This study reports substantial variation in the swimming ability of river-resident fish at three hierarchical levels: species, population and individual. Differences in swimming ability were related to a range of morphological and physiological traits. The results highlight the dangers of using average swimming speeds as a basis for barrier mitigation.
Keywords: barrier passage, fish pass, metabolism, morphology, respirometry, selective effects
Abstract
Artificial barriers cause widespread impacts on freshwater fish. Swimming performance is often used as the key metric in assessing fishes’ responses to river barriers. However, barrier mitigation is generally based on the swimming ability of salmonids and other strong swimmers because knowledge of swimming ability for most other freshwater fish is poor. Also, fish pass designs tend to adopt a ‘one size fits all’ approach because little is known about population or individual variability in swimming performance. Here, we assessed interspecific and intraspecific differences in the sustained swimming speed (Usus) of five freshwater fish with contrasting body sizes, morphologies and swimming modes: topmouth gudgeon, European minnow, stone loach, bullhead and brown trout. Significant Usus variation was identified at three organizational levels: species, populations and individual. Interspecific differences in Usus were as large as 64 cm s−1, upstream populations of brown trout showed mean Usus 27 cm s−1 higher than downstream populations, and species exhibited high individual variation (e.g. cv = 62% in European minnow). Sustained swimming speed (Usus) increased significantly with body size in topmouth gudgeon, European minnow and brown trout, but not in the two benthic species, bullhead and stone loach. Aerobic scope had a significant positive effect on Usus in European minnow, stone loach and brown trout. Sustained swimming speed (Usus) decreased with relative pectoral fin length in European minnow and brown trout, whereas body fineness was the best predictor in stone loach and bullhead. Hence, swimming performance correlated with a diverse range of traits that are rarely considered when predicting fish passage. Our study highlights the dangers of using species’ average swimming speeds and illustrates why a ‘one size fits all’ approach often fails to mitigate for barrier effects. We call for an evidence-based approach to barrier mitigation, one that recognizes natural variability at multiple hierarchical levels.
Introduction
Artificial barriers such as dams, weirs and culverts are ubiquitous in rivers worldwide (Lehner et al., 2011; Januchowski-Hartley et al., 2013; Grill et al., 2019; Belletti et al., 2020) and cause numerous impacts on freshwater fish populations, including habitat fragmentation (Morita and Yamamoto, 2002; Santucci Jr et al., 2005), disrupted migrations (Lucas and Baras, 2008) and reduced connectivity (Wofford et al., 2005), which can make populations more vulnerable to other anthropogenic pressures (Fagan, 2002). To mitigate barrier impacts on fish, natural resource managers should identify those that are causing the most severe impacts (Kemp and O’Hanley, 2010). Passage through velocity barriers, such as culverts and sloping ramps, is highly dependent on fish swimming speed (Haro et al., 2004; Peake, 2004; Castro-Santos, 2005; Castro-Santos, 2006; Weibel and Peter, 2013). Swimming performance data are also critical for the design of effective fish passes to provide passage over vertical barriers such as dams and weirs (Katopodis, 1992; Clay, 1995).
There has been historical bias in fish passage research, which has tended to focus on large, commercially important salmonids (Clay, 1995; Roscoe and Hinch, 2010). Crucially, salmonids are the ‘elite athletes’ of river fish communities (Webb, 1975), well known for their high swimming speeds and jumping ability (Stuart, 1962), and hence represent an exception, rather than a fair characterization of the wider river fish community (Birnie-Gauvin et al., 2019). A lack of swimming performance data for most non-salmonid species is likely to be one of the underlying reasons why salmonids appear to be three times more likely to pass the average fish pass (Noonan et al., 2012). There has also been a tendency to focus on diadromous species when considering barrier effects, while river-resident taxa have largely been ignored (Lucas and Batley, 1996). This is perhaps due to the misconception that species that complete their lifecycles in rivers are sedentary and their longitudinal movements are negligible (Gerking, 1959). However, it is increasingly recognized that river-resident species regularly undertake long-distance movements for spawning (e.g. Lucas and Batley, 1996) and foraging (e.g. Schoby and Keeley, 2011) and these movements are also important to maintain gene flow between populations (Wofford et al., 2005). Hence, river-resident fish are also impacted by barriers, and knowledge of these species’ swimming abilities is crucial for predicting barrier effects, as well as identifying effective mitigation options (Kemp and O’Hanley, 2010).
Barriers (both natural and artificial) can affect colonization by invasive species (e.g. Townsend and Crowl, 1991; Vitule et al., 2012; Robinson et al., 2019), and in some cases, selective barriers have been used as a management tool (Rahel and McLaughlin, 2018). Consequently, there is often a trade-off between preventing the spread of invasive species and ensuring population connectivity of native species. Where invasive species are present, effective barrier management therefore requires detailed knowledge of the swimming ability of invasive species, as well as native taxa.
Fish passage guidelines tend to prescribe maximum flow velocities and barrier heights that should not be exceeded, and these are deemed suitable for broad groups of fish. For example, the UK Environment Agency fish pass guidelines suggest maximum flow velocities of 1.4–2.0 m s−1, and differential heads of 0.1–0.2 m, in a pool pass to ensure passage of ‘coarse fish’ (any freshwater fish other than salmonids; Armstrong et al., 2010). Such broad generalizations ignore potential variability in swimming performance, both at inter- and intraspecific levels (Taylor and McPhail, 1985; Tudorache et al., 2008).
River fish communities consist of species with different body shapes, physiological traits and swimming modes that define their realized niches (Willis et al., 2005; Poff and Allan, 1995; Montaña et al., 2014; Pang et al., 2020). Additionally, individuals of the same species can show substantial trait variation at the population level due to adaptation to local environmental conditions (e.g. Taylor and McPhail, 1985; Pakkasmaa and Piironen, 2001; Webster et al., 2011). Riverine habitats show predictable longitudinal changes (Vannote et al., 1980), with headwater streams tending to be more turbulent and fast flowing, while lower catchment reaches tend to provide more slow-flowing habitat. These conditions should select for higher swimming ability in upstream headwater populations compared to downstream lowland populations. Even similar-sized individuals from the same population can vary 2-fold in swimming speed (Ojanguren and Braña, 2003), as well as differing markedly in functionally relevant morphological (Boily and Magnan, 2002) and physiological traits (Metcalfe et al., 2016).
Here, we examined the extent of the interspecific and intraspecific variation in swimming performance of five species belonging to four contrasting families: two cyprinids (topmouth gudgeon Pseudorasbora parva and European minnow Phoxinus phoxinus), one nemacheilid loach (stone loach Barbatula barbatula), one cottid (bullhead Cottus gobio) and one salmonid (brown trout Salmo trutta). These species were chosen because they occupy contrasting habitat types (Maitland and Linsell, 2006), vary widely in body size and shape, and differ in swimming mode. Moreover, topmouth gudgeon, brown trout and European minnow have established invasive populations outside their native ranges, often with severe ecological impacts (Pinder et al., 2005; Museth et al., 2007; Jones and Closs, 2018), and swimming performance data for these species are important for invasive species management (Rahel and McLaughlin, 2018).
