employees with cancer. The Macmillan survey found that only one-third of line managers feel well equipped to support employees with cancer [71]. Caregivers are also affected. In the UK, caregivers have the right to request flexible working and emergency time off for dependents and are protected against discrimination. Caregivers in Germany are entitled to similar privileges under the Pflegejahr scheme.
Both the Macmillan at Work scheme and Barbara Wilson’s Working with Cancer® in the UK support employees and employers, by providing a range of services including advice and information, toolkits and e-learning modules, newsletters, best practice guides, coaching sessions and consultancy advice.
Similar initiatives to support employers across Europe could be introduced. The preferred and most feasible solution identified by the Expert Panel was a ‘Seal of Approval’ accreditation scheme that creates a supportive work environment for patients with mBC and their caregivers (Table 2). The scheme, which realistically would cover all cancers, not just mBC, would target workplaces and employers to encourage the establishment of a cancer-friendly work environment for patients and caregivers, with benefits such as flexible working hours, guidance for managers, and tax or social security benefits for the employee. This would be a national-level initiative and could start with a pilot scheme involving the state (as the largest employer) setting an example, with other major businesses being part of the pilot. The pilot scheme would collect evidence to ensure that this is a workable idea, e.g. by gathering data on the number of employees affected, and collating information on the legal position and outlining the role of different stakeholders, including managers and human resource professionals. A charter or checklist of criteria that workplaces need to meet would be used to assess workplace qualification for accreditation. If successful, and cost-effective, the scheme could potentially be rolled out to smaller businesses. The scheme should also consider implications for employers and inform patients of their legal rights. PAGs would be the main drivers of this initiative.
This type of initiative does come with some limitations, for example, taking account of disparities in social security and health care insurance at national level across Europe. There may also be a need for legislation to flexibly balance social security benefits and part-time employee salaries (especially if that employer is that state itself), without an adverse impact on either employee or employer finances. In fact, the scheme should be financially neutral (subsidized) for employers with, for example, compensation for the enhanced administration and cost of hiring two part-time workers instead of one full-time person. Self-employed workers would also need to be considered and benefit from equivalent measures.
4. Discussion
Improving outcomes for patients with mBC across Europe, and indeed the world, remains one of the biggest healthcare challenges in oncology. Mortality rates for metastatic disease remain high, so, from a certain point of view, all patients with mBC could be considered underserved. However, for those patients that are underserved owing to either individual factors, such as age, ethnicity, socioeconomic status and others, and/or persistent disparities in cancer care, the unmet needs are even greater. Nevertheless, these needs are increasingly being recognized by people and organizations, such as the ABC Global Alliance, the mBC Alliance, the EU and may other national or local organizations and initiatives, who are striving to develop, promote and support raising awareness, sharing best practices and, hopefully, addressing the specific needs of UPPs to ultimately improve and extend the lives of those living with mBC.
Working to these same goals, the Expert Panel identified and prioritized six key challenges that could feasibly be addressed in order to improve the situation of UPPs with mBC in Europe. They then conceived proposals and ideas that could most readily be developed and measured by multi-stakeholder groups and authorities to address those challenges. This included raising awareness of the mBC voice, better and wider implementation of mBC guidelines, improved cooperation between the different HCPs, adequate tailoring of support, enhancing local and international data exchange, and tackling issues faced in the workplace.
Taken individually, each of the proposals outlined above should be considered a starting point for individual countries to review and assess the impact of existing initiatives and either build on them or establish something new, based on the ideas presented here. Given the importance of multidisciplinary stakeholder participation to ensure a holistic approach to any of these projects, it is recommended that each country should utilize (or convene, if one is not already in existence) a “National Multidisciplinary mBC Working Group” to be responsible for the supervision, management and measurement of the impact of any actions undertaken. These national working groups could then partner with each other, as well as with existent multinational organizations, specifically the ABC Global Alliance, to move towards a pan-European approach to tackling these challenges. mBC working groups should also operate in harmony with wider cancer care initiatives, including NCCPs and the European Cancer Patient’s Bill of Rights.
