Abstract
Background
There is need to identify novel markers that lead to an early occurrence of myocardial infarction (MI) in young South Asian population. This population has different risk profile as compared with others. Telomere length is known to be a marker of aging, and shorter telomeres have been reported in cardiovascular diseases (CVDs). We aimed to identify the association of telomere length in young nonsmokers and non-diabetic MI patients.
Methods
In a case–control study of 154 subjects (n = 77 cases (ages 18–45 years, non-diabetic, non-smoker patients with MI) and n = 77, age and sex matched healthy controls), DNA extraction from peripheral blood leukocytes was carried out and the relative telomere length was estimated by quantitative PCR. The results were adjusted with various demographic parameters like age, gender and body mass index (BMI). The correlation studies were carried out between telomere length, sex and type of MI.
Results
The relative telomere length was significantly shorter in young MI patients (31–45 years) compared with matched healthy controls (p < 0.0001). Interestingly, in a gender-based comparison, the female patients had shorter telomere length (p < 0.01).
Conclusion
In this pilot study, we found that the telomere length was shorter among young, non-diabetic, non-smoker MI patients as compared with similar young controls without MI in a South Asian cohort. Thus, telomere length may be a potential screening tool for young patients who don't have conventional risk factors. Larger studies are needed to confirm these findings.
Keywords: Telomere, Young, Acute myocardial infarction, South Asian
1. Introduction
It is only over the last few decades that the impact of coronary artery disease (CAD) among very young South Asian population has been recognized. Although of significant importance, there are scarcity of data regarding risk factors and outcomes in young individuals (<45 years of age) suffering from an acute myocardial infarction (MI).1 Moreover, there is a growing interest in the increased risk and etiology of CAD in South Asians.2 Recent report suggests a close relationship between the biological age and atherosclerotic process indicating that the mechanisms involved in cellular senescence might have a role in endothelial dysfunction with consequent development of CAD.3,4 It may therefore be important to identify markers of cellular biological aging that are able to predict the risk of early onset of atherosclerosis, and consequently premature CAD. Eukaryotic chromosomes end in tandem repeats of the deoxyribonucleic acid (DNA) sequence TTAAGGG, which are known as telomeres, and their length can be used as surrogate markers for cellular aging, vascular aging, and inflammation.5 Accelerated telomere shortening has previously been demonstrated in patients with coronary artery disease (CAD), with recent studies supporting their causal role in CAD and other disorders.6 The present pilot study was carried out in young (≤45 years), non-smoker, non-diabetic patients with MI to analyze their demographic, clinical, and angiographic profile, and telomere length to evaluate its potential as an important marker of premature CAD.
2. Materials and methods
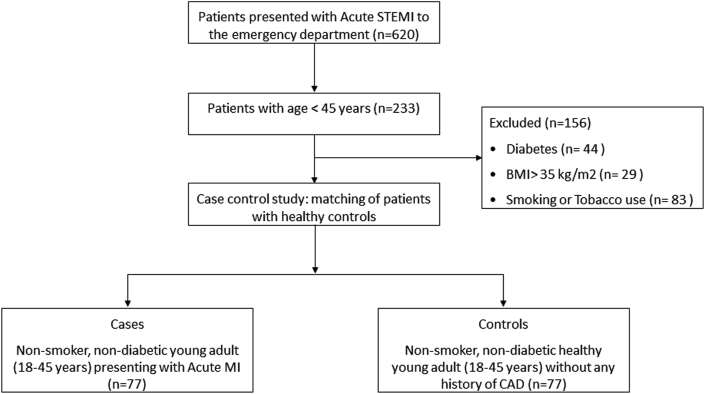
The study compared telomere lengths in 77 non-smoker, non-diabetic young adult patients (18–45 years of age) who presented with acute MI (cases), with 77 healthy, age- and sex-matched controls without a prior history of CAD. At the time of participation, all case subjects were 24–72 h from their acute event, and in a clinically stable condition. In order to isolate the impact of non-traditional risk factors, patients who were diabetic, had a body mass index (BMI) > 35 kg/m2, history of tobacco use, or were suspected of having an infectious disease or myocarditis were excluded from an original cohort of 233 patients (Fig. 1). Genomic DNA was extracted from peripheral blood leucocytes, quantified, and assessed for telomere length by quantitative reverse transcription polymerase chain reaction (RT-qPCR) method utilizing a validated protocol.7 This technique allows measurement of relative telomere length as a ratio of telomeric repeat copy (T) DNA to a single-copy gene (S) DNA (T/S ratio).
Fig. 1.
Consort diagram for the present study.
2.1. Statistical analysis
The statistical differences between cases and controls were calculated by SPSS version 16.0 using general linear models and applying univariate ANOVA. The data were adjusted for various demographic parameters like age, gender, and body mass index (BMI) to determine their effect on telomere length.