Materials and methods
Study species
Between 26 and 36 individuals of each species were collected by electric fishing (HT-2000 backpack machine, Halltech Aquatic Inc., Ontario, Canada) from populations in rivers and lakes in Wales (Table S1) in summer 2017 when water temperatures were between 15°C and 18°C. Topmouth gudgeon, European minnow, stone loach and bullhead were each collected from a single population, whereas brown trout was collected from two distinct catchments, each sampled from an upstream headwater and a downstream lowland site, to assess population-level variability in swimming performance. Brown trout was chosen for the population level study because they occur in a wide range of fluvial habitat types, ranging from small headwater streams to large slow-flowing rivers. Upstream sites were high elevation, steeply sloping, second-order streams characterized by turbulent fast flow, while downstream sites were lower catchment, low gradient, fifth-order rivers that offered more slow-flowing habitat (Table S1).
Fish were housed in separate 200 l cylindrical tanks in a 2500 l recirculating aquaculture system (TMC System 5000P, Tropical Marine Centre Ltd, Hertfordshire, UK). Individuals were marked using unique combinations of visual implant elastomer tags (Northwest Marine Technology, Anacortes, USA) and left to acclimatize for at least two weeks before swimming tests. Housing water temperature was maintained at 15 ± 1°C and photoperiod was set to 12 h:12 h light/dark cycle. Fish were fed daily (9 am) to satiation on pellet food (Atlantic Gold, Pacific Trading Aquaculture Ltd, Dublin, Ireland), supplemented with live maggots and frozen bloodworm.
Swimming performance and metabolism
Swimming performance and metabolic rate (MR) were measured in one of four different sized swim tunnel respirometers (Loligo Systems, Viborg, Denmark), three Blaska-type tunnels and one Steffensen-type swim tunnel (Table S2; Fig. S1). The use of different tunnels ensured a suitable fish volume:water volume ratio for accurate measurement of MR (Svendsen et al., 2016). We followed best practice recommendations for allocating fish to different tunnels according to body weight (www.loligosystems.com; Table S2). Because fish size to tunnel size was kept as constant as possible, we are confident that potential side wall effects on swimming were kept to a minimum. Water velocities for each tunnel were carefully calibrated either using a purpose-built AC10000 flow meter (Loligo Systems, Viborg, Denmark) or using a proven dye tracing technique (Poulsen et al., 2012). Swimming speeds were also corrected for solid blocking effects (the increase in water velocity surrounding the fish caused by the fish body blocking a portion of the tunnel) following standard methodologies (Bell and Terhune, 1970). Water temperature was maintained at 15 ± 0.1°C in ambient water tanks using a temperature control set (Model AC10150; Loligo Systems, Viborg, Denmark). Air stones in ambient tanks ensured dissolved oxygen was always near saturation (>95%). Weekly cleaning of equipment and UV treatment of water ensured that bacterial respiration (measured at the end of each experiment) was negligible.
Test fish were weighed (±0.1 g) and measured for total body length (BL, mm) and maximum body girth (MBG, mm; see Fig. S2), before being introduced individually into the respirometers at 5 pm daily. AutoResp software (Loligo Systems, Viborg, Denmark) was used to automate the flush (180 s), wait (60 s) and measurement periods (420 s). Preliminary trials indicated that this flushing rate was sufficient to ensure dissolved oxygen never fell below 80%, and measurement periods were long enough to ensure an R2 > 0.9 for accurate measurement of O2 consumption (Genz et al., 2013). Flow velocities were set to 1 cm s−1 to ensure adequate mixing of test water, and fish were left to acclimatize overnight. Oxygen partial pressure (kPa) in the test chambers was measured using fibre optic sensors (OX11250; Loligo Systems, Denmark) and mass-specific oxygen consumption rates (MO2; mgO2 kg−1 h−1) were calculated for each measurement phase using AutoResp software. Mass-specific oxygen consumption rates were used as a proxy for MR (Norin and Malte, 2011; Svendsen et al., 2013). Standard MR (SMR) was recorded at 9 am the following morning, calculated as the mean of the 10 lowest MR values during the 16 h test period (Norin and Malte, 2011).
Immediately after measurement of SMR, velocity was incrementally increased to measure maximum MR (MMR) and sustained swimming speed (Usus). Sustained swimming speed (Usus) is a measure of the aerobic swimming ability of fish (Brett, 1965), shows individual repeatability (Oufiero and Garland Jr, 2009) and is one of the most widely used metrics used in fish pass design (e.g. Clough et al., 2004; Laborde et al., 2016). The upstream half of the swim tunnels was covered to encourage a rheotactic response against the current (Fig. S1). Test velocities started at 5 cm s−1 and were increased in 5 cm s−1 increments every 9 min, while measuring MO2 (180 s flush, 60 s wait, 300 s measure), until fish stopped swimming effectively against the current. For the species that predominantly swam higher in the water column (topmouth gudgeon, European minnow and to a lesser extent brown trout), Usus was defined as the point at which fish switched from a steady to an unsteady locomotory gait (Drucker, 1996). This point, known as ‘gait transition speed’, is recognizable in a range of fish species and is a reliable point at which to measure MMR and Usus (Peake, 2008). Gait transition was not appropriate to measure Usus in bullhead and stone loach because preliminary trials indicated that they did not show consistent active swimming but rather tended to use their pectoral fins and occasional tail beats to hold a benthic position at the upstream end of the swim tunnels. For these two species, Usus was recorded at the point at which fish failed to maintain position at the upstream end of the chamber for over 10 s. Fish were observed constantly during swimming trials to identify the endpoints described above. MMR was estimated as the highest MO2 recorded (over a full 300 s measurement period), and aerobic scope (AS) was calculated as MMR minus SMR (Metcalfe et al., 2016).
Morphology
After testing in the respirometer, fish were euthanized via an overdose of 2-phenoxyethanol (following Home Office Schedule 1 procedures) and standardized photos (dorsal and lateral views) were taken using an overhead camera (Panasonic Lumix G2). Total BL, MBG, pectoral fin length (PL), caudal fin height (CL) and caudal fin area (CA) were measured (±1 mm; Fig. S2) using ImageJ (Schneider et al., 2012). Three metrics of body morphology were calculated due to their relevance for swimming ability (Fig. S2). Aspect ratio (AR) is a metric derived from the height and surface area of the caudal fin, and individuals with higher AR generally show higher swimming performance (Sambilay, 1990). Fineness ratio (FR) is a measure of how streamlined fish are, and more streamlined individuals tend to show higher swimming performance (Baktoft et al., 2016). Pectoral fin length ratio (PFLR) is a measure of pectoral fin length relative to BL, and individuals with longer pectoral fins tend to show higher swimming performance (Ojanguren and Braña, 2003).