It is important to see how the proposals outlined in this review are interrelated, to examine the role of and benefits to the different stakeholders, as well as considering how they could be implemented and assessed for effectiveness.
Raising public awareness of the mBC voice, through initiatives such as the evidence package and the work accreditation scheme, would improve the representation of this group in society. Promoting the mBC voice locally means it would be heard in national political circles, potentially leading to development of laws and policies that would benefit patients, caregivers, employers and healthcare providers.
An organized and principled HCP education system that balances robust scientific evidence, a compassionate approach to treatment, economically viable practices and effective communication between all stakeholders is key for providing effective care. Therefore, ensuring implementation of high-quality mBC treatment guidelines in all countries, driven by patient empowerment, coupled with targeted HCP training and interventions to support management of non-oncology-related healthcare, would both assure a more consistent treatment approach and facilitate appropriate dialogue between patients, oncologists, PCPs and other HCPs for enhanced understanding and shared treatment decision making. To implement these measures, establishment of local patient education groups, in which patients would be able liaise with local HCPs to learn about and discuss treatment guidelines and available treatments, would be encouraged.
However, healthcare education and implementation of guidelines should consider the multicultural and diverse nature of the modern population inhabiting Europe. Patients with mBC belonging to different cultures, subcultures and societal groups require an individualized approach. Therefore, stakeholders, including HCPs, social workers and politicians, should work with representatives of each subpopulation to ensure that the right support is available to everyone regardless of their culture, ethnicity, creed, level of acculturation and/or sexual orientation.
Finally, HCPs are at the center of healthcare and by providing them with best resources and educational opportunities available, the authorities can ensure the best level of care. Implementation of guidelines, promoting the mBC voice and educating PCPs could help HCPs who provide oncology services to perform better. Underpinning this is the need to capture a comprehensive and accurate picture of the scope of the mBC population in each country, through national registries and real-world data collection. Furthermore, this, coupled with the proposed mBC HCP exchange program, would broaden the international cooperation on mBC treatment, allowing HCPs to make better-informed decisions and have a stronger understanding of the current clinical landscape.
5. Conclusions
The challenges faced by UPPs with mBC are wide-ranging and varied and, therefore, addressing them successfully requires a coordinated, multidisciplinary approach that involves all stakeholders, from patients to politicians. The disparities that exist across Europe must be tackled by cooperation and collaboration both within and between countries. Effective interventions must focus not only on the patients but also address the system, to improve care across the continuum of mBC evaluation and treatment. We have highlighted some of the high priority access barriers to healthcare facing UPPs with mBC and suggest actionable goals that countries and health systems can aim to implement through multifunctional stakeholder groups, in accordance with local needs and in synergy with existing initiatives. It is hoped that this could encourage a move towards building a revised approach to the treatment and care of one of the biggest burdens on women’s health in the 21st century.
Role of funder
The funders had no role in preparation of the manuscript.
Funding
Financial support for this review was provided by Pfizer Inc.
The meeting upon which this manuscript was based was instigated and funded by Pfizer and organized by WPP Health Practice (London, UK) on behalf of Pfizer.
Medical writing support was provided by Lindsay Queen of Darwin Healthcare Communications (London, UK) and was funded by Pfizer.