3. Results
The baseline characteristics of all the cases are given in Table 1. In this pilot study, we found that the mean age (±standard deviation) of cases (35.33 ± 6.22) and controls (34.38 ± 5.86), and BMI were similar (p > 0.05). The mean levels of low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and total cholesterol (TC) were significantly higher, and high-density lipoprotein cholesterol (HDL-C) levels were significantly lower among cases than controls (Table 1).
Table 1.
Characteristics of the study population.
| Variable | Cases (mean ± SD) | Controls (mean ± SD) | p value |
|---|---|---|---|
| Age (years) | 35.33 ± 6.22 | 34.38 ± 5.86 | 0.258 |
| Age group | |||
| (18–30 years) | 21 | 28 | 0.226 |
| (31–45 years) | 56 | 49 | |
| Gender | |||
| Male | 65 | 58 | 0.159 |
| Female | 12 | 19 | |
| SBP (mm Hg) | 123.40 ± 17.41 | 113.66 ± 12.43 | <0.001 |
| DBP (mm Hg) | 76.32 ± 8.65 | 71.58 ± 6.15 | <0.001 |
| BMI (kg/m2) | 23.994 ± 2.87 | 24.365 ± 3.23 | 0.374 |
| LV EF (%) | 39.50 ± 8.02 | 60.00 | <0.001 |
| LDL (mg/dl) | 92.33 ± 15.76 | 81.39 ± 7.60 | <0.001 |
| HDL (mg/dl) | 44.50 ± 7.90 | 52.72 ± 4.73 | <0.001 |
| TC (mg/dl) | 165.39 ± 13.23 | 159.48 ± 8.34 | <0.001 |
| TG (mg/dl) | 137.98 ± 11.17 | 125.68 ± 11.56 | <0.001 |
| Type of AMI | |||
|
52 (67.5%) | NA | NA |
|
18 (23.5%) | ||
|
7 (9%) | ||
| Treatment Strategy | |||
|
21 (27.5%) | NA | NA |
|
38 (49.5%) | ||
|
18 (23%) | ||
SD: standard deviation, SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index, LVEF: left ventricular ejection fraction, LDL: low density lipoprotein, HDL: high density lipoprotein, TC: total cholesterol, TG: triglycerides, AMI: acute myocardial infarction, AWMI: anterior wall myocardial infarction, IWMI: inferior wall myocardial infarction, LWMI: lateral wall myocardial infarction, PCI: percutaneous coronary intervention, NA: not applicable.
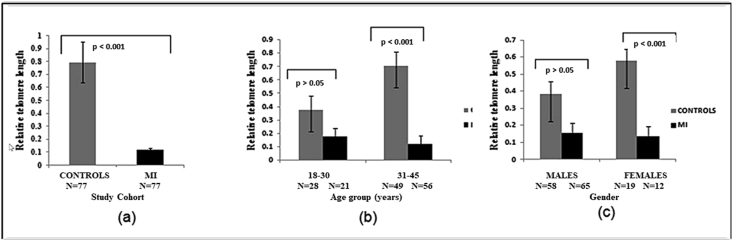
The age-, gender-, and BMI-adjusted relative telomere length (T/S ratio) was significantly higher among controls (0.792) compared with that of cases (0.115) (P < 0.001) (Fig. 2 a);
Fig. 2.
a) The age, gender and BMI adjusted relative telomere length (T/S ratio) expressed as (mean ± SE) among cases and controls. b) The gender and BMI-adjusted relative telomere length (mean ± SE) comparison among controls and cases in age group 18–30 and 31–45. c) The age and BMI-adjusted relative telomere length (mean ± SE) comparison among controls and cases in males and females.
The differences in relative telomere length were observed separately in different age groups, gender, and BMI, since they are known to affect telomeres. The gender- and BMI-adjusted relative telomere length was significantly higher among controls than cases in the age group 31–45 years (in the age group 18–30 years, relative telomere length was numerically higher among controls but didn't attain statistical significance (Fig. 2b));
The age- and BMI-adjusted relative telomere length was significantly higher among female controls than female cases (the relative telomere length was higher in controls in males as well, but didn't achieve statistical significance (Fig. 2c));
The age-, gender-, and BMI-adjusted relative telomere length was numerically lowest in cases with anterior wall MI than inferior wall/posterior wall MI followed by lateral wall MI, although this relationship did not meet statistical significance (p > 0.05).