Statistical analysis
Interspecific differences in Usus were tested by ‘ANCOVA’, with Usus as the response variable, and ‘Species’ as the predictor, while statistically controlling for the effect of BL. Slope comparisons were examined using the ‘emtrends’ function in R package ‘emmeans’ (Lenth, 2019) to calculate Bonferroni corrections for multiple pairwise comparisons. Intercept comparisons were carried out using the ‘emmeans’ function in the same package. Interspecific differences in physiological and morphological traits were evaluated by general linear models with SMR, AS, MMR, BL, FR, PFLR and AR as the response variables and ‘Species’ as the explanatory variable. Trait values were log or square root transformed to stabilize variances and normalize residuals, where necessary.
Interspecific differences in the relationship between MR and swimming speed were explored using a linear mixed-effects model (LMM), with MR as the response variable, ‘Swimming speed’ and ‘Species’ and their interaction as fixed factors, and individual ‘FishID’ as a random factor to account for multiple measurements (at different swimming speeds) at the individual level. Pairwise comparisons of slope and intercept were carried out using the ‘emtrends’ and ‘emmeans’ functions.
Inter-population differences in Usus, SMR, MMR, AS, BL, FR, PFLR and AR in brown trout were examined using separate LMMs, with ‘Location’ (i.e. upstream or downstream) and BL as fixed effects and ‘Catchment’ as a random factor. The ‘lmerTest’ package (Kuznetsova et al., 2017) was used to estimate the statistical significance of model coefficients using the Satterthwaite’s approximation to calculate degrees of freedom.
Relationships between individual Usus and traits were assessed using separate LMs for each species, fitting Usus as the response variable and traits (BL, SMR, AS, FR, PFLR and AR) as explanatory variables. Model selection was undertaken using the ‘dredge’ function in the R package ‘MuMIn’ (Barton, 2018) to identify the most parsimonious model by minimizing corrected Akaike Information Criteria (AICc). Where more than one candidate model had similar levels of support (∆AICc < 2), the ‘model.avg’ function in ‘MuMIn’ was used to calculate parameter estimates across the ‘top model set’ (Grueber et al., 2011). All statistics were carried out using R statistical software (Version 3.6.1; R Core Team, 2019).
Results
Interspecific variation
Sustained swimming ability showed significant interspecific differences (F4,144 = 53.97, P < 0.001), when the effect of BL (F1,144 = 74.50, P < 0.001) was accounted for (Fig. 1), and there was a significant interaction between ‘Species’ and BL (F4,144 = 5.84, P < 0.001). Mean Usus ranged from a minimum of 35 ± 5 cm s−1 in topmouth gudgeon to 99 ± 10 cm s−1 in brown trout (mean ± 95% confidence interval (CI); Fig. 2). European minnow showed a significantly higher slope than all other species (pairwise differences: ∆β ≥ 1.22, t.ratio144 ≥ 2.86, P < 0.039), except topmouth gudgeon (pairwise difference: ∆β = 0.99 ± 0.53, t.ratio144 = 1.87, P = 0.336). No other interspecific differences in slope were statistically significant (β ≤ 0.63, t4,144 ≤ 1.32, P ≥ 0.679). Controlling for the effect of BL, two pairwise species comparisons were significant: European minnow showed significantly higher Usus than both stone loach (α = 46 ± 14, t.ratio144 = 3.31, P = 0.010) and trout (α = 43 ± 11, t.ratio144 = 3.98, P = 0.001). There was little indication that interspecific variation in Usus was related to any of the other traits measured (Table 1).
Figure 1.
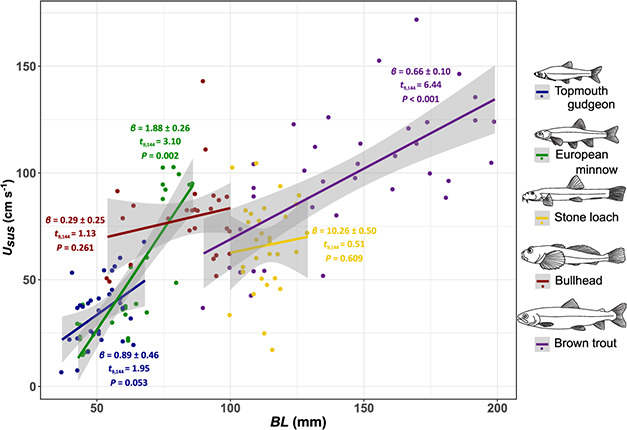
Interspecific variation in sustained swimming speed (Usus) with BL; slope estimates (β), t values and P values provided for each species.
Figure 2.
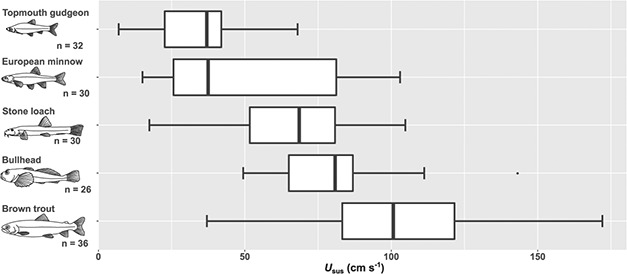
Interspecific differences in sustained swimming speed (Usus).
Table 1.
Interspecific differences in physiological and morphological traits and Usus for comparison (mean ± SE).
Topmouth gudgeon  n = 32 n = 32 |
European minnow  n = 30 n = 30 |
Stone loach  n = 30
n = 30 |
Bullhead  n = 26 n = 26 |
Brown trout  n = 36 n = 36 |
|
|---|---|---|---|---|---|
| Physiological trait | |||||
| SMR (mgO2 kg−1 h−1) | 138 ± 7A | 199 ± 13B | 109 ± 5C | 108 ± 5C | 122 ± 3A |
| MMR (mgO2 kg−1 h−1) | 473 ± 27A | 712 ± 37B | 403 ± 21C | 360 ± 11C | 534 ± 12D |
| AS (mgO2 kg−1 h−1) | 334 ± 24A | 512 ± 41B | 294 ± 20AC | 252 ± 13C | 411 ± 12D |
| Morphological trait | |||||
| BL (mm) | 53 ± 1A | 64 ± 3B | 116 ± 1C | 86 ± 3D | 147 ± 6E |
| FR (ratio) | 0.210 ± 0.003AD | 0.204 ± 0.003AB | 0.155 ± 0.005C | 0.216 ± 0.007D | 0.197 ± 0.003B |
| PFLR (ratio) | 0.137 ± 0.004A | 0.174 ± 0.003B | 0.153 ± 0.003C | 0.247 ± 0.007D | 0.176 ± 0.003B |
| AR (ratio) | 2.65 ± 0.10A | 2.34 ± 0.12B | 1.28 ± 0.04C | 1.19 ± 0.06C | 2.10 ± 0.04B |
SMR, standard metabolic rate; MMR, maximum metabolic rate; AS, aerobic scope; BL, body length; FR, fineness ratio; PFLR, pectoral fin length ratio; AR, aspect ratio; significant differences are denoted by different letters.