Declaration of competing interest
Emilio Alba: None. Concepción Biurrún: None. Fatima Cardoso:. Consultancy role for Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Sanofi, Samsung Bioepis, Seattle Genetics, Teva. PierFranco Conte: Speaker (Novartis, Roche, AstraZeneca); Travel grant (Novartis, Celgene, Tesaro); Institutional research grant (Novartis, Merck KGa, Roche, Bristol Myers Squibb) Rosanna D’Antona: None. Jacques De Grève: None. Joseph Gligorov: None. Françoise Meunier: None. Carlo Palmieri: Advisory boards and funding for clinical studies (Pfizer). Oriol Sola-Morales: Dr. Sola-Morales has consulted for most of the multinational pharmaceutical companies, including all ‘top 10’. He has received fees and honoraria for such consultancies and is currently engaged in several projects with many of these companies. He holds ownership of several start-up companies in the healthcare field and has had in the past stock ownership of ‘Top 10’ pharmaceutical companies. Luzia Travado: None. Catherine Ubaysi: Compensation for representing Patients en réseau at events. Roberta Ventura: None. Eduard Vrdoljak: Support for clinical trials and scientific projects (Pfizer, Roche, Bristol Myers Squibb, AstraZeneca); Speaker fees and consulting (Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Novartis, PharmaSwiss, Pfizer, Roche, Sanofi, MSD, Merck). Andrew Walker: Pharmaceutical & biotechnology companies (Abbvie, Akcea, Albireo, Alexion, Allergan, Astellas, AstraZeneca, Autolus, Avexis, Biocrysta, Bristol Myers Squibb, Calico, Celgene, Chiesi, Daiichi-Sankyo, Deciphera, Eli Lilly, Ferring, Galapagos, GSK, GW Pharma, Intercept, Ipsen, Janssen, Jazz, Kite/Gilead, Les Laboratories Servier, Lundbeck, Merck Serono, Mundibiopharma, Mylan, Norgine, Novartis, NovoNordisk, Pfizer, Pierre Fabre, RegenXBio, Takeda, UCB, Vertex). Consultancy companies (Adeptfield, Atheneum Partners, Bresmed, CB Partners, Charles Rivers Associates, Creativ Ceutical, Datamonitor, de Facto Research, Dolon, DRG, Evidera, Executive Insight, Fiecon, Fingerpost Consulting, Galbraith Wight, Guidehouse, Health Advances, HiTT, Huron Consulting Group, Inbeeo, Informa/Data Monitor, IQVIA, M2Econ, Market Access Transformation, Miller Economics, Mirador, Mtech Access, Navigant, Open Access, Pagoda S&L Int. Services, Parexel, Partners 4 Access, Plich Advisory Services, Precision Xtract, PRMA, PWC, QualWorld, Remap, Research Partnership, RJW Partners, SAI Med Partners, Simon Kucher, Therapeutic Challenges, Two Labs, Valid Insight, Verto, Wellmera, Windrose, ZS Associates). Public sector: University of Aberdeen. Theresa Wiseman: None. Lieve Wierinck: None. Rachel Wuerstlein: Agendia, Amgen, Aristo, AstraZeneca, Boehringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi-Sankyo, Eisai, Genomic Health, GlaxoSmithKline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Onkowissen, Paxman, Palleos, Pfizer, Pierre Fabre, Puma Biotechnology, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro, Teva.
Acknowledgements
The authors would like to extend sincere thanks to Richard Price, EU Affairs Policy Manager at ECCO, the European CanCer Organization, Jane Maher, Clinical Advisor at Macmillan Cancer Support, UK, and Arianna Stabile from Susan G. Komen Italia, for their valuable contributions to the meeting discussions and outputs.
Contributor Information
Eduard Vrdoljak, Email: edo.vrdoljak@gmail.com.
Joseph Gligorov, Email: joseph.gligorov@tnn.aphp.fr.
Lieve Wierinck, Email: lievewierinck@icloud.com.
PierFranco Conte, Email: pierfranco.conte@unipd.it.
Jacques De Grève, Email: jacques.degreve@uzbrussel.be.
Françoise Meunier, Email: doctormeunier@fmeunier.eu.
Carlo Palmieri, Email: C.Palmieri@liverpool.ac.uk.
Luzia Travado, Email: luzia.travado@fundacaochampalimaud.pt.
Andrew Walker, Email: andrew@salusalba.com.
Theresa Wiseman, Email: Theresa.Wiseman@rmh.nhs.uk.
Rachel Wuerstlein, Email: Rachel.Wuerstlein@med.uni-muenchen.de.
Emilio Alba, Email: ealbac@uma.es.