4. Discussion
To the best of our knowledge, this is the first such study from South Asian population to compare telomere lengths in young individuals presenting with MI, who did not have other traditional risk factors for CAD such as diabetes mellitus, obesity, or smoking8 (Fig. 3). South Asians have different risk profile and there is urgent need to identify newer potential markers for premature CAD beyond conventional risk factors. Telomeres are altered under conditions of oxidative stress, and have long been considered a marker of cellular aging. Aging itself is a risk factor for increased susceptibility for cardiovascular diseases, but chronological age may often not indicate the extent of biological aging witnessed by accelerated cellular stress—a function of genetic and environmental factors.3,5,6 In that sense, telomere length could be an important marker of one's true risk for cardiovascular diseases. Moreover, atherosclerosis occurs in the setting of chronic inflammation with associated rapid white cell turnover, and consequently, may be associated with shorter telomere lengths.3,5,6 Thus, the extent of telomere shortening may be considered as a marker of the burden of atherosclerosis in an individual. The finding of longer telomeres in female patients compared with male patients may be attributed to the beneficial effect of estrogen-dependent activation of endothelial telomerase via phosphoinositol 3-kinase (PI3K)/Akt and nitric oxide signaling, and addition of hexametric repeats to telomeres.9 It is consistent with the traditionally observed increased risk of CAD in males as compared with females, and further strengthens the correlation of telomere length and increased cardiovascular risk. Smoking leads to increased oxidative stress and inflammation and is associated with shortened telomere length,10 in part why we performed this study only on non smokers. The telomere length was lower in cases with anterior wall MI than inferior wall/posterior wall MI followed by lateral wall MI, suggesting a possible relation between severity of clinical presentation and telomere length. An interesting finding was higher telomere length among patients with recanalised vessels than single-vessel disease followed by multivessel disease. Though it didn't achieve statistical significance, it suggests that telomere length might be a potential predictor of severity of the disease. It would be worthwhile investigating this in a larger population. The findings of our study align well with those of a limited few that show shorter leucocyte telomere length to be associated with increased cardiovascular risk,8,11 but are novel in that they highlight this correlation in a young South Asian population that otherwise did not have traditional cardiovascular risk factors. Reports are available to support that telomere length may be inversely correlated with modifiable risk factors including body mass index and total serum lipids.12 These findings when coupled with those of our study suggest further investigating telomere length as a putative tool for screening lower-risk individuals, and could have significant public health implications.
Fig. 3.
Telomere length and myocardial infarction.
Although unique, our pilot study was limited by its small sample size, cross-sectional nature, which do not imply causality. Moreover, adjusting for all factors that may play a role in affecting telomere length is beyond the scope of our study, and must be kept in mind while designing future studies.
Despite all of these limitations, this pilot study in South Asians identifies a potential novel marker in a younger population presenting with MI without conventional risk factors. Our findings reveal that shorter telomere length associate significantly with young MI. Telomere length might be used as an independent marker for predicting young MI. There is a need to have better understanding of telomeric behaviors in young MI patients to warrant future studies for looking at better therapeutics and diagnostics.
5. Conclusions
The assessment of telomere length in young MI patients, especially among cases without conventional risk factors, could lead to the development of screening tools for patients at risk for CAD, and warrant further large-scale studies to confirm these findings.
Source of funding
None.
Declaration of competing interest
None declared for all authors.
References
- 1.Fournier J.A., Cabezon S., Cayuela A., Ballesteros S.M., Cortacero J.A., Diaz De La Llera L.S. Long-term prognosis of patients having acute myocardial infarction when </=40 years of age. Am J Cardiol. 2004 Oct 15;94(8):989–992. doi: 10.1016/j.amjcard.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 2.Pais P., Pogue J., Gerstein H. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet. 1996 Aug 10;348(9024):358–363. doi: 10.1016/s0140-6736(96)02507-x. [DOI] [PubMed] [Google Scholar]
- 3.Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123(7):849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Codd V., Nelson C.P., Albrecht E. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013 Apr;45(4):422–427. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.S Olovnikov A.M. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996 Jul-Aug;31(4):443–448. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.V Samani N.J., Boultby R., Butler R., Thompson J.R., Goodall A.H. Telomere shortening in atherosclerosis. Lancet. 2001 Aug 11;358(9280):472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 7.Cawthon Richard M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouilette S., Singh R.K., Thompson J.R., Goodall A.H., Samani N.J. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 9.Edo M.D., Andrés V. Aging, telomeres, and atherosclerosis. Cardiovasc Res. 2005;66(2):213–221. doi: 10.1016/j.cardiores.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Babizhayev M.A., Yegorov Yegor E. Smoking and Health: association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fund Clin Pharmacol. 2011;25(4):425–442. doi: 10.1111/j.1472-8206.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H., Ozono R., Suyama C., Sueda T., Kambe M., Oshima T. Telomere attrition in white blood cell correlating with cardiovascular damage. Hypertens Res. 2004;27(5):319–325. doi: 10.1291/hypres.27.319. [DOI] [PubMed] [Google Scholar]
- 12.Karimi B., Yunesian M., Nabizadeh R., Mehdipour P. Serum level of total lipids and telomere length in the male population: a cross-sectional study. Am J Men's Health. 2019 Apr;13(2) doi: 10.1177/1557988319842973. 1557988319842973. [DOI] [PMC free article] [PubMed] [Google Scholar]