There were significant interspecific differences in the relationship between MR and swimming speed (F9,1220 = 128.6, P < 0.001; Fig. 3). European minnow and topmouth gudgeon showed substantially higher mass-specific MRs (typically 200–300 mgO2 kg−1 h−1) at low swimming speeds (<20 cm s−1) compared to stone loach, bullhead and brown trout (typically 100–150 mgO2 kg−1 h−1). Bullhead and brown trout showed significantly lower slopes in the relationship between MR and swimming speed compared to European minnow and topmouth gudgeon (t.ratio173–215 > −4.01, P < 0.001). These differences in swimming energetics were largely in line with behavioural observations during swimming trials: European minnow and topmouth gudgeon actively swam even at very low current speeds, whereas bullhead, brown trout and stone loach tended to maintain position using their pectoral fins at lower current speeds (<50 cm s−1), generally only swimming actively at current speeds exceeding 50 cm s−1.
Figure 3.
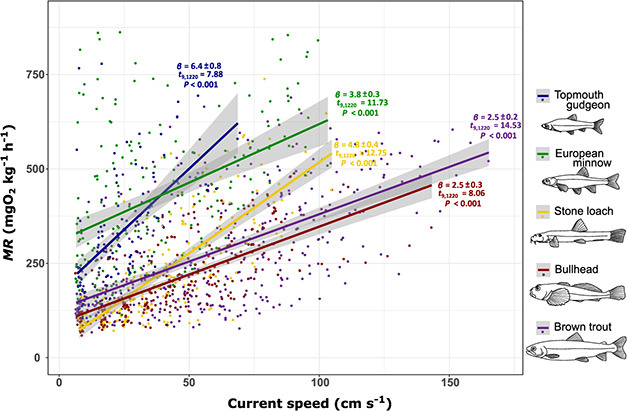
Interspecific variation in MR with current speed in swimming respirometers; slope estimates, t values and P values for each species.
Population variation in brown trout
Brown trout from upstream populations showed significantly higher Usus (mean ± standard error (SE) = 115 ± 7 cm s−1) than those from downstream populations (mean ± SE = 88 ±7 cm s−1; Fig. 4), when the effects of BL and ‘Catchment’ were controlled for (t32 = 2.97, α = 21.84 ± 7.35, P = 0.006). No upstream–downstream population trait differences were observed, except for upstream populations of brown trout showing significantly lower PFLR (t34 = −2.36, b = −0.007 ± 0.003, P = 0.024; Table S3).
Figure 4.
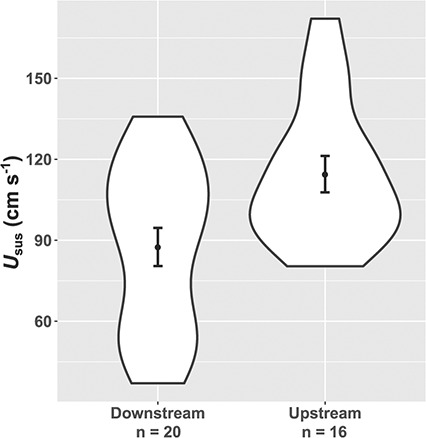
Differences in sustained swimming speed (Usus) between brown trout from upstream headwater and downstream lowland populations (mean ± SE).
Individual variation
There was substantial intraspecific variation in Usus (Fig. 2), with bullhead varying the least (cv = 25%) and European minnow the most (cv = 62%). Sustained swimming speed (Usus) increased significantly with BL in topmouth gudgeon, European minnow and brown trout, but not in bullhead or stone loach (Table 2). AS showed a significant positive relationship with Usus in European minnow, stone loach and brown trout, but not in bullhead or topmouth gudgeon. Sustained swimming speed (Usus) increased significantly with FR only in stone loach (P = 0.014).
Table 2.
Model averaged parameter estimates for best performing models (∆AICc < 2) predicting relationship between intraspecific variation in Usus and the various morphological and physiological traits examined
| Species | Trait | β ± SE | z or (t) value | P value |
|---|---|---|---|---|
Topmouth gudgeon (n = 32) 
|
BL AS | 0.90 ± 0.32 0.02 ± 0.02 | 2.67 0.45 | 0.008 0.655 |
European minnow (n = 30) 
|
BL AS PFLR | 1.74 ± 0.20 0.04 ± 0.01 −454 ± 165 | (8.77) (3.05) (−2.75) | <0.001 0.005 0.011 |
Stoneloach (n = 30) 
|
AS FR SMR | 0.09 ± 0.03 315 ± 122 0.14 ± 0.14 | 2.78 2.47 1.00 | 0.005 0.014 0.316 |
Bullhead (n = 26) 
|
FR AR BL | 226 ± 122 19 ± 13 0.29 ± 0.26 | 1.77 1.40 1.06 | 0.077 0.160 0.288 |
Trout (n = 36) 
|
AS BL PFLR AR SMR | 0.14 ± 0.05 0.66 ± 0.12 −924 ± 426 −22 ± 13 −0.27 ± 0.18 | 2.57 5.15 2.09 1.61 1.43 | 0.010 <0.001 0.037 0.106 0.151 |
BL, body length; SMR, standard metabolic rate; AS, aerobic scope; FR, fineness ratio; PFLR, pectoral fin length ratio; AR, aspect ratio.
Discussion
Our study reveals that a ‘one size fits all’ approach for estimating fish swimming performance in relation to barrier passability is not tenable. Substantial interspecific variation in swimming ability was observed, with mean Usus differing by as much as 64 cm s−1 even among species of similar body size. As barrier impacts are related to swimming ability (Castro-Santos, 2006; Makrakis et al., 2007; Castro-Santos and Haro, 2010), our study indicates considerable potential for velocity barriers to select against weak swimmers. Moreover, as the interspecific differences in Usus were strongly influenced by body size, barriers may have size-selective effects (Volpato et al., 2009; Noonan et al., 2012).
Barriers associated with road crossings (e.g. culverts) tend to be abundant in river systems globally (Januchowski-Hartley et al., 2013; Mantel et al., 2017; Jones et al., 2019; Belletti et al., 2020), so, based on our data, selective effects based on swimming ability and body size are likely to be widespread. Passage of culverts by weaker swimming fish can be facilitated by adding baffles (Newbold et al., 2014), and decreasing baffle spacing can improve passage of small-bodied fish (Cabonce et al., 2019). Our results also serve to highlight the challenge of designing efficient fish passes for diverse groups of fish. Fish pass hydraulics should be designed with flow velocities low enough to accommodate the weakest-swimming target fish. However, faster-swimming fish can sometimes be deterred from entering fish passes with insufficient attraction flows (Williams et al., 2012). In this sense, fish passes that provide diverse flow conditions (e.g. nature-like fishways) are likely to be most successful in allowing passage of groups of fish with contrasting swimming abilities (Bunt et al., 2012; Williams et al., 2012).