Concepción Biurrún, Email: cbiurrun57@gmail.com.
Rosanna D’Antona, Email: rosannadantona@europadonna.it.
Oriol Sola-Morales, Email: osola@fhitt.org.
Catherine Ubaysi, Email: catherine.ubaysi@gmail.com.
Roberta Ventura, Email: rventura@abcglobalalliance.org.
Fatima Cardoso, Email: fatimacardoso@fundacaochampalimaud.pt.
Appendix A. Premeeting survey questions and key findings
Survey questions
1. How would you describe the characteristics of underserved patient populations with breast cancer in your country? Please tick any of the factors below that you feel represent this population:
-
•
Low socio-economic status
-
•
Low levels of education
-
•
Low levels of health literacy/access to information
-
•Geography
-
oLocated in a rural setting and/or area with poor transport infrastructure
-
oLocated in an area where access to services is poor
-
o
-
•Ethnicity/culture
-
oFrom a ‘hard-to-reach’ community such as traveler groups
-
oLanguage barriers
-
oCultural beliefs/behaviors
-
o
-
•
Age-related issues
-
•
Suffer from comorbidities including mental health
-
•
Workplace issues
-
•
Other – please specify
2. Are any of these factors particularly relevant to patients with metastatic breast cancer? If yes, please outline which ones.
3. What are the biggest unmet needs for underserved patient populations with metastatic breast cancer?
4. What are the challenges in addressing the unmet needs of the UPPs in metastatic breast cancer or making these a priority in your country/the EU?
5. Are you aware of any specific examples such as papers or initiatives developed to address the UPPs needs in metastatic breast cancer? Please specify examples based on the following categories:
-
•
From a policy perspective
-
•
From a clinical perspective
-
•
From a patient/advocacy perspective
Survey responses
The principal unmet needs identified by the survey for the UPP with mBC were:
-
•
Better treatments, including equitable early access to new drugs
-
•
Access to quality specialized care (e.g. mBC-specific guidelines, early diagnosis, culturally sensitive care), especially a clinical specialist team with good communication skills
-
•
Access to disease and treatment information, including clinical trial recruitment
-
•Psychological/emotional support
- oGreater recognition of mBC and its impact (not just on patients but also on their caregivers and employers) at a national level (e.g. mBC Awareness Day)
The challenges in addressing the unmet needs of the UPPs in mBC or in making these a priority identified by the survey were:
-
•
A need for more multidisciplinary teams, incorporating psychologists, nutritionists, specialist nurses and community-based HCPs, as well as more specialist breast units and referral centers
-
•
A need for better communication between HCPs and patients and a greater understanding of the needs of diverse cultural populations by physicians
-
•
There is a lack of adequate cancer-control policies, and a need for more action by politicians to highlight the burden of mBC and promote initiatives to address unmet needs
-
•
Separate systems of governance and ‘postcode lotteries’ within countries may result in disparities in treatment between different regions
-
•
There is a bias towards prioritizing messages about early breast cancer over metastatic disease in many national awareness campaigns (e.g. Pink October)
Appendix B.
Challenges associated with mBC UPPs identified during the workshops at the expert meeting
| Category | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Healthcare availability and access | Lack of access to integrated and specialized care for patients with BC. | Lack of trained medical staff dedicated to cancer care. | Lack of specialists (and education of) with an interest in mBC. |
| Access to treatments. | Lack of early access to treatments. | Lack of access and knowledge of clinical trials. | |
| Organization of medical systems. | Need for improved access to clinical trials. | Lack of data and registries – no accurate counting of patients with mBC. | |
| Access to diagnosis and genetic testing. | Access in rural locations. | ||
| Education and awareness needs | Lack of training and knowledge of citizens. | Lack of awareness of mBC. | Lack of mBC awareness. |
| Lack of knowledge of and access to existing clinical trials. | Healthcare team training and education. | Information needed on treatment options. | |
| Lack of focus on PROs + QoL. | Poor workplace understanding. | ||
| Better physician–patient communication. | |||
| Ethnicity and cultural issues | Need for better communication between HCPs and patients. | Access to clinical trials for broader ethnic groups. | Age discrimination. |
| Religious and cultural barriers. | Lack of understanding of cultural needs and norms. | Cultural/ethnic minority challenges. | |
| Mismatch between patient and HCP perspectives. | |||
| Socioeconomic and workplace issues | Low socioeconomic status. | Socioeconomic status dictates levels of access to care. | Financial burden. |
| Social and economic support and flexible working policies. | Lack of workplace support and policies. | ||
| Including both guidelines and economic support in national cancer plans. | Overtreatment of patients. |
BC, breast cancer; HCP, healthcare professional; mBC, metastatic breast cancer; PRO, patient-reported outcome; QoL, quality of life; UPP, underserved patient population.