MR increased rapidly with flow velocity in most species, suggesting that even swimming at speeds considerably lower than Usus requires substantial energetic expenditure, which has considerable implications for predicting barrier effects on fish movement. For example, while passage of a single instream structure might be well within the maximum swimming speed of fish, the presence of multiple structures will likely have a cumulative effect that may be beyond their energetic scope (Armstrong et al., 2010; Roscoe and Hinch, 2010). Additionally, the energetic cost of passage may leave fish with insufficient energy reserves to reproduce or complete other basic life history functions (Caudill et al., 2007; Thiem et al., 2016). There are also clear implications for the provision of resting pools in fish pass design, which are added in an effort to prevent fatigue (Katopodis, 1992; Castro-Santos and Haro, 2010; Williams et al., 2012). If flow speeds within resting pools are not sufficiently low, fish may be unable to negotiate other parts of the fish pass (Castro-Santos and Haro, 2010). Our study indicates that more benthic-swimming species (bullhead, stone loach and to a lesser extent brown trout) were able to maintain position in low flow velocities (5–15 cm s−1) with relative ease (close to SMR values) by holding position using pectoral fins, while the more pelagic species (European minnow and topmouth gudgeon) had to spend substantially more energy by active swimming. Thus, flow velocities in resting pools may need to be lower for some pelagic-swimming species than for benthic fish.
Velocity barriers rarely present uniform flow conditions, and fish passes generally offer resting places with slow flows, pinch points where maximum flows are found and a range of flow speeds between these extremes (Katopodis, 1992; Clay, 1995). The Environment Agency (UK) fish pass guidelines (Armstrong et al., 2010) suggest maximum flows of 1.1 m s−1 in culverts to allow passage of course fish and less than 1.25 m s−1 to allow passage of brown trout. These values were higher than the Usus of 98% of course fish and 86% of brown trout in our study. Prescribed flow speeds for pool passes are also higher than the Usus of the vast majority of fish in our study (1.4–2.0 m s−1 for coarse fish and 1.7–2.4 m s−1 for brown trout; Armstrong et al., 2010). Fish use a combination of anaerobic burst (at pinch points), sustained (moderate velocity areas) and endurance (in rest areas) swimming types to negotiate obstacles (Castro-Santos, 2006) so the guideline flow speeds would not necessarily prevent passage. However, our data do suggest that even culverts and fish passes built to best practice guidelines are likely to be energetically demanding for many fish and a large proportion of fish are likely to be excluded from upstream passage. The poor performance of fish passes globally (Noonan et al., 2012) is likely to be at least in part due to overestimation of swimming performance and underestimation of the energetic demands of passage. Some options for improving passage efficiency include reducing flow speeds, increasing rest areas and limiting the number of pinch points where energetically demanding burst swimming is required.
The significantly higher Usus observed in upstream populations of brown trout compared to downstream populations is consistent with a priori predictions, based on higher flow velocities in headwater areas selecting for higher swimming ability (Taylor and McPhail, 1985; Páez et al., 2008; Leavy and Bonner, 2009). Brown trout can inhabit a much wider range of hydrological conditions than that covered by our study (Lobón-Cerviá and Sanz, 2017) so it is likely that population-level variation may be much greater than observed here. There was no evidence that the observed population-level differences in Usus were due to body size, but individuals from the upstream populations had shorter pectoral fins relative to their body size, which has previously been associated with higher swimming ability (Rouleau et al., 2010). The upstream–downstream population differences could be due to local adaptation (Garcia de Leaniz et al., 2007) or phenotypic plasticity (Oufiero and Whitlow, 2016). Irrespective of the drivers, the results indicate river managers also need to take population location into account when considering barrier effects and mitigation options.
The extent of intraspecific variation in Usus was unexpected (e.g. 37–172 cm s−1 in brown trout) and highlights the importance of working with the full range of swimming abilities that species exhibit, rather than using mean values. To effectively mitigate barrier impacts, fish passes should aim to provide passage for all individuals (Baras and Lucas, 2001), but using mean swimming speeds as benchmarks would inevitably select against the weakest-swimming individuals. This highlights the need to explicitly consider potential selective pressures of barriers and fish passes on fish communities (e.g. Volpato et al., 2009).
At the intraspecific level, Usus showed a positive association with BL in European minnow, brown trout and topmouth gudgeon. In contrast, Usus was unrelated to BL in stone loach and bullhead, perhaps indicating other traits are more important in benthic species. The positive relationship identified between Usus and AS in European minnow, stone loach and brown trout is consistent with other studies (Reidy et al., 2000; Killen et al., 2012) and shows the importance of considering metabolism in fish passage. The negative relationships we observed between Usus and PFLR in brown trout and European minnow was unexpected as longer pectoral fins have been previously shown to confer better station-holding ability and faster swimming speeds (Arnold et al., 1991; Ojanguren and Braña, 2003). However, our findings are in agreement with Rouleau et al. (2010) who found salmonids with shorter pectoral fins swam faster, possibly because short fins reduce drag. Overall, our results indicate that the drivers of intraspecific variation in swimming speed vary between species and are more complex than simple size-related variation.
The potential use of velocity barriers to prevent passage of invasive fish has been put forward by several studies (Newbold et al., 2016; Rahel and McLaughlin, 2018; Zielinski et al., 2019). Dispersal along river catchments is a major pathway for secondary invasions in topmouth gudgeon (Pinder et al., 2005), but the wide range of Usus observed emphasizes the difficulties in designing effective selective barriers to prohibit their passage. The maximum Usus of topmouth gudgeon was 68 cm s−1, which was above the mean Usus for many of the native taxa. To be effective, selective barriers need to prevent all invasive individuals passing, without disrupting the passage of native species. In this case, using a threshold of > 68 cm s−1 to prevent passage of topmouth gudgeon would clearly impair the passage of native species. Hence, the use of velocity barriers in controlling invasive fish will often be challenging, needs to be carefully considered, and requires detailed knowledge of the full range of swimming performance of both invasive and native species.
We used four different swim tunnels to test Usus in a range of fish sizes to ensure accurate measurement of MRs. While we followed best practice to minimize any potential tunnel size effect (e.g. keeping fish volume:water volume relatively consistent, carefully calibrating current velocities, and correcting for solid blocking effects) we cannot be absolutely sure that the use of different tunnels did not affect swimming behaviour. Unfortunately, controlling for any such effect statistically was not possible as tunnel size was completely confounded by fish body size so this approach would have led to erroneous conclusions. Ultimately, we are confident that we used the best possible approach to simultaneously test Usus and measure MRs across a range of fish sizes.
Longitudinal migrations have been documented in river-resident brown trout (Clapp et al., 1990), bullhead (Knaepkens et al., 2005), European minnow (Nunn et al., 2010) and stone loach (Maerten et al., 2007). These movements are crucial for spawning, foraging, accessing refugia, counteracting downstream displacements in high flows and allowing recolonization of vacant habitat patches following disturbance (Lucas and Baras, 2008). Even where such movements are rare, they are very important to support gene flow between populations (Junker et al., 2012). Free movement is therefore essential to the maintenance of healthy river fish communities, but velocity barriers and ineffective fish passes are disrupting these movements. It is crucial that river managers worldwide base decisions on representative swimming data for the whole target fish community.