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F., Spence D., Mertz S. Global analysis of advanced/metastatic breast cancer: decade report (2005–2015) Breast. 2018;39:131–138. doi: 10.1016/j.breast.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F., Costa A., Norton L. ESO-ESMO 2nd International Consensus Guidelines for advanced breast cancer (ABC2) Breast. 2014;23:489–502. doi: 10.1016/j.breast.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Gobbini E., Ezzalfani M., Dieras V. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Canc. 2018;96:17–24. doi: 10.1016/j.ejca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Fietz T., Tesch H., Rauh J. Palliative systemic therapy and overall survival of 1,395 patients with advanced breast cancer – results from the prospective German TMK cohort study. Breast. 2017;34:122–130. doi: 10.1016/j.breast.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Sundquist M., Brudin L., Tejler G. Improved survival in metastatic breast cancer 1985–2016. Breast. 2017;31:46–50. doi: 10.1016/j.breast.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute SEER stat fact sheets: female breast cancer. 2016. http://seer.cancer.gov/statfacts/html/breast.html Available from.
- 8.Hartkopf A.D., Huober J., Volz B. Treatment landscape of advanced breast cancer patients with hormone receptor positive HER2 negative tumors – data from the German PRAEGNANT breast cancer registry. Breast. 2018;37:42–51. doi: 10.1016/j.breast.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Lux M.P., Nabieva N., Hartkopf A.D. Therapy landscape in patients with metastatic HER2-positive breast cancer: data from the PRAEGNANT real-world breast cancer registry. Cancers. 2019;11:10. doi: 10.3390/cancers11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCann K.E., Hurvitz S.A. Advances in the use of PARP inhibitor therapy for breast cancer. Drugs Context (US) 2018;7:212540. doi: 10.7573/dic.212540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy S.M., Caroll E., Nanda R. Atezolizumab for the treatment of breast cancer. Expert Rev Anticancer Ther. 2020:1–8. doi: 10.1080/14737140.2020.1732211. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso F., Senkus E., Costa A. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo H.S., Rumble R.B., Macrae E. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 14.Waks A.G., Winer E.P. Breast cancer treatment: a review. J Am Med Assoc. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 15.Avendaño C., Benn K., Biganzoli L. 2017. A policy roadmap on addressing metastatic breast cancer.https://lillypad.eu/WP/wp-content/uploads/MBC-Policy-Roadmap-Report-digital-version-17.09.2017.pdf Available from. [Google Scholar]
- 16.National Comprehensive Cancer Network NCCN breast cancer guidelines. https://www.nccn.org/professionals/physician_gls/default.aspx#site Available from.
- 17.Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol. 2014;5:465–477. doi: 10.5306/wjco.v5.i3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Cancer Patient Coalition (ECPC) Challenging the Europe of Disparities in Cancer. A framework for improved survival and better quality of life for European cancer patients. 2015. https://ecpc.org/wp-content/uploads/2019/08/ECPC-White-Paper-Europe-of-disparities-EN-3.pdf Available from.
- 19.Dixit N., Crawford G., Lemonde M. Left behind: cancer disparities in the developed world. Support Care Canc. 2016;24:3261–3264. doi: 10.1007/s00520-016-3192-4. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan R., Aggarwal A. Putting a price on cancer. Nat Rev Clin Oncol. 2016;13:137–138. doi: 10.1038/nrclinonc.2016.12. [DOI] [PubMed] [Google Scholar]
- 21.Byrne J., Capbell H., Gilchrist M. Barriers to care for breast cancer: a qualitative study in Ireland. Eur J Canc Care. 2017;27 doi: 10.1111/ecc.12876. [DOI] [PubMed] [Google Scholar]
- 22.Ginsburg O., Bray F., Coleman M.P. The global burden of women’s cancers: an unmet grand challenge in global health. Lancet. 2017;389:847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean L.T., Gehlert S., Neuhouser M.L. Social factors matter in cancer risk and survivorship. Cancer Causes Control. 2018;29:611–618. doi: 10.1007/s10552-018-1043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thrift-Perry M., Cabanes A., Cardoso F. Global analysis of metastatic breast cancer policy gaps and advocacy efforts across the patient journey. Breast. 2018;41:93–106. doi: 10.1016/j.breast.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Boyce K., White C., Hunt P. Inequalities in health? An update on the effect of social deprivation for patients with breast cancer in South East Wales. Surgeon. 2019;17:88–96. doi: 10.1016/j.surge.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Ren J.-X., Gong Y., Ling H. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Canc Res Treat. 2019;173:225–237. doi: 10.1007/s10549-018-4956-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breast Cancer Foundation New Zealand (BCFNZ) “I’m still here”. Insights into living - and dying – with advanced breast cancer in New Zealand. 2018. https://breastcancerfoundation.org.nz/Images/Assets/21894/1/BCFNZ-ABC-Report-2018-Executive-Summary.pdf Available from.
- 28.Allemani C., Matsuda T., Di Carlo V. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrdoljak E., Bodoky G., Jassem J. Cancer control in Central and Eastern Europe: current situation and recommendations for improvement. Oncol. 2016;21:1183–1190. doi: 10.1634/theoncologist.2016-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yedjou C.G., Sims J.N., Miele L. Health and racial disparity in breast cancer. Adv Exp Med Biol. 2019;1152:31–49. doi: 10.1007/978-3-030-20301-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyle G., Hendrie G.A., Hendrie D. Understanding the effects of socioeconomic status along the breast cancer continuum in Australian women: a systematic review of evidence. Int J Equity Health. 2017;16:182. doi: 10.1186/s12939-017-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreyer M.S., Nattinger A.B., McGinley E.L. Socioeconomic status and breast cancer treatment. Breast Canc Res Treat. 2018;167:1–8. doi: 10.1007/s10549-017-4490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teo I., Vilardaga J.P., Tan Y.P. A feasible and acceptable multicultural psychosocial intervention targeting symptom management in the context of advanced breast cancer. Psycho Oncol. 2020;29:389–397. doi: 10.1002/pon.5275. [DOI] [PubMed] [Google Scholar]
- 34.Travado L., Reis J.C., Watson M. Psychosocial oncology care resources in Europe: a study under the European partnership for action against cancer (EPAAC) Psycho Oncol. 2017;26:523–530. doi: 10.1002/pon.4044. [DOI] [PubMed] [Google Scholar]
- 35.European Cancer Patient Coalition (ECPC) Transforming Breast Cancer Together. White paper on a new collaborative initiative to improve breast cancer prevention, diagnosis and care across Europe. 2018. https://ecpc.org/wp-content/uploads/2019/08/ecpc-white-paper-transforming-breast-cancer-together-2.pdf Available from.
- 36.ABC Global Charter 2018. https://www.abcglobalalliance.org/abc-global-charter/ Available from.