Conclusions
Our study shows substantial variability in Usus among species, among populations and among individuals within populations. Swimming speed is a major determinant of passage success (Haro et al., 2004; Castro-Santos, 2006) and migration rates (Eliason et al., 2011). There is a general consensus that traditional methods in fish pass design are failing (Noonan et al., 2012; Kemp, 2016; Birnie-Gauvin et al., 2019), and new approaches are needed. There is a need to move away from a ‘one size fits all’ approach to address natural variability in swimming performance within river fish communities. Barrier removal should always be considered, but in cases where removal is not feasible, we suggest that fish passes affording diverse and spatially heterogeneous flows (e.g. nature-like fish passes; Katopodis et al., 2001; Calles and Greenberg, 2005) offer the option that best embraces the variability in swimming performance existing in natural populations.
Funding
This study was funded by the EC Horizon 2020 Research & Innovation Programme (AMBER Project, grant agreement No. 689682).
Supplementary Material
Acknowledgements
Fish were collected under permit from National Resources Wales (ref. numbers: EP/CW032-H-845/12719/01; EP/CW004-I-558/10598/01; EP/CW004-J-559/10632/01). We thank the staff at Centre for Sustainable Aquatic Research for help with animal husbandry. Thanks also to Jessica Vevers, Edward Miller, Matteo Rolla and Ben Overland who helped with fish collection. All methods were undertaken with approval from Swansea University Animal Ethics Review Board (SU-Ethics-Staff-231017/28; SU-Ethics-Staff-071217/30; SU-Ethics-Staff-061017/18; SU-Ethics-Staff-061017/18; SU-Ethics-Staff-270917/19).
References
- Armstrong G, Apahamian M, Fewings G, Gough P, Reader N, Varallo P (2010) Environment Agency Fish Pass Manual. Environment Agency, Bristol, England [Google Scholar]
- Arnold GP, Webb PW, Holford BH (1991) The role of the pectoral fins in station-holding of Atlantic salmon parr (Salmo salar L.). J Exp Biol 156: 625–629. [Google Scholar]
- Baktoft H, Jacobsen L, Skov C, Koed A, Jepsen N, Berg S, Mikkel B, Aarestrup K, Svendsen JC (2016) Phenotypic variation in metabolism and morphology correlating with animal swimming activity in the wild: relevance for the OCLTT (oxygen- and capacity-limitation of thermal tolerance), allocation and performance models. Conserv Physiol 4: doi:10.1093/conphys/cov055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baras E, Lucas MC (2001) Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: a mechanistic perspective. Ecohydrol Hydrobiol 1: 291–304. [Google Scholar]
- Barton K. (2018) MuMIn: Multi-Model Inference. R package version 1.42.1. https://CRAN.R-project.org/package=MuMIn.
- Bell WH, Terhune LDB (1970) Water tunnel design for fisheries research. Fisheries Research Board Canadian Technical Report 195: 1–69.
- Belletti B, Garcia de Leaniz C, Jones J, Bizzi S, Börger L, Segura G, Castelletti A, van de Bund W, Aarestrup K, Barry J et al. (2020) More than one million barriers fragment Europe’s rivers. Nature, 588: 436–441. [DOI] [PubMed] [Google Scholar]
- Birnie-Gauvin K, Franklin P, Wilkes M, Aarestrup K (2019) Moving beyond fitting fish into equations: progressing the fish passage debate in the Anthropocene. Aquat Conserv 29: 1095–1105. [Google Scholar]
- Boily P, Magnan P (2002) Relationship between individual variation in morphological characters and swimming costs in brook charr (Salvelinus fontinalis) and yellow perch (Perca flavescens). J Exp Biol 205: 1031–1036. [DOI] [PubMed] [Google Scholar]
- Brett JR. (1965) The relation of size to rate of oxygen consumption and sustained swimming speed of sockeye salmon (Oncorhynchus nerka). J Fish Board Canada 22: 1491–1501. [Google Scholar]
- Bunt C, Castro-Santos T, Haro A (2012) Performance of fish passage structures at upstream barriers to migration. River Res Appl 28: 457–478. [Google Scholar]
- Cabonce J, Fernando R, Wang H, Chanson H (2019) Using small triangular baffles to facilitate upstream fish passage in standard box culverts. Environ Fluid Mech 19: 157–179. [Google Scholar]
- Calles EO, Greenberg LA (2005) Evaluation of nature-like fishways for re-establishing connectivity in fragmented salmonid populations in the river Emån. River Res Appl 21: 951–960. [Google Scholar]
- Castro-Santos T. (2005) Optimal swim speeds for traversing velocity barriers: an analysis of volitional high-speed swimming behavior of migratory fishes. J Exp Biol 208: 421–432. [DOI] [PubMed] [Google Scholar]
- Castro-Santos T. (2006) Modelling the effect of varying swim speeds on fish passage through velocity barriers. Trans Am Fish Soc 135: 1230–1237. [Google Scholar]
- Castro-Santos T, Haro A (2010) Fish guidance and passage at barriers. In P Domenici, ed, Fish Locomotion: An Eco-ethological Perspective. Science Publishers, Enfield, NH, pp. 62–89. [Google Scholar]
- Caudill CC, Daigle WR, Keefer ML, Boggs CT, Jepson MA, Burke BJ, Zabel RW, Peery CA (2007) Slow dam passage in adult Columbia River salmonids associated with unsuccessful migration: delayed negative effects of passage obstacles or condition-dependent mortality? Can J Fish Aquat Sci 64: 979–995. [Google Scholar]
- Clapp DF, Clark RD Jr, Diana JS (1990) Range, activity, and habitat of large, free-ranging brown trout in a Michigan stream. Trans Am Fish Soc 119: 1022–1034. [Google Scholar]
- Clay CH. (1995) Design of Fishways and Other Fish Facilities. Lewis Publishers, Boca Raton, FL. [Google Scholar]
- Clough SC, Lee-Elliott IE, Turnpenny AWH, Holden SDJ, Hinks C (2004) Swimming speeds in fish: Phase II. R&D Technical Report W2-049/TR1. Environment Agency, Bristol. [Google Scholar]
- Drucker EG. (1996) The use of gait transition speed in comparative studies of fish locomotion. Am Zool 36: 555–566. [Google Scholar]
- Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. [DOI] [PubMed] [Google Scholar]
- Fagan WF. (2002) Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83: 3243–3249. [Google Scholar]
- Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, Lajus D, Letcher BH et al. (2007) A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biol Rev 82: 173–211. [DOI] [PubMed] [Google Scholar]
- Genz J, Jyde M, Svendsen JC, Steffensen JF, Ramløv H (2013) Excess post-hypoxic oxygen consumption is independent from lactate accumulation in two cyprinid fishes. Comp Biochem Physiol A 165: 54–60. [DOI] [PubMed] [Google Scholar]
- Gerking SD. (1959) The restricted movement of fish populations. Biol Rev 34: 221–242. [Google Scholar]