- 37.Atun R., Jaffray D.A., Barton M.B. Expanding global access to radiotherapy. Lancet Oncol. 2015;16:1153–1186. doi: 10.1016/S1470-2045(15)00222-3. [DOI] [PubMed] [Google Scholar]
- 38.Economist Intelligence Unit (EIU) and European Society for Medical Oncology (ESMO) Cancer medicines shortages in Europe. Policy recommendations to prevent and manage shortages. 2017. http://www.eiu.com/graphics/marketing/pdf/ESMO-Cancer-medicines-shortages.pdf Available from.
- 39.All-Party Parliamentary Group on Breast Cancer A mixed picture: an inquiry into geographical inequalities and breast cancer breast. 2018. https://breastcancernow.org/sites/default/files/appgbc_a_mixed_picture.pdf Available from.
- 40.Blackwood O., Deb R. Multidisciplinary team approach in breast cancer care: benefits and challenges. Indian J Pathol Microbiol. 2020;63(Supplement):S105–S112. doi: 10.4103/IJPM.IJPM_885_19. [DOI] [PubMed] [Google Scholar]
- 41.Kesson E.M., Allardice G.M., George W.D. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344 doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Funding Guide . 2015. Requirements to join the Erasmus Programme.http://www.european-funding-guide.eu/articles/financing-tips/requirements-join-erasmus-programme Available from. [Google Scholar]
- 43.Corr M. So you want to be an erasmus medical student? Ulster Med J. 2016;85:60–61. [PMC free article] [PubMed] [Google Scholar]
- 44.European Commission Europe’s beating cancer plan. 2020. https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/12154-Europe-s-Beating-Cancer-Plan Available from.
- 45.Jelenc M., Albreht T., Budewig K. Cancer control joint action. European guide on quality improvement in comprehensive cancer control. Policy paper on national cancer control programmes (NCCPs) https://cancercontrol.eu/archived/uploads/PolicyPapers27032017/Policy_Paper_2_NCCP.pdf Available from.
- 46.Eicher M., Kadmon I., Claassen S. Training breast care nurses throughout Europe: the EONS post-basic curriculum for breast cancer nursing. Eur J Canc. 2012;48:1257–1262. doi: 10.1016/j.ejca.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Bechold R. How do I say this nicely? Your oncologist wasn’t following guidelines. https://www.cancernetwork.com/blog/how-do-i-say-nicely-your-oncologist-wasnt-following-guidelines Cancer Network. March 26, 2013. Available from.
- 48.André F., Neven P., Marinsek N. Disease management patterns for postmenopausal women in Europe with hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer. Curr Med Res Opin. 2014;30:1007–1016. doi: 10.1185/03007995.2014.887002. [DOI] [PubMed] [Google Scholar]
- 49.Bošković L., Gašparić M., Petković M. Bone health and adherence to vitamin D and calcium therapy in early breast cancer patients on endocrine therapy with aromatase inhibitors. Breast. 2017;31:16–19. doi: 10.1016/j.breast.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Lawler M., Le Chevalier T., Murphy M.J., Jr. A catalyst for change : the European cancer patient’s Bill of rights. Oncol. 2014;19:217–224. doi: 10.1634/theoncologist.2013-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawler M., Banks I., Law K. The European cancer patient’s Bill of rights, update and implementation 2016. ESMO Open. 2016;1 doi: 10.1136/esmoopen-2016-000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klabunde C.N., Ambs A., Keating N.L. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton W., Barrett J., Stapley S. Clinical features of metastatic cancer in primary care: a case–control study using medical records. Br J Gen Pract. 2015:e516. doi: 10.3399/bjgp15X686077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallner L.P., Li Y., Furgal A.K.C. Patient preferences for primary care provider roles in breast cancer survivorship care. J Clin Oncol. 2017;35:2942–2948. doi: 10.1200/JCO.2017.73.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudson S.V., Miller S.M., Hemler J. Adult cancer survivors discuss follow-up in primary care: “not what I want, but maybe what I need”. Ann Fam Med. 2012;10:418–427. doi: 10.1370/afm.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein R.M., Street R.L., Jr. National Cancer Institute, NIH Publication No. 07-6225; Bethesda, MD: 2007. Patient-centered communication in cancer care: promoting healing and reducing suffering. [Google Scholar]
- 57.Thomssen C., Lüftner D., Untch M. International consensus conference for advanced breast cancer, Lisbon 2019: ABC5 Consensus – assessment by a German Group of Experts. Breast Care. 2020;15:82–95. doi: 10.1159/000505957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clayton J.M., Hancock K.M., Butow P.N. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust. 2007;186:S77–S105. doi: 10.5694/j.1326-5377.2007.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 59.Gilligan T., Coyle N., Frankel R.M. Patient-clinician communication: American society of clinical oncology consensus guideline. J Clin Oncol. 2017;35:3618–3632. doi: 10.1200/JCO.2017.75.2311. [DOI] [PubMed] [Google Scholar]
- 60.Stover A.M., Tompkins Stricker C., Hammelef K. Using stakeholder engagement to overcome barriers to implementing patient-reported outcomes (PROs) in cancer care delivery. Med Care. 2019;57:S92–S99. doi: 10.1097/MLR.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 61.Wheeler S.B., Reeder-Hayes K.E., Carey L.A. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncol. 2013;18:986. doi: 10.1634/theoncologist.2013-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeder-Hayes K.E., Anderson B.O. Breast cancer disparities at home and abroad: a review of the challenges and opportunities for system-level change. Clin Canc Res. 2017;23:2655–2664. doi: 10.1158/1078-0432.CCR-16-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fontana F., Bischoff A. Uptake of breast cancer screening measures among immigrant and Swiss women in Switzerland. Swiss Med Wkly. 2008;138:752–758. doi: 10.4414/smw.2008.12328. [DOI] [PubMed] [Google Scholar]
- 64.Francovich L., Di Napoli A., Rossi P.G. Cervical and breast cancer screening among immigrant women resident in Italy [Article in Italian] Epidemiol Prev. 2017;41(3–4 Suppl 1):18–25. doi: 10.19191/EP17.3-4S1.P018.061. [DOI] [PubMed] [Google Scholar]
- 65.Graetz V., Rechel B., Groot W. Utilization of health care services by migrants in Europe - a systematic literature review. Br Med Bull. 2017;121:5–18. doi: 10.1093/bmb/ldw057. [DOI] [PubMed] [Google Scholar]
- 66.Schwachenwalde S., Sauzet O., Razum O. The role of acculturation in migrants’ use of gynecologic emergency departments. Int J Gynecol Obstet. 2020;149:24–30. doi: 10.1002/ijgo.13099. [DOI] [PubMed] [Google Scholar]
- 67.Brown M.T., McElroy J.A. Unmet support needs of sexual and gender minority breast cancer survivors. Support Care Canc. 2018;26:1189–1196. doi: 10.1007/s00520-017-3941-z. [DOI] [PubMed] [Google Scholar]
- 68.Forsea A.M. Cancer registries in Europe-going forward is the only option. Ecancermedicalscience. 2016;10:641. doi: 10.3332/ecancer.2016.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flowers M., Birkey Reffey S., Mertz S.A. Obstacles, opportunities and priorities for advancing metastatic breast cancer research. Canc Res. 2017;77:3386–3390. doi: 10.1158/0008-5472.CAN-17-0232. [DOI] [PubMed] [Google Scholar]
- 70.Lee J.Y., Lim S.H., Lee M.-Y. The impacts of inclusion in clinical trials on outcomes among patients with metastatic breast cancer (MBC) PloS One. 2016;11 doi: 10.1371/journal.pone.0149432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smerald G., Coaker R., Bloom E. Working through Cancer. Surveying experiences of cancer and work. https://www.macmillan.org.uk/_images/working-through-cancer_tcm9-341781.pdf Available from.
- 72.Wilson B. Five common misconceptions about Cancer at work. 2019. https://www.thehrdirector.com/features/health-and-wellbeing/five-common-misconceptions-cancer-work3292019/ Available from.