- Grill G, Lehner B, Thieme M, Greenen B, Tickner D, Antonelli F, Babu S, Borrelli P, Cheng L, Crochetiere H, Macedo H et al. (2019) Mapping the world’s free-flowing rivers. Nature 569: 215–221. [DOI] [PubMed] [Google Scholar]
- Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24: 699–711. [DOI] [PubMed] [Google Scholar]
- Haro A, Castro-Santos T, Noreika J, Odeh M (2004) Swimming performance of upstream migrant fishes in open-channel flow: a new approach to predicting passage through velocity barriers. Can J Fish Aquat Sci 61: 1590–1601. [Google Scholar]
- Januchowski-Hartley SR, McIntyre PB, Diebel M, Doran PJ, Infante DM, Joseph C, Allan JD (2013) Restoring aquatic ecosystem connectivity requires expanding inventories of both dams and road crossings. Front Ecol Environ 11: 211–217. [Google Scholar]
- Jones J, Börger L, Tummers J, Jones P, Lucas M, Kerr J, Kemp P, Bizzi S, Consuegra S (2019) A comprehensive assessment of stream fragmentation in Great Britain Science of the total environment 673: 756–762. [DOI] [PubMed] [Google Scholar]
- Jones P, Closs G (2018) The introduction of brown trout to New Zealand and their impact on native fish communities. In Brown Trout: Biology, Ecology and Management. John Wiley and Sons Ltd, Hoboken, New Jersey, USA, pp. 545–567. [Google Scholar]
- Junker J, Peter A, Wagner CE, Mwaiko S, Germann B, Seehausen O, Keller I (2012) River fragmentation increases localized population genetic structure and enhances asymmetry of dispersal in bullhead (Cottus gobio). Conserv Genet 13: 545–556. [Google Scholar]
- Katopodis C. (1992) Introduction to Fishway Design. Department of Fisheries and Oceans, Winnipeg, Manitoba. [Google Scholar]
- Katopodis C, Kells JA, Acharya M (2001) Nature-like and conventional fishways: alternative concepts? Can Water Resour J 26: 211–232. [Google Scholar]
- Kemp PS. (2016) Meta-analyses, metrics and motivation: mixed messages in the fish passage debate. River Res Appl 32: 2116–2124. [Google Scholar]
- Kemp PS, O'Hanley JR (2010) Procedures for evaluating and prioritising the removal of fish passage barriers: a synthesis. Fish Manag Ecol 17: 297–322. [Google Scholar]
- Killen SS, Marras S, Steffensen JF, McKenzie DJ (2012) Aerobic capacity influences the spatial position of individuals within fish schools. Proc R Soc B 279: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaepkens G, Baekelandt K, Eens M (2005) Assessment of the movement behaviour of the bullhead (Cottus gobio), an endangered European freshwater fish. Anim Biol 55: 219–226. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82: 1–26. [Google Scholar]
- Laborde A, González A, Sanhueza C, Arriagada P, Wilkes M, Habit E, Link O (2016) Hydropower development, riverine connectivity, and non-sport fish species criteria for hydraulic design of fishways. River Res Appl 32: 1949–1957. [Google Scholar]
- Leavy TR, Bonner TH (2009) Relationships among swimming ability, current velocity association, and morphology for freshwater lotic fishes. N Am J Fish 29: 72–83. [Google Scholar]
- Lehner B, Liermann CR, Revenga C, Vörösmarty C, Fekete B, Crouzet P, Döll P, Endejan M, Frenken K, Magome J et al. (2011) High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front Ecol Environ 9: 494–502. [Google Scholar]
- Lenth R. (2019) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.3.01.
- Lobón-Cerviá J, Sanz N (2017) Brown Trout: Biology, Ecology and Management. John Wiley & Sons Ltd. , Hoboken, NJ, USA. [Google Scholar]
- Lucas MC, Baras E (2008) Migration of Freshwater Fishes. John Wiley & Sons Ltd. , London, UK. [Google Scholar]
- Lucas MC, Batley E (1996) Seasonal movements and behaviour of adult barbel Barbus barbus, a riverine cyprinid fish: implications for river management. J Appl Ecol 33: 1345–1358. [Google Scholar]
- Maitland PS. (2006) Guide to Freshwater Fish of Britain and Europe. Hamlyn, London, UK. [Google Scholar]
- Makrakis S, Makrakis MC, Wagner RL, Dias JHP, Gomes LC (2007) Utilization of the fish ladder at the Engenheiro Sergio Motta Dam, Brazil, by long distance migrating potamodromous species. Neotrop Ichthyol 5: 197–204. [Google Scholar]
- Mantel SK, Rivers-Moore N, Ramulifho P (2017) Small dams need consideration in riverscape conservation assessments. Aquat Conserv 27: 748–754. [Google Scholar]
- Maerten E, Eens M, Knaepkens G (2007) Performance of a pool-and-weir fish pass for small bottom-dwelling freshwater fish species in a regulated lowland river. Anim Biol 57: 423–432. [Google Scholar]
- Metcalfe NB, Van Leeuwen TE, Killen SS (2016) Does individual variation in metabolic phenotype predict fish behaviour and performance? J Fish Biol 88: 298–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaña CG, Winemiller KO, Sutton A (2014) Intercontinental comparison of fish ecomorphology: null model tests of community assembly at the patch scale in rivers. Ecol Monogr 84: 91–107. [Google Scholar]
- Morita K, Yamamoto S (2002) Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conserv Biol 16: 1318–1323. [Google Scholar]
- Museth J, Hesthagen T, Sandlund OT, Thorstad EB, Ugedal O (2007) The history of the minnow Phoxinus phoxinus (L.) in Norway: from harmless species to pest. J Fish Biol 71: 184–195. [Google Scholar]
- Newbold LR, Karageorgopoulos P, Kemp PS (2014) Corner and sloped culvert baffles improve the upstream passage of adult European eels (Anguilla anguilla). Ecol Eng 73: 752–759. [Google Scholar]
- Newbold LR, Shi X, Hou Y, Han D, Kemp PS (2016) Swimming performance and behaviour of bighead carp (Hypophthalmichthys nobilis): application to fish passage and exclusion criteria. Ecol Eng 95: 690–698. [Google Scholar]
- Noonan MJ, Grant JWA, Jackson CD (2012) A quantitative assessment of fish passage efficiency. Fish Fish 13: 450–464. [Google Scholar]
- Norin T, Malte H (2011) Repeatability of standard metabolic rate, active metabolic rate and aerobic scope in young brown trout during a period of moderate food availability. J Exp Biol 214: 1668–1675. [DOI] [PubMed] [Google Scholar]
- Nunn AD, Copp GH, Vilizzi L, Carter MG (2010) Seasonal and diel patterns in the migrations of fishes between a river and a floodplain tributary. Ecol Freshw Fish 19: 153–162. [Google Scholar]
- Ojanguren AF, Braña F (2003) Effects of size and morphology on swimming performance in juvenile brown trout (Salmo trutta L.). Ecol Freshw Fish 12: 241–246. [Google Scholar]
- Oufiero CE, Garland T Jr (2009) Repeatability and correlation of swimming performances and size over varying time-scales in the guppy (Poecilia reticulata). Funct Ecol 23: 969–978. [Google Scholar]
- Oufiero CE, Whitlow KR (2016) The evolution of phenotypic plasticity in fish swimming. Curr Zool 62: 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páez DJ, Hedger R, Bernatcher L, Dodson JJ (2008) The morphological plastic response to water current velocity varies with age and sexual state in juvenile Atlantic salmon, Salmo salar. Freshw Biol 53: 1544–1554. [Google Scholar]
- Pakkasmaa S, Piironen J (2001) Morphological differentiation among local trout (Salmo trutta) populations. Biol J Linn Soc 72: 231–239. [Google Scholar]
- Pang X, Shao F, Ding SH, Fu SJ, Zhang YG (2020) Interspecific differences and ecological correlations of energy metabolism traits in freshwater fishes. Funct Ecol 34: 616–630. [Google Scholar]
- Peake SJ. (2004) An evaluation of the use of critical swimming speed for determination of culvert water velocity criteria for smallmouth bass. Trans Am Fish Soc 133: 1472–1479. [Google Scholar]
- Peake SJ. (2008) Gait transition speed as an alternate measure of maximum aerobic capacity in fishes. J Fish Biol 72: 645–655. [Google Scholar]
- Pinder AC, Gozlan RE, Britton JR (2005) Dispersal of the invasive topmouth gudgeon, Pseudorasbora parva in the UK: a vector for an emergent infectious disease. Fish Manag Ecol 12: 411–414. [Google Scholar]
- Poff NL, Allan JD (1995) Functional organization of stream fish assemblages in relation to hydrological variability. Ecology 76: 606–627. [Google Scholar]
- Poulsen SB, Jensen LF, Schulz C, Deacon M, Meyer KE, Jäger-Kleinicke T et al. (2012) Ontogenetic differentiation of swimming performance and behaviour in relation to habitat availability in the endangered North Sea houting (Coregonus oxyrinchus). Aquat Living Resour 25: 241–249. [Google Scholar]
- R Core Team (2019) R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Rahel FJ, McLaughlin RL (2018) Selective fragmentation and the management of fish movement across anthropogenic barriers. Ecol Appl 28: 2066–2081. [DOI] [PubMed] [Google Scholar]
- Reidy SP, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203:347–357. [DOI] [PubMed] [Google Scholar]
- Robinson CV, Garcia de Leaniz C, Consuegra S (2019) Effect of artificial barriers on the distribution of the invasive signal crayfish and Chinese mitten crab. Sci Rep 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe DW, Hinch SG (2010) Effectiveness monitoring of fish passage facilities: historical trends, geographic patterns and future directions. Fish Fish 11: 12–33. [Google Scholar]
- Rouleau S, Glémet H, Magnan P (2010) Effects of morphology on swimming performance in wild and laboratory crosses of brook trout ecotypes. Funct Ecol 24: 310–321. [Google Scholar]
- Sambilay V., Jr (1990) Interrelationships between swimming speed, caudal fin aspect ratio and body length of fishes. Fishbyte 8: 16–20. [Google Scholar]
- Santucci VJ Jr, Gephard SR, Pescitelli SM (2005) Effects of multiple low-head dams on fish, macroinvertebrates, habitat, and water quality in the Fox River, Illinois. N Am J Fish 25: 975–992. [Google Scholar]
- Schoby GP, Keeley ER (2011) Home range size and foraging ecology of bull trout and westslope cutthroat trout in the upper Salmon River Basin, Idaho. Trans Am Fish Soc 140: 636–645. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart TA. (1962) The leaping behaviour of salmon and trout at falls and obstructions. Her Majesty’s Stationery Office, Freshwater and Salmon Fisheries Research Paper 28, Edinburgh.
- Svendsen JC, Banet AI, Christensen RH, Steffensen JF, Aarestrup K (2013) Effects of intraspecific variation in reproductive traits, pectoral fin use and burst swimming on metabolic rates and swimming performance in the Trinidadian guppy (Poecilia reticulata). J Exp Biol 216: 3564–3574. [DOI] [PubMed] [Google Scholar]
- Svendsen MBS, Bushnell PG, Steffensen JF (2016) Design and setup of intermittent-flow respirometry system for aquatic organisms. J Fish Biol 88: 26–50. [DOI] [PubMed] [Google Scholar]
- Taylor EB, McPhail JD (1985) Variation in body morphology among British Columbia populations of coho salmon, Oncorhynchus kisutch. Can J Fish Aquat Sci 42: 2020–2028. [Google Scholar]
- Thiem JD, Dawson JW, Hatin D, Danylchuk AJ, Dumont P, Gleiss AC, Wilson RP, Cooke SJ (2016) Swimming activity and energetic costs of adult lake sturgeon during fishway passage. J Exp Biol 219: 2534–2544. [DOI] [PubMed] [Google Scholar]
- Townsend CR, Crowl TA (1991) Fragmented population structure in a native New Zealand fish: an effect of introduced brown trout? Oikos 61: 347–354. [Google Scholar]
- Tudorache C, Viaene P, Blust R, Vereecken H, De Boeck G (2008) A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol Freshw Fish 17: 284–291. [Google Scholar]
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37: 130–137. [Google Scholar]
- Vitule JRS, Skóra S, Abilhoa V (2012) Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Divers Distrib 18: 111–120. [Google Scholar]
- Volpato G, Barreto R, Marcondes A, Moreira P, Ferreira M (2009) Fish ladders select fish traits on migration–still a growing problem for natural fish populations. Mar Freshwater Behav Physiol 42: 307–313. [Google Scholar]
- Webb PW. (1975) Hydrodynamics and energetics of fish propulsion. Bull Fish Res Board Canada 190: 1–158. [Google Scholar]
- Webster MM, Atton N, Hart PJ, Ward AJ (2011) Habitat-specific morphological variation among threespine sticklebacks (Gasterosteus aculeatus) within a drainage basin. PLoS One 6: e21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel D, Peter A (2013) Effectiveness of different types of block ramps for fish upstream movement. Aquat Sci 75: 251–260. [Google Scholar]
- Williams JG, Armstrong G, Katopodis C, Larinier M, Travade F (2012) Thinking like a fish: a key ingredient for development of effective fish passage facilities at river obstructions. River Res Appl 28: 407–417. [Google Scholar]
- Willis SC, Winemiller KO, Lopez-Fernandez H (2005) Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142: 284–295. [DOI] [PubMed] [Google Scholar]
- Wofford JE, Gresswell RE, Banks MA (2005) Influence of barriers to movement on within-watershed genetic variation of coastal cutthroat trout. Ecol Appl 15: 628–637. [Google Scholar]
- Zielinski DP, McLaughlin R, Castro-Santos T, Paudel B, Hrodey P, Muir A (2019) Alternative sea lamprey barrier technologies: history as a control tool. Rev Fish Sci Aquac 27: 438–457